Abstract
Tobacco smoking is globally far more widespread than use of any other substance of abuse. Nicotine is an important tobacco constituent that is responsible for addictive properties of smoking. The currently available medications for the treatment of nicotine addiction have limited efficacy. A challenging novel therapeutic concept is vaccination against nicotine. An efficient vaccine would generate antibodies that sequester nicotine in the blood and prevent its access to the brain. The vaccine would have great potential for treating nicotine addiction and for relapse prevention. We reviewed the current status of vaccines against nicotine addiction that are undergoing clinical trials or are in preclinical development. We discuss problems associated with the development of nicotine vaccines, their efficacy in addiction treatment, challenges and ethical concerns. Existing evidence indicates that nicotine vaccination is well tolerated and capable of inducing an immune response but its effectiveness in increasing smoking abstinence has not been shown so far.
Keywords: nicotine, vaccines, nicotine addiction, drugs of abuse, cigarette smoking, smoking cessation, relapse prevention, dopamine
Introduction
Global burden of substance abuse
Most of the drugs which are prohibited for non-medical use are believed, or have been proven to be addictive. These drugs include amphetamines, cannabis, cocaine, MDMA (ecstasy), benzodiazepines, and opioids. Although illegality of most of these drugs precludes the accurate estimation of their prevalence, it has been estimated that 149–271 million people worldwide, aged 15–64 (3.3–6.1%) had used illicit drugs at least once in 2009.1 Many people who use drugs are addicted not just to one substance, but many types including legally available psychoactive substances like alcohol or tobacco. Use of addictive substances is a significant global cause of premature mortality and morbidity. Global burden of drug use is not only caused by their fatal overdose but includes mental disorders, HIV, hepatitis C and B infections, road-traffic and home accidents, suicides and violence.2 There is evidence that global illicit drug consumption has increased since 1990.2 Generally, rates of drug dependence are higher in more developed countries, in males than in females, and people from more disadvantaged backgrounds.3-5 Other risk factors include poverty, early age of drug initiation, poor school performance, parental or sibling drug use, parental conflict, sensation seeking, and social norms for the toleration of drug use (for a review see ref. 2).
Drug addiction is considered as a lifelong, chronic, relapsing brain disorder in which physical dependence on a substance leads to its compulsive and repetitive use. It has been estimated that in the US on average 20% of people who use drugs meet the criteria of drug dependence as defined by the International Classification of Diseases (ICD 10th Revision) and American Psychiatric Association.6 These classifications require the presence of three or more indicators for at least a month within previous year. These indicators include the following: (1) a strong desire to take the drug; (2) an impaired control over use; (3) a withdrawal syndrome after cessation or reduction of use; (4) tolerance to the effects of the substance; (5) the need for larger doses to achieve the desired psychological effect; (6) a disproportionate amount of time spent by the drug user to obtain, use, and recover from drug use; and (7) continuing to take the drug despite the problems that occur.7,8
The major drugs of abuse depend on access to the brain for their psychological and reward effects. Dopamine (DA) transmission has been shown to be affected by all types of drugs of abuse. Release of DA after administration of the drug is associated with pleasurable experience and is critical for promotion of drug self-administration. Basic studies have documented a reduction in the activity of DA neurons in alcohol, opiate, cannabinoid, and nicotine dependent rats (for a review see ref. 9). DA release in the Nucleus accumbens (a part of a brain that plays an important role in reward, pleasure, laughter, aggression, and fear) is decreased in drug-dependent rodents. For example, nicotine has been shown to bind to nicotinic cholinergic receptors in brains. By stimulating these receptors, nicotine releases a variety of neurotransmitters, including dopamine (see below). With repeated exposure to a drug, tolerance to its effects develops. With the increasing numbers of binding sites on receptors, higher doses of a drug are required to cause the same effect. Finally, the symptoms of craving and withdrawal appear in drug addicts during periods of abstinence.
Despite the devastating consequences of drug abuse, the majority of drug dependent users receive no treatment at all.10 The dynamic progress of medicine, biochemistry, pharmacology and biotechnology over the last decade has led to increasing numbers of drug addiction therapies. Those therapies often include behavioral support and counseling combined with pharmacotherapy. The majority of medications used in addiction treatment affect dopaminergic, GABA-ergic, serotonergic, and glutamatergic systems. As discussed above, dopamine plays a key role in the addiction process. However, significant side-effects have limited the use of medications that work directly on the dopaminergic system.9 Methadone (an opioid agonist) and buprenorphine (a partial opioid agonist) maintenance therapies are currently recommended for the treatment of opioid dependence. Naltrexone (a long-acting opioid antagonist) is used primarily in the management of alcohol dependence and opioid dependence. However, the use of existing pharmacotherapy in addiction treatment is limited in many cases and is often associated with several problems, including limited effectiveness, adverse reactions, narrow therapeutic index, possible overdose and illicit use of the drug, and high costs of therapy.10-13 Currently, there are no medications approved by the US Food and Drug Administration (FDA) to treat cocaine and methamphetamine addictions. Because of the limitations of existing treatments, there is an urgent need for novel approaches of substance abuse treatment.
A challenging novel therapeutic concept is vaccination against addictive substances. Vaccines against substances of abuse may help addicts achieve initial abstinence and prevent relapse, but also enhance behavioral therapies when combined with other anti-addiction drugs and potentially prevent addictions in high-risk populations and children.14
New perspectives in addiction treatment—vaccines
The idea of vaccines as a cure for addiction comes from the same concept which was discovered years ago in order to handle infectious diseases. It underlines the significance of our self-secure inborn resources capable of recognizing unwanted particles, and thus being able to inactivate them. The immune system has now been taken under consideration again in the case of pharmacokinetic inactivation of certain agents known to be responsible for physical and behavioral addiction, such as methamphetamine, heroin, and eventually nicotine which is now in the III Phase of clinical trials.15
Most addictive substances can work only after reaching certain areas in the brain, so the idea of blocking this access was successfully developed in order to “catch” and inactivate the addictive substances when they are in the blood. By blocking or at least slowing the drugs entry into the brain, antibodies may be effective in reducing the pharmacological effects of this drug on the brain, and in consequence reducing its behavioral reinforcement effect. The antibodies generated after administration of a vaccine against a specific drug can bind to the drug and form the antibody-drug complex molecules that are too large to cross the blood-brain barrier. This can be used as well in the case of methamphetamine (METH), morphine/heroin and nicotine (Table 1). For example, a novel strategy uses anti-METH antibodies of high affinity to prevent the access of the methamphetamine to the central nervous system. This is possible due to the immunization with METH-conjugated vaccines (MCV).16,17 The novel morphine/heroin vaccine using a 6-glutaratemorphine as a hapten, reduces behavioral/psychoactive effects of heroin in rats.18 However, it has been suggested that nicotine addiction is a better candidate to immunotherapy because the maximum daily dose of nicotine which is consumed through cigarette smoking is lower than the dose of cocaine that is used in serious addiction, so that the predicted effect of immunization can be achieved.15,19
Table 1. Potential vaccines against substances of abuse.
