Abstract
Dendritic cells (DCs) are professional antigen-presenting cells that comprise several subsets with distinct phenotypes and functions, including inflammatory DCs that appear during inflammation. By analyzing human inflammatory fluids (arthritic synovial fluid and tumor ascites), we have identified the human equivalent of inflammatory DCs.
Keywords: dendritic cells, human, inflammation, macrophages, TH17
Dendritic cells (DCs) are a rare and heterogeneous population of immune cells that is essential for the initiation of immune responses and the maintenance of self-tolerance. In mice, several distinct DC subpopulations are found in steady-state lymphoid organs and peripheral tissues. During inflammation, an additional DC population, termed “inflammatory DCs,” appears. These DCs differentiate in situ from monocytes that are recruited to the site of inflammation.1 Human DCs also comprise several subsets, but an equivalent of mouse inflammatory DCs have remained elusive for a long time. We have recently identified and characterized a novel population of human DCs that is found in inflammatory microenvironments, and we propose that these cells represent the human equivalent of murine inflammatory DCs.
Distinct subsets of DCs have been characterized in human blood, skin and lymphoid organs. Although the ontogeny of human DCs remains poorly understood, it is now clear that these DC subsets differ considerably from widely employed monocyte-derived DCs, which are differentiated in the presence of granulocyte macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4.2 Therefore, although it has been known for many years that monocytes have the potential to differentiate into DCs in vitro, the in vivo counterpart of human monocyte-derived DCs remained elusive.
In order to identify potential inflammatory DCs in humans, we analyzed myeloid cell populations in two different inflammatory microenvironments: the synovial fluid of rheumatoid arthritis patients and inflammatory tumor ascites from breast and ovarian cancer patients.3 In both series of samples, we detected cells expressing markers commonly found on antigen presenting cells (i.e., CD11c and MHC class II molecules). These cells could be divided into two main populations: CD16+BDCA1− cells, which exhibited features typical of macrophages (i.e., a vacuolar morphology and poor T-cell stimulatory functions) and CD16-BDCA1+ cells, which displayed characteristics typical of DCs (i.e., a dendritic morphology and robust T-cell stimulatory capacities) (Fig. 1A). This population of DCs expressed surface markers that differed from those of circulating DCs and DCs found in steady-state lymphoid organs. Of note, inflammatory DCs expressed surface molecules commonly considered as macrophage markers, like CD14 and the mannose receptor (CD206).
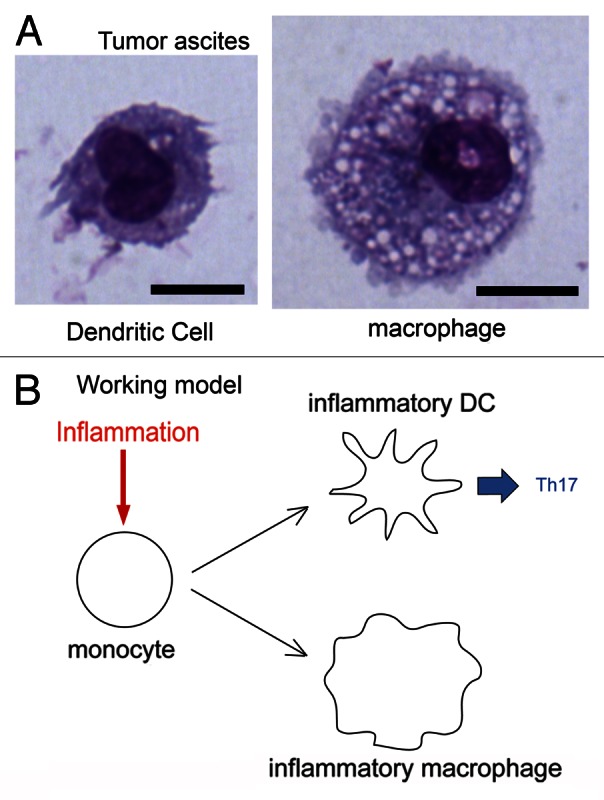
Figure 1. Identification of human inflammatory dendritic cells. (A) Human tumor ascites contain two populations of antigen-presenting cells: dendritic cells (DCs) and macrophages. Giemsa/May–Grünwald staining. Bar = 10 μm. (B) Like their murine counterparts, human inflammatory DCs differentiate from monocytes that are recruited by inflammatory microenvironments. These DCs induce TH17 responses, whereas inflammatory macrophages do not.
