Abstract
The identification of growth and differentiation pathways that are responsible for the proliferation and survival of cancer stem cells (CSCs) has opened avenues for the discovery of novel therapeutic targets. In the initial phase of an anticancer immune response, T cells specific for tumor-associated antigens develop in patients and, at least under selected circumstances, are able to eliminate malignant cells. However, it remains unknown whether CSC-specific T cells are also operational. We found naturally occurring multifunctional CD4+ and CD8+ T cells specific for the stem cell marker OCT4 among the peripheral blood mononuclear cells (PBMCs) of both healthy individuals and ovarian cancer patients. Moreover, lymphocytes isolated from the ascites of patients affected by ovarian malignancies also contained OCT4-specific T cells. OCT4-reactive CD4+ T cells did not produce interferon γ (IFNγ) and IFNγ-inducible protein 10 (IP-10) but were capable of proliferation upon stimulation with dendritic cells (DCs) loaded with an OCT4-derived peptide or OCT4 mRNA. OCT4-reactive CD8+ cells did not proliferate in response to a similar challenge, yet produced IP-10 as well as sufficient amounts of IFNγ to induce IP-10 . Furthermore, CD8+ cytotoxic T cells were able to release their lysosomal components, as indicated by the mobilization of CD107a. These results demonstrate the existence of anti-CSC specific T cells in ovarian cancer patients.
Keywords: CD4, CD8, IP-10, ovarian cancer, proliferation, stem cell markers
Introduction
The immune system has the potential to recognize and destroy aberrant cells before they form large tumors (reviewed in ref. 1). However, cancer cells often avoid immunological elimination, either as the immune system fails to recognize them or as they actively stimulate immunosuppressive processes.2 Tumor-infiltrating T cells specific for tumor-associated antigens (TAAs) are frequently detected in cancer patients.3 During disease progression, different types of T cells are recruited to the neoplastic lesion, including CD4+ T cells, CD8+ T cells, CD3+CD56+ T cells, Th17 cells, natural killer (T) T cells and regulatory T cells (Tregs).4,5 An effective antitumor immune response consists of the direct killing of tumor cells by CD8+ cytotoxic T lymphocytes (CTLs), which recognize TAAs presented in complex with MHC class I molecules. Spontaneous tumor-targeting CD8+ T cells have been identified in various malignancies, including melanoma, adenocarcinoma and leukemia (reviewed in ref. 6). The antigenic targets of these T cells include mutated proteins, embryonic proteins that are selectively re-expressed by malignant cells as well as overexpressed proteins that - when expressed at normal levels - are not presented on MHC Class I molecules.7 Several TAAs that can be recognized by T cells have also been identified in ovarian tumors.8 However, most of these TAAs are not tumor-specific bur rather shared with normal tissues.
Upon antigenic stimulation, T cells secrete cytokines, proliferate and proceed to develop into CTLs. Activated CD4+ and CD8+ T cells express newly synthesized activation markers such as CD137, a co-stimulatory molecule that is induced as a result of antigen-specific T-cell activation. The expression of CD137 correlates with the functional activation of CD8+ T cells as well as with the differentiation of effector and memory CD8+ T cells. Moreover, tumor-lytic T cells are enriched in the CD137+ cell population.9-11 The predominant mechanism of tumor killing by CTLs is perforin-granzyme dependent (reviewed in ref. 12). The acquisition of cell surface CD107a by CD8+ T cells indicates that these cells have secreted intracellular perforin as a result of activation-induced degranulation.13 Alongside, antigen-reactive T cells secrete multiple cytokines including (but not limited to) interferon γ (IFNγ) and tumor necrosis factor α (TNFα), which can stimulate MHC Class I expression on tumor cells and hence increase their sensitivity to the effector functions of CTLs. IFNγ may also activate macrophages to directly kill tumor cells (reviewed in ref. 14).
The recent identification of growth and differentiation pathways that are responsible for the proliferation and survival of cancer stem cells (CSCs) has unveiled novel therapeutic targets. CSCs (or tumor-initiating cells) are rare tumor cells with the ability to self-renew and give rise to phenotypically diverse tumor-cell populations. Increasing experimental evidence suggests that CSCs are responsible for initiating tumor growth and promoting metastasis in many different types of cancer, including ovarian carcinoma.15-26 However, evidence supporting the existence of CSC-targeting adaptive immune responses is scarce. We have previously shown that OCT4+MYC+NANOG+ cells constitute putative ovarian CSCs. OCT4 is an embryonic stem cell-specific transcription factor that is expressed at high levels by undifferentiated human embryonic stem cells (ESCs) and is downregulated upon differentiation.27-29 Furthermore, patients affected by germ cell tumors appear to harbor OCT4+ cells,30 and T cells specific for this CSC-specific antigen have been documented in a high percentage of healthy individuals.31,32 The hypothetical absence of OCT4-specific T cells from the circulation of ovarian cancer patients might hence reflect another mechanisms whereby ovarian tumor escape immunosurveillance.
As reported here, we detected low frequencies of CD4+ and CD8+ T cells specific for OCT4 in a high percentage of healthy donors as well as all ovarian cancer patients tested in this respect. Moreover, we found that these OCT4-specific T cells, which could also be isolated from tumor-associated ascitic fluids, are cytotoxic and can be expanded in vitro.
