Abstract
Elderly people are at increased risk of influenza and pneumococcal diseases. Influenza increases clinical pneumococcal disease incidence. Pneumococcal vaccination could therefore be a supplement to influenza vaccination. This study evaluated all-cause mortality and antibiotic consumption according to elderly people’s influenza and pneumococcal vaccination status. Its goal was to demonstrate that vaccination with both Influenza and pneumococcal vaccines decrease all-cause mortality and antibiotic consumption. From 2004-10-01 to 2004-12-31 (3 mo), elderly people (≥ 65 y) who lived in the Gard department (South of France) were offered both vaccinations. Among the 68,897 subjects followed-up one year after this vaccination campaign, 21,303 (30.9%) were vaccinated with both vaccines, 18,651 (27.1%) with influenza vaccine alone, 3,769 (5.5%) with pneumococcal vaccine alone; 25,174 (36.5%) subjects were unvaccinated. Mortality rate (per 1,000 inhabitants-year) adjusted on gender, age and prior underlying chronic disease was 17.9 (95% CI: 16.3–19.6), 20.8 (19.0–22.8), 22.5 (19.0–26.6) and 24.7 (22.7–26.8), respectively. It was 42.1 (38.8–45.8) in elderly people with underlying chronic disease who received both vaccines vs. 58.1 (53.7–62.9) in unvaccinated elderly people. The decrease in mortality rate was 27.0% (20.0–34.0) in subjects who received both vaccines and 16.0% (6.0–24.0) in those who received influenza vaccine. No significant reduction in mortality rate was seen with the pneumococcal vaccine alone. Influenza and/or pneumococcal vaccinations did not decrease antibiotic consumption that drastically increases during the winter period. An additive effect was observed in the prevention of all-cause mortality with influenza and pneumococcal vaccines given together in elderly people, including in those with underlying chronic disease.
Keywords: 23-valent pneumococcal capsular polysaccharide vaccine, additive effect, cohort study, elderly, influenza vaccine, treatment outcome
Introduction
Influenza can have serious consequences in the elderly. Annual influenza vaccination is the most effective method for preventing influenza infection and its complications.1–3 It has been shown to be effective in preventing influenza virus infection and reducing the risk of pneumonia, hospital admission and death.1,3 In 2005, annual vaccination was recommended for elderly people by the World Health Organization (WHO)1 and for individuals ≥ 65 y of age by the French national health authorities.4 In France, a mail-based household survey found an influenza vaccine coverage rate of 70% in elderly people during the 2005–06 influenza season5 and the estimate influenza vaccine coverage was 62.6% and 62.7% for the 2008–2009 and 2009–2010 seasons according to a retrospective cross-sectional survey.6
Streptococcus pneumonia is the most common cause of both pneumonia and severe pneumonia. In industrialized countries, the incidence of pneumococcal disease is particularly high in young children (< 2 y) and elderly people (≥ 65 y).7 The 23-valent pneumococcal vaccination is recommended by the WHO and in some industrialized countries8 for selected groups of subjects, including elderly people, in particular those living in institutions. In 2005, the French national health authorities4 recommended pneumococcal vaccination every 5 y for elderly people only if they are at high risk of pneumococcal invasive infections (e.g., chronic organ failure, immunodepression, diabetes) or when they are being admitted in institutions.4,9 Efficacy of pneumococcal vaccine to prevent pneumococcal bacteremia and pneumonia in elderly people has been shown in case-control10–14 and cohort15–17 studies. In contrast, prospective randomized studies have provided inconclusive evidence for the benefits of the 23-valent pneumococcal vaccine in elderly people in reducing pneumonia, hospitalization for pneumonia and death18–22 whereas other demonstrated its efficacy.23 This lack of clear efficacy evidence and the absence of reimbursement by the national health insurance in France probably partly explain the low vaccination coverage rate reported in the elderly, including in those at higher risk of pneumococcal disease (i.e., residents of nursing homes).24, 25
Influenza increases the risk of pneumococcal disease incidence.26 Pneumococcal vaccination could therefore be a supplement to influenza vaccination for the elderly. However, before 2005, little data on combined effect of both vaccines were available and most of these data were issued from studies with major methodological shortcomings.10 For ethical considerations, placebo-controlled trials are not possible to assess the efficacy of both these vaccines in the prevention of severe influenza, pneumonia and deaths in elderly people. Studies in several countries were therefore needed to estimate sustained additive preventive effects of both vaccines as performed in the North of Europe.27,28 An observational cohort pilot study was thus implemented in one French department to assess the efficacy of each of the two vaccines and their additive effect among elderly people. Its primary evaluation criterion was all-cause mortality. Its secondary evaluation criterion was the antibiotic consumption within the year following the vaccination campaign: it was expected that antibiotic consumption would decrease during the winter period with the vaccine-related decrease in pulmonary infections or complications. Results of this study have been partly presented as a poster at the 7th Journées Nationales d’Infectiologie, Bordeaux, France (June 6–7, 2006), but were never published. A recent retrospective cohort study performed in Taiwan29 showed that pneumococcal vaccine provides additional protection to the elderly who have already been vaccinated with influenza vaccine: all-cause mortality and hospitalization decrease in elderly subjects vaccinated by both vaccines as compared with influenza vaccine alone. In addition, a literature review concluded that 8 of 9 clinical studies found that a concomitant vaccination program conferred clinical benefits.30 The aim of this article is thus to present results of this pilot study, because they confirm results of the recent studies and provide additional information.
