Abstract
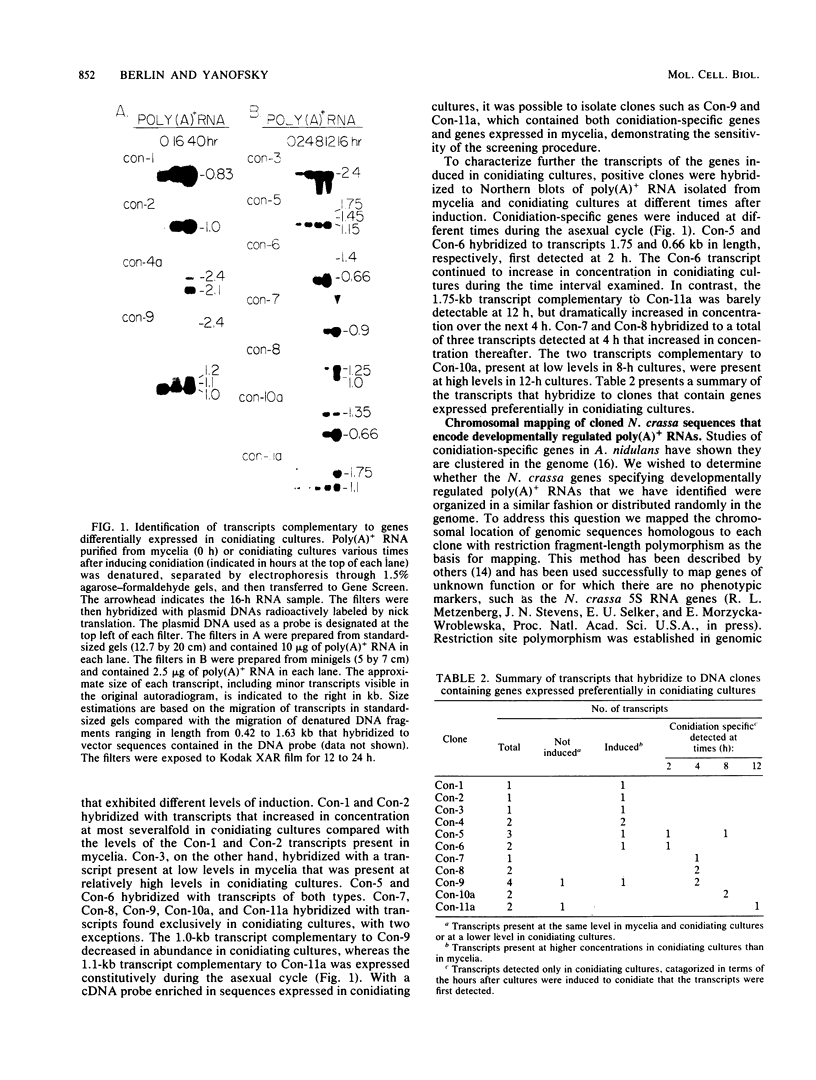
A Neurospora crassa genomic DNA library was screened with a cDNA probe enriched in sequences expressed in conidiating cultures. Clones were isolated that preferentially hybridized to this probe versus a second cDNA probe complementary to polyadenylated RNA isolated from mycelia. Twelve clones contained unique sequences that hybridized to 22 transcripts, 19 of which accumulated preferentially in conidiating cultures. Eight transcripts were present in higher levels in conidiating cultures than in mycelia. Eleven transcripts were detected only in conidiating cultures and first appeared at different times during the asexual cycle. We mapped genomic sequences homologous to the 11 clones by conventional crosses using restriction fragment-length polymorphisms as genetic markers. The sequences homologous to genes expressed preferentially in conidiating cultures are distributed on six of the seven chromosomes. Clones that map to the same chromosome are linked. No recombination occurred between genomic sequences homologous to three clones, suggesting that the genes contained in these clones may constitute a gene cluster.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berlin V., Yanofsky C. Protein changes during the asexual cycle of Neurospora crassa. Mol Cell Biol. 1985 Apr;5(4):839–848. doi: 10.1128/mcb.5.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M., Pelham H. R. Expression of a Drosophila heat-shock protein in Xenopus oocytes: conserved and divergent regulatory signals. EMBO J. 1982;1(12):1583–1588. doi: 10.1002/j.1460-2075.1982.tb01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunke K. J., Anthony J. G., Sternberg E. J., Weeks D. P. Repeated consensus sequence and pseudopromoters in the four coordinately regulated tubulin genes of Chlamydomonas reinhardi. Mol Cell Biol. 1984 Jun;4(6):1115–1124. doi: 10.1128/mcb.4.6.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Enquist L. W., Madden M. J., Schiop-Stanley P., Vande Woude G. F. Cloning of herpes simplex type 1 DNA fragments in a bacteriophage lambda vector. Science. 1979 Feb 9;203(4380):541–544. doi: 10.1126/science.216076. [DOI] [PubMed] [Google Scholar]
- Garnjobst L., Tatum E. L. A survey of new morphological mutants in Neurospora crassa. Genetics. 1967 Nov;57(3):579–604. doi: 10.1093/genetics/57.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hinnebusch A. G., Fink G. R. Repeated DNA sequences upstream from HIS1 also occur at several other co-regulated genes in Saccharomyces cerevisiae. J Biol Chem. 1983 Apr 25;258(8):5238–5247. [PubMed] [Google Scholar]