Results
OCT4-specific IP-10 production by peripheral blood mononuclear cells of healthy subjects
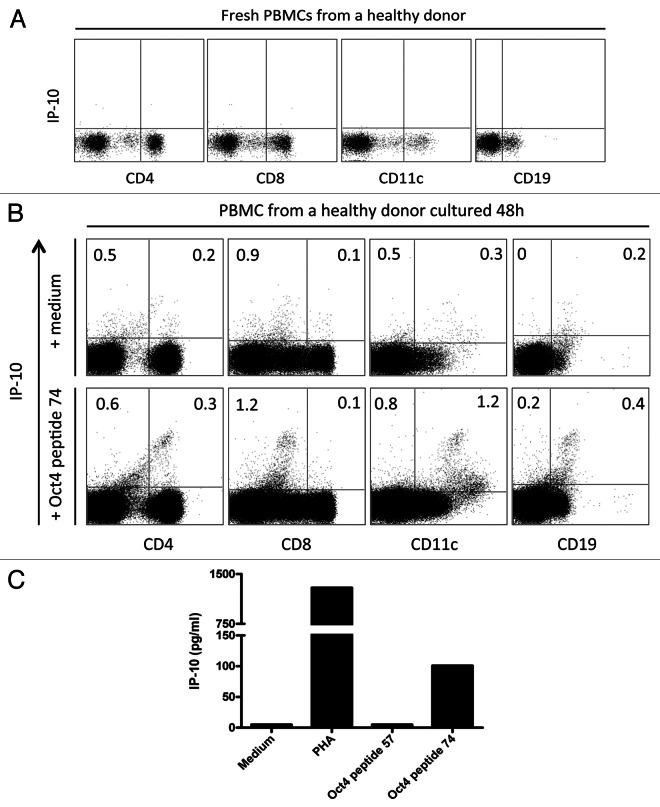
To assess the capacity of T cells to recognize OCT4, peripheral blood mononuclear cells (PBMCs) from healthy donors were either cultured with medium only, either stimulated with phytohemagglutinin (PHA, as a positive control) or stimulated with the immunoreactive OCT4-derived peptide 74 from Dhodapkar’s OCT4 peptide library.32 Endogenous CXCL10 (also known as IFNγ-inducible protein-10, IP-10) was not observed in CD4+ T cells, CD8+ T cells, B cells and monocytes prior to stimulation (Fig. 1A), as cells cultured with medium only showed only background staining. Conversely, upon stimulation with the immunoreactive OCT4-derived peptide for 48 h, CD11c+ cells expressed high levels of intracellular IP-10. Of note, IP-10 was hardly detectable in CD8+ cells exposed to an OCT4-derived peptide (Fig. 1B). Next, we omitted brefeldin A from the experimental setting to assess IP-10 secretion. After 48 h of stimulation with the immunoreactive OCT4-derived peptide, IP-10 was detectable in the culture supernatants corresponding to the PBMCs of 7 out of 10 (70%) healthy donors tested (Fig. 1C). To determine the specificity of the anti-OCT4 response, the non-immunoreactive OCT4-derived peptide 57 from Dhodapkar’s library was also used to stimulate PBMCs. As expected, no IP-10 secretion was detected in these conditions (Fig. 1C). Collectively, these data indicate the presence of readily detectable OCT4-specific cells among the PBMCs of healthy individuals.
Figure 1. OCT4-specific IP-10 production in peripheral blood mononuclear cells from healthy subjects. (A) Freshly isolated peripheral blood mononuclear cells (PBMCs) were labeled for intracellular interferon γ (IFNγ)-inducible protein 10 (IP-10). (B and C) Alternatively, PBMCs were stimulated with an immunoreactive OCT4-derived peptide for 48 h, followed by the assessment of intracellular IP-10 by flow cytometry (B) or IP-10 secretion by ELISA (C). Non-stimulated PBMCs were included as a negative control. No brefeldin A was added to the culture, and a minimum of 1×105 cells was analyzed.
Detection of functional OCT4-specific CD4+ T cells in vitro
To analyze the functional capacity of OCT4-reactive CD4+ T cells in vitro, MACS-sorted CD4+ T cells were stimulated with autologous dendritic cells (DCs) loaded with the immunoreactive OCT4-derived peptide 74. CD4+ T cells cocultured with unloaded autologous DCs and CD4+ T cells stimulated with anti-CD3/anti-CD28 beads were used as a negative and positive control, respectively.
IP-10 production
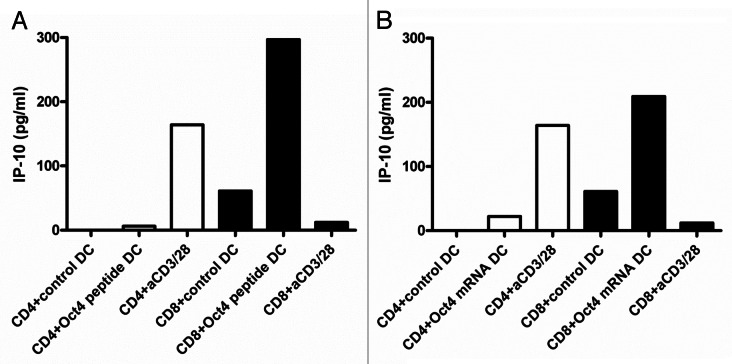
After 2 d of T cell-DC co-culture, IP-10 secretion in culture supernatants was assessed. Very low levels of IP-10 were secreted by all donors tested (n = 12) in this respect (Fig. 2A) To exclude the non-reactivity to the single OCT4-derived peptide, CD4+ T cells were also stimulated with autologous DCs that had previously been electroporated with the OCT4 mRNA. In this case, full-length OCT4 was synthesized within DCs and processed for presentation on MHC class I molecules. Of note, OCT4 was hardly detectable 2 h upon electroporation. However, as we observed a response in transfected DCs, we concluded that OCT4 was subjected to very rapid degradation. mRNA-transfected DCs had a similar functional impact on IP-10 than their peptide-loaded counterparts. Thus, IP-10 was not secreted by CD4+ T cells exposed to OCT4 mRNA-transfected DCs (Fig. 2B).
Figure 2. IP-10 production by OCT4-specific T cells. (A and B) MACS-sorted CD4+ and CD8+ cells were stimulated with autologous dendritic cells (DCs) loaded as indicated, and after 2 d of co-culture interferon γ (IFNγ)-inducible protein 10 (IP-10) secretion was assessed by ELISA. (A) IP-10 production by T cells exposed to autologous DCs loaded with an immunoreactive OCT4-derived peptide (representative data from one donor). (B) IP-10 production by T cells exposed to autologous DCs loaded with the OCT4 mRNA (representative data from the same donor).
IFNγ secretion and proliferation
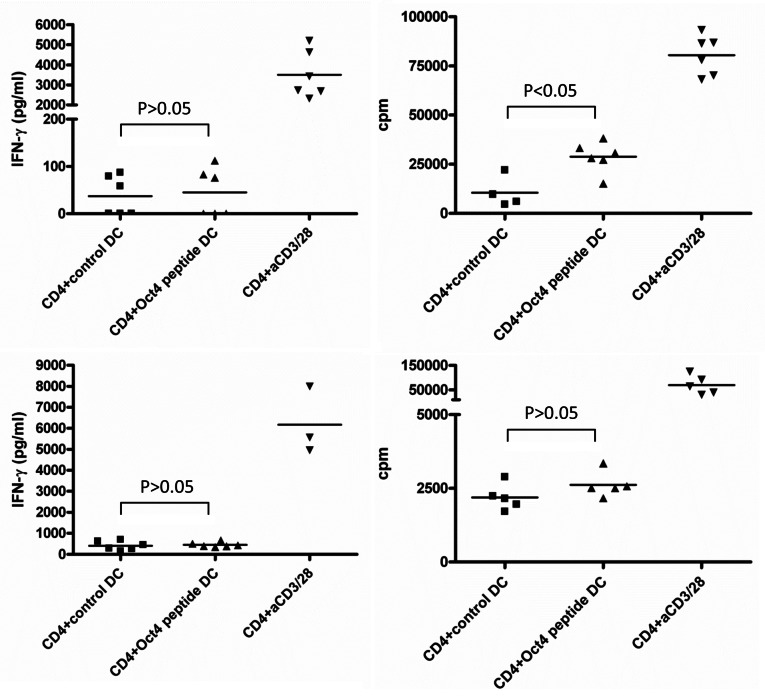
As we failed to detect IP-10 secretion by CD4+ cells exposed to OCT4-presenting DCs, we tested if CD4+ cells produced IFNγ under these conditions. After 3 d of co-colture between T cells and DCs loaded with an immunoreactive OCT4-derived peptide, IFNγ secretion was assessed (Table S1). In a first experiment we pooled the supernatants from 6 parallel wells, but we then decided to measure each well individually, owing to the variability of results. We found that the frequency of IFNγ-producing cells in these conditions was very low. Indeed, for some subjects, only 1 out of 6 wells was found to be IFNγ-positive. Globally, IFNγ production was detectable in the culture supernatants corresponding to 6 out of 12 donors. However, IFNγ levels were never increased to statistically significant extents (p > 0.05, according to one-way ANOVA Tukey test) when CD4+ cells were stimulated with peptide-loaded DCs (Fig. 3). In addition, low levels of IFNγ correlated with undetectable IP-10 secretion.
