Abstract
Myeloid-derived suppressor cells (MDSC) from mice bearing bone metastases differentiate into functional osteoclasts in vitro and in vivo, through a signaling pathway that relies on nitric oxide. In addition, MDSC-targeting drugs have been shown to robustly inhibit osteolysis. Thus, MDSC stand out as novel osteoclast progenitors and hence as candidate targets for the control of osteolytic bone disease.
Keywords: bone metastasis, breast cancer, myeloid-derived suppressor cells, osteoclasts
Myeloid-derived suppressor cells (MDSC) constitute a major immunosuppressive cell population that has been correlated with poor prognosis in multiple neoplasms. MDSC are a heterogeneous population of myeloid precursors that fail to undergo maturation. MDSC exert immunosuppressive effects through a variety of mechanisms, many of which are mediated by the upregulation of arginase 1 and production of reactive oxygen and nitrogen species (ROS and RNS, respectively).1
In healthy individuals, MDSC constitute about 0.5% of peripheral blood mononuclear cells. In patients at early stages of neoplastic diseases, this proportion can increase by more than 5-fold.2 About 30% of cells in the bone marrow (BM) of normal mice can be considered as MDSC. Following the metastatic spread of experimental cancers to the bone, the amount of MDSC increases by 2–3-fold, promoting local immunosuppression and hence allowing for the growth of metastatic cells. Considering such a high influx of MDSC to the bone, we hypothesized that MDSC may contribute to metastasis in more than one way. Hence, we investigated if MDSC directly contribute to osteolysis by differentiating into osteoclasts.
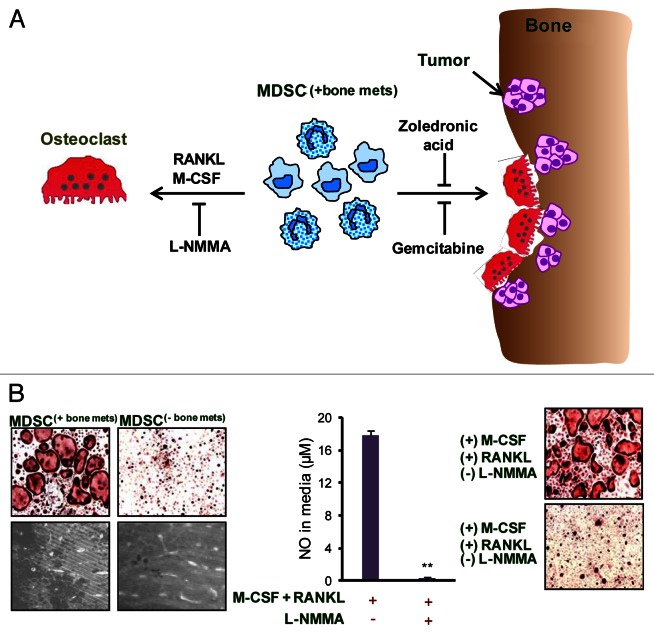
Using a pre-clinical model of breast cancer (BCa) bone dissemination, we demonstrated that MDSC can function as osteoclast precursors.3 MDSC isolated from mice bearing bone metastases underwent osteoclast differentiation in vitro in the presence of receptor activator of nuclear factor-κB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF). In addition, MDSC gave rise to bone-resorbing osteoclasts, causing increased osteolysis in vivo. As MDSC exert immunosuppression via arginase 1-, ROS- and RNS-dependent pathways, we investigated if any of these mechanisms would also be involved in the differentiation of MDSC into osteoclasts. Our data indicated a possible role of nitric oxide (NO) signaling in the differentiation of bone-derived MDSC into osteoclasts (Fig. 1).3
Figure 1. Role and mechanism of MDSC-mediated osteoclastogenesis in bone metastases. (A) As osteolytic cancers grow within bone, an increased influx of myeloid derived suppressor cells (MDSC(+bone mets)) is noted. When these MDSC(+bone mets) are cultured in the presence of receptor activator of nuclear factor-κB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF), they undergo differentiation into multi-nucleated, acid phosphatase 5, tartrate resistant (ACP5)+ osteoclasts. Nitric oxide (NO) signaling is crucial for MDSC differentiation into osteoclasts and can be blunted using NG-monomethyl-L-arginine acetate (L-NMMA), an inducible NO synthase (iNOS) inhibitor. The adoptive transfer of MDSC(+bone mets) results in increased osteolytic lesions and tumor growth in the bone of recipient mice. MDSC-mediated osteolysis can also be controlled in vivo by gemcitabine or zoledronic acid. The administration of either these agents reduces MDSC abundance, hence limiting osteolytic lesions and tumor growth. (B) Data show that MDSC isolated from mice bearing bone metastases (MDSC(+bone mets)) are capable of differentiating into osteoclasts and resorbing bone, whereas MDSC from mice bearing non-metastatic tumors (MDSC(-bone mets)) failed to differentiate into osteoclasts or exert bone-resorbing functions. NO levels are elevated in MDSC(+bone mets) and NO production can be inhibited with L-NMMA. In the presence of L-NMMA, MDSC(+bone mets) fail to differentiate into osteoclasts, proving the critical role of NO in this process.