- Jones C. W., Kafatos F. C. Structure, organization and evolution of developmentally regulated chorion genes in a silkmoth. Cell. 1980 Dec;22(3):855–867. doi: 10.1016/0092-8674(80)90562-0. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Matsuyama S. S., Nelson R. E., Siegel R. W. Mutations specifically blocking differentiation of macroconidia Neurospora crassa. Dev Biol. 1974 Dec;41(2):278–287. doi: 10.1016/0012-1606(74)90306-6. [DOI] [PubMed] [Google Scholar]
- Mitchell M. B., Mitchell H. K. A PARTIAL MAP OF LINKAGE GROUP D IN NEUROSPORA CRASSA. Proc Natl Acad Sci U S A. 1954 Jun;40(6):436–440. doi: 10.1073/pnas.40.6.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr W. C., Timberlake W. E. Clustering of spore-specific genes in Aspergillus nidulans. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5976–5980. doi: 10.1073/pnas.79.19.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Bienz M. A synthetic heat-shock promoter element confers heat-inducibility on the herpes simplex virus thymidine kinase gene. EMBO J. 1982;1(11):1473–1477. doi: 10.1002/j.1460-2075.1982.tb01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins D. D., Radford A., Newmeyer D., Björkman M. Chromosomal loci of Neurospora crassa. Microbiol Rev. 1982 Dec;46(4):426–570. doi: 10.1128/mr.46.4.426-570.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert W. R., Patel V. B., Giles N. H. Genetic regulation of the qa gene cluster of Neurospora crassa: induction of qa messenger ribonucleic acid and dependency on qa-1 function. Mol Cell Biol. 1981 Sep;1(9):829–835. doi: 10.1128/mcb.1.9.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schechtman M. G., Yanofsky C. Structure of the trifunctional trp-1 gene from Neurospora crassa and its aberrant expression in Escherichia coli. J Mol Appl Genet. 1983;2(1):83–99. [PubMed] [Google Scholar]
- Selitrennikoff C. P., Nelson R. E., Siegel R. W. Phase-specific genes for macroconidiation in Neurospora crassa. Genetics. 1974 Oct;78(2):679–690. doi: 10.1093/genetics/78.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Struhl K. The new yeast genetics. 1983 Sep 29-Oct 5Nature. 305(5933):391–397. doi: 10.1038/305391a0. [DOI] [PubMed] [Google Scholar]
- Terenzi H. F., Reissig J. L. Modifiers of the cot gene in Neurospora: the gulliver mutants. Genetics. 1967 Jun;56(2):321–329. doi: 10.1093/genetics/56.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timberlake W. E. Developmental gene regulation in Aspergillus nidulans. Dev Biol. 1980 Aug;78(2):497–510. doi: 10.1016/0012-1606(80)90349-8. [DOI] [PubMed] [Google Scholar]
- Zimmermann C. R., Orr W. C., Leclerc R. F., Barnard E. C., Timberlake W. E. Molecular cloning and selection of genes regulated in Aspergillus development. Cell. 1980 Oct;21(3):709–715. doi: 10.1016/0092-8674(80)90434-1. [DOI] [PubMed] [Google Scholar]