Abstract
Epidermal growth factor receptor (EGFR) expression levels appear to modulate the efficacy of EGFR-targeting monoclonal antibodies. More specifically, we observed that high EGFR densities negatively affect the effects of EGFR-specific antibodies on EGFR phosphorylation yet exacerbate Fc-mediated tumor-cell killing. These results suggest that the predominant mode of action of EGFR-targeting antibodies depend on EGFR expression levels.
Keywords: antibody, epidermal growth factor receptor, expression levels
In 2004, the first monoclonal antibody targeting the epidermal growth factor receptor (EGFR), cetuximab (a chimeric IgG1 also known as Erbitux®, from Merck-Serono S.A.), was approved by the U.S. Food and Drug Administration (FDA) for the therapy of metastatic colorectal carcinoma (mCRC). Two years later, the U.S. FDA extended the approved indications of cetuximab to squamous cell cancer of the head and neck (SCCHN). In addition to cetuximab, a second EGFR-targeting monoclonal antibody, panitumumab (a human IgG2 also known as Vectibix®, from Amgen Inc.), has been approved for the therapy of mCRC. However, although both these EGFR-targeting antibodies modestly prolong the survival rates of patients, complete remissions are rarely achieved. Therefore, several approaches are under investigation to identify biomarkers that may allow for the prediction of the clinical efficacy of EGFR-targeting anticancer strategies and hence for a more precise selection of patients that may truly benefit from these therapeutics.1
Many studies have linked the clinical response of cancer patients to EGFR-targeting antibodies with the expression level of EGFR ligands, mutations in the downstream mediators of the EGFR signaling cascade as well as with polymorphisms of EGFR or the Fc γ receptors (FcγRs) IIa (131H/R) and IIIa (158V/F). Oncogenic mutations in the gene coding for the GTPase v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) have been unravelled as the most prevalent factors underpinning the resistance of mCRC patients to EGFR-targeting antibodies. Accordingly, patients bearing KRAS-mutated CRC are currently excluded from EGFR-directed antibody-based therapies.1
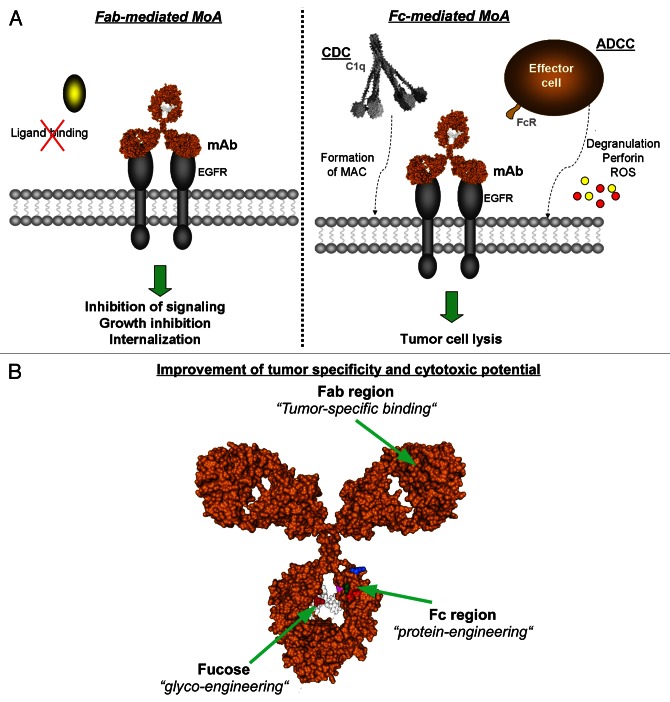
Interestingly, a recent retrospective analysis of a randomized, multicenter, phase III study of cetuximab in combination with cisplatin/vinorelbine (CV) vs. CV alone in the first-line treatment of patients with advanced non-small cell lung cancer (NSCLC) (FLEX study) has demonstrated a positive correlation between the clinical response to cetuximab and EGFR expression levels, as determined by quantitative immunohistochemistry.2 These observations have led to a controversial discussion about the impact of EGFR expression levels on the efficacy of EGFR-targeting antibodies, in the setting of NSCLC as well as in other clinically-relevant scenarios such as CRC and SCCHN.3 In this context, our group initiated a systematic analysis to unravel how different expression levels of EGFR at the cell surface affect the mode of action of EGFR-specific antibodies (Fig. 1A). Importantly, a positive correlation was observed between EGFR expression levels and the Fc-dependent antineoplastic effects of EGFR-targeting antibodies, including antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). Conversely, the Fab-dependent effects of EGFR-specific antibodies such as the inhibition of ligand-induced receptor phosphorylation negatively correlated with EGFR expression levels.4 Based on these data, it may be hypothesized that the Fc-dependent anticancer activity of EGFR-targeting antibodies is predominant in tumors expressing high EGFR levels, provided that neoplastic lesions are accessible for effector cells and/or the complement system, whereas Fab-mediated effect may be most relevant in tumors that express reduced EGFR levels. As oncogenic KRAS signaling downregulates the expression of EGFR on the cell surface, oncogenic KRAS mutations not only interfere with the cancer cell-intrinsic effects of EGFR-targeting antibodies but also are expected to limit their Fc-dependent antineoplastic effects.5 Furthermore, data from the Human Protein Atlas (www.proteinatlas.org/ENSG00000146648/normal) and own unpublished observations indicate that EGFR is expressed to lower levels in the normal colon than in the lung. Taken together, these observations may explain why KRAS mutations are associated with resistance to EGFR-targeting antibodies in CRC but not in NSCLC patients.