Figure 3. Detection of OCT4-reactive CD4+ T cells. MACS-sorted CD4+ cells were stimulated with autologous dendritic cells (DCs) loaded with an immunoreactive OCT4-derived peptide. CD4+ cells stimulated with anti-CD3/anti-CD28 beads and CD4+ cells exposed to unloaded DCs served as a positive and negative control condition, respectively. Results from 2 representative donors showing interferon γ (IFNγ) production (on day 3) and antigen-dependent CD4+ T-cell proliferation (on day 6) are reported. CD4+ cells from both donors did not exhibit high IFNγ production by in response to OCT4-derived peptide-loaded DCs (p > 0.05), while cells from one donor underwent a significant proliferative response (p < 0.05).
Nevertheless, on day 6 of culture, an OCT4-dependent proliferative response was detected in CD4+ T cells from 7 out of 12 healthy donors (Table S1). We observed that not all the wells of the 6-well plates employed in this assay exhibited measurable cell proliferation, indicating that the donors also differed in terms of OCT4-reactive precursor cell frequency. Of the 12 healthy donors tested, 3 exhibited high frequencies of OCT4-reactive precursor cells, 4 a low frequency, and 5 had no or a minimal amount of OCT4-reactive precursors. No clear correlation between IFNγ production and cell proliferation was observed (Fig. 3). Although there was some degree of inter-donor variability with regard to OCT4-specific IFNγ production and proliferation, these results demonstrate the existence of memory OCT4-reactive CD4+ T cells in the circulation of healthy subjects, which are able to proliferate in vitro upon antigenic stimulation. Comparable results were obtained when CD4+ T cells were stimulated with autologous DCs loaded with the OCT4-coding mRNA (data not shown).
Detection of activated OCT4-specific CD8+ T cells in vitro
To analyze the functional characteristics of OCT4-reactive CD8+ T cells in vitro, MACS-sorted CD8+ T cells were stimulated with autologous DCs loaded with the immunoreactive OCT4-derived peptide 74. CD8+ T cells stimulated with unloaded autologous DCs and CD8+ T cells exposed to anti-CD3/anti-CD28 beads were used as a negative and positive control, respectively.
IP-10 production
CD8+ T cells were co-cultured for 2 d in the presence of autologous DCs that had been loaded with an immunoreactive OCT4-derived peptide, followed by the assessment of IP-10 secretion. IP-10 was detected in the co-culture supernatants corresponding to 10 out of 12 healthy donors (Fig. 2A). IP-10 concentrations ranged from 210 to 564 pg/mL. Comparable results were obtained when CD8+ cells were stimulated by DCs loaded with the OCT4 mRNA (Fig. 2B). Remarkably, CD8+ T cells did not produce IP-10 in response to anti-CD3/anti-CD28 beads (Fig. 2), which is in accordance with data highlighting fundamental differences in the immunobiology of CD4+ and CD8+ cells.33 Further supporting this notion, it has recently been demonstrated that optimal TNFα and IFNγ responses by CD8+ cells exposed to anti-CD3 monoclonal antibodies require the simultaneous activation of CD26-transduced signals.34
IFNγ secretion and proliferation
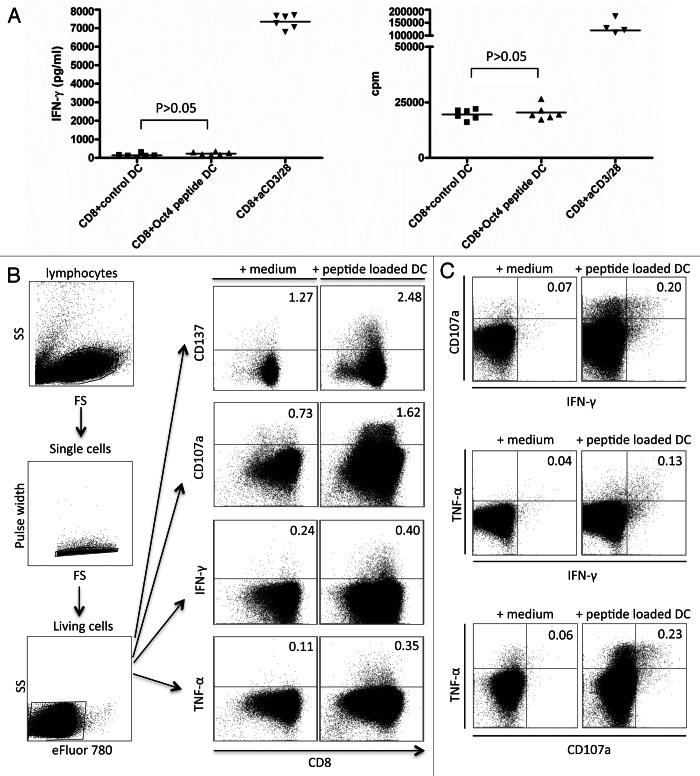
Similar to CD4+ T cells, CD8+ T cells exposed to OCT4-derived peptide-loaded DCs failed to exhibit a significantly increased secretion of IFNγ. This held true for the CD8+ T cells of all 12 healthy donors (Fig. 4A). Given the high levels of IP-10 observed in culture supernatants, we assume that IFNγ was secreted but rapidly consumed to stimulate IP-10 production. Antigen-dependent T-cell proliferation was examined 5 or 6 d upon stimulation, and CD8+ T cells exposed to autologous DCs loaded with an immunoreactive OCT4-derived peptide invariably failed to incorporate a significantly greater amount of 3[H]-thymidine than CD8+ T cells maintained in control conditions (Fig. 4A and Table S2). To exclude the non-reactivity to the single OCT4-derived peptide, CD8+ T cells were also exposed to autologous DCs that had previously been electroporated with the OCT4 mRNA, yielding similar results (data not shown). Taken together, these findings indicate that OCT4-specific CD8+ T cells are rare in healthy individuals and that the proliferative capacity of these cells upon in vitro stimulation is poor (Table S2). These results also indicate that CD8+ T cells can be activated in vitro by specific OCT4-derived peptides, leading to the production of IP-10.
