Abstract
Receptor activator of NFκB ligand (RANKL) is mainly known for its role in bone metabolism, constituting a target for therapeutic interventions. Increasing evidence suggests that RANKL is also involved in oncogenesis and tumor progression, including a prominent role in host-tumor interaction. Our data suggest that targeting RANKL may reinforce natural killer (NK) cell-mediated antitumor responses in patients affected by hematological malignancies.
Keywords: leukemia, NK cells, RANK, RANKL
The tumor necrosis factor (TNF) family member receptor activator of NFκB ligand (RANKL) is a key regulator of bone metabolism. The binding of RANKL to its receptor (RANK) stimulates osteoclastogenesis and activates mature osteoclasts, resulting in increased bone resorption. A second RANKL-binding protein, osteoprotegerin (OPG), acts as a soluble decoy receptor. OPG competes with RANK for RANKL binding, thereby preventing its biological effects and acting as an inhibitor of bone resorption. Increased RANKL levels are associated with osteolytic lesions, and the RANKL/RANK/OPG triad is largely involved in both normal and pathological bone metabolism. Based on this prominent role in bone turnover, a RANKL-neutralizing antibody (Denosumab) has been developed and shown to be effective in treatment of non-malignant and malignant osteolysis (reviewed in ref. 1).
Initially, RANKL was described as modulator of immune cell function, as it was found to influence the survival and T cell-stimulatory capacity of dendritic cells (DCs).2,3 More recently, several studies revealed the important role of RANKL in the pathophysiology of malignant diseases. Provided by regulatory T cells, RANKL contributes to metastatic spread of cancer cells.4 Other investigators reported on the expression and pathophysiological involvement of RANKL in hematopoietic malignancies like chronic lymphoid leukemia (CLL) and Multiple Myeloma (MM).5,6 Its counterpart RANK was found to be expressed on NK cells which are central components of innate immunity, but its involvement in NK function remained elusive so far. NK cells play an important role in tumor immunosurveillance, in particular with regards to acute myeloid leukemia (AML), as demonstrated by multiple lines of evidence including data from allogenic stem cell transplantation studies. Driven by these premises, we analyzed the expression and function of RANKL in this disease as well as the involvement of the RANK/RANKL signaling axis in the interaction of AML and NK cells.7
The analysis of primary samples from 78 AML patients revealed substantial expression levels of RANKL on the cell surface in about 70% of investigated cases. Similar to many other members of the TNF family, RANKL itself is capable to transduce signals (reverse signaling), as documented in T cells and CLL cells.5,8 In case of AML cells, RANKL signaling was found to stimulate metabolic activity as well as the release of cytokines that are known to act as growth and survival factors in this disease. The exposure of NK cells to RANKL-elicited factors impaired the antileukemic reactivity of NK cells, as revealed by experiments involving RANKL-negative targets to exclude potential effects of direct RANK/RANKL interactions. In addition, the factors released by AML cells in response to RANKL signaling (the exact nature of which remains to be elucidated) were found to induce RANK on NK cells, and substantially higher expression of RANK was detected on the surface of NK cells from AML patients as compared with those from healthy donors. Finally, we demonstrated that RANK, upon interaction with its counterpart on target cells, directly impairs the antileukemic functions of NK cells.
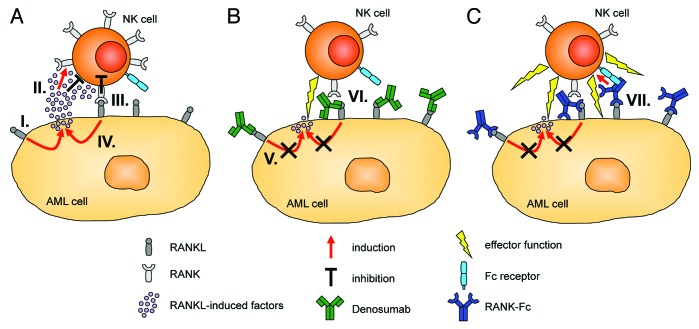
Based on our findings, we hypothesize that RANKL influences the interaction of NK and AML cells by mediating a feedback loop that involves the release of factors by the latter which upregulate RANK on the former. In addition to the immediate inhibitory effects of RANKL-induced factors, RANK is then readily available to interact with RANKL expressed by AML cells. This results in the activation of a bidirectional signal transduction cascade that causes the delivery of RANK-mediated inhibitory signals to NK cells and perpetuates RANKL reverse signaling in AML cells. It is therefore tempting to speculate that RANKL sustains a “vicious cycle” that facilitates the evasion of leukemia cells from NK cell-mediated immunosurveillance (Fig. 1A). In this context, it is worth noting that members of the TNF family such as CD137 ligand may transduce signals in the absence of their cognate receptor.9 It remains to be determined whether this is also the case for RANKL.
