Abstract
The unfolded protein response (UPR) has been established as a cell-intrinsic mechanism of survival for malignant cells facing microenvironmental stressors. Recent evidence indicates that the UPR also modulates antitumor immunity. Here, we discuss the bi-faced role of the UPR as it both promotes and antagonizes antitumor T-cell immunity.
Keywords: T cell immunity, antigen presentation, immune surveillance, tumor ploidy, tumor unfolded protein response
The immunosurveillance hypothesis posits that tumor cells are controlled by the immune system based on the recognition by T cells of tumor-associated antigens (TAAs) presented in the context of MHC molecules. Operationally, this requires the uptake of malignant cells (or their debris) by host antigen-presenting cells (APC), such as macrophages and dendritic cells, leading to the generation of TAA-specific T cells that have the capacity of eliminating tumor cells. While autochthonous and vaccine-induced T-cell responses can be generated in vivo and ex vivo, multiple lines of preclinical and clinical evidence indicate that tumor cells normally escape antitumor immunity.1 Key effectors of such an escape are tumor-infiltrating APCs—predominantly macrophages and dendritic cells—which are recruited and aberrantly polarized by neoplastic cells. Recent evidence suggests these tumor-educated myeloid cells exhibit a mixed pro-inflammatory/immunosuppressive phenotype and are able to provoke tumorigenic inflammation, as they secrete cytokines including interleukin (IL)-6, IL-23 and tumor necrosis factor α (TNFα), while concomitantly suppressing the generation of effective antitumor T-cell immunity.2 T cells activated within the tumor microenvironment do not readily proliferate, fail to eliminate malignant cells, suppress the activity of TAA-specific cytotoxic T cells and may sustain tumorigenic inflammation.3 However, the tumor cell-derived signals that deregulate the functions of tumor-infiltrating myeloid cells, and ultimately of T cells, remain to be fully elucidated.
The tumor microenvironment harbors various noxae including hypoxia and low nutrient availability, which elicit an endoplasmic reticulum (ER) stress. To cope with these conditions, tumor cells activate a conserved set of adaptive intracellular signaling pathways collectively known as the unfolded protein response (UPR). Under conditions of ER stress, the chaperone molecule glucose-regulated protein, 78 kDa (GRP78) disassociates from three sensors located at the ER membrane, namely inositol requiring enzyme 1α (IRE1α), activating transcription factor 6 (ATF6) and PKR-related ER kinase (PERK), inducing their activation. The signaling cascades elicited by these sensors ameliorate ER stress via several mechanisms, including a selective inhibition of protein translation as well as the upregulation of enzymes that assist protein folding, maturation and degradation. Depending on the strength and duration of ER stress, the UPR can also promote apoptosis.4
The UPR is activated in tumor cells of diverse histological origin, but not in peritumoral areas, and has been demonstrated to constitute a cell-intrinsic tumorigenic signaling pathway that correlates with tumor progression as it promotes the survival and proliferation of malignant cells, angiogenesis, tumorigenic inflammation and chemoresistance.5 In addition, UPR-associated signals in tumor cells and APCs have been shown to impinge upon antigen presentation and host antitumor immune responses. Early studies demonstrated that GRP78-deficient fibrosarcoma cells evoke a more robust memory T-cell response, resulting in rejection of poorly immunogenic tumor cells. Mouse thymoma cells experiencing palmitate- or glucose deprivation-induced ER stress exhibit a decreased presentation of transgenic ovalbumin on MHC class I molecules. We have shown that the induction of the UPR in lymphoma cells is associated with the transcriptional downregulation of tapasin, a chaperone molecule involved in the quality control of MHC class I/peptide complexes in the ER (reviewed in ref. 2). Lastly, recent results indicate that IRE1α-transduced signals upregulate miR-346, in turn downregulating antigen peptide transporter 1 (TAP1) and hence ostensibly decreasing MHC class I-associated antigen presentation.6 These findings suggest that cancer cells and APCs that experience ER stress are subjected to a remodeling of the antigen-processing machinery, yielding decreased presentation of high affinity immunodominant peptides.
