Abstract
The healthcare workers having seroprotection at 3 weeks (n = 127) following Pandemic H1N1 2009 influenza vaccination were followed up for antibody persistence. Seroprotection at 12 mo (60.2%) was significantly lower as compared with 3 weeks (74.7%), 3 mo (77.8%) and 6 mo (75.4%). The vaccine provided seroprotection up to one year.
Keywords: antibody persistence, healthcare workers, immune response, influenza vaccination, Pandemic H1N1 2009, post-licensure
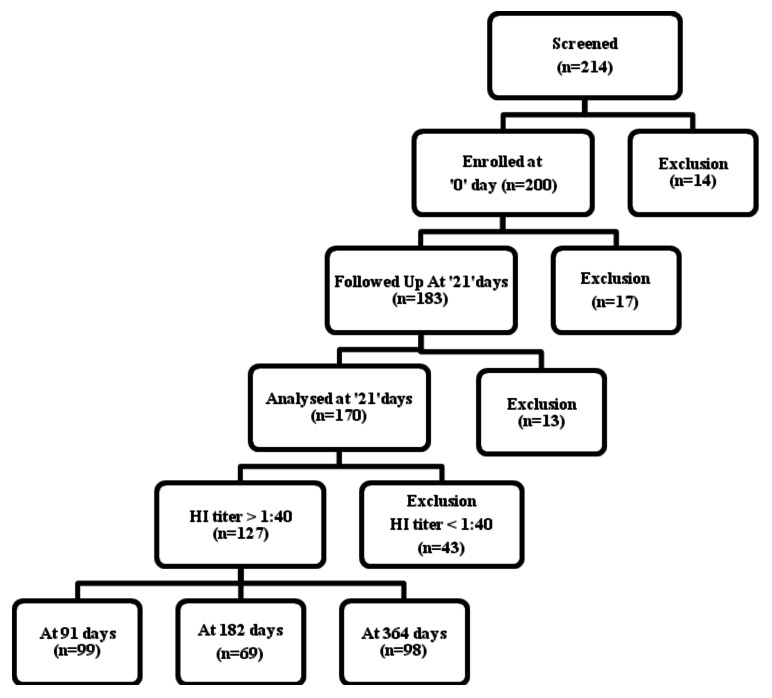
India experienced severe Pandemic H1N1 2009 influenza.1 It caused widespread community transmission, mostly among school-aged children, with the most infections being asymptomatic or mild.2 The Government of India approved a split-virion inactivated unadjuvanted monovalent Pandemic H1N1 2009 influenza vaccine (Panenza) for intramuscular administration of 0.5 ml single dose containing 15 µg of haemagglutinin (HA) units for vaccination of healthcare workers in India in April 2010. The vaccine was reported to be highly immunogenic and safe among 170 vaccinees during the field use in India.3 We studied only 127 vaccinees detected with seroprotection at 3 weeks for antibody persistence at 3, 6 and 12 mo. Participants were allowed to drop out and then be included again at a later time point as indicated in the flowchart (Fig. 1).
Figure 1. Flowchart of vaccinees followed up at different time points.
Healthcare workers included doctors, nurses, counselors, hospital staff and attendants. The ethical approval was obtained from the Institutional Human Ethical Committee. We obtained the written informed consent for participation. Demographic details and co-morbid conditions were recorded at baseline and past or current illness at follow up visits. The vaccinees deposited 5 ml blood samples at 3 weeks and at 3, 6 and 12 mo.
Sera were subjected to haemagglutination inhibition (HI) assay using 0.5% turkey red blood cells and as per WHO recommended protocols.4 Pandemic H1N1 2009 influenza virus A/Jalna/NIV9436/2009(H1N1) (GenBank accession numbers-HM204573; HM241701–07) isolated at the National Institute of Virology was used in the study. It was similar to the A/California/7/2009 vaccine strain of pandemic H1N1 2009 influenza virus. The HI antibody titer of ≥ 1:40 was defined as seroprotection.5 The primary outcome was the percentage of healthcare workers having seroprotection at 3, 6 and 12 mo.
Seroprotection was calculated by considering only the vaccinees that could be followed up at the specified time points. A sample size of 138 subjects was estimated assuming 90% immunogenicity with 5% precision and 95% desired confidence level. Geometric mean titers (GMTs) were also calculated. Seroprotection percentages and GMTs were reported with the 95% confidence intervals (95% CIs). We used Chi-square tests for comparing seroprotection percentages and t-tests for comparing GMTs of antibody levels at 3, 6 and 12 mo follow up with reference to 3 weeks following vaccination.