Figure 1. Involvement of RANKL in the crosstalk of acute myeloid leukemia (AML) and natural killer (NK) cells and potential points of therapeutic intervention. (A) Receptor activator of NFκB ligand (RANKL) signaling induces the release of immunomodulatory factors by AML cells (I), which directly inhibit NK-cell reactivity and upregulate RANK expression on their cell surface (II). RANK transduces inhibitory signals to NK cells upon interaction with RANKL expressed by AML cells (III), and perpetuates RANKL reverse signaling in the latter (IV). (B) The RANKL-neutralizing antibody Denosumab blocks RANK/RANKL interactions. This reduces the release of RANKL-induced immunomodulatory factors by AML cells and their above described immunomodulatory effects (V). In addition, Denosumab prevents inhibitory RANK signaling into NK cells (VI), which results in enhanced NK cell antitumor reactivity. (C) In contrast to Denosumab, an Fc-optimized RANK-Ig fusion protein not only neutralizes RANKL (signaling), but also potently induces NK cell-mediated antibody-dependent cellular cytotoxicity (ADCC) against RANKL-expressing malignant cells the Fcγ receptor IIIa (VII).
We further demonstrated that the clinically employed RANKL-targeting antibody Denosumab can block both the release of the NK cell-inhibitory factors by AML cells and prevent RANK signaling in NK cells, resulting in enhanced NK cell-mediated antileukemic reactivity (Fig. 1B). Thus, therapeutic targeting of RANKL may serve to reinforce NK-cell effector functions in leukemia patients.
In a very recent study addressing, among others, the expression of RANKL in CLL and MM, we introduced an Fc-optimized RANK-Fc fusion protein for the targeting of RANKL.10 This construct enables the neutralization of RANKL with an efficacy that is similar to that of Denosumab. However, in contrast to Denosumab, an IgG2 antibody that does not promote antibody-dependent cellular cytotoxicity (ADCC), our fusion protein (based on an IgG1 scaffold) also targets the malignant cells for NK cell-reactivity (Fig. 1C). The NK cell-mediated antitumor response elicited by our fusion protein was further enhanced by the introduction of two amino acid substitutions, namely S239D and I332E, in its CH2 domain, resulting in increased affinity for the Fcγ receptor IIIa. A characterization of the efficacy and potential side effects of this construct in vivo is currently ongoing.
The crosstalk of tumor cells with the immune system in general and with NK cells in particular and its consequences for oncogenesis and tumor progression are far from being fully understood. Various members of the TNF/TNFR family appear to participate in this crosstalk, and RANKL seems to play an important role in this setting. A better understanding of the multifaceted role of RANKL in tumor biology could pave the way to rational therapeutic approaches, among others therapeutically strengthening anti-tumor immunity and specifically--as suggested by our data--strengthening the reactivity of NK cells, resulting in better therapeutic options for cancer patients.
Disclosure of Potential Conflicts of Interest
The authors were supported by grants from Deutsche Forschungsgemeinschaft (SA1360/7-1) and Deutsche Krebshilfe (109620).
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23850
References
- 1.Lacey DL, Boyle WJ, Simonet WS, Kostenuik PJ, Dougall WC, Sullivan JK, et al. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11:401–19. doi: 10.1038/nrd3705. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–9. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 3.Wong BR, Josien R, Lee SY, Sauter B, Li HL, Steinman RM, et al. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med. 1997;186:2075–80. doi: 10.1084/jem.186.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470:548–53. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Secchiero P, Corallini F, Barbarotto E, Melloni E, di Iasio MG, Tiribelli M, et al. Role of the RANKL/RANK system in the induction of interleukin-8 (IL-8) in B chronic lymphocytic leukemia (B-CLL) cells. J Cell Physiol. 2006;207:158–64. doi: 10.1002/jcp.20547. [DOI] [PubMed] [Google Scholar]
- 6.Farrugia AN, Atkins GJ, To LB, Pan B, Horvath N, Kostakis P, et al. Receptor activator of nuclear factor-kappaB ligand expression by human myeloma cells mediates osteoclast formation in vitro and correlates with bone destruction in vivo. Cancer Res. 2003;63:5438–45. [PubMed] [Google Scholar]
- 7.Schmiedel BJ, Nuebling T, Steinbacher J, Malinovska A, Wende CM, Azuma M, et al. Receptor activator for NF-κB ligand in acute myeloid leukemia: expression, function, and modulation of NK cell immunosurveillance. J Immunol. 2013;190:821–31. doi: 10.4049/jimmunol.1201792. [DOI] [PubMed] [Google Scholar]
- 8.Chen NJ, Huang MW, Hsieh SL. Enhanced secretion of IFN-gamma by activated Th1 cells occurs via reverse signaling through TNF-related activation-induced cytokine. J Immunol. 2001;166:270–6. doi: 10.4049/jimmunol.166.1.270. [DOI] [PubMed] [Google Scholar]
- 9.Kang YJ, Kim SO, Shimada S, Otsuka M, Seit-Nebi A, Kwon BS, et al. Cell surface 4-1BBL mediates sequential signaling pathways ‘downstream’ of TLR and is required for sustained TNF production in macrophages. Nat Immunol. 2007;8:601–9. doi: 10.1038/ni1471. [DOI] [PubMed] [Google Scholar]
- 10.Schmiedel BJ, Scheible CA, Nuebling T, Kopp HG, Wirths S, Azuma M, et al. RANKL expression, function, and therapeutic targeting in multiple myeloma and chronic lymphocytic leukemia. Cancer Res. 2013;73:683–94. doi: 10.1158/0008-5472.CAN-12-2280. [DOI] [PubMed] [Google Scholar]