To address whether these DCs represented a distinct DC subset or an activated form of conventional DCs, we used Affymetrix microarrays and we compared the transcriptional profiles of DCs and macrophages purified from 5 inflammatory ascites to those of CD14+ and CD16+ cell monocytic populations and BDCA1+ DCs purified from the blood of four healthy individuals. This transcriptomic analysis revealed that inflammatory DCs represent a distinct DC subtype exhibiting molecular features of both conventional DCs and inflammatory macrophages. Interestingly, inflammatory DCs expressed transcription factors involved in DC differentiation, including ZBTB46, which has recently been shown to be specific of the DC lineage in both mice and humans.4
To investigate whether inflammatory DCs are the in vivo equivalents of monocyte-derived DCs, we designed a two-step strategy. First, we identified gene signatures for human macrophages, BDCA1+ DCs, circulating CD16+ or CD14+ monocytes, and monocyte-derived DCs generated in vitro using different publicly available human gene expression data sets.2,5 Then, we analyzed the transcriptomic profiles of the 5 cell populations that we had isolated for the expression of these genetic signatures. Gene set enrichment analysis revealed that inflammatory DCs are specifically enriched in the monocyte-derived DC gene signature and are therefore most likely derived from monocytes rather than from DC precursors.
Finally, we analyzed the functional properties of inflammatory DCs. When cultured with allogeneic naive CD4+ T cells, inflammatory DCs, but not inflammatory macrophages, potently stimulated TH17 responses. TH17 polarization could be inhibited by means of antibodies blocking transforming growth factor β (TGFβ) IL-6, IL-1β and IL-23. Of note, both inflammatory DCs and macrophages secreted IL-6 and IL-1β, but only the former secreted IL-23.
We propose that inflammatory DCs are the human equivalents of murine monocyte-derived inflammatory DCs and are involved in the initiation and maintenance of TH17 responses (Fig. 1B). TH17 cells have been implicated in the pathogenesis of several autoimmune or inflammatory diseases, including rheumatoid arthritis, multiple sclerosis, asthma, inflammatory bowel disease and psoriasis,6 as well as in tumor-associated inflammation.7 Our study shows that inflammatory DCs are the main inducers of TH17 cells in arthritic joints and possibly in other inflammatory settings. Given that IL-23 signaling has recently been shown to promote tumor growth,8 inflammatory DCs could represent viable targets for antitumor immunotherapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23851
References
- 1.León B, López-Bravo M, Ardavín C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007;26:519–31. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 2.Robbins SH, Walzer T, Dembélé D, Thibault C, Defays A, Bessou G, et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008;9:R17. doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segura E, Touzot M, Bohineust A, Cappuccio A, Chiocchia G, Hosmalin A, et al. Human inflammatory dendritic cells induce th17 cell differentiation. Immunity. 2013;38:336–48. doi: 10.1016/j.immuni.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 4.Satpathy AT, Kc W, Albring JC, Edelson BT, Kretzer NM, Bhattacharya D, et al. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med. 2012;209:1135–52. doi: 10.1084/jem.20120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crozat K, Guiton R, Contreras V, Feuillet V, Dutertre CA, Ventre E, et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207:1283–92. doi: 10.1084/jem.20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu Y, Shen F, Crellin NK, Ouyang W. The IL-17 pathway as a major therapeutic target in autoimmune diseases. Ann N Y Acad Sci. 2011;1217:60–76. doi: 10.1111/j.1749-6632.2010.05825.x. [DOI] [PubMed] [Google Scholar]
- 7.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–8. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]


