Abstract
Human papillomavirus (HPV) types associated with head and neck cancer generally induce a robust immune response. Despite the establishment of such an inflammatory microenvironment, HPV is able to persist and promote malignant transformation. The PD-1:PD-L1 immune checkpoint may play a critical role in the creation of an immunoprivileged site for viral persistence and the subsequent development of cancer.
Keywords: head and neck cancer, HPV, immune checkpoints, PD-1, PD-L1
Human papillomavirus (HPV) is recognized as the causative agent of a growing subset of head and neck cancers.1,2 HPV-associated head and neck squamous cell carcinomas (HPV-HNSCC) arise in the lymphoid tissue of the tonsils and the tongue base and the majority of these lesions can be discriminated from tobacco-related neoplasms by a characteristic infiltration of lymphocytes in the stroma and tumor nests.3 HPV-infected cells as well as HPV-associated cancer cells express foreign viral proteins such as E6 and E7, which are highly immunogenic and hence would be expected to induce strong anti-tumor immune responses. In fact, a high frequency of tumor-infiltrating lymphocytes (TILs) is found within these tumors, reflecting their inherent immunogenicity. Nevertheless, HPV-HNSCCs are able to evade immune surveillance, persist and grow.
We were interested in understanding how HPV can infect lymphoid organs such as the palatine and lingual tonsils and still evade immune recognition and clearance as virus-infected cells undergo malignant transformation. Interestingly, we observed that the strong immunological responses generated against HPV-HNSCC cells can trigger the programmed cell death 1 (PD-1)/PD-1 ligand 1 (PD-L1) immune checkpoint, which abolish their capacity to eliminate tumor cells in the absence of therapeutic interventions.4 To evaluate the relevance of the PD-1/PD-L1 pathway in HPV-HNSCC, we compared PD-1 expression by TILs and peripheral blood mononuclear cells (PBMCs) in patients with HPV-HNSCCs and from patients with a nonmalignant tonsil process. We found that the majority of activated CD8+ TILs isolated from HPV-HNSCC lesions express high levels of PD-1 as compared with CD8+ T cells isolated from benign, chronically inflamed tonsils.
The engagement of PD-1 on activated T cells by its ligand, PD-L1, can promote robust immunosuppressive effects. Therefore, we investigated the expression levels of PD-L1 in the tumor microenvironment and found that up to 70% of HPV-HNSCC cells expressed PD-L1 on their membrane, and that PD-L1 was often juxtaposed to CD8+ T cells.4 In normal tissues, PD-L1 is induced in response to inflammatory cytokines such as interferon γ (IFNγ). This system constitutes a major mechanism of tissue protection in the course of T-cell mediated inflammation. Driven by these premises, we evaluated IFNγ expression in HPV-HNSCC lesions using quantitative RT-PCR. We found a significant increase in the expression of IFNγ-coding mRNAs, which correlated with increased amounts of CD8+ TILs, in PD-L1-expressing as compared with PD-L1 non-expressing tumors, suggesting that the CD8+ T cells that infiltrate PD-L1+ HPV-HNSCCs are activated and secrete IFNγ, which in turn may drive the expression of PD-L1. Subsequent functional assays demonstrated that the CD8+PD-1+ TILs isolated from PD-L1+ tumors are functionally anergic and unable to produce effector cytokines in adequate amounts. These findings support a model in which the PD-1:PD-L1 system is activated during the development of HPV-HNSCCs as an adaptive mechanism of resistance to local IFNγ secretion, hence protecting cancer cells from immune elimination. Furthermore, we observed a localized expression of PD-L1 within the deep crypts of tonsils, where initial HPV infection occurs. This suggests that HPV may infect an immunoprivileged site within the tonsils and perhaps explains how a virus can evade immune recognition and clearance within a lymphoid organ.