The role of MDSC as osteoclast progenitors in BCa was also demonstrated in an independent study by Danilin et al.4 This study indicated MDSC as osteoclast precursors using the human BCa cell line MDA-MB-231. MDA-MB-231 cells cultured with tumor-derived MDSCs express high levels of GLI family zinc finger 2 (GLI2)- and parathyroid hormone-like hormone (PTHLH)-coding mRNAs. Both these factors are important in osteoclast activation and in the induction of osteolysis. MDSC-mediated osteolysis was also observed in multiple myeloma (MM).5 Thus, using a murine syngenic MM model, Zhuang et al. demonstrated that MDSC are capable of mediating bone resorption in vitro and in vivo. Both BCa and MM metastases are characterized by increased MDSC infiltration as neoplastic cells grow in the bone, and both these carcinomas are associated with osteolytic bone lesions. Osteolysis is a prominent feature of metastatic cancer as well as of rheumatoid arthritis (RA). MDSC isolated from a murine RA model also functioned as primary osteoclast progenitors and were capable of differentiating into functional osteoclasts in a NO-dependent fashion.6
One of the major findings of our study is that only MDSC obtained from the bone microenvironment of tumor-bearing mice can undergo osteoclast differentiation. Thus, neither MDSC from other metastatic sites nor MDSC from the bone of tumor-bearing mice devoid of bone metastases differentiated into osteoclasts. Similar observations were also reported by Danilin et al. and Zhuang et al. Hence, it is clear that a cross-talk between MDSC, tumor cells and the bone microenvironment is necessary for the differentiation of MDSC into osteoclasts. MDSC from mice bearing bone metastases also induced osteolysis in syngenic animals, indicating that these cells are primed as osteoclast progenitors and that the bone microenvironment triggers their differentiation into functional osteoclasts in vivo. It is possible that soluble factors secreted by tumor cells in the bone “prime” these cells to be osteoclast progenitors. BCa cells secrete a variety of pro-osteoclastogenic growth factors. For instance, the chemokines chemokine (C-C motif) ligand 2 (CCL2, also known as monocyte chemoattractant protein 1) and CCL5 (best known as RANTES), secreted by BCa bone metastases are known stimulators of osteoclastogenesis. MDSC express CCR2, the receptor for CCL2, implying that they are responsive to this chemokine. Secreted phosphoprotein 1 (SSP1, best known as osteopontin) is expressed by a majority of Bca metastatic lesions and promote the expression of cathepsin K and matrix metallopeptidase 9 (MMP9), thus enhancing osteoclast functions.7 The BM in MM lesions contains high levels of interleukin (IL)-1, IL-3, IL-6, IL-7, CCL20 and activin, all of which are osteoclast activating factors.8 Moreover, factors secreted by cancer cells as well as mediators released by immune cells in the bone microenvironment also contribute to osteolysis. Both BCa and MM bone metastases are infiltrated by plasmacytoid dendritic cells (pDC), which promote immunosuppressive effects.9,10 In both these settings, robust pDC infiltration is associated with high levels of IL-3, IL-6, IL-10, IL-15, CCL2, CCL5 and chemokine (C-X-C motif) ligand 10 (CXCL10). Some of these cytokines can induce osteoclastogenesis, either directly or indirectly by promoting the expression of RANKL. So, the presence of a pro-osteoclastogenic milieu in the metastatic bone microenvironment may drive the differentiation of MDSCs into osteoclasts.