Figure 1. EGFR expression levels affect the mode of action of EGFR-targeting monoclonal antibodies. (A) Modes of action of EGFR-targeting antibodies in tumor therapy. Epidermal growth factor receptor (EGFR) targeting monoclonal antibodies are able to elicit distinct effector mechanisms leading to tumor cell destruction. Fab-mediated effects (left panel) include the inhibition of ligand binding, and hence of proliferation, the induction of apoptosis as well as EGFR internalization. Fc-mediated mechanisms (right panel) are triggered as the Fc region binds either the complement component C1q to induce complement-dependent cytotoxicity (CDC), or Fc receptors on effector cells to trigger antibody-dependent cell-mediated cytotoxicity (ADCC). (B) Structural model of human IgG1. Amino acid substitutions and fucose residues in heavy chains are highlighted. Image generated with the 3D-molecule viewer package of NTI Vector (Life Technologies). MAC, membrane attack complex. Pdb file from Clark, MR, Chem Immunol 1997; 65:88–110.
In order to improve the modest efficacy of EGFR-directed antibodies, many approaches have been developed (Fig. 1B).1 Among these, improving the binding affinity of the Fc portion of IgG1 molecules for FcγRIIIa by glycosylation or protein engineering has been shown to successfully enhance ADCC as mediated by NK cells, irrespective of KRAS mutational status. In line with this notion, a glyco-engineered EGFR-targeting antibody has demonstrated some clinical efficacy in a Phase I clinical study.6 However, enhancing the affinity of EGFR-directed antibodies for FcγRIII (CD16) limits the recruitment of polymorphonuclear leukocytes (PMNs) as effector cells. Myeloid cells—like monocytes/macrophages and PMNs—are the predominant effector cell population for the human IgG2 antibody panitumumab. The recruitment of myeloid cells can be improved by employing EGFR-targeting antibodies of the IgA isotype.1 In order to recruit T cells as the main effectors of EGFR-directed monoclonal antibodies, EGFR- and CD3-targeting molecules, so-called bispecific T-cell-engagers (BiTes), have been generated, resulting in significant antineoplastic effects in mouse xenograft models.7 Besides engineering approaches, combination strategies involving antibodies that target non-overlapping epitopes in the extracellular domain III of EGFR have been investigated. Thus, combinations of two non cross-blocking EGFR-directed monoclonal antibodies have been demonstrated to initiate CDC against tumor cells in vitro, while the same effect was not observed in the presence of single EGFR-directed antibodies.1 Of note, this concept has also been successfully demonstrated to enhance tumor growth inhibition by EGFR-targeting antibodies in vitro and in vivo, and has already entered a Phase II clinical trial.8
Since EGFR is also expressed by non-malignant, epithelial tissues (www.proteinatlas.org/ENSG00000146648/normal), enhancing the efficacy of EGFR-targeting antibodies may be accompanied by increased toxicity. For example, high EGFR expression levels in the skin have been associated with skin rashes, the most common side-effect of EGFR-directed antibody therapy. That is why antibodies have been developed that exclusively target tumor-specific EGFR epitopes, such as the EGFR variant III (EGFRvIII). Interestingly, a tumor-specific increase in cytotoxicity was observed when EGFRvIII- and EGFR-directed antibodies were combined, which was further enhanced by Fc protein-engineering.9 Alternatively, the selectivity of these antibodies for cancer cells can be enhanced by the masking of antigen-binding sites with peptides that are specifically cleaved off by tumor-specific proteases.10