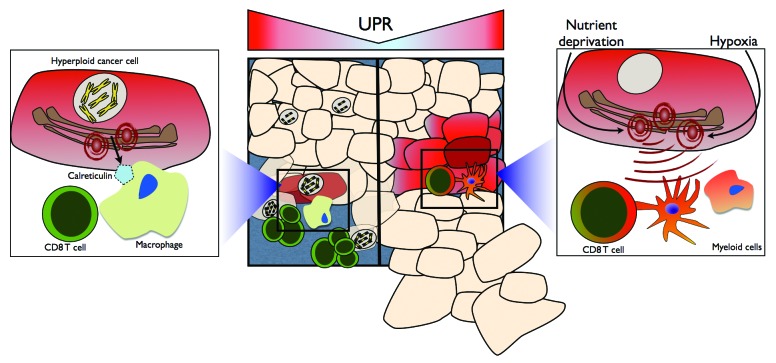
Recent reports have begun to elucidate the mechanisms whereby the UPR modulates antitumor immunity. For instance, hyperploid cancer cells translocate the ER chaperone calreticulin to the cell surface in a UPR-dependent manner, promoting their phagocytosis by APCs and ultimately initiating a hyperploid cell-specific immune response.7 In addition, the UPR may exert a novel cell-extrinsic role that synthesizes its functions in tumorigenic inflammation and aberrant antigen presentation, ultimately resulting in the subversion of antitumor immunity. This paradigm finds support in the demonstration that the ER stress is transmissible from malignant to myeloid cells, including macrophages and dendritic cells.8 Thus, transmissible ER stress (TERS)-imprinted myeloid cells display a mature, pro-inflammatory/immunosuppressive phenotype as they secrete IL-6, IL-23 and TNFα and upregulate the immunosuppressive enzyme arginase.8 TERS-imprinted dendritic cells exhibit a decreased ability to cross-present high-affinity antigens, and fail to efficiently cross-prime antigen-specific CD8+ T cells, resulting in T-cell activation in the absence of proliferation. Importantly, TERS-imprinted dendritic cells appear to suppress the cross-priming ability of normal bystander dendritic cells in an antigen-independent manner. CD8+ T cells cross-primed by TERS-imprinted dendritic cells upregulate FOXP3, CD25 and IL-2, and exhibit a decreased cytotoxic activity (ref. 9 and unpublished data). A similar T-cell immunosuppressive phenotype has recently been described in colon cancer patients as well as in experimental models of colon cancer.10 Finally, TERS-imprinted dendritic cells promote the growth of immunogenic tumor cells in vivo.
The role of the UPR is expanding from that of a merely cell-intrinsic homeostatic mechanism to that of a process that regulates tumor growth in both cell-intrinsic and cell-extrinsic manners. Not only does the UPR aid tumor cells in surviving ER stress-inducing stimuli, such as hypoxia, nutrient deprivation and chemo/radiotherapy, but it also may play a Janus-faced role in antitumor immunity, favoring at once the immunosurveillance of hyperploid cells and tumorigenic inflammation. In this latter scenario, the UPR appears indeed to promote the polarization of tumor-infiltrating myeloid cells toward an inflammatory/immunosuppressive phenotype, leading to T-cell dysfunction and, ultimately, to the evasion of tumor cells from immunosurveillance (Fig. 1). Future work is needed to characterize the precise role of the UPR in different compartments of the tumor microenvironment in vivo, further define the phenotype of CD4+ and CD8+ T cells that develop in the presence of UPR-experiencing malignant cells, and evaluate the prognostic and diagnostic value of cancer-associated UPR in patients.
Figure 1. The bi-faced role of the unfolded protein response on antitumor T-cell immunity. Hyperploid cancer cells are capable of inducing an antitumor immune response via the unfolded protein response (UPR)-dependent translocation of calreticulin to the cell surface. Cell surface calreticulin promotes macrophage-mediated phagocytosis, ultimately leading to the selective elimination of hyperploid cancer cells by CD8+ T cells (left). UPR-experiencing malignant cells polarize tumor-infiltrating myeloid cells toward a pro-inflammatory/immunosuppressive phenotype characterized by inefficient antigen presentation and CD8+ T-cell cross-priming, ultimately derailing antitumor T-cell immunity and facilitating tumor outgrowth.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23901
References
- 1.Klebanoff CA, Acquavella N, Yu Z, Restifo NP. Therapeutic cancer vaccines: are we there yet? Immunol Rev. 2011;239:27–44. doi: 10.1111/j.1600-065X.2010.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahadevan NR, Zanetti M. Tumor stress inside out: cell-extrinsic effects of the unfolded protein response in tumor cells modulate the immunological landscape of the tumor microenvironment. J Immunol. 2011;187:4403–9. doi: 10.4049/jimmunol.1101531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blatner NR, Mulcahy MF, Dennis KL, Scholtens D, Bentrem DJ, Phillips JD, et al. Expression of RORγt marks a pathogenic regulatory T cell subset in human colon cancer. Sci Transl Med. 2012;4:ra159. doi: 10.1126/scitranslmed.3004566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–6. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 5.Luo B, Lee AS. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene. 2013;32:805–18. doi: 10.1038/onc.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartoszewski R, Brewer JW, Rab A, Crossman DK, Bartoszewska S, Kapoor N, et al. The unfolded protein response (UPR)-activated transcription factor X-box-binding protein 1 (XBP1) induces microRNA-346 expression that targets the human antigen peptide transporter 1 (TAP1) mRNA and governs immune regulatory genes. J Biol Chem. 2011;286:41862–70. doi: 10.1074/jbc.M111.304956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Senovilla L, Vitale I, Martins I, Tailler M, Pailleret C, Michaud M, et al. An immunosurveillance mechanism controls cancer cell ploidy. Science. 2012;337:1678–84. doi: 10.1126/science.1224922. [DOI] [PubMed] [Google Scholar]
- 8.Mahadevan NR, Rodvold J, Sepulveda H, Rossi S, Drew AF, Zanetti M. Transmission of endoplasmic reticulum stress and pro-inflammation from tumor cells to myeloid cells. Proc Natl Acad Sci U S A. 2011;108:6561–6. doi: 10.1073/pnas.1008942108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahadevan NR, Anufreichik V, Rodvold JJ, Chiu KT, Sepulveda H, Zanetti M. Cell-extrinsic effects of tumor ER stress imprint myeloid dendritic cells and impair CD8⁺ T cell priming. PLoS One. 2012;7:e51845. doi: 10.1371/journal.pone.0051845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–8. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]