| Drug / Vaccine | Construction of the vaccine | Company |
|---|---|---|
|
Opiates (morphine/heroin) | ||
| - |
Ester-linked morphine tetanus toxoid; Conjugate with keyhole limpet hemocyanin (KLH) |
- |
|
Methamphetamine | ||
| - |
Various conjugates with keyhole limpet hemocyanin (KLH) or outer membrane protein complex (OMPC) (e.g., KLH-S-Meth, OMPC-S-Meths) |
- |
|
Cocaine | ||
| TA-CD |
Conjugate with deactivated cholera toxin B subunit protein combined with human adjuvant alum |
Celtic Pharmaceuticals |
|
Nicotine | ||
| NicVAX |
3-AmNic-rEPA (3′-aminomethylnicotine Pseudomonas aeruginosa r-exoprotein A) |
GlaxoSmithKline/Nabi Biopharmaceuticals |
| TA-NIC |
Nicotine butyric acid covalently linked to recombinant cholera toxin B |
Celtic Pharmaceuticals |
| NIC002 |
Recombinantly produced virus-like protein |
Cytos Biotechnology/Novartis |
| Niccine |
N/A |
Independent Pharmaceutica |
| SEL-068 | Synthetically engineered nanoparticle | Selecta Biosciences Inc. |
Tobacco use as a leading cause of death
Tobacco smoking is globally far more widespread than use of other addictive substances, including amphetamines, cannabis, cocaine, and opioids. Thus, its contribution to disease burden is greater than that for alcohol or illicit drugs.2 The World Health Organization estimates there are 1.3 billion smokers worldwide and each year 5 million smokers die because of tobacco-related diseases.20 Tobacco is a major cause of death from cancers, cardiovascular and pulmonary diseases. If current smoking patterns continue, smoking will cause 10 million deaths each year by 2020.21 Cigarette smoking is also a risk factor for many diseases and disorders, including infections, osteoporosis, delayed wound healing, gastric ulcers, and diabetes.
Neuropharmacology of nicotine
Smoking is a highly effective form of drug administration and nicotine is a key addictive component of tobacco (Fig. 1). Inhaled nicotine from tobacco smoke enters the circulation through the lungs. The accumulation of the nicotine in the brain starts approximately 7 sec after inhalation.22 Nicotine binds to nicotinic acetylcholine receptors in brain that leads to a release of adrenaline and dopamine. This improves mood and reinforces the behavior. Since nicotine is the main factor responsible for the addiction to cigarette smoking, its rapid rates of absorption and entry to the brain are believed to be key factors responsible for the high abuse potential of this drug. Unlike cigarettes, nicotine replacement therapy (NRT) products (patches, gums, lozenges) used for smoking cessation, deliver nicotine slowly, and the risk of abuse is low.23 The half-life of nicotine is 2 h and it is mostly metabolized in the liver by the cytochrome P450 isoenzyme CYP2A6 into cotinine (Fig. 1).
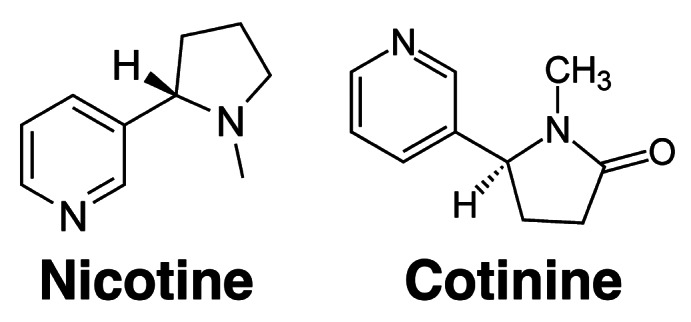
Figure 1. Chemical structures of nicotine (left) and its major metabolite cotinine (right).
Nicotine induces pleasure and reduces stress and anxiety (for a review see ref. 24). Smokers use nicotine to control their mood and for arousal. Smoking improves concentration and reaction times. When a person who is addicted to nicotine stops smoking, he experiences unpleasant withdrawal symptoms. Those symptoms include: depressed mood, irritability, anxiety, anhedonia (the absence of pleasure or the ability to experience it), and restlessness.
Current therapies for nicotine addiction and future perspectives
Nicotine dependence is considered a chronic and relapsing disorder. The currently available medications for the treatment of nicotine addiction have only limited success. Contemporary therapy of nicotine addiction contains nicotine replacement therapy (NRT), bupropion and varenicline as first-line therapies, and clonidine and nortriptyline as a second-line therapy which is recommended for patients who do not respond or are unable to tolerate first-line medicaments. There are also additional treatments like monoamine oxidase inhibitors, selective serotonin reuptake inhibitors, nicotine receptor antagonists, opioid receptor antagonists, bromocriptine, anti-anxiety drugs, and others under investigation, like inhibitors of the isoforms of the cytochrome P-450 and cannabinoid-1 receptor antagonists.25,26
To date, three medications are FDA-approved for smoking cessation: nicotine replacement therapies (NRT), sustained-release bupropion (an atypical antidepressant), and varenicline (a partial agonist of α4β2 nicotinic receptors). The Centers for Disease Control and Prevention (CDC) estimates that almost 75% smokers in the US want to quit.27 Despite the relative efficacy of existing pharmacotherapies, less than 5% of those who try to quit remain smoke-free after one year. Thus, new effective smoking cessation medications are urgently needed. A challenging novel therapeutic concept is vaccination against nicotine.
Nicotine vaccines aim to elicit antibodies that block the pharmacological effects of nicotine. They have great potential for treating nicotine addiction and for relapse prevention. The total population of smokers worldwide is very large and motivation to quit smoking is relatively strong. Smokers who would be vaccinated against nicotine are typically very motivated to stop smoking and do not have the ambivalence about abstinence that is common among other drug users.14 Taking into account that, at first, nicotine binds to the antibody, and then dissociates from it, some amount may reach the brain. However, the reinforcement might be attenuated because of the reduction of the rate with which nicotine enters the brain. Therefore, vaccines may not prevent nicotine from nicotine replacement therapy products (NRT) from reaching levels in the brain that are able to relieve withdrawal symptoms. That would give potential to combine vaccine treatment with NRT which may be compatible or synergistic.28 As noted by Shen et al.,14 the low cost of a nicotine vaccine will facilitate widespread distribution of the vaccine for public health purposes to a wide range of less wealthy populations in both developed and developing nations’ healthcare systems.