Figure 4. Detection of OCT4-reactive CD8+ T cells. (A–C) MACS-sorted CD4+ cells were stimulated with autologous dendritic cells (DCs) loaded with an immunoreactive OCT4-derived peptide. CD4+ cells stimulated with anti-CD3/anti-CD28 beads and CD4+ cells exposed to unloaded DCs served as a positive and negative control condition, respectively. (A) Results from one representative donor showing no significant interferon γ (IFNγ) production (on day 3) and no antigen-dependent CD8+ T-cell proliferation (on day 6) (p > 0.05) are reported. (B) After 12 d of co-culture, a second stimulation was performed with -derived peptide-loaded DCs. Thereafter, peptide-specific CD8+ T cells were analyzed for their cytotoxic functions by intracellular cytokine staining and flow cytometry. The expression of activation markers by cells from one representative healthy donor is shown, together with the gating strategy. (C) Results from one representative donor showing the mobilization of CD107a and the expression of IFNγ and tumor necrosis factor α (TNFα) by the same cells [gating strategy as in (B)] are shown.
Functionality after re-stimulation
Approximately 12 d after the first stimulation, CD8+ T cells were restimulated with DCs loaded with an immunoreactive OCT4-derived peptide, in order to examine the existence of multifunctional CD8+ T cells. Staphylococcal enterotoxin B (SEB)-exposed CD8+ T cells and previously stimulated CD8+ T cells cultured in standard conditions served as a positive and negative control, respectively. After 5 h of stimulation, CD8+ T cells were assessed for CD107a mobilization as well as for the expression of IFNγ and TNFα, while CD137 expression was measured upon overnight stimulation. CD137-expressing CD8+ T cells were for 8 out of 9 donors investigated in this respect, while CD107a mobilization coupled to the expression of IFNγ and TNFα was detected in the CD8+ T cells of 4 out of 6 donors tested. The gating strategy and results from a representative donor are shown in Figure 4B. The data depicted in Figure 4C indicate that CD107a mobilization and the expression of TNFα and IFNγ occurred in the same cells.
OCT4-specific IP-10 production by cells isolated from ovarian cancer patients
We have previously demonstrated that ovarian cancer cells expressing both OCT4 and MYC constitute putative ovarian CSCs. As we detected OCT4-specific memory cells in healthy donors, we prospectively analyzed the presence of OCT4-specific memory cells in a cohort of ovarian cancer patients (Table S3). No patient pre-selection was made, and tests were run on subjects for which both ascites and PBMC samples were available. Both ascites-derived mononuclear cells (MNCs) and autologous PBMCs were stimulated for 48 h with the immunoreactive OCT4-derived peptide 74, followed by the assessment of IP-10 secretion in culture supernatants (Fig. 5). PHA and non-stimulated cells served as positive and negative controls, respectively. Similar to healthy donors, all 5 ovarian cancer patients tested exhibited measurable OCT4-specific IP-10 production, indicating the existence of OCT4-specific memory T cells in these individuals.
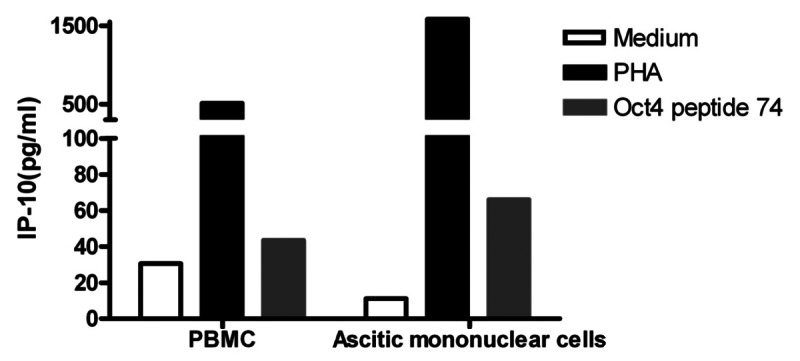
Figure 5. Immunity to OCT4 in ovarian cancer patients. Peripheral blood mononuclear cells (PBMCs) and ascitic fluid-derived mononuclear cells from the same patient were cultured in the presence or in the absence of an immunoreactive OCT4-derived peptide for 48 h, followed by the quantification of interferon γ (IFNγ)-inducible protein 10 (IP-10) secretion in co-culture supernatants. Phytohemagglutinin PHA-stimulated cells were used as a positive control. Results from one representative patients are shown.
Discussion
The current study demonstrates that most healthy subjects, as well as patients bearing ovarian tumors, have naturally occurring memory T cells specific for OCT4 in their peripheral blood. Furthermore, we show that CD4+ OCT4-reactive T cells from healthy donors can be expanded and their CD8+ counterparts can be activated by specific OCT4 peptides in vitro. Upon stimulation with autologous DCs loaded with OCT4-derived peptides or with the OCT4-coding mRNA, OCT4-specific CD4+ and CD8+ T cells secrete cytokines. Although it is tempting to assume that individuals would be completely tolerant to self proteins, the elimination of autoreactive T cells during negative selection in the thymus is not fool-proof, as demonstrated by the emergence of autoimmune diseases. These autoreactive T-cell clones are generally kept under control by Tregs or fail to be stimulated in vivo owing to low levels of antigen expression, in turn resulting in minimal degrees of presentation of the corresponding peptides on MHC molecules.35 However, during oncogenesis, several of these low-abundance proteins are overexpressed, which may drive the activation of T cells that actively infiltrate neoplastic lesions.36-41 Such autoreactive T cells can also be activated using different approaches in the context of anticancer immunotherapy.42-46 The importance of expression levels for the recognition of a given antigen by the immune system has recently been demonstrated.47 In particular, it has been shown that tumor-specific T cells fail to exert antineoplastic effects as melanoma cells dedifferentiate, resulting in the decreased expression of TAAs including gp100 and tyrosinase.47 The antigen expression level is hence of the utmost importance for determining whether T cells become reactive against malignant cells or remain quiescent. This concept has further been exemplified in a study that evaluated the immune responses to teratomas evoked by induced pluripotent stem cells, as it appeared that the increased expression of several normal proteins initiated adaptive immunity. However, not all overexpressed proteins elicited an immune response,7 suggesting that factors other than the expression level, such as the host MHC alleles, the subcellular location of TAAs and their degree of proteosomal degradation, also play a critical role in determining the intensity of immune responses.