To conclude, the expression level of EGFR on the surface of cancer cells may be connected to distinct modes of action of EGFR-targeting antibodies and hence may cause significant differences in their clinical efficacy. The major limitations of EGFR-directed antibody therapy may be overcome by novel promising approaches that improve the specificity of these agents for tumor cells while increasing their capacity to recruit effector cells and the complement system. This said, a full understanding of the interplay between EGFR expression levels and the clinical efficacy of therapeutic EGFR-targeting monoclonal antibodies has not yet been reached.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24052
References
- 1.Peipp M, Dechant M, Valerius T. Sensitivity and resistance to EGF-R inhibitors: approaches to enhance the efficacy of EGF-R antibodies. MAbs. 2009;1:590–9. doi: 10.4161/mabs.1.6.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pirker R, Pereira JR, von Pawel J, Krzakowski M, Ramlau R, Park K, et al. EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the phase 3 FLEX study. Lancet Oncol. 2012;13:33–42. doi: 10.1016/S1470-2045(11)70318-7. [DOI] [PubMed] [Google Scholar]
- 3.Licitra L, Störkel S, Kerr KM, Van Cutsem E, Pirker R, Hirsch FR, et al. Predictive value of epidermal growth factor receptor expression for first-line chemotherapy plus cetuximab in patients with head and neck and colorectal cancer: Analysis of data from the EXTREME and CRYSTAL studies. Eur J Cancer. 2012 doi: 10.1016/j.ejca.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Derer S, Bauer P, Lohse S, Scheel AH, Berger S, Kellner C, et al. Impact of epidermal growth factor receptor (EGFR) cell surface expression levels on effector mechanisms of EGFR antibodies. J Immunol. 2012;189:5230–9. doi: 10.4049/jimmunol.1202037. [DOI] [PubMed] [Google Scholar]
- 5.Derer S, Berger S, Schlaeth M, Schneider-Merck T, Klausz K, Lohse S, et al. Oncogenic KRAS impairs EGFR antibodies’ efficiency by C/EBPβ-dependent suppression of EGFR expression. Neoplasia. 2012;14:190–205. doi: 10.1593/neo.111636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paz-Ares LG, Gomez-Roca C, Delord JP, Cervantes A, Markman B, Corral J, et al. Phase I pharmacokinetic and pharmacodynamic dose-escalation study of RG7160 (GA201), the first glycoengineered monoclonal antibody against the epidermal growth factor receptor, in patients with advanced solid tumors. J Clin Oncol. 2011;29:3783–90. doi: 10.1200/JCO.2011.34.8888. [DOI] [PubMed] [Google Scholar]
- 7.Lutterbuese R, Raum T, Kischel R, Hoffmann P, Mangold S, Rattel B, et al. T cell-engaging BiTE antibodies specific for EGFR potently eliminate KRAS- and BRAF-mutated colorectal cancer cells. Proc Natl Acad Sci U S A. 2010;107:12605–10. doi: 10.1073/pnas.1000976107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koefoed K, Steinaa L, Søderberg JN, Kjær I, Jacobsen HJ, Meijer PJ, et al. Rational identification of an optimal antibody mixture for targeting the epidermal growth factor receptor. MAbs. 2011;3:584–95. doi: 10.4161/mabs.3.6.17955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klausz K, Berger S, Lammerts van Bueren JJ, Derer S, Lohse S, Dechant M, et al. Complement-mediated tumor-specific cell lysis by antibody combinations targeting epidermal growth factor receptor (EGFR) and its variant III (EGFRvIII) Cancer Sci. 2011;102:1761–8. doi: 10.1111/j.1349-7006.2011.02019.x. [DOI] [PubMed] [Google Scholar]
- 10.Donaldson JM, Kari C, Fragoso RC, Rodeck U, Williams JC. Design and development of masked therapeutic antibodies to limit off-target effects: application to anti-EGFR antibodies. Cancer Biol Ther. 2009;8:2147–52. doi: 10.4161/cbt.8.22.9765. [DOI] [PMC free article] [PubMed] [Google Scholar]