Among a total of 127 vaccinees considered for the follow up study (Table 1), majority were males (59.8%) and young adults (64.6% aged 18–39 y). We used the age of 40 y as the cut-off for younger vs. older adults as reported in influenza vaccine trials.5 Underlying co-morbid conditions were reported by 34 vaccinees including obesity (body mass index ≥ 30 Kg/m2) among 18 vaccinees. Baseline seroprotection before vaccination was minimal. None of the vaccinee received the seasonal influenza vaccination in the past. Pandemic H1N1 2009 influenza illness confirmed by RT-PCR was reported by 5 vaccinees before vaccination. Only 2 of these 5 vaccinees had baseline seroprotection.
Table 1. Demographic data and co-morbidity status of 127 followed up vaccinees.
| Characteristics | No. (%) of vaccinees |
|---|---|
| Gender |
|
| Males |
76 (59.8) |
| Females |
51 (40.2) |
| Age (years) |
|
| 18–39 |
82 (64.6) |
| 40–59 |
45 (35.6) |
| Co-morbidity |
34 (26.8) |
| Obesity |
18 (14.2) |
| Baseline seroprotection |
11 (8.7) |
| PCR-confirmed Pandemic H1N1 2009 illness | 5 (3.9) |
PCR, polymerase chain reaction.
The follow up could be achieved in 99, 69 and 98 vaccinees at 3, 6 and 12 mo respectively (Fig. 1). These included 65 vaccinees sampled at all three time points. The percentage seroprotection and GMTs with 95% CIs at 3 weeks and at 3, 6 and 12 mo are presented in Table 2. The seroprotection at 3 mo and 6 mo was not significantly different than at 3 weeks. Whereas, only 60.2% (95% CI 50.5–69.8) vaccinees had seroprotection at 12 mo as compared with 74.7% (95% CI (68.2–81.2) vaccinees at 3 weeks (p < 0.05). However, GMTs of HI antibody titers at 3 mo, 6 mo and 12 mo were not significantly different than at 3 weeks.
Table 2. Seroprotection levels and geometric mean titers at different follow up time points.
| Time points | No. with seroprotection/No. investigated* | Seroprotection % (95% confidence interval) | P value | Geometric mean titers (95% confidence interval) | P value |
|---|---|---|---|---|---|
| 3 weeks |
127/170 |
74.7 (68.2–81.2) |
Reference |
84.7 (67.3 - 106.5) |
Reference |
| 3 mo |
77/99 |
77.8 (69.6 - 86.0) |
> 0.05 |
76.2 (60.3 - 96.3) |
> 0.05 |
| 6 mo |
52/69 |
75.4 (65.2 - 85.5) |
> 0.05 |
64.1 (48.0 - 85.7) |
> 0.05 |
| 12 mo | 59/98 | 60.2 (50.5 - 69.8) |
< 0.05 | 42.9 (33.9 - 54.3) |
> 0.05 |
Indicates the vaccinees available for follow up at different time points after vaccination.
In a subset of 65 vaccinees sampled at all three follow up time points, seroprotection was 61.5% (95% CI 49.3–72.7) at 12 mo as against 75.4% (95% CI 63.9–84.7) at 3 mo and 76.9% (95% CI 65.6–86.0) at 6 mo. We did not find significant difference in seroprotection between males and females; and between young adults (18–39 y) and older adults (40–59 y) at all the time points. Influenza like illness was not reported by any vaccinee during the follow up.
The present study provides information about the antibody persistence following a single dose of Pandemic H1N1 2009 influenza vaccine among healthcare workers during the year 2010 in Pune, India. There was a slightly higher seroprotection at 3 mo (77.8%) as compared with 3 weeks (74.7%). This could be due to boosting of immunity by natural subclinical or asymptomatic infections during the ongoing influenza season. The seroprotection at 6 mo was 75.4%. Similar findings were also noted in a subset of 65 vaccinees sampled at all three follow up time points in our study. In a clinical trial using a similar vaccine, 76.8% adults had seroprotection at 6 mo.6 Similarly, 87% seroprotection was reported at 6 mo in a clinical trial involving 53 adults.7 However, only 35.1% vaccinees had seroprotection at 9 mo in a field evaluation in China.8 Similarly, only 34% of 86 vaccinees had seroprotection at 6 mo in China.9 The antibody persistence in 75.4% vaccinees at 6 mo in our study is acceptable during field use.