In our study, the frequency of OCT4-reactive cells was quite low, indicating that these cells are not (or poorly) stimulated in vivo. Thus, OCT4 appears to be either not expressed or expressed at very low levels. In line with this notion, healthy individuals were shown to lack tolerance to some OCT4 epitopes. Interestingly, ovarian cancer patients harbor a small cancer cell population that expresses OCT4. As most, if not all, patient-derived peripheral lymphocytes were able to detect OCT4, OCT4+ ovarian cancer cells presumably do not reflect a lack of anti-OCT4 immune responses in these patients.
OCT4-specific T cells were detected even in the ascites of ovarian cancer patients. Apparently, these T cells are counterbalanced by strong suppressive mechanisms that operate in the abdomen.48-52 Natural immunity against proteins involved in pluripotency has previously been demonstrated. For example, Dhodapkar et al. have shown that OCT4-responsive T cells can be detected in the PBMCs of approximately 83% healthy donors and that these OCT4-specific cells mainly are CD4+ T cells. The same authors also found 38% of germ cell tumor patients to display measurable OCT4-specific T-cell immunity before chemotherapy, a fraction that increased to 83% upon treatment.53 Natural immune responses targeting the pluripotency-related protein SOX2 have also been reported. In particular, it has been shown that CD8+ SOX2-specific T cells are frequent in patients with monoclonal gammopathy of undetermined significance (MGUS). MGUS often precedes myeloma, but SOX2-specific T-cell immunity was not detectable in myeloma patients.54 Nevertheless, anti-SOX2 autoantibodies were detected in multiple myeloma patients upon allogeneic stem cell transplantation.55 OCT4 and SOX2 are crucial in regulating the self-renewal and pluripotency of ESCs, and both have recently been involved in the development of CSCs in various types of cancer.56-66 In some cases, OCT4 and SOX2 have been detected in the cytoplasm, a setting that is compatible with robust presentation on MHC class I molecules.61 Although OCT4-specific cells were detectable in both healthy subjects and ovarian cancer patients, their reactivity was not comparable to that of normal memory T cells to recall antigens. As a result of very limited re-stimulation in vivo, OCT4-specific cells are hence likely remain in a slumbered state. Recently, a similar status has been reported for antitumor CTLs capable of inhibiting the expansion of naïve and memory CD8+ T cells,67 and it has been suggested that a temporary blockade of these perforin-expressing antitumor CTLs could have huge effects on the DC-based enforcement of antitumor responses as well as on the adoptive transfer of T cells equipped with appropriate T-cell receptors.
The presence of OCT4-specific T cells in healthy individuals (without any signs of autoimmune disease) indicates that OCT4 is normally expressed at such low levels that it fails to stimulate T-cell activation and expansion. In line with this notion, the frequency of these cells was very low. Therefore, it seems safe to use OCT4 as a target antigen for the elicitation of therapeutic immune response. The fact that OCT4-specific T cells were detected in cancer-free individuals comforts the notion that the reactivity of their counterparts in cancer patients has not shifted to de novo epitopes. This said, the mechanisms that inhibit immune responses in the abdomen of cancer patients need to be overcome to enable the use of OCT4-targeting immunotherapeutic approaches.
Materials and Methods
OCT4 peptides
Dhodapkar et al. synthesized an OCT4-derived peptide library and tested T-cell reactivity to individual peptides. This library consisted of 95 peptides (15-mer overlapping by 11 amino acids).32 A highly T-cell immunoreactive peptide (number 74, DVVRVWFCNRRQKGK), a non-immunoreactive peptide (number 57, WVEEADNNENLQEIC) as well as an elongated version of peptide 74 (NRRDVVRVWFCNRRQKGKVWFC) were purchased from JPT Peptide Technologies.
In vitro OCT4 mRNA synthesis
The plasmid pGEM4Z-5′UT-OCT4–3′UT-A64 was constructed by digestion of the full-length OCT4-containing plasmid pCR-TOPO-OCT4 (ImaGenes GmbH, BC117435) and pGEM4Z-5′UT-gp100–3′UT-A64 with EcoRI (Bioke, R3101L), followed by insertion of the OCT4-coding sequence into the empty pGEM4Z vector. Plasmids were amplified in Escherichia coli and isolated using the Nucleospin Plasmid QuickPure reagent (Macherey-Nagel, 740 615.250). This plasmid was linearized with SpeI (New England Biolabs, R0133S), purified by phenol/chloroform extraction plus ethanol precipitation and used as the template for mRNA synthesis with the mMESSAGE mMACHINE kit (T7 promotor, Applied Biosystems, AM1344), according to the manufacturer’s instructions.
Stimulation and maintenance of OCT4-specific T cells
Buffy coats were obtained from the local blood bank from anonymous healthy donors after informed consent was obtained. Blood was donated in the afternoon, tested and processed. The next morning, the buffy coat was collected and immediately processed. PBMCs were isolated by density-gradient centrifugation using Lymphoprep (Axis-Shield PoC AS, N-1114547). Monocytes were purified from PBMCs following a 1 h adherence step at 37°C in X-VIVO 15 medium (Lonza, BE04-418Q) supplemented with 2% human serum (HS, Sigma, H1513, pre-tested for mycoplasma and virus). Non-adherent peripheral blood lymphocytes (PBLs) were washed with PBS and cryopreserved in X-VIVO 15 medium containing 2% HS and 10% DMSO for subsequent use. Adherent cells were cultured in X-VIVO 15 medium supplemented with 2% HS and 450 U/mL granulocyte macrophage colony-stimulating factor (GM-CSF, from Cellgenix, 1412-050) and 300 U/mL IL-4 (Cellgenix, 1403-050). After 5–6 d of culture, DCs were matured for 2 d upon by the administration of 2 μg/mL polyinosinic:polycytidylic acid (polyIC, from Sigma Aldrich, P0913) and 4 μg/mL (R848, from Enzo Life Sciences-Axxora, ALX-420-038-MO25). On day 7 or 8, 2 × 106 mature DCs were pulsed for 3 h with 15 μg OCT4-derived peptides, or 10 × 106 mature DCs were electroporated with the OCT4 mRNA as described below. Then, DCs were co-cultured with MACS-sorted cryopreserved autologous CD4+ or CD8+ T cells at a DC:responder cell ratio of 1:30 (2×105 responder cells per well) in Iscove's modified Dulbecco's medium (IMDM, from Invitrogen, 21980-065) supplemented with 10% HS in 96-well plates. IL-2 (100 U/mL, Proleukin®) was added to the DC:T-cell co-cultures on day 6 and was subsequently added at 50 U/mL every 2–3 d. IL-7 (R&D Systems, 207-IL) and IL-15 (5 ng/mL, Biomol, 52619.10)) were also added to the DC:CD8+ T cell cocultures from the beginning of the co-culture period and every 2–3 d thereafter. T cells co-cultured with unloaded autologous DCs were used as negative controls, and T cells stimulated with anti-CD3/anti-CD28 beads (Invitrogen, 111.31D) served as positive controls. To test the presence of OCT4-reactive CD8+ T cells, cells were restimulated with peptide-loaded autologous DCs and analyzed for functionality.