Considering the complications and poor prognosis that are associated with metastatic disease, there is an urgent need to develop novel specific therapeutic strategies. Targeting MDSC can be one of such approaches. For instance, the inhibition of NO signaling in MDSC may reduce MDSC-mediated osteolysis. Nitroaspirin is known to inhibit the inducible nitric oxide synthease (iNOS) in MDSC. Targeting the NO pathway in MDSC may also inhibit MDSC-mediated immunosuppression. MDSC can be targeted using chemotherapeutics such as gemcitabine, which selectively and efficiently suppresses MDSC. In a pre-clinical BCa model, the administration of gemcitabine reduced indeed MDSC levels, controlled the extent of bone metastases and overall tumor growth.3 Bisphosphonate, which is the primary therapeutic option for patients with bone metastases, also reduces the proliferation of MDSC. The treatment of MDSC isolated from MM-bearing mice with zoledronic acid significantly inhibited MDSC-mediated bone destruction in vivo.5 Hence, a carefully designed combination therapy may reduce MDSC-mediated bone destruction and immunosuppression (Fig. 1).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24064
References
- 1.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–89. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 3.Sawant A, Deshane J, Jules J, Lee CM, Harris BA, Feng X, et al. Myeloid-derived suppressor cells function as novel osteoclast progenitors enhancing bone loss in breast cancer. Cancer Res. 2013;73:672–82. doi: 10.1158/0008-5472.CAN-12-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danilin S, Merkel AR, Johnson JR, Johnson RW, Edwards JR, Sterling JA. Myeloid-derived suppressor cells expand during breast cancer progression and promote tumor-induced bone destruction. Oncoimmunology. 2012;1:1484–94. doi: 10.4161/onci.21990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhuang J, Zhang J, Lwin ST, Edwards JR, Edwards CM, Mundy GR, et al. Osteoclasts in multiple myeloma are derived from Gr-1+CD11b+myeloid-derived suppressor cells. PLoS One. 2012;7:e48871. doi: 10.1371/journal.pone.0048871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charles JF, Hsu LY, Niemi EC, Weiss A, Aliprantis AO, Nakamura MC. Inflammatory arthritis increases mouse osteoclast precursors with myeloid suppressor function. J Clin Invest. 2012;122:4592–605. doi: 10.1172/JCI60920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das S, Samant RS, Shevde LA. Hedgehog signaling induced by breast cancer cells promotes osteoclastogenesis and osteolysis. J Biol Chem. 2011;286:9612–22. doi: 10.1074/jbc.M110.174920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raje N, Roodman GD. Advances in the biology and treatment of bone disease in multiple myeloma. Clin Cancer Res. 2011;17:1278–86. doi: 10.1158/1078-0432.CCR-10-1804. [DOI] [PubMed] [Google Scholar]
- 9.Sawant A, Hensel JA, Chanda D, Harris BA, Siegal GP, Maheshwari A, et al. Depletion of plasmacytoid dendritic cells inhibits tumor growth and prevents bone metastasis of breast cancer cells. J Immunol. 2012;189:4258–65. doi: 10.4049/jimmunol.1101855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chauhan D, Singh AV, Brahmandam M, Carrasco R, Bandi M, Hideshima T, et al. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer Cell. 2009;16:309–23. doi: 10.1016/j.ccr.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]