Development of Vaccines Against Nicotine
General principles of the nicotine vaccine
Nicotine vaccines aim to eliciting antibodies that block the pharmacological effects of nicotine. Nicotine particles are too small to induce the immune system (Fig. 1). When conjugated with a bigger carrier (for example protein) it can be presented by antigen presenting cells (APC) to lymphocytes by association with major histocompatibility complex (MHC). Lymphocytes recognize the peptide antigens using T-cell receptors. Then, throughout participation of cytokines, begins the humoral response in which the antibodies are produced. Plasmatic cells produce nicotine-specific antibodies (immunoglobulins) which circulate in the blood stream in readiness to bind to nicotine. After delivery of nicotine by smoking, it is bound by nicotine-specific antibodies and the formed complex is too large to cross the blood-brain barrier which results in the decrease of nicotine action. The nicotine particle alone is too small to be recognized by the immune system and to stimulate the immune memory (Fig. 1), so that the repeated administration of the nicotine-conjugate in the form of vaccination is needed to maintain the efficient antibody concentration in serum. Each time the vaccination is administered, the immune memory is engaged in the same way, similar to when the organism has had contact with an infectious antigen. The difference is that the nicotine vaccination acts first like a typical vaccination and each booster in the future acts as a “planned infection.” In that way the first vaccination causes the primary immune response as a consequence of the first contact with an antigen, and each subsequent administration of the nicotine-conjugate results in the production of antibodies in the base of specific acquired resistance, which uses the memory about the antigens. Thus, the response to the consequent vaccinations should be faster and more effective.
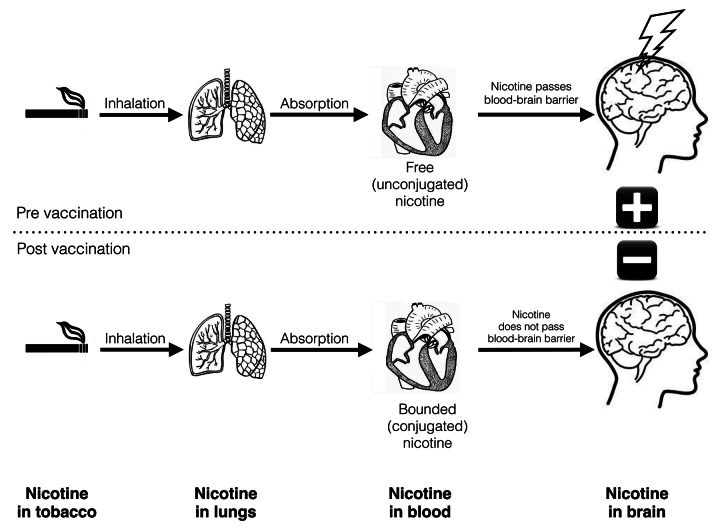
The mechanism of action is a pharmacokinetic antagonism and is based on blocking the effects of nicotine peripherally before they can act centrally (Fig. 2).29 The blockade of drug function by vaccination, provided by nicotine-specific antibodies, depends on both their affinity (how well they bind to the drug) and thus, the total amount of the antibody that is in circulation must be known when we want to determine the percentage of drug amount that can be bound.30 The time in which elicited antibodies can work is strictly correlated with the pharmacokinetics of nicotine. After inhalation of nicotine from a cigarette, it is absorbed by the pulmonary circulation. It then enters the arterial blood and travels to the brain. This arterial blood is a place of action that can prevent the reinforcing effects of nicotine, so that the critical parameter is the time between a puff of cigarette smoke and nicotine reaching the brain. The time of rise and fall in arterial concentration of nicotine after each puff is well over 60 sec. The rate of brain uptake of nicotine is highly correlated with the rate of its elimination from the lungs. The washout half-life (t1/2) of the inhaled nicotine dose in the lung averages 89 sec and nicotine remains there for several minutes, whereas the rise in nicotine concentration in the brain has a half-life of 33 sec.28
Figure 2. Mechanism of action of a vaccine against nicotine addiction. In the absence of vaccine (above), nicotine readily passes through blood-brain barrier and causes reinforcement in brain. If a vaccine is administered (below), the antibodies bind to the drug and prevent nicotine from entering the brain.
Developments of nicotine conjugate vaccine
Because nicotine is too small (167 kD; Figure 1) to induce the immune system by itself, it has to become a part of a bigger structure. Thus, nicotine or structurally related compounds which are haptens, are connected with the immunogenic carrier protein throughout the linker (e.g., succinid acid). The structure of this formation is a complete immunogen named further as a conjugated vaccine.31,32 The construction of this complex has a very big influence on the property of the produced antibodies. The structure of the linker or its attachment to the nicotine molecule may change the selectivity of the antibodies. Longer linkers as well as the linker’s attachment to the 6-position of the nicotine molecule results in higher selectivity of the antibodies to the nicotine. In the case of nicotine, which is in human in 70% metabolized to cotinine of longer half-life, it is very important to reduce this cross-reactivity.33 Some limited cross-reactivity of nicotine-specific antibodies with cotinine elicited by vaccination is about 3%.34 It was also investigated on rats to identify how elicited nicotine antibodies can influence addiction to cocaine. Results show that while nicotine self-administration was reduced among vaccinated rats in about 38% of the sample, cocaine self-administration was not affected. Moreover, no compensatory increase in nicotine self-administration was found. This indicates that vaccination can be useful in reducing the behavioral effects of nicotine administration.35
NicYKQGGFLGLYSFKPMPLaR is a peptide-based nicotine vaccine which contains a conformationally biased agonist of human C5a (YSFKPMPLaR), used as a molecular adjuvant and B cell epitope of human MUC1 glycoprotein (YKQGGFLGL). Important is here the assistance of B cell epitope which is delivered to APCs by YSFKPMPLaR. Presentation of the Nic-modified YKQGGFLGL by APC would cause production of anti-nicotine antibodies. Construction of the vaccine that contains multiple points of nicotine hapten attachments, can deliver more nicotine hapten epitopes to the APC, what can result in increased production of antibodies. This vaccine is able to attenuate the effect of nicotine in vaccinated rats by inducing a significant increase in the antibody titers. Elicited antibodies were specific for nicotine, and as a result, immunized rats were significantly less sensitive to behavioral effects that are induced by high concentrations of nicotine (0.4 mg/kg).36
Another vaccine was constructed from the nicotine hapten 6-(carboxymethylureido)-(±)-nicotine (CMUNic) that was coupled to keyhole limpet hemocyanin (KLH). Vaccination reduced the brain nicotine concentration in rats by 30–46% (the fifth dose of nicotine). And this concentration was reduced even after repeated nicotine dosing (reduction for cumulative dosing by 23%). It is also possible that antibodies bounded up to 76% of the nicotine dose. A decrease in total clearance and the steady-state volume of distribution was also observed. Moreover the terminal half-life (t1/2) was longer compared with controls.37
It has been suggested that to generate additive antibody responses we have to use two concurrently structurally distinct nicotine immunogens. The idea of using a third structurally distinct immnogen was conducted with the new 1’-SNic immunogen (2S)-N,N′-(disulfanediyldiethane-2,1-diyl)bis[4-(2-pyridin-3-ylpyrrolidin-1-yl)butanamide] conjugated to keyhole limpet hemocyanin (KLH). In rats vaccinated with this compound, antibodies of high affinity for nicotine were produced and did not show a cross-reactivity in ELISA with either 3′AmNic-rEPA or 6-CMUNic-BSA. Vaccination resulted in increased retention of nicotine in serum and reduction of the nicotine distribution to the brain. Both were comparable to effects caused by 3′AmNic-rEPA. The new formulation can activate B cell populations that are non-overlapping and distinct from B-cell population induced by other above mentioned immunogens.38
Recently it has been reported that the first synthetically engineered nanoparticle nicotine vaccine—SEL-068, designed by Selecta Biosciences Inc. entered the Phase I clinical trials to evaluate it’s safety, tolerability and pharmacodynamic profile. The company indicates that the greatest advantage of the vaccine is that because it’s fully synthetic, the immune response will focus on nicotine, thus avoiding responses to biological carriers.39
Active vs. passive immunization against nicotine