Electroporation of DCs
Mature DCs were washed twice with PBS and once with phenol-red free OptiMEM medium (Invitrogen, 11058-021). Twenty micrograms of mRNA were transferred to a 4-mm cuvette (Bio-Rad, 65-2089), and 8–10 × 106 cells were added in 180 μL of OptiMEM and incubated for 3 min before being pulsed in a Genepulser Xcell (Bio-Rad) with an exponential decay pulse of 300 V and capacitance of 150 μF. After electroporation, DCs were allow to rest for at least 2 h at 37°C in phenol red-free X-VIVO 15 medium (Lonza, BE04-744Q) supplemented with 6% HS before further treatment.
Detection of CD137-expressing CD8+ T cells
To evaluate the CD137 expression by CD8+ T cells stimulated with OCT4-derived peptide-loaded DCs, 2 × 106 previously stimulated T cells were re-stimulated with autologous DCs loaded with an immunoreactive OCT4-derived peptide and cultured in 1 mL IMDM supplemented with 10% HS and 5 μg/mL anti-CD28/anti-CD49d co-stimulatory reagents (BD Biosciences, 347690). In every experiment, T cells activated with 1 μg/mL SEB (Sigma S4881) were used as a positive control, and a negative control (medium only) was included to monitor the spontaneous expression of CD137. After 5 h of stimulation, 3 μg/mL brefeldin A (BFA, Sigma, B6542) were added. After 16–18 h, cells were stained with anti-CD8-FITC conjugates (BD Biosciences, 555366), fixed with 4% paraformaldehyde, permeabilized with PBS containing 0.5% saponin plus 2% HS and stained with anti-CD137-APC conjugates (BD Biosciences, 550890). Flow cytometry was performed using a CyAnTM ADP cytometer (Beckman Coulter), and data were analyzed using the FlowJo software v. 9.2.
CD8+ T-cell intracellular cytokine detection and CD107a mobilization assay
To test the functionality of CD8+ T cells, 2 × 106 CD8+ cells previously exposed to autologous DCs loaded with an immunoreactive OCT4-derived peptide were re-exposed to the same conditions and incubated in 1 mL IMDM containing 10% HS, 5 μg/mL anti-CD28/anti-CD49d reagents, 1 μl/mL monensin (eBioscience, 00-4505-51) and 10 μg/mL BFA. The same positive and negative controls described above were used. Anti-CD107a-Alexa Fluor® 700 conjugates (BD, 561340) were added at the beginning of the co-culture, and 5 h later cells were collected and stained with the Fixable Viability Dye eFluor® 780 (eBioscience, 65-0865-18) and anti-CD8-APC conjugates (BD, 555369). Finally, cells were fixed, permeabilized and stained with anti-TNFα-PE-Cy7 (eBioscience, 25-7349-82) and anti-IFNγ-PerCP-Cy5.5 (BD Biosciences, 560704) conjugates and analyzed by flow cytometry.
ELISA
The concentration of IFNγ in co-culture supernatants was measured by using of conventional ELISA approach. Briefly, ELISA plates (NUNC, 155382) were coated overnight with a monoclonal antibody specific for human IFNγ (Thermo Scientific Pierce Antibodies, M700A), blocked with 1% bovine serum albumin (BSA, Sigma-Aldrich, A3294) and washed with PBS containing 0.05% Tween 20 (Merck, 8.22184.0500). Then, cell culture supernatant were added and incubated at room temperature for 1 h. After washing, a biotinylated detection antibody (Thermo Scientific Pierce Antibodies, M701B) was added and allowed to incubate for 1 h at room temperature. Finally, plates were washed and a streptavidin-conjugated horseradish peroxidase (Thermo Scientific Pierce Antibodies, C21135) was added for 30 min, followed by incubation with the substrate 3,3′,5,5′-tetramethylbenzidine (Sigma, T2885). The reaction was stopped with 0.8 M H2SO4 and the absorbance at 450 nm was measured using an ELISA plate reader (Bio-Rad). IP-10 levels in co-culture supernatants were measured using the human CXCL10/IP-10 DuoSet ELISA kit (R&D Systems, DY266), according to the manufacturer’s instructions.
Detection of OCT4-reactive T cells in PBMCs from healthy donors and patients
The ascites of patients with ovarian cancer were obtained during debulking surgery. The ascitic fluid was processed immediately at room temperature or—when the surgery was performed in the afternoon—was stored overnight at 4°C and then processed the next morning. Institutional guidelines for the collection of human material were followed. Blood was obtained from healthy donors and cancer patients after informed consent was obtained. Blood samples were taken during or shortly after surgery and were also processed at room temperature within 24 h of collection. Ascites-derived mononuclear cells and PBMCs (from both healthy donors and patients) were isolated by density-gradient centrifugation using Lymphoprep. Then, 2 × 105 cells were cultured in IMDM supplemented with 10% HS and 10 μg/mL peptide 74, 10 μg/mL peptide 57 or 5 μg/mL PHA. Cells cultured in medium only provided negative control conditions. After 48 h, supernatants were collected and measured for the production of IP-10 by ELISA. In some experiments, the production of intracellular IP-10 was also measured. In this case, cells were collected, washed, labeled with anti-CD8-FITC conjugates and then fixed, permeabilized and labeled with anti-IP-10-PE conjugates (R&D Systems, IC266P) prior to analysis by flow cytometry.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Dutch government to the Netherlands Institute for Regenerative Medicine (NIRM, FES0908).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental materials may be found here:
http://www.landesbioscience.com/journals/oncoimmunology/article/24271/
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24271
References
- 1.Rutkowski MR, Stephen TL, Conejo-Garcia JR. Anti-tumor immunity: myeloid leukocytes control the immune landscape. Cell Immunol. 2012;278:21–6. doi: 10.1016/j.cellimm.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Preston CC, Goode EL, Hartmann LC, Kalli KR, Knutson KL. Immunity and immune suppression in human ovarian cancer. Immunotherapy. 2011;3:539–56. doi: 10.2217/imt.11.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galon J, Pagès F, Marincola FM, Thurin M, Trinchieri G, Fox BA, et al. The immune score as a new possible approach for the classification of cancer. J Transl Med. 2012;10:1. doi: 10.1186/1479-5876-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bamias A, Koutsoukou V, Terpos E, Tsiatas ML, Liakos C, Tsitsilonis O, et al. Correlation of NK T-like CD3+CD56+ cells and CD4+CD25+(hi) regulatory T cells with VEGF and TNFalpha in ascites from advanced ovarian cancer: Association with platinum resistance and prognosis in patients receiving first-line, platinum-based chemotherapy. Gynecol Oncol. 2008;108:421–7. doi: 10.1016/j.ygyno.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Papamichail M, Perez SA, Gritzapis AD, Baxevanis CN. Natural killer lymphocytes: biology, development, and function. Cancer Immunol Immunother. 2004;53:176–86. doi: 10.1007/s00262-003-0478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagorsen D, Scheibenbogen C, Marincola FM, Letsch A, Keilholz U. Natural T cell immunity against cancer. Clin Cancer Res. 2003;9:4296–303. [PubMed] [Google Scholar]