The relevance of the PD-1:PD-L1 immune checkpoint in anticancer immunity is highlighted by several reports demonstrating that the blockade of PD-1 or PD-L1 by specific monoclonal antibodies can reverse the anergic state of tumor-specific T cells and hence enhance antitumor immune responses.5,6 The blockade of immune checkpoints is being investigated as a novel therapeutic approach to cancer in patients. In particular, cytotoxic T-lymphocyte antigen 4 (CTLA-4) and PD-1, two major regulators of these checkpoints, are actively being targeted by therapeutic interventions. Ipilimumab, a monoclonal antibody specific for CTLA-4, has been shown to provide a survival benefit to advanced metastatic melanoma patients in a randomized Phase III clinical trial, though it was associated with significant immune-related toxicities.7 In a first-in-human clinical trial, a PD-1-blocking antibody (MDX-1106, Nivolumab) has been evaluated in patients affected by advanced metastatic melanoma, colorectal cancer, castrate-resistant prostate cancer, non-small-cell lung cancer, and renal cell carcinoma. In this study, the antibody was well tolerated and clinical activity was recorded in all settings but prostate cancer.8 In a subset of patients, the expression of PD-L1 on the surface of tumor cells appeared to correlate with the likelihood of response to therapy. Extended Phase I clinical studies with anti-PD-1 (MDX-1106, Nivolumab) and anti-PD-L1 (BMS-936559) antibodies confirmed objective clinical responses in patients affected by multiple tumor types as well as a link between PD-L1 expression on the tumor cell surface and the rate of objective responses.9,10
As HPV-HNSCC cells express high levels of PD-L1 on their membrane, our study supports a rationale for administering PD-1- or PD-L1-targeting therapies to head and neck cancer patients (Fig. 1).
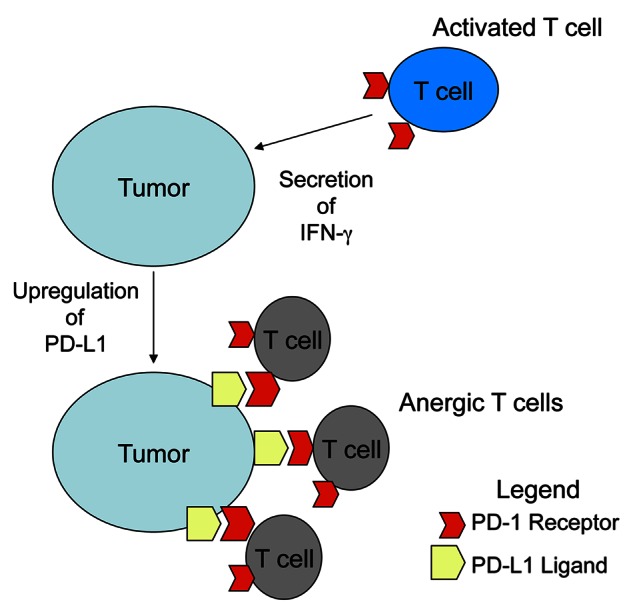
Figure 1. Mechanism by which tumor cells resist adaptive immune responses. Activated CD8+ T cells expressing the PD-1 receptor are recruited to the tumor and become activated to secrete interferon γ (IFNγ). The local production of IFNγ induces the expression of PD-L1 on the surface of tumor cells, resulting in the delivery of a PD-1-transduced, immunosuppressive signal to CD8+ T cells that drives anergy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24065
References
- 1.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–20. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Westra WH. The changing face of head and neck cancer in the 21st century: the impact of HPV on the epidemiology and pathology of oral cancer. Head Neck Pathol. 2009;3:78–81. doi: 10.1007/s12105-009-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–96. [PubMed] [Google Scholar]
- 6.Porichis F, Kwon DS, Zupkosky J, Tighe DP, McMullen A, Brockman MA, et al. Responsiveness of HIV-specific CD4 T cells to PD-1 blockade. Blood. 2011;118:965–74. doi: 10.1182/blood-2010-12-328070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brahmer JR, Tykodi SS, Chow LQM, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]