The mechanism of immunization against nicotine can be achieved by active or passive immunization. The active immunization is based on the development of antibodies in response to the presentation of particles like nicotine to the immune system. Nicotine vaccines belong to the active immunization which consist of repeated administration of the immunogen (a compound containing the nicotine particle) in order to make the immune system produce nicotine-specific antibodies. Passive immunization depends on the administration of previously produced nicotine-specific antibodies in other subjects, such as rabbits, and subsequent purification of these antibodies.41
The antibodies can be put to use by both active and passive immunization. Active immunization requires only a few injections to reach the effective serum level of the antibodies that lasts for several months (e.g., one injection per month during the 3 or 4 mo period) which results in low costs and better acceptance from the patients point of view. However the time needed to reach this level (2–3 mo) is longer and it is difficult to control this level in comparison to the passive immunization. Passive immunization gives us the possibility to control the antibody dose and, therefore, see the dose-response relationship as far as it reaches the high antibody levels that are unlikely to be reached by vaccination alone. However, among the disadvantages of passive immunization are higher costs, frequency of the injections, shorter elimination half-life, and the risk that faster initial binding of free nicotine will result in intensification of earlier withdrawal stages.18,31,40 Furthermore, passive immunotherapy is connected with the risk of serum sickness in case of foreign protein injection to humans, as well as the risk of viral transfer. An important difference is that passive immunization does not produce immunological memory against the abused drug in comparison to active immunization (for a review see ref. 42). Some data suggest that greater efficacy can be gained by combined use of active vaccination with passive immunization.40
Passive immunization may be an alternative among patients with immune deficiency, such as people with HIV infections.29 Both types of immunization can cause some level of allergic reactions.29 It was also suggested that active immunization is potentially irreversible.43
Efficacy of nicotine vaccines
The efficacy of the nicotine vaccine depends on the antibody serum concentration. This is affected by quality of the vaccine, dose, frequency, and time interval between vaccinations. As suggested by Kinsey et al.,44 an effective nicotine vaccine will rapidly elicit large quantities of antibody that can steadily bind the drug in circulation and prevent its transportation through the blood-brain barrier. Sufficient immune response is gained after a period of one to six months with frequent booster injections in order to achieve efficient antibody levels.29 The individual variable serum antibody concentration partly depends on some genetic factors that control HLA, cytokine and the T cell surface receptor expression, and it is unclear whether individuals who have a poor response to one vaccine have responded poorly to another vaccine.45 However, serum antibody concentrations may not be the crucial factor for efficacy of the therapy, as was shown in vaccinated rats which had reduced nicotine distribution to the brain even when the doses of nicotine where twice the estimated binding antibody’s capacity. It suggests that there may be other mechanisms that keep nicotine away from the brain than sequestration of nicotine by antibodies.
The binding equilibrium constant (Ka), also referred to as affinity, is defined by Ka = [NicAb]/[Nic][Ab] where [NicAb] is the plasma volume concentration of bound nicotine-antibody complexes, and [Nic] and [Ab] are the plasma volume concentrations of unbound drug and unbound antibody, respectively. It refers to the strength with which the antibodies bind to the drug. The Ka should be high enough to bind the nicotine and low enough to release it and allow its elimination.37 Although in one trial the nicotine dose in the chronic infusion experiment exceeded the antibodies binding capacity 33-fold, this chronic dosing did not compromise the ability of antibodies to reduce nicotine brain concentration.46
Nicotine antibodies that have moderate affinities for nicotine might avoid the saturation effect of antibody binding sites.28 In rats vaccinated with 3′-AmNic-rEPA, the nicotine distribution was reduced to the muscles, kidney, liver, heart, spleen and testes, however to a lesser extent than to the brain. Distribution of nicotine to the fat (in the single nicotine dose protocol) and to the lung (in the chronic nicotine infusion protocol) were increased. Reduction of the nicotine distribution to the brain was also tested in a time-dependent manner. The reduction was about 64% at 1 min while at 25 min was about 45%. In this experiment rats received a single i.v. nicotine dose that was equal to twice the estimated binding capacity of antibodies that were produced in response to vaccination. The fact that the total and bound serum nicotine concentration was higher and the unbound nicotine serum concentration was lower in vaccinated rats in comparison to control, suggest that vaccination with 3′-AmNic-rEPA reduces the distribution to the brain not only because of sequestration of nicotine in serum. It happens also by redirecting tissue distribution of nicotine disproportionately away from the brain. It may be possible that retention of nicotine in some tissue is connected with extravascular nicotine-specific IgG. The nicotine-specific antibody concentration is higher in fat than in other tissue. Despite the observed increase in nicotine distribution in fat, this is probably not very important in case of overall disposition of nicotine, because only about 3% of nicotine dose accounts for nicotine in fat.47 The experiment model in rats inhaled with cigarette smoke suggests the participation of pulmonary mucosal antibodies, principally IgA in the alteration of nicotine distribution in comparison to i.v. administration. However, the brain nicotine concentrations which are the most important measure of immunization efficacy, were comparable.48
Some data underline the advantage of a bivalent vaccine as it was in the example of 3′-AmNic-rEPA served together with 6-CMUNic-KLH to rats. This resulted in additive total antibodies concentration. However, the result was that the bivalent vaccine was more effective than use of the 6-CMUNic-KLH alone but was not more effective than application of the 3′-AmNic-rEPA alone. It is most likely because of the higher affinity for nicotine of the antibodies produced by 3′-AmNic-rEPA.45
Whereas the nature of antibody binding to nicotine is reversible, it is possible that some of the nicotine particles will eventually reach the receptor. This would happen with a significant delay, while the dependence-inducing stimulation happens instantaneously after application. The nicotine-induced cerebral stimulation would not work and the smoker could not satisfy the craving.31,32 As far as the antibody level is increased, more nicotine is captured and sequestered in the blood, which leads to the reduction of the reinforcing effects of nicotine.49
The efficacy of each therapy is grandly altered by good or bad compliance. In the case of vaccination, due to the type of application, the compliance is rather good and easy to control. This is important when trying to assess the value of therapy in this context. Improved patient compliance led to a lack of major side effects and relatively minimal dosing requirements.15 Moreover the mechanism of action allows simultaneous combination of other pharmacotherapies.31,32
Safety of nicotine vaccines
Antibodies that are elicited by vaccination are specific for nicotine and do not bind to other molecules, receptors or neurotransmitters. Therefore, vaccination against nicotine should not have many adverse effects. Data from clinical trials of the NicVax vaccine (see Table 2) indicate that the most frequently reported local events were tenderness (67%, ache (50%), swelling or induration (38%). The severity of local reactions was mild (81%) and moderate (23%). Systemic reactogenicity events were graded as mild (85%) and moderate (15%) in severity. Among them, the most frequent were muscle ache (38%), headache (25%) and general discomfort or malaise (25%). The most frequent adverse events were bad taste, dry mouth, increase in weight and tenderness at the injection site.50
Table 2. Review of completed and ongoing clinical trials of nicotine vaccines.