- 7.Zhao T, Zhang Z-N, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–5. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 8.Renkvist N, Castelli C, Robbins PF, Parmiani G. A listing of human tumor antigens recognized by T cells. Cancer Immunol Immunother. 2001;50:3–15. doi: 10.1007/s002620000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolfl M, Kuball J, Ho WY, Nguyen H, Manley TJ, Bleakley M, et al. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood. 2007;110:201–10. doi: 10.1182/blood-2006-11-056168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cannons JL, Lau P, Ghumman B, DeBenedette MA, Yagita H, Okumura K, et al. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J Immunol. 2001;167:1313–24. doi: 10.4049/jimmunol.167.3.1313. [DOI] [PubMed] [Google Scholar]
- 11.Wen T, Bukczynski J, Watts TH. 4-1BB ligand-mediated costimulation of human T cells induces CD4 and CD8 T cell expansion, cytokine production, and the development of cytolytic effector function. J Immunol. 2002;168:4897–906. doi: 10.4049/jimmunol.168.10.4897. [DOI] [PubMed] [Google Scholar]
- 12.Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol. 2002;2:735–47. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- 13.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/S0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 14.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–89. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 15.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 17.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–51. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 18.Curley MD, Therrien VA, Cummings CL, Sergent PA, Koulouris CR, Friel AM, et al. CD133 expression defines a tumor initiating cell population in primary human ovarian cancer. Stem Cells. 2009;27:2875–83. doi: 10.1002/stem.236. [DOI] [PubMed] [Google Scholar]
- 19.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–63. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dieter SM, Ball CR, Hoffmann CM, Nowrouzi A, Herbst F, Zavidij O, et al. Distinct types of tumor-initiating cells form human colon cancer tumors and metastases. Cell Stem Cell. 2011;9:357–65. doi: 10.1016/j.stem.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–37. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 22.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–23. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–8. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasheed Z, Wang Q, Matsui W. Isolation of stem cells from human pancreatic cancer xenografts. J Vis Exp. 2010 doi: 10.3791/2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- 26.Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–20. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Looijenga LH, Stoop H, de Leeuw HP, de Gouveia Brazao CA, Gillis AJ, van Roozendaal KE, et al. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. 2003;63:2244–50. [PubMed] [Google Scholar]
- 28.Medvedev SP, Shevchenko AI, Mazurok NA, Zakiian SM. [OCT4 and NANOG are the key genes in the system of pluripotency maintenance in mammalian cells] Genetika. 2008;44:1589–608. [PubMed] [Google Scholar]
- 29.Zangrossi S, Marabese M, Broggini M, Giordano R, D’Erasmo M, Montelatici E, et al. Oct-4 expression in adult human differentiated cells challenges its role as a pure stem cell marker. Stem Cells. 2007;25:1675–80. doi: 10.1634/stemcells.2006-0611. [DOI] [PubMed] [Google Scholar]
- 30.Jones TD, Ulbright TM, Eble JN, Baldridge LA, Cheng L. OCT4 staining in testicular tumors: a sensitive and specific marker for seminoma and embryonal carcinoma. Am J Surg Pathol. 2004;28:935–40. doi: 10.1097/00000478-200407000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Dhodapkar MV. Immunity to stemness genes in human cancer. Curr Opin Immunol. 2010;22:245–50. doi: 10.1016/j.coi.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhodapkar KM, Feldman D, Matthews P, Radfar S, Pickering R, Turkula S, et al. Natural immunity to pluripotency antigen OCT4 in humans. Proc Natl Acad Sci U S A. 2010;107:8718–23. doi: 10.1073/pnas.0915086107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Kurlander RJ. Comparison of anti-CD3 and anti-CD28-coated beads with soluble anti-CD3 for expanding human T cells: differing impact on CD8 T cell phenotype and responsiveness to restimulation. J Transl Med. 2010;8:104. doi: 10.1186/1479-5876-8-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hatano R, Ohnuma K, Yamamoto J, Dang NH, Morimoto C. CD26-mediated co-stimulation in human CD8(+) T cells provokes effector function via pro-inflammatory cytokine production. Immunology. 2013;138:165–72. doi: 10.1111/imm.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang HY, Wang RF. Enhancing cancer immunotherapy by intracellular delivery of cell-penetrating peptides and stimulation of pattern-recognition receptor signaling. Adv Immunol. 2012;114:151–76. doi: 10.1016/B978-0-12-396548-6.00006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bakker AB, Schreurs MW, de Boer AJ, Kawakami Y, Rosenberg SA, Adema GJ, et al. Melanocyte lineage-specific antigen gp100 is recognized by melanoma-derived tumor-infiltrating lymphocytes. J Exp Med. 1994;179:1005–9. doi: 10.1084/jem.179.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nijman HW, Van der Burg SH, Vierboom MP, Houbiers JG, Kast WM, Melief CJ. p53, a potential target for tumor-directed T cells. Immunol Lett. 1994;40:171–8. doi: 10.1016/0165-2478(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 38.Kawakami Y, Eliyahu S, Jennings C, Sakaguchi K, Kang X, Southwood S, et al. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J Immunol. 1995;154:3961–8. [PubMed] [Google Scholar]
- 39.Salgaller ML, Afshar A, Marincola FM, Rivoltini L, Kawakami Y, Rosenberg SA. Recognition of multiple epitopes in the human melanoma antigen gp100 by peripheral blood lymphocytes stimulated in vitro with synthetic peptides. Cancer Res. 1995;55:4972–9. [PubMed] [Google Scholar]
- 40.Disis ML, Knutson KL, McNeel DG, Davis D, Schiffman K. Clinical translation of peptide-based vaccine trials: the HER-2/neu model. Crit Rev Immunol. 2001;21:263–73. doi: 10.1615/CritRevImmunol.v21.i1-3.170. [DOI] [PubMed] [Google Scholar]