Results
Baseline characteristics
Out of the 117,229 inhabitants of the French department aged 65 y and older, 68,897 (58.8%) were identified by the health insurance database and included in this cohort after GP consultation. Of them, 60.2% were female and 43.7% lived with at least one underlying chronic disease (ALD30). For further details, please see Material and Methods and Appendix). Their mean age was 75.2 ± 7 y (range: 65−102). Table 1 shows the distribution of the study population by gender, presence of at least one ALD30, age group and vaccination status. Vaccination rate was 58.0% for influenza vaccine (27.1% + 30.9%), 36.4% for pneumococcal vaccine (5.5% + 30.9%) and 30.9% for both vaccines.
Table 1. Baseline characteristics of the cohort during the vaccination campaign, from 04-10-01 to 2004-12-31 (n = 68,897).
| (%) | ALL | BOTH | IV | PV | NONE | p-value |
|---|---|---|---|---|---|---|
| Total |
100.0 |
30.9 |
27.1 |
5.5 |
36.5 |
< 0.0001 |
| Gender |
|
|
|
|
|
< 0.0001 |
| Men |
39.8 |
33.6 |
25.9 |
5.8 |
34.7 |
|
| Women |
60.2 |
29.1 |
27.8 |
5.3 |
37.8 |
|
| Presence at least one ALD30 |
< 0.0001 |
|||||
| Yes |
43.7 |
36.8 |
27.4 |
6.3 |
29.5 |
|
| No |
56.3 |
26.4 |
26.8 |
4.8 |
42.0 |
|
| Age-group (years) |
|
|
|
|
|
< 0.0001 |
| 65–69 |
25.1 |
26.3 |
23.6 |
5.2 |
44.9 |
|
| 70–74 |
26.6 |
30.7 |
26.2 |
5.2 |
37.9 |
|
| 75–80 |
25.3 |
33.6 |
28.2 |
5.6 |
32.6 |
|
| > 80 | 23.0 | 33.2 | 30.6 | 5.9 | 30.3 | |
ALD30, 30 long-lasting affections (e.g., chronic organ failure, diabetes, immunodepression); ALL, all groups together; BOTH, Influenza and Pneumococcal vaccine; IV, influenza vaccine alone; NONE, unvaccinated; PV, pneumococcal vaccine alone.
Vaccination rate was statistically significantly higher in men than in women and in persons living with at least one ALD30. A statistically significant increasing vaccination rate with age was observed.
Incidence of mortality
Between 2005-01-01 and 2005-12-31, 2,261 persons died from all causes. The all-cause mortality rate in this cohort was 34.1/1,000 PY [95% CI: 32.7–35.6]. Mortality rate adjusted on gender, living with at least one ALD30 and age-group was 24.7/1,000 PY [22.7–26.8] for the unvaccinated group, 22.5/1,000 PY [19.0–26.6] for the pneumococcal vaccine group, 20.8/1,000 PY [19.0- 22.8] for the influenza vaccine group and 17.9/1,000 PY [16.3–19.6] for influenza + pneumococcal group.
Table 2 also shows adjusted mortality rate in each of the vaccine groups according to gender, existing of underlying disease (ALD30) and age.
Table 2. All cause-mortality rate per 1,000 PY [95% CI] according to vaccine groups between 2005-01-01 and 2005-12-31 (n = 68,897).