| Vaccine/Phase | Design | Outcomes |
|---|---|---|
|
NIC002 (formerly CYT002-NicQB, Cytos Biotechnology) | ||
| Phase I57 |
Randomized, placebo-controlled. 40 healthy, non-smoking volunteers; 10 subjects in each of four dose groups: • group 1: 50µg NicQb • group 2: 100µg NicQb (both without adjuvants) • group 3: 100µg NicQb (in the presence of Alum dissolved in PBS) • group 4: 100µg NicQb (in the presence of Alum dissolved in sodium acetate). In each group, 8 of the 10 received vaccine and 2 received placebo. |
• Vaccine reported to be safe, good toleration, high immunogenicity, immunological response rate of 100%, antibody responses were long-lasting but declined over time. • 93% of adverse effects were rated as mild, 7% as moderate, none as severe, the most frequent side effects were local reactions (anesthesia, bruising, discoloration, erythema, induration, edema, pain, paraesthesia, swelling, tenderness or warmth) headache or flu-like symptoms. • Antibodies affinity of 33 nM (for group 4) • The half-life of nicotine-specific IgG was 47 d (absence of Alum) and 67 d (in the presence of Alum). • Nicotine-specific IgG antibodies were apparent after day 7, the 100% responder rate was observed by day 14. |
| Phase II58 |
Randomized, double-blinded, placebo-controlled. 341 smokers, five injections, monthly intervals between injections. Data were available from 239 study subjects who were divided into low, medium, and high responders according to their nicotine antibody levels. |
• Vaccine was safe and generally well-tolerated, immunological response rate of 100%. • Incidence of fever nearly 40% (in new formulation reduced to almost zero), incidence of flu-like symptoms up to 70% (in new formulation reduced to about 10%) • No difference in abstinence rates among low and medium respondents. • 33.3% of vaccinated smokers were high respondents: 6-mo abstinence was achieved by 56.6% of them, and 12-mo abstinence was achieved by 41.5% of them. |
| Phase II51 |
Double-blinded, placebo-controlled, multi-center study. Repeated administration of 100µg NIC002. 200 cigarette smokers who were motivated to quit smoking. Primary endpoint: statistically significant difference in continuous abstinence from smoking determined from weeks 8 to 12 after start of treatment compared with placebo. |
• Primary endpoint not achieved (interim analysis). • Treatment was safe and well tolerated but failed to induce sufficiently high antibody titers, which may have led to the negative outcome. |
|
TA-NIC (Celtic Pharmaceuticals) | ||
| Phase II59,60 |
60 subjects who smoked between 10 and 75 cigarettes a day, divided into three cohorts of 20 smokers. In each cohort 16 received the active vaccine and 4 received the placebo. Doses of TA-NIC: 50µg, 250µg and 1000 µg. Intramuscular injection at weeks 0, 2, 4, 6, 8 and 12 with a booster at 32 weeks, recording the number of cigarettes smoked per day, determining the time to first cigarette and the time to relapse following a quit attempt at week 12 and if necessary, another quit attempt at week 32, these quit rates were then assessed again after 12 mo. |
• No drug-related serious adverse events were seen in any cohort, minimal injection-site effects. • Anti-nicotine antibody responses were dose dependent. • At week six, 19 of the 44 (43%) subjects receiving TA-NIC voluntarily gave up smoking or reported reduced pleasure when smoking compared with only 1 out of 11 (9%) receiving the placebo • 12 mo self-reported quit rates were substantially greater among those receiving TA-NIC than those receiving placebo; in the placebo group, 1 out of 12 participants (8%) reported being abstinent at their last visit or at 12 mo compared with 3 out of 16 (19%) and 6 out of 16 (38%) in the two groups receiving the higher doses of TA-NIC. • The proportion of participants who successfully made a quit attempt was 95% among those receiving TA-NIC and 73% among those receiving the placebo. • A booster, given at 32 weeks, produced a substantial and sustained increase in nicotine specific antibodies in both groups receiving the higher doses of TA-NIC. |
| Phase II61 |
Double-blinded, randomized, placebo-controlled, multicenter, dose-ranging study, 100 or 250 μg of TA-NIC |
No results (ongoing) |
|
NicVAX (Nabi Biopharmaceuticals/GlaxoSmithKline) | ||
| Phase I/II50,52 |
Double-blinded, placebo controlled. Four injections containing either NicVAX 100 μg or a placebo (days 0, 14, 28, 182). Follow-up: 6 mo. 21 healthy smoking and 9 healthy non-smoking volunteers. |
• Good toleration. • Three doses produced significant levels of antibodies, which declined slowly over the next several months. • A fourth dose (given on day 182) boosted nicotine specific antibodies to even higher levels, which then declined more slowly over time. • Localized reactions: tenderness, aching and redness at the injection site, systemic reactions: myalgia (muscle pain), headache and malaise (weakness) |
| Five phase I/II clinical trials among more than 475 subjects. |
• Good toleration, high immunogenicity, dose-dependent increase in antibody concentrations. • Clinical proof-of-concept for efficacy in smoking cessation, clinical response rates highly correlated with antibody concentration |
|
| Phase II (US)63 |
Double-blinded, placebo-controlled, randomized study. 68 smokers not intending to quit. Four injections; three doses (50, 100, 200 μg). |
• Very good toleration. • At the 200 μg dose, 33% of smokers quit smoking (defined as no smoking for at least 30 consecutive days) vs. 9% in the placebo group. • Substantial reduction in average cigarette consumption in smokers who received the highest dose of NicVAX vs. lower doses or placebo. • Side effects were similar between the active dose levels and the placebo group. |
| Phase II (Europe)62 |
Randomized, dose-ranging study in smokers. 51 healthy smokers, who were randomized to receive one of four dose levels (100, 200, 300 and 400 μg), with either of two Alum adjuvant formulations. Five injections over a 6 mo period. A total of 20 subjects received the 200 μg dose, with 10 subjects receiving each of the other dose levels. |
• The study was undertaken to assess the tolerability and antibody response at higher doses (300 and 400 μg). • All formulations of NicVAX were well tolerated and immunogenic, antibody concentrations increased with each dose administration. • The lower adjuvant formulations did not appear to compromise the antibody response. • Nicotine dependency exhibited a trend downward from the baseline, and tended to be better sustained in the higher dose formulations. |
| Phase II62 |
Administered dose of 400 μg. Six-dose immunization schedule. |
• Good toleration. • Antibody levels achieved at 14 weeks were more than 2-fold higher than those achieved at the same time point in the Phase IIb proof-of-concept study as a result of the added injection. • Over 80% of subjects who completed the six-dose 400 µg NicVAX regimen had anti-nicotine antibody levels above the target threshold at the planned week 14 “quit date.” • Higher antibody levels can be generated three months earlier and in a much higher percentage of subjects for sustained periods of time than observed in previous studies. |
| Phase IIb49 |
Double-blinded, placebo-controlled, dose-ranging. 301 heavy smokers who smoked an average of 24 cigarettes per day. Primary endpoint: continuous abstinence from smoking after 6 mo (during weeks 19–26 after first vaccination). Secondary endpoints: abstinence rate at 12 mo, daily cigarette consumption, antibody levels, safety and nicotine dependency. |