- 41.Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A. 2001;98:14565–70. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lesterhuis WJ, Aarntzen EH, De Vries IJ, Schuurhuis DH, Figdor CG, Adema GJ, et al. Dendritic cell vaccines in melanoma: from promise to proof? Crit Rev Oncol Hematol. 2008;66:118–34. doi: 10.1016/j.critrevonc.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 43.Schuurhuis DH, Verdijk P, Schreibelt G, Aarntzen EH, Scharenborg N, de Boer A, et al. In situ expression of tumor antigens by messenger RNA-electroporated dendritic cells in lymph nodes of melanoma patients. Cancer Res. 2009;69:2927–34. doi: 10.1158/0008-5472.CAN-08-3920. [DOI] [PubMed] [Google Scholar]
- 44.Aarntzen EH, Bol K, Schreibelt G, Jacobs JF, Lesterhuis WJ, Van Rossum MM, et al. Skin-test infiltrating lymphocytes early predict clinical outcome of dendritic cell-based vaccination in metastatic melanoma. Cancer Res. 2012;72:6102–10. doi: 10.1158/0008-5472.CAN-12-2479. [DOI] [PubMed] [Google Scholar]
- 45.Van Nuffel AM, Benteyn D, Wilgenhof S, Pierret L, Corthals J, Heirman C, et al. Dendritic cells loaded with mRNA encoding full-length tumor antigens prime CD4+ and CD8+ T cells in melanoma patients. Mol Ther. 2012;20:1063–74. doi: 10.1038/mt.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oshita C, Takikawa M, Kume A, Miyata H, Ashizawa T, Iizuka A, et al. Dendritic cell-based vaccination in metastatic melanoma patients: phase II clinical trial. Oncol Rep. 2012;28:1131–8. doi: 10.3892/or.2012.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landsberg J, Kohlmeyer J, Renn M, Bald T, Rogava M, Cron M, et al. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature. 2012;490:412–6. doi: 10.1038/nature11538. [DOI] [PubMed] [Google Scholar]
- 48.Labidi-Galy SI, Sisirak V, Meeus P, Gobert M, Treilleux I, Bajard A, et al. Quantitative and functional alterations of plasmacytoid dendritic cells contribute to immune tolerance in ovarian cancer. Cancer Res. 2011;71:5423–34. doi: 10.1158/0008-5472.CAN-11-0367. [DOI] [PubMed] [Google Scholar]
- 49.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–81. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yigit R, Massuger LF, Figdor CG, Torensma R. Ovarian cancer creates a suppressive microenvironment to escape immune elimination. Gynecol Oncol. 2010;117:366–72. doi: 10.1016/j.ygyno.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 51.Kryczek I, Wei S, Zhu G, Myers L, Mottram P, Cheng P, et al. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. 2007;67:8900–5. doi: 10.1158/0008-5472.CAN-07-1866. [DOI] [PubMed] [Google Scholar]
- 52.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 53.Dhodapkar KM, Feldman D, Matthews P, Radfar S, Pickering R, Turkula S, et al. Natural immunity to pluripotency antigen OCT4 in humans. Proc Natl Acad Sci U S A. 2010;107:8718–23. doi: 10.1073/pnas.0915086107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spisek R, Kukreja A, Chen LC, Matthews P, Mazumder A, Vesole D, et al. Frequent and specific immunity to the embryonal stem cell-associated antigen SOX2 in patients with monoclonal gammopathy. J Exp Med. 2007;204:831–40. doi: 10.1084/jem.20062387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kobold S, Tams S, Luetkens T, Cao Y, Sezer O, Bartels BM, Reinhard H, Templin J, Bartels K, Hildebrandt Y, Lajmi N, Marx A, Haag F, Bokemeyer C, Kroger N, Atanackovic D. Patients with multiple myeloma develop SOX2-specific autoantibodies after allogeneic stem cell transplantation. Clin Dev Immunol 2011; 2011:302145. [DOI] [PMC free article] [PubMed]
- 56.Zhao P, Liu C, Xu K, Zheng S, Li H, Xu Y, et al. [Expression of OCT4 protein in bladder cancer and its clinicopathological implications] Nan Fang Yi Ke Da Xue Xue Bao. 2012;32:643–6. [PubMed] [Google Scholar]
- 57.Xu K, Zhu Z, Zeng F. Expression and significance of Oct4 in bladder cancer. J Huazhong Univ Sci Technolog Med Sci. 2007;27:675–7. doi: 10.1007/s11596-007-0614-z. [DOI] [PubMed] [Google Scholar]
- 58.Singh S, Trevino JG, Bora-Singhal N, Coppola D, Haura E, Altiok S, et al. EGFR/Src/Akt signaling modulates Sox2 expression and self-renewal of stem-like side-population cells in non-small cell lung cancer. Mol Cancer. 2012;11:73. doi: 10.1186/1476-4598-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bourguignon LY, Wong G, Earle C, Chen L. Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes miR-302 expression leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells from head and neck squamous cell carcinoma. J Biol Chem. 2012;287:32800–24. doi: 10.1074/jbc.M111.308528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Riggi N, Suvà ML, De Vito C, Provero P, Stehle JC, Baumer K, et al. EWS-FLI-1 modulates miRNA145 and SOX2 expression to initiate mesenchymal stem cell reprogramming toward Ewing sarcoma cancer stem cells. Genes Dev. 2010;24:916–32. doi: 10.1101/gad.1899710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao S, Yuan Q, Hao H, Guo Y, Liu S, Zhang Y, et al. Expression of OCT4 pseudogenes in human tumours: lessons from glioma and breast carcinoma. J Pathol. 2011;223:672–82. doi: 10.1002/path.2827. [DOI] [PubMed] [Google Scholar]
- 62.Guo Y, Liu S, Wang P, Zhao S, Wang F, Bing L, et al. Expression profile of embryonic stem cell-associated genes Oct4, Sox2 and Nanog in human gliomas. Histopathology. 2011;59:763–75. doi: 10.1111/j.1365-2559.2011.03993.x. [DOI] [PubMed] [Google Scholar]
- 63.Prud’homme GJ. Cancer stem cells and novel targets for antitumor strategies. Curr Pharm Des. 2012;18:2838–49. doi: 10.2174/138161212800626120. [DOI] [PubMed] [Google Scholar]
- 64.Tsai L-L, Yu C-C, Chang Y-C, Yu C-H, Chou M-Y. Markedly increased Oct4 and Nanog expression correlates with cisplatin resistance in oral squamous cell carcinoma. J Oral Pathol Med. 2011;40:621–8. doi: 10.1111/j.1600-0714.2011.01015.x. [DOI] [PubMed] [Google Scholar]
- 65.Hu L, McArthur C, Jaffe RB. Ovarian cancer stem-like side-population cells are tumourigenic and chemoresistant. Br J Cancer. 2010;102:1276–83. doi: 10.1038/sj.bjc.6605626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gao MQ, Choi YP, Kang S, Youn JH, Cho NH. CD24+ cells from hierarchically organized ovarian cancer are enriched in cancer stem cells. Oncogene. 2010;29:2672–80. doi: 10.1038/onc.2010.35. [DOI] [PubMed] [Google Scholar]
- 67.Zhi-Iong Ma J, Yang J, Qin JS, Richter A, Perret R, El-Deiry WS, et al. Inefficient boosting of antitumor CD8(+) T cells by dendritic-cell vaccines is rescued by restricting T-cell cytotoxic functions. Oncoimmunology. 2012;1:1507–16. doi: 10.4161/onci.22128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.