| BOTH | IV | PV | NONE | |
|---|---|---|---|---|
| Deaths |
702 |
638 |
150 |
771 |
| Mortality Rate (a) |
17.9 [16.3–19.6] (b) |
20.8 [19.0–22.8] (b) |
22.5 [19.0–26.6] (c) |
24.7 [22.7–26.8] |
| Gender |
|
|
|
|
| Women |
346 |
358 |
84 |
426 |
| |
14.4 [13.0–15.9] |
16.7 [15.1–18.5] |
18.1 [15.2–21.5] |
19.8 [18.1–21.7] |
| Men |
356 |
280 |
66 |
345 |
| |
22.3 [20.2–24.6] |
26.0 [23.4–28.4] |
28.1 [23.6–33.4] |
30.8 [28.0–33.8] |
| ALD30 |
|
|
|
|
| Yes |
632 |
544 |
131 |
633 |
| |
42.1 [38.8–45.8] |
49.0 [45.0–53.5] |
53.0 [45.0–62.4] |
58.1 [53.7–62.9] |
| No |
70 |
94 |
19 |
138 |
| |
7.6 [6.7–8.7] |
8.8 [7.8–10.1] |
9.6 [7.9–11.6] |
10.5 [9.3–11.8] |
| Age-group |
|
|
|
|
| 65–70 y |
61 |
42 |
16 |
93 |
| |
9.3 [7.9–10.8] |
10.8 [9.2–12.6] |
11.7 [9.5–14.4] |
12.8 [11.1–14.8] |
| 70–74 y |
107 |
78 |
16 |
123 |
| |
12.4 [10.9–14.2] |
14.4 [12.6–16.5] |
15.6 [12.8–18.9] |
17.1 [15.1–19.4] |
| 75–80 y |
147 |
137 |
34 |
171 |
| |
18.4 [16.3–20.6] |
21.4 [19.0–24.0] |
23.1 [19.2–27.7] |
25.3 [22.6–28.3] |
| > 80 y |
387 |
381 |
84 |
384 |
| 48.6 [44.1–53.6] | 56.4 [51.2–62.4] | 61.1 [51.4–72.6] | 67.0 [61.0–73.6] |
ALD30, 30 long-lasting affections (e.g., chronic organ failure, diabetes, immunodepression); BOTH, Influenza and Pneumococcal vaccines group; IV, influenza vaccine alone; NONE, unvaccinated; PV, pneumococcal vaccine alone; PY, (inhabitants) per year; (a) mortality rate per 1,000 PY, adjusted on gender, existing of ALD30 and age [95% CI]; (b) p < 0.05, compared with the group of unvaccinated subjects; (c) p > 0.05, compared with the group of unvaccinated subjects; n, number of deaths; CI, 95% confidence interval
The lowest adjusted mortality rate was observed in healthy elderly people vaccinated with both influenza and pneumococcal vaccines (7.6/1,000 PY) whereas the highest adjusted mortality rate was seen in unvaccinated elderly people aged 80 y and older (67.0/1,000 PY). Regardless of the vaccine group, adjusted mortality rate was lower in women than in men and in healthy than elderly people with at least one ALD30. Adjusted mortality rate increased gradually across high age-class.
Risk of dying and reduction of all-cause mortality
The risk of dying within one year adjusted on gender, ALD30 and age was 0.91 [0.77–1.08] for persons receiving pneumococcal vaccine, 0.84 [0.76–0.94] for influenza vaccine and 0.73 [0.66–0.80] for those receiving the both vaccines, according to the multivariate Cox regression analysis. Thus, the reduction of all-cause mortality associated with pneumococcal, influenza and the both vaccines was respectively 9.0% [-8.0–23.0], 16.0% [6.0–24.0] and 27.0% [20.0–34.0]. Table 3 shows the adjusted risk of dying according to the gender, existing of chronic diseases and age-class.
Table 3. Risk* of all-cause mortality according to vaccine groups between 2005-01-01 and 2005-12-31.
| Risk | Hazard Ratio | p-value | Mortality decrease |
|---|---|---|---|
| |
[95% CI] |
|
[95% CI] |
| Vaccination |
|
|
|
| No vaccination |
1 |
|
|
| Pneumococcal vaccination |
0.91 [0.77–1.08] |
0.30 |
9.0 [-8.0–23.0] |
| Influenza vaccination |
0.84 [0.76–0.94] |
0.001 |
16.0 [6.0–24.0] |
| Influenza + Pneumococcal vaccination |
0.73 [0.66–0.80] |
< 0.001 |
27.0 [20.0–34.0] |
| Existing of chronic diseases |
|
|
|
| Healthy elderly persons |
1 |
|
|
| Chronic disease (ALD 30) |
5.52 [4.90–6.23] |
< 0.001 |
|
| Gender |
|
|
|
| Female |
1 |
|
|
| Male vs. female |
1.55 [1.43–1.69] |
< 0.001 |
|
| Age (in years) |
|
|
|
| 70–74 |
1.34 [1.12–1.59] |
< 0.001 |
|
| 75–79 |
1.98 [1.68–2.32] |
< 0.001 |
|
| ≥ 80 | 5.20 [4.49–6.04] | < 0.001 |
Note: Multivariate Cox regression analysis in all patients adjusted for all variables listed in the table. ALD30, 30 long-lasting affections; CI, confidence interval. *Adjusted on gender, existing of chronic diseases and age.
Antibiotic consumption
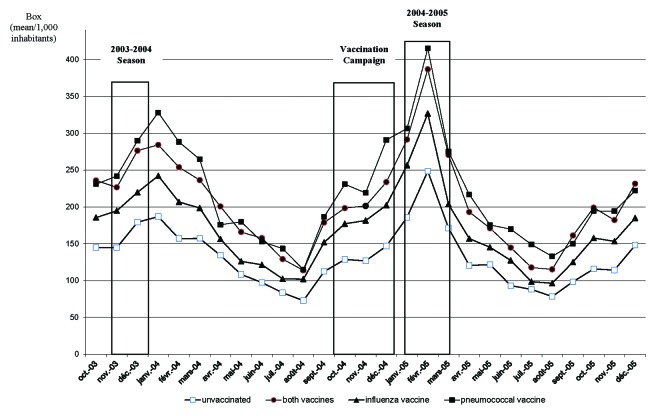
Figure 1 shows antibiotic consumption according to the vaccine groups from one year before to one year after the vaccination campaign (2003-10-01 to 2005-12-31) of vaccinated and unvaccinated elderly.
Figure 1. Antibiotic use one year before and one after vaccination campaign per vaccine groups (from 2003-10-01 to 2005-12-31).
Antibiotic consumption increased during influenza outbreaks. The influenza epidemic peak was observed during the week 49 for the 2003/2004 season and the week 6 for the 2004/2005 season (http://ww.grog.org/documents/Semaine_franchissement_seuil_epidemique.pdf). The influenza peak preceded shortly that of antibiotic consumption. No difference in antibiotic consumption was seen between vaccine groups before and after the vaccination campaign (p > 0.05).
Discussion
This study demonstrated that vaccination with influenza vaccine in the fall of 2004 was associated with a decrease of all-cause mortality in elderly people during the year following vaccination. An additive effect was observed in the prevention of all-cause mortality when elderly people were vaccinated with both influenza and pneumococcal vaccines. No significant reduction in mortality was seen with the pneumococcal vaccine alone. The additive effect was observed including in frail elderly people (i.e., those with underlying chronic disease or aged 80 y and older). This study failed to demonstrate that vaccination with influenza and/or pneumococcal vaccines decreased antibiotic consumption during the year following vaccination.
In our knowledge, this study is one of the largest observational studies on influenza and pneumococcal vaccinations in elderly people in France. Administrative data were used to prospectively identify deaths one year after the vaccination period and to retrospectively and prospectively record antibiotic consumption within the year before and after the vaccination period. This design helped to avoid some methodological bias observed in case control or in population-based cross-sectional studies. However, this study presents some limitations. First, as it was a cohort study evaluating a few numbers of variables (and not a randomized study), it can be hypothesized that non-identified and/or non-identifiable variables could interfere with the present results. For example, the vaccination status among seniors before the vaccination campaign was not collected during this study. One could hypothesize that a history of flu during the preceding season can lead some seniors to be vaccinated the following year; or conversely to be unvaccinated (“I am protected against the flu because I got it last year”). In addition, it must be noted that in France, each year, only half of seniors are vaccinated against influenza.
Second, this study evaluated all-cause mortality and third, although the seasonal periodicity of influenza and pneumococcal deaths in temperate countries is well documented, the study period lasted one year. The effectiveness (i.e., prevention of illness in vaccinated population) of influenza vaccines depends on the outcome being measured.3 Multiple outcome measures, including the prevention of medically attended acute respiratory illness, prevention of laboratory-confirmed influenza virus illness, prevention of influenza or pneumonia-associated hospitalizations or deaths, prevention of all-cause hospitalizations or deaths, are possible. Effectiveness for non-specific outcomes will be lower than for more specific outcomes as they can be due to other causes that influenza and/or pneumococcal vaccination would not be expected to prevent. Conversely, results might be biased if healthier persons are more likely to be vaccinated, for example. However, we thought that specific mortality assessment during the influenza season would be too restrictive. Indeed, first, a diagnosis of influenza virus (or pneumococcal) infection is rarely confirmed with sensitive and specific laboratory methods and rarely recorded on death certificate. Second, it has been shown that infection increases the risk of myocardial infarction31 and that impaired respiratory function increases the risk of cardiovascular mortality.32 Third, whereas influenza infection increases the risk of complications and deaths in elderly people3, vaccination reduces the need for hospitalization in chronic respiratory conditions, cardiac disease and stroke.28 Finally, the drastic increase in antibiotic consumption during the 2004/2005 influenza outbreak followed by its progressive decrease tended to prove that influenza led to complications that lasted at least several months.
Finally, the intensity of the additive effect of the influenza and pneumococcal vaccinations found during this study probably reflects the matching between the vaccine strains and the circulating influenza viruses. It would be different for another year with other circulating and vaccine influenza strains. During the 2004/2005-influenza season, the matching between the vaccine strains (A/New Caledonia/20/99(H1N1)-like virus; A/Fujian/411/2002(H3N2)-like virus and B/Shanghai/361/2002-like virus) and the circulating viruses was not optimal in particular for the H3N2 strain. Moreover, influenza A (H3N2) viruses were associated with widespread outbreaks in several countries33. A better matching would probably induce a better additive effect.
In this study, the influenza vaccination coverage rate was 58.0%. This rate was slightly lower than that reported in more recent studies in France5, 6. This difference can be partly explained by the fact that most of the studies evaluating influenza vaccine were mail or telephone based. In addition, this study was performed before the H5N1 epidemic alert in 2005 and the H1N1 pandemic in 2009. As in many European countries, the influenza vaccination rate in elderly people has been low in France despite the implementation of age-based recommendations 10 y ago33,34. In recent studies, a trend of increasing vaccination coverage rate was observed in Europe and France34,35. The pneumococcal vaccination coverage rate in France is less than 20% probably because the pneumococcal vaccine has not been reimbursed by the health insurance, except for frail elderly24. In this study, the pneumococcal vaccine was reimbursed by the local health insurance and therefore the vaccination coverage rate was higher (36.4%), even if inferior to the influenza coverage rate.
The large number of individuals receiving one of the two vaccines permitted to evaluate the effectiveness of each vaccine separately. The influenza vaccine was associated with all-cause mortality reduction of 16.0% (95% CI: 6.0–24.0). This result supports previous findings35–39. The pneumococcal vaccination alone in this study was not significantly associated with reduction of all-cause mortality. In the group who received influenza and pneumococcal vaccines, the mortality reduction compared with the unvaccinated group was 27.0%. Thus, the two vaccines had clearly additive effect in the prevention of all-cause mortality when taking into account of age and chronic medical conditions, which corroborate the findings of previous studies28,29.
Regarding antibiotic use, overall, elderly people obviously took more antibiotics during the 2004/2005 than the 2003/2004-influenza season. The difference between the two influenza outbreaks possibly explained this difference. Whereas both influenza outbreaks were due to the same influenza virus type AH3N2 strain and had similar amplitude (approximately 4,000,000 cases), they differed by their targets. Influenza virus targeted younger people during the 2003/2004 than the 2004/2005-influenza season (http://www.grog.org/documents/Bilan_GROG_03_04.pdf; http://www.grog.org/documents/Bilan_GROG_04_05.pdf). The attack rate in the elderly population was 2.4% for the 2003/2004 season and 4.6% for the 2004/2005-influenza season (http://www.grog.org/documents/Impact_grippe_0109.pdf). Moreover, no significant difference was observed between vaccine groups in the number of boxes of antibiotics delivered to the elderly people. This result is not surprising as influenza vaccine effectiveness in preventing influenza-like illness (that can be due to other pathogens and are frequent during the winter season) is low in elderly people even when its effectiveness is satisfactory in preventing influenza-related death. In the study by Jefferson et al.39,40, in elderly individuals living in the community, influenza vaccines were not significantly effective against influenza, influenza-like illness or pneumonia but prevented hospital admission for influenza and pneumonia and all-cause mortality.
Finally, it can be concluded from the results of this study that influenza and pneumococcal vaccines have an additive effect, reducing all-cause mortality in elderly people, including in those with underlying disease. It can also be concluded that provision of a voucher for free vaccination improves pneumococcal vaccination coverage. These findings should improve pneumococcal vaccination: e.g., free voucher for pneumococcal vaccination in elderly people, concomitant administration of influenza and pneumococcal vaccines, specific vaccination programs with scheduled appointment in elderly people.
Material and Methods
Vaccination campaign
A vaccination campaign was conducted from 2004-10-01 to 2004-12-31 in the Gard, part of the Languedoc-Roussillon region, situated in the southeast of France, which comprised 623,058 inhabitants according to the census of 1999 (http://www.insee.fr/fr/themes/document.asp?ref_id=3231). Out of them, 117,229 inhabitants (18.8%) were ≥ 65 y of age. This vaccination campaign was organized by the departmental health insurance of Gard with the help of the GPs, pharmacists and the French medical association. It was advertised in the daily newspapers and on the local radio.
In addition, all people aged ≥ 65 y of age living in the Gard and identified in the health insurance database received a letter explaining the campaign and the opportunity to receive both influenza and pneumococcal vaccines. People aged ≥ 65 y of age were invited to receive influenza and pneumococcal vaccines during a period of 3 mo.
Study design
The study was an observational cohort study.
All elderly people seen by their GP received a voucher for free-vaccination if they had no contraindication to influenza or pneumococcal vaccination. Both vaccines were delivered by a pharmacist. The elderly people agreeing to be vaccinated were vaccinated by their GP during a second visit. Four groups of subjects were identified: subjects vaccinated with both influenza and pneumococcal vaccines (BOTH), subjects vaccinated with influenza vaccine alone (IV), subjects vaccinated with pneumococcal vaccine alone (PV) and unvaccinated subjects (NONE). Deaths were recorded during the year following the beginning of the study and antibiotic consumption within the year before and after the vaccination period for all the elderly people initially seen by the GPs. Mortality rate and antibiotic consumption were defined according to vaccination status.
The study was approved by the Commission Nationale de l’Informatique et des Libertés, the French information protection commission and by the Nîmes University hospital Institutional Review Board of Nimes.
Vaccination and vaccine groups
According to the WHO recommendations, the influenza vaccines for the 2004/2005-influenza season (Northern Hemisphere) contained the following strains: A/New Caledonia/20/99(H1N1) like, A A/Fujian/411/2002(H3N2) like and B/Shanghai/361/2002 like. Pneumococcal vaccine was 23-valent pneumococcal polysaccharide (Pneumo 23, Marcy l’Etoile, Sanofi Pasteur MSD Aventis, France).
In France, since 2000, influenza vaccination is reimbursed for all elderly people by the French national health insurance. Pneumococcal vaccination is indicated for immunization against pneumococcal infections for all patients older than 2 y, but only reimbursed for persons with underlying chronic diseases. In this study, the pneumococcal vaccine was reimbursed for elderly people (≥ 65 y) by the departmental health insurance regardless of the presence of underlying chronic disease.
Data collection
Information on individual’s name was recorded into the departmental health insurance database. For each vaccinated and unvaccinated subjects, the departmental health assurance had information on age, sex, underlying chronic diseases and antibiotic consumption.
Information on underlying chronic diseases was recorded by the French health insurance about 30 long-lasting affections (ALD30, for further details please see Appendix). Diagnosis concerning each ALD30 was coded according to the International Classification of Disease, 10th revision (ICD-10-CM).
The consumption of antibiotics known to be used in lower respiratory tract infection in the Elderly (e.g., penicillins or penicillin combinations as first-line treatment and macrolides, fluoroquinolones and cephalosporins as second-line treatment) was retrospectively and prospectively collected one year before and one year after the vaccination campaign period using the departmental health insurance database. Mortality was prospectively collected using the same database.
Statistical analysis
The primary endpoint was the all-cause mortality rate within one year after the vaccination period (2004-10-01 to 2005-12-31). The second endpoint was antibiotic use one year before and one year after vaccination period (2003-10-01 to 2005-12-31). All collected data of the study were anonymized before statistical analysis.
Statistical analyses were conducted using SAS (SAS Institute, V 8.01). Baselines characteristics between the four vaccine groups were compared using chi2-test for qualitative variables. Poisson regression (GENMOD procedure) was used to compute in each vaccine group, all-cause mortality rate per 1,000 PY with their 95% CIs adjusted on gender, ALD30 status and age. Cox hazard model was used to compute HR of all-cause mortality in the three vaccinated groups taking unvaccinated group as reference. The Cox hazard model was proportional hazard for all included variables [graphical (Log-Log) and one time-dependent variable]. The reduction in all-cause mortality was calculated from the HR as (1-HR) x 100%. The adjusted mean difference in antibiotic consumption before and after the campaign was compared between vaccine groups using the general linear model.
Appendix
-
30 underlying chronic disease called 30 long-lasting affections (ALD30) and coded according to the ICD-10-CM.
Sequel of cerebrovascular disease (I69)
Aplastic anemia (D6.19)
Chronic and progressive arteriosclerosis vascular disease (I70) includes coronary disease (I25.1) with clinical ischemic manifestations.
Complicated schistosomiasis (B659)
Serious congenital malformation of heart (Q24.9), congestive heart failure (I50.0), valve diseases (I39.8)
Liver cirrhosis (K74.6)
Primary severe immunodeficiency or acquired severe immunodeficiency (D80-D84)
Insulin-dependent (E10) or non-insulin-dependent (E11) diabetes mellitus.
Severe diseases of myoneural junction and muscle include myopathy (G70-G73)
Homozygous hemoglobinopathy (D55-D59)
Hereditary factor VIII or factor IX deficiency (D66-D67)
Severe arterial hypertension (I10)
Acute myocardial infarction (I21)
Chronic respiratory failure (J96.1)
Severe Hansen’s disease (Leprosy) (A30.9)
Parkinson disease (G20)
Hereditary metabolic disorders requiring a specific long treatment (E70-E90)
Cystic fibrosis (E84)
Primary nephritic syndrome and severe chronic nephropathy (N04)
Paraplegia (G82.2)
Inflammatory polyarthritis (M30.0), systemic lupus erythematous (M32.9), progressive systemic sclerosis (M34.0)
Serious rheumatoid arthritis (M06.9)
Psychosis (F29), mental retardation (F70-F79), disorders of adult personality and behavior (F69)
Progressive ulcerative colitis (K51.9) and Crohn's disease (K50.9)
Serious multiple sclerosis (G35)
Active scoliosis (M41.9)
Severe ankylosing spondylitis (M45)
Transplanted organ and tissue status (Z94)
Active tuberculosis (A15-A19)
Malignant neoplasms of lymphoid, hematopoietic and related tissue (C96.9)
Acknowledgments
We thank Mr. Stéphane Vergne from the Departmental health insurance for his helpful assistance in data management and Mrs. Fabienne Péretz, independent medical writer.
Glossary
Abbreviations:
- ALD30
30 long-lasting affections (e.g., chronic organ failure, diabetes or immunosuppression) identified by the French health insurance (for further information, please see the list of affections in appendix)
- CI
confidence interval
- GP
general practitioner
- HR
hazard ratio
- PY
(inhabitants) per year
- WHO
World Health Organization
Funding/Support
This study has been funded by the Departmental health insurance of Gard (financial support) and Sanofi Pasteur MSD (vaccines provision). The funders had no role in the study design, data collection, statistical analysis, decision to publish or preparation of the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/22550
References
- 1.World Health Organization. Influenza vaccines. Wkly Epidemiol Rec 2005; 80(33): 279-87. Available: http://www.who.int/wer Accessed: 01 October 2012.
- 2.Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza --- United States, 1976-2007. MMWR Morb Mortal Wkly Rep 2010; 59:1057-62; http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5933a1.htm Accessed 01October 2012;. [PubMed]
- 3.Fiore AE, Shay DK, Haber P, Iskander JK, Uyeki TM, Mootrey G, et al.; Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC). Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007; 56(RR-6):1-54; http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5606a1.htm Accessed 01 October 2012;. [PubMed]
- 4.Vaccination schedule for 2005. Recommendations from the “Conseil supérieur d’hygiène publique de France. BEH. 2005;29-30:142–56. [Google Scholar]
- 5.Lina B, Holm MV, Szucs TD. [Evolution of influenza vaccination coverage in France from 2001 to 2006] Med Mal Infect. 2008;38:125–32. doi: 10.1016/j.medmal.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Vaux S, Van Cauteren D, Guthmann JP, Le Strat Y, Vaillant V, de Valk H, et al. Influenza vaccination coverage against seasonal and pandemic influenza and their determinants in France: a cross-sectional survey. BMC Public Health. 2011;11:30. doi: 10.1186/1471-2458-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ortqvist A, Hedlund J, Kalin M. Streptococcus pneumoniae: epidemiology, risk factors, and clinical features. Semin Respir Crit Care Med. 2005;26:563–74. doi: 10.1055/s-2005-925523. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Global surveillance for severe acute respiratory syndrome (SARS). Wkly Epidemiol Rec 2003; 78(14):110-19. Available: http://www.who.int/wer Accessed: 18 December 2011.
- 9.Rosenheim M. Comité technique des vaccinations. Rapport. Efficacité du vaccin polysaccharidique pneumococcique chez les sujets âgés. 2002 [Article in French] Available: http://www.hcsp.fr/docspdf/cshpf/r_mt_021212_rapport.pdf Accessed 01 October 2012.
- 10.Assendelf WJ, Scholten RJPM, Offringa M. Pneumococcal vaccination for the elderly in The Netherlands? Assessment of the quality and content of available comparative studies. Neth J Med. 2004;62:36–44. [PubMed] [Google Scholar]
- 11.Sims RV, Steinmann WC, McConville JH, King LR, Zwick WC, Schwartz JS. The clinical effectiveness of pneumococcal vaccine in the elderly. Ann Intern Med. 1988;108:653–7. doi: 10.7326/0003-4819-108-5-653. [DOI] [PubMed] [Google Scholar]
- 12.Farr BM, Johnston BL, Cobb DK, Fisch MJ, Germanson TP, Adal KA, et al. Preventing pneumococcal bacteremia in patients at risk. Results of a matched case-control study. Arch Intern Med. 1995;155:2336–40. doi: 10.1001/archinte.1995.00430210086013. [DOI] [PubMed] [Google Scholar]
- 13.Vila-Córcoles A, Ochoa-Gondar O, Hospital I, Ansa X, Vilanova A, Rodríguez T, et al. EVAN Study Group Protective effects of the 23-valent pneumococcal polysaccharide vaccine in the elderly population: the EVAN-65 study. Clin Infect Dis. 2006;43:860–8. doi: 10.1086/507340. [DOI] [PubMed] [Google Scholar]
- 14.Domínguez A, Izquierdo C, Salleras L, Ruiz L, Sousa D, Bayas JM, et al. Working Group for the Study of Prevention of CAP in the Elderly Effectiveness of the pneumococcal polysaccharide vaccine in preventing pneumonia in the elderly. Eur Respir J. 2010;36:608–14. doi: 10.1183/09031936.00171309. [DOI] [PubMed] [Google Scholar]
- 15.Butler JC, Breiman RF, Campbell JF, Lipman HB, Broome CV, Facklam RR. Pneumococcal polysaccharide vaccine efficacy. An evaluation of current recommendations. JAMA. 1993;270:1826–31. doi: 10.1001/jama.1993.03510150060030. [DOI] [PubMed] [Google Scholar]
- 16.Nichol KL, Baken L, Wuorenma J, Nelson A. The health and economic benefits associated with pneumococcal vaccination of elderly persons with chronic lung disease. Arch Intern Med. 1999;159:2437–42. doi: 10.1001/archinte.159.20.2437. [DOI] [PubMed] [Google Scholar]
- 17.Ansaldi F, Turello V, Lai P, Bastone G, De Luca S, Rosselli R, et al. Effectiveness of a 23-valent polysaccharide vaccine in preventing pneumonia and non-invasive pneumococcal infection in elderly people: a large-scale retrospective cohort study. J Int Med Res. 2005;33:490–500. doi: 10.1177/147323000503300503. [DOI] [PubMed] [Google Scholar]
- 18.Alfageme I, Vazquez R, Reyes N, Muñoz J, Fernández A, Hernandez M, et al. Clinical efficacy of anti-pneumococcal vaccination in patients with COPD. Thorax. 2006;61:189–95. doi: 10.1136/thx.2005.043323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaillat J, Zmirou D, Mallaret MR, Rouhan D, Bru JP, Stahl JP, et al. [Clinical trial of an antipneumococcal vaccine in elderly subjects living in institutions] Rev Epidemiol Sante Publique. 1985;33:437–44. [PubMed] [Google Scholar]
- 20.Simberkoff MS, Cross AP, Al-Ibrahim M, Baltch AL, Geiseler PJ, Nadler J, et al. Efficacy of pneumococcal vaccine in high-risk patients. Results of a Veterans Administration Cooperative Study. N Engl J Med. 1986;315:1318–27. doi: 10.1056/NEJM198611203152104. [DOI] [PubMed] [Google Scholar]
- 21.Koivula I, Stén M, Leinonen M, Mäkelä PH. Clinical efficacy of pneumococcal vaccine in the elderly: a randomized, single-blind population-based trial. Am J Med. 1997;103:281–90. doi: 10.1016/S0002-9343(97)00149-6. [DOI] [PubMed] [Google Scholar]
- 22.Ortqvist A, Hedlund J, Burman LA, Elbel E, Höfer M, Leinonen M, et al. Swedish Pneumococcal Vaccination Study Group Randomised trial of 23-valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle-aged and elderly people. Lancet. 1998;351:399–403. doi: 10.1016/S0140-6736(97)07358-3. [DOI] [PubMed] [Google Scholar]
- 23.Maruyama T, Taguchi O, Niederman MS, Morser J, Kobayashi H, Kobayashi T, et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ. 2010;340:c1004. doi: 10.1136/bmj.c1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubois G. [Pneumococcal vaccination in France among adults] Bull Acad Natl Med. 2002;186:1461–76, discussion 1476-7. [PubMed] [Google Scholar]
- 25.Risso K, Naqvi A, Pillet S, Leplatois A, Pulcini C. [Insufficient pneumococcal vaccine coverage in adult inpatients at risk] Med Mal Infect. 2010;40:341–6. doi: 10.1016/j.medmal.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Kuster SP, Tuite AR, Kwong JC, McGeer A, Fisman DN, Toronto Invasive Bacterial Diseases Network Investigators Evaluation of coseasonality of influenza and invasive pneumococcal disease: results from prospective surveillance. PLoS Med. 2011;8:e1001042. doi: 10.1371/journal.pmed.1001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hedlund J, Christenson B, Lundbergh P, Ortqvist A. Effects of a large-scale intervention with influenza and 23-valent pneumococcal vaccines in elderly people: a 1-year follow-up. Vaccine. 2003;21:3906–11. doi: 10.1016/S0264-410X(03)00296-2. [DOI] [PubMed] [Google Scholar]
- 28.Christenson B, Hedlund J, Lundbergh P, Ortqvist A. Additive preventive effect of influenza and pneumococcal vaccines in elderly persons. Eur Respir J. 2004;23:363–8. doi: 10.1183/09031936.04.00063504. [DOI] [PubMed] [Google Scholar]
- 29.Chang YC, Chou YJ, Liu JY, Yeh TF, Huang N. Additive benefits of pneumococcal and influenza vaccines among elderly persons aged 75 years or older in Taiwan--a representative population-based comparative study. J Infect. 2012;65:231–8. doi: 10.1016/j.jinf.2012.04.014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Gilchrist SA, Nanni A, Levine O. Benefits and effectiveness of administering pneumococcal polysaccharide vaccine with seasonal influenza vaccine: an approach for policymakers. Am J Public Health. 2012;102:596–605. doi: 10.2105/AJPH.2011.300512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clayton TC, Thompson M, Meade TW. Recent respiratory infection and risk of cardiovascular disease: case-control study through a general practice database. Eur Heart J. 2008;29:96–103. doi: 10.1093/eurheartj/ehm516. [DOI] [PubMed] [Google Scholar]
- 32.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127:1952–9. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. Recommended composition of influenza virus vaccines for use in 2005/2006-influenza season. Wkly Epidemiol Rec 2005; 80(8): 71-. Available: http://www.who.int/wer Accessed: 01 October 2012. [PubMed]
- 34.Blank PR, Schwenkglenks M, Szucs TD. Vaccination coverage rates in eleven European countries during two consecutive influenza seasons. J Infect. 2009;58:446–58. doi: 10.1016/j.jinf.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Blank PR, Schwenkglenks M, Szucs TD. Influenza vaccination coverage rates in five European countries during season 2006/07 and trends over six consecutive seasons. BMC Public Health. 2008;8:272. doi: 10.1186/1471-2458-8-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mullooly JP, Bennett MD, Hornbrook MC, Barker WH, Williams WW, Patriarca PA, et al. Influenza vaccination programs for elderly persons: cost-effectiveness in a health maintenance organization. Ann Intern Med. 1994;121:947–52. doi: 10.7326/0003-4819-121-12-199412150-00008. [DOI] [PubMed] [Google Scholar]
- 37.Fedson DS, Wajda A, Nicol JP, Hammond GW, Kaiser DL, Roos LL. Clinical effectiveness of influenza vaccination in Manitoba. JAMA. 1993;270:1956–61. doi: 10.1001/jama.1993.03510160074032. [Erratum in: JAMA 1994; 271] [20] [DOI] [PubMed] [Google Scholar]
- 38.Nichol KL, Margolis KL, Wuorenma J, Von Sternberg T. The efficacy and cost effectiveness of vaccination against influenza among elderly persons living in the community. N Engl J Med. 1994;331:778–84. doi: 10.1056/NEJM199409223311206. [DOI] [PubMed] [Google Scholar]
- 39.Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann Intern Med. 1995;123:518–27. doi: 10.7326/0003-4819-123-7-199510010-00008. [DOI] [PubMed] [Google Scholar]
- 40.Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet. 2005;366:1165–74. doi: 10.1016/S0140-6736(05)67339-4. [DOI] [PubMed] [Google Scholar]