• Primary endpoint: statistically significant number of subjects in the high anti-nicotine antibody responder group who met the trial's primary endpoint of eight weeks of continuous abstinence between weeks 19–26. • 12-mo data confirmed the highly significant trends seen in the previous data at six months and nine months for both smoking cessation and long-term smoking abstinence, high anti-nicotine response was defined as the top 30% of antibody responders (61 of the total 201). • Subjects having the highest serum anti-nicotine antibody levels achieved higher rates of 8 weeks of continuous abstinence compared with subjects who received placebo. 24.6% of these subjects (p = 0.024) showed continuous abstinence between weeks 19–26 compared with 12.0% for the 100 subjects receiving placebo. • The quit rate of those subjects who did not have a high antibody response was not statistically different from placebo. • 12-Month continuous abstinence: NicVAX 400 μg, 5 injections = 16% (8/51), Placebo = 6% (6/100), p = 0.038 (intent to treat population) • 12-Month continuous abstinence: NicVAX 200 μg, 5 injections = 14% (7/50), Placebo = 6% (6/100), p = 0.056 (intent to treat population) |
| Phase IIb (NicVax+ Varenicline)62 |
Double-blinded, placebo-controlled parallel-arm study. Approximately 600 subjects randomized in a 1:1 ratio to one of two treatment groups: • one group receives NicVAX and varenicline, • the other group receives placebo and varenicline. Primary endpoint: long-term smoking abstinence rate at one year. Secondary endpoints include the abstinence rate at various interim periods, smoking lapse and relapse, safety, immunogenicity and withdrawal symptoms. |
No data (ongoing) |
| Phase III64* |
Double-blinded, placebo-controlled trial. Approximately 1,000 subjects. Primary endpoint of the study: abstinence rate for 16 weeks ending at 12 mo. Secondary endpoints: abstinence rate at various time intervals, safety and immunogenicity, withdrawal symptoms, cigarette consumption, smoking satisfaction and nicotine dependency. |
• Primary end point was not met and there was no statistical difference between the NicVAX and placebo groups.* |
| |
A clinical study testing NicVAX in combination with varenicline (Chantix) |
• No data (ongoing; results are expected in the second half of 2012). |
|
SEL-068 (Selecta Biosciences) | ||
| Phase I39 |
Double-blinded, placebo-controlled. Ascending dose. Healthy, non-smoking and smoking volunteers. Evaluation of vaccine's potency. |
• No data (ongoing, results were expected in the first half of 2012). |
|
Niccine (Independent Pharmaceutica) | ||
| Phase II65 | The ability of the vaccine to prevent relapse over one year. 355 smokers that have recently stopped smoking with the aid of smoking cessation medication and counseling. |
• No data |
Note: * the detailed data and conclusions from the trial have not been published yet in peer-reviewed journals
Data from the Phase I of clinical trials of NIC002 (see Table 2) revealed that the adverse events included local reactions at the injection site, flu-like symptoms, muscle ache and increased body temperature. The phase II of clinical trials showed that up to 70% of study participants reported side effects. These included local injection site reactions and flu-like symptoms. Between month 6 and 12, no side effects related with the vaccine were reported.51
Nicotine vaccine for smoking cessation
Nicotine vaccines can be used both for relapse prevention or as preparation for a quit attempt. The development of vaccines against nicotine addiction to support smoking cessation has been of great interest to the pharmaceutical industry. Results from a few clinical trials are available so far (Table 2). The majority of those trials were designed as smoking-cessation studies with target quit days rather than relapse prevention trials. The results from Phase II clinical trials published so far indicated only modest efficacy of the nicotine vaccine for smoking cessation. The abstinence rates among vaccinated smokers do not surpass those in the placebo control groups. Abstinence rates were significantly higher than placebo only in those smokers who achieved higher therapeutic antibody levels. The detailed data and conclusions from two Phase III clinical trials of the NicVAX have not been published in peer-reviewed journals yet. However, according to press releases, both trails failed to show efficacy of the vaccine vs. control, despite success in Phase I and II trials.62,64
A correlation was found between the total serum antibody concentration and the nicotine levels as far as the locomotor sensitization to nicotine.45,52 For example the mean antibody level that was induced by NIC002 in healthy volunteers was four times higher with the dose of 300 µg than with the dose of 100 µg of the vaccine.51 The far-reaching effects of the 3-AmNic-rEPA are promising when taking into account the higher continuous abstinence rates, whereas other currently available nicotine cessation medications are associated with the 12 mo continuous abstinence that is not higher than 30%.49,53
The social orientation for nicotine vaccines was examined in one investigation. Among asked smokers, 55% declared that they would be likely or very likely to try the new vaccine if it would become available for smoking cessation, whereas 20% of them declared that they are unlikely or very unlikely to try the vaccine. Smokers who never tried to quit smoking were least likely to try vaccine (28%), while most favorable to the vaccine were those who had tried 5 or more times in the past to quit smoking (66%).54
A possible limitation of the vaccination may be compensatory smoking (i.e., smoking more cigarettes or taking deeper and more frequent puffs) when the titer of antibodies is insufficient, because due to this incomplete inactivation, nicotine can finally reach the brain.55 It can also cause another problems, as the effect of vaccination can be circumvented by using a higher dose of nicotine. On this basis, vaccination could be counterproductive, as young people could smoke more to try its efficacy.56
Nicotine vaccine for relapse prevention
As noted by Raupach et al.,42 smokers who have already quit may represent an ideal target group for relapse prevention. Relapse prevention may be helpful in situations when a vaccinated person who had been smoking previously goes into a space which is full of cigarette smoke. The nicotine inhaled from the second-hand smoke would be sequestered by antibodies, so that the amount of nicotine reaching the brain would be too small to develop the reinforcing effect of craving, and they would not be tempted to smoke a cigarette.44 Unfortunately, as discussed above, the formation of sufficient amounts of antibodies takes more than two weeks, much longer than the time when most smokers relapse. A possible use of vaccination can also be prevention of smoking among young people at high-risk, who currently smoke occasionally and those who are not yet addicted but have already tried cigarettes.55
Cotinine vaccine
All nicotine vaccines discussed above have been shown to be highly specific to the drug. Specificity refers to the extent to which the elicited antibodies bind nicotine in preference to other compounds including its metabolite cotinine (Fig. 1). In general, greater specificity reduces competition from other compounds for binding capacity, improves safety, and reduces the likelihood of adverse side effects.31,32 Although vaccines against nicotine are at the advanced stage of clinical evaluation, alternative approaches to improving pharmacotherapy outcomes have also been considered and evaluated.
The point of departure to the idea of cotinine vaccine that could increase the efficacy of nicotine replacement therapy was the fact that cotinine (the main nicotine metabolite with a much longer half-life) is a weak nicotinic agonist and decreases responses to nicotine through desensitization of the receptor (nAChR). Trans-4-thiol cotinine coupled to tetanus toxoid (TT-CotSH) was used to vaccinate rats and to elicit cotinine antibodies that would sequester cotinine in blood-stream thus preventing it from reaching the brain. This would contribute to the reduction of the antagonism by cotinine in the central nervous system.66,67 That may decrease the number of cigarettes which are needed to achieve the “normal” levels of nicotine in smokers. That is a contrast to the situation in which smokers may try to increase the nicotine dose by increasing the number of cigarettes to overcome the effect of immunization, what can be possible in the case of nicotine vaccination.68 After vaccination with TT-CotSH, serum nicotine levels were unchanged, which is evidence that elicited antibodies are specific for cotinine and do not bind to nicotine.66
Challenges and Ethical Concerns
Improving efficacy and safety of nicotine vaccines
The data from clinical trials suggest that many patients may not produce a sufficient antibody response. All clinical trials conducted so far (Table 2) have shown inconsistency in the degree of antibody response in all study groups. Proof-of-concept has been established in that individuals who achieved higher levels of anti-nicotine antibodies had higher smoking cessation and abstinence rates. According to Fahim et al.,69 there are several possibilities to increase efficacy of the vaccines. First, vaccine potency has to be improved for example by introducing novel carriers and/or adjuvants to stimulate higher immune response. Alternatively, personalized medicine approach should be applied to target subjects who have a robust immunological response to vaccination. Finally, vaccines may be combined with existing or novel pharmacotherapies for maintenance of smoking abstinence and relapse prevention.
Two Phase III clinical trials failed to show efficacy of the nicotine vaccine vs. control, despite success in Phase I and II trials (NicVAX, see Table 2).62,64 These data surely cast a long shadow on the field, as has happened in other fields where a high-profile drug has failed in Phase III clinical trials. Despite early successful trials, only a well-controlled and large Phase III study will provide the data that a given vaccine is effective in reducing smoking.
According to Shen et al.,14 the nicotine vaccine would have an important advantage over existing pharmacotherapies in that it has a prolonged effect. The relapse rates after vaccination may be substantially lower as it requires only limited cooperation from patients. When compared with currently approved smoking cessation drugs, there is no need for daily administration of the drug. Since only bimonthly booster shots are required to achieve high levels of antibodies in the blood, patients’ adherence to drug therapy may be significantly improved. On the other hand, increasing the number of doses may improve immunogenicity and increase the rate of adverse effects of vaccination. As suggested previously by Cerny et al.,32 patient adherence may be also improved by replacing painful intra muscular injections with subcutaneous application of the vaccine, especially if it requires as much as six injections. A proper balance between safety and efficacy of new dosage schedules need to be carefully assessed in future clinical trials and selection of adjuvants with highly immunogenic properties will be critical for the development of an efficacious conjugate vaccine.
Nicotine vaccine and its influence on fetus
Maternal vaccination of rats with 3′-AmNic-rEPA contributed to the reduction in distribution of nicotine to the fetal brain, from 17% to 40% (with vaccination) and 60% (with passive immunization) and to the maternal brain in 44–47%.34,70 It is not known whether such reduction is large enough to minimize the adverse effects of gestational exposure of the fetal brain to nicotine (such as premature delivery, increased neonatal mortality, sudden infant death syndrome and low birth weight).70 Both maternal and fetal serum nicotine-specific antibody concentrations were highly correlated, and vaccination acted in the fetus analogous to the mother by binding nicotine in serum, reducing the concentration of unbound nicotine and reducing distribution of nicotine to the brain.34
Passive immunization at the 21mg/kg Nic-IgG dose resulted in reduction of nicotine distribution to the whole fetus.70 In contrast to the reduction of nicotine distribution to the fetal brain, the distribution of nicotine to the whole fetus was generally not altered by vaccination. The concentration of nicotine-specific antibodies in fetal serum was about 10% of the concentration in maternal serum after vaccination.34 Other authors report however that the nicotine vaccine causes a decrease of the nicotine transfer across the placenta to the fetus circulation.71 It was also suggested that it may be possible that the lower Kd (the dissociation constant) of the antibodies for nicotine from passive immunization (Nic-IgG), compared with the antibodies in the serum of vaccinated rats, resulted in the larger effect of passive immunization in reduction of nicotine distribution to the fetal brain in rats.70
Maternal antibodies in vaccinated rats had 100-fold higher transfer to the fetus than antibodies from passive-immunized rats. This transfer of antibodies in vaccinated rats may also have escorted bound nicotine into the fetus, allowing the accumulation of nicotine in the fetus. The lower maternal serum concentration of nicotine in vaccinated rats did not cause a lower total nicotine transfer to the fetus. However, within the fetus, effects of nicotine-specific antibodies as in the mother resulted in reduction of nicotine distribution to the brain. Both maternal vaccination and passive immunization resulted in the reduction of a single nicotine dose to the fetal brain, suggesting that immunization may be a way of protecting the fetus against the adverse effects of cigarette smoking.70
Ethical and economic challenges
In theory, vaccines against addictive substances could be used not only by addicts to achieve abstinence or prevent relapse, but also to prevent addiction in high-risk populations. This causes questions about the plausibility of vaccine use as a prevention of smoking uptake in adolescents. For certain, the negative side is that introducing vaccination at an early age, taking into account that it may be irreversible, limits the option of using nicotine as a therapy in the future. This is risky as it cannot be denied that use of nicotine (aside from cigarette smoking) might at one time be wanted.72
An important issue is the economic aspect of such a therapy. According to Shen at al,14 vaccines against drug abuse are generally considered by pharmaceutical industry as high-risk products with low profit margins. Vaccination against substances of abuse has to be compared with other healthcare interventions, including prevention programs and health promotion, to assess if it is cost-effective. The cost-effectiveness of vaccination against nicotine was evaluated in a cohort of 100,000 smokers and non-smokers aged 20 (equal number of males and females) in Australia.73 The results indicate that the cost to avert a smoker at age 20 was $43,659 (under the most optimistic assumption) and increased to $296,019 (a more plausible scenario). Authors suggest that the vaccine program was not cost-effective under any scenario, indicating that it is unlikely to be publicly funded in Australia or another developed country. That was assuming the high immune response to the vaccine in the population at 80%, and the cost of the vaccine at about $100, $150 and $200 per vaccination with a schedule of 6 vaccinations per year. Moreover, the authors point out that the threshold analysis found that there was no level of efficacy of the vaccine at which the prevention would be cost effective. That is, for a dose schedule of 6 vaccinations and a cost of > $70 per vaccination, even assuming 90% immune response and 0% dropout. The authors present that the maximum decrease in smoking prevalence that would be achieved at age 20 in the examined cohort was 14% and this outcome would cost, depending on the cost of the vaccine, from $1,668 M and $2,801 M. The other solution is to reduce the costs of the program by targeting the vaccination to the subjects that are at highest risk, but it is difficult to find the acceptable screening test. It may also lead to stigmatization of young people.73
Conclusions
Tobacco smoking is globally far more widespread than use of other addictive substances, including amphetamines, cannabis, cocaine, and opioids. Nicotine vaccines aim to elicit antibodies that block the pharmacological effects of nicotine. They have great potential for treating nicotine addiction and for relapse prevention.
Vaccine efficacy depends on many factors, such as antibody specificity, affinity, and antibody blood levels which are affected by the design of the vaccine conjugate, the dose of the vaccine, the adjuvant selection, and the frequency of vaccinations. The data from clinical trials suggest that many patients may not produce a sufficient antibody response. However, for those who do attain high levels of antibodies, vaccination has been shown to be effective in achieving and maintaining abstinence. Since many smokers are highly motivated to quit, they are quite unlikely to try to deliberately override the antibody capacity. The development of a highly specific vaccine with high affinity against nicotine is likely to further enhance the effectiveness of smoking-cessation pharmacotherapy. Future strategies may include examining additional ways to increase antibody levels to improve vaccine efficacy. Ethical and economic challenges are the barriers to implementation of nicotine vaccines.
Acknowledgments
The authors declare that they did not receive any funding for the preparation of this review. M.L. Goniewicz has received grants to conduct investigator-initiated studies from the Ministry of Science and Higher Education of Poland, UK Centre for Tobacco Control Studies, and Pfizer (manufacturer of smoking cessation medicines). M. Delijewski has no conflicts of interest to declare.
Glossary
Abbreviations:
- 3-AmNic-rEPA
3′-aminomethylnicotine Pseudomonas aeruginosa r-exoprotein A
- APC
antigen presenting cells
- DA
dopamine
- FDA
the US Food and Drug Administration
- GABA
gamma-aminobutyric acid
- HIV
human immunodeficiency virus
- HLA
human leucocyte antigen
- KLH
keyhole limpet hemocyanin
- METH
methamphetamine
- MHC
major histocompatibility complex
- nAChR
nicotine acetylcholine receptor
- OMPC
outer membrane protein complex
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/22060
References
- 1.UN Office on Drugs and Crime. World drug report 2011. [http://www.humansecuritygateway.com/showRecord.php?RecordId=35492 (accessed on March 15, 2012)]
- 2.Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379:55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- 3.Kessler R, Üstün T. The WHO World Mental Health Surveys: global perspectives on the epidemiology of mental disorders. Cambridge: Cambridge University Press, 2008. [Google Scholar]
- 4.Degenhardt L, Chiu WT, Sampson N, Kessler RC, Anthony JC, Angermeyer M, et al. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO World Mental Health Surveys. PLoS Med. 2008;5:e141. doi: 10.1371/journal.pmed.0050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniel JZ, Hickman M, Macleod J, Wiles N, Lingford-Hughes A, Farrell M, et al. Is socioeconomic status in early life associated with drug use? A systematic review of the evidence. Drug Alcohol Rev. 2009;28:142–53. doi: 10.1111/j.1465-3362.2008.00042.x. [DOI] [PubMed] [Google Scholar]
- 6.Glantz MD, Anthony JC, Berglund PA, Degenhardt L, Dierker L, Kalaydjian A, et al. Mental disorders as risk factors for later substance dependence: estimates of optimal prevention and treatment benefits. Psychol Med. 2009;39:1365–77. doi: 10.1017/S0033291708004510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. The ICD-10 classification of mental and behavioural disorders – diagnostic criteria for research. Geneva: World Health Organization, 1993. [Google Scholar]
- 8.APA. Diagnostic and statistical manual of mental disorders: DSM-IV-TR, 4th edn. Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
- 9.Diana M. The dopamine hypothesis of drug addiction and its potential therapeutic value. Front Psychiatry. 2011;2:64. doi: 10.3389/fpsyt.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stotts AL, Dodrill CL, Kosten TR. Opioid dependence treatment: options in pharmacotherapy. Expert Opin Pharmacother. 2009;10:1727–40. doi: 10.1517/14656560903037168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahan M, Srivastava A, Ordean A, Cirone S. Buprenorphine: new treatment of opioid addiction in primary care. Can Fam Physician. 2011;57:281–9. [PMC free article] [PubMed] [Google Scholar]
- 12.Vadivelu N, Hines RL. Buprenorphine: a unique opioid with broad clinical applications. J Opioid Manag. 2007;3:49–58. doi: 10.5055/jom.2007.0038. [DOI] [PubMed] [Google Scholar]
- 13.Kreek MJ, Borg L, Ducat E, Ray B. Pharmacotherapy in the treatment of addiction: methadone. J Addict Dis. 2010;29:200–16. doi: 10.1080/10550881003684798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen XY, Orson FM, Kosten TR. Vaccines against drug abuse. Clin Pharmacol Ther. 2012;91:60–70. doi: 10.1038/clpt.2011.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreno AY, Janda KD. Immunopharmacotherapy: vaccination strategies as a treatment for drug abuse and dependence. Pharmacol Biochem Behav. 2009;92:199–205. doi: 10.1016/j.pbb.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carroll FI, Blough BE, Pidaparthi RR, Abraham P, Gong PK, Deng L, et al. Synthesis of mercapto-(+)-methamphetamine haptens and their use for obtaining improved epitope density on