At 12 mo, 60.2% vaccinees had seroprotection in our study. The lung transplant patients maintained seroprotection for approximately 11 mo between seasons.10 Seroprotection rate in all age groups declined markedly over the 12-mo period in a study of seasonal trivalent vaccine in Korea.11 Such evidence is needed in guiding the vaccination policies for optimal timing of vaccination campaigns in relation to influenza seasonality. Influenza like illness was not reported by the vaccinees during the follow up in our study. However, under-reporting of influenza like illnesses could not be ruled out in the absence of active surveillance. In addition, this study has a weakness in terms of a very small data set available at follow up. We did not find any gender and age-group wise difference in seroprotection as reported in earlier studies.8,9 We could not compare obese and non-obese groups due to inadequate sample size.
In conclusion, a single dose of the monovalent Pandemic H1N1 2009 influenza vaccine provided seroprotection up to one year among adults indicating the expected performance of the vaccine in Indian population during field use.
Acknowledgments
We acknowledge the Pune District Health Authorities for the help and support during field activities. We are also thankful to Mr. P.A. More and Miss S. Pagar for their technical contributions in field activities and data management respectively.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The Indian Council of Medical Research provided the funding.
Contributors
B.V.T. planned and executed the study, managed and analyzed data, wrote the manuscript and approved the final submission as corresponding author. S.D.P. managed specimen-testing protocols, analyzed and interpreted data, revised and approved the manuscript. Y.K.G. contributed in planning, execution and data management and reviewed and approved the final version of the manuscript. S.S.P. managed the assay protocols and performed assays. A.C.M. provided administrative support, provided inputs for study design, reviewed and approved the manuscript for submission.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/22421
References
- 1.Mishra AC, Chadha MS, Choudhary ML, Potdar VA. Pandemic influenza (H1N1) 2009 is associated with severe disease in India. PLoS ONE. 2010;5:e10540. doi: 10.1371/journal.pone.0010540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tandale BV, Pawar SD, Gurav YK, Chadha MS, Koratkar SS, Shelke VN, et al. Seroepidemiology of pandemic influenza A (H1N1) 2009 virus infections in Pune, India. BMC Infect Dis. 2010;10:255. doi: 10.1186/1471-2334-10-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tandale BV, Pawar SD, Mishra AC. Immunogenicity and safety of pandemic H1N1 2009 vaccine among adults in field use, India. Vaccine. 2012;30:2043–4. doi: 10.1016/j.vaccine.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Manual on animal influenza diagnosis and surveillance. WHO/CDS/CSR/NCS; 2002.
- 5.Sanofi Pasteur. Panenza Pandemic influenza vaccine (H1N1) (split-virion, inactivated). Public Assessment Report. FR/H/447/01-02/DC. Available at: http://afssaps-prd.afssaps.fr/html/par eu/20091113 fr447 panenza par.pdf (accessed on 25.01.10).
- 6.Lai YC, Yang KC, Hsieh SM, Yao CA, Lee LT, Huang KC. Persistence of immunogenicity of a monovalent influenza virus A/H1N1 2009 vaccine in healthy volunteers. Clin Vaccine Immunol. 2012;19:429–35. doi: 10.1128/CVI.05528-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crum-Cianflone NF, Iverson E, Defang G, Blair PJ, Eberly LE, Maguire J, et al. Durability of antibody responses after receipt of the monovalent 2009 pandemic influenza A (H1N1) vaccine among HIV-infected and HIV-uninfected adults. Vaccine. 2011;29:3183–91. doi: 10.1016/j.vaccine.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan KH, To KK, Hung IF, Zhang AJ, Chan JF, Cheng VC, et al. Differences in antibody responses of individuals with natural infection and those vaccinated against pandemic H1N1 2009 influenza. Clin Vaccine Immunol. 2011;18:867–73. doi: 10.1128/CVI.00555-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M, Yuan J, Li T, Liu Y, Wu J, Di B, et al. Antibody dynamics of 2009 influenza A (H1N1) virus in infected patients and vaccinated people in China. PLoS ONE. 2011;6:e16809. doi: 10.1371/journal.pone.0016809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moran JJ, Rose WE, Darga AJ, Rohde KA, Hayney MS. Persistence of influenza vaccine-induced antibodies in lung transplant patients between seasons. Transpl Infect Dis. 2011;13:466–70. doi: 10.1111/j.1399-3062.2011.00654.x. [DOI] [PubMed] [Google Scholar]
- 11.Song JY, Cheong HJ, Hwang IS, Choi WS, Jo YM, Park DW, et al. Long-term immunogenicity of influenza vaccine among the elderly: Risk factors for poor immune response and persistence. Vaccine. 2010;28:3929–35. doi: 10.1016/j.vaccine.2010.03.067. [DOI] [PubMed] [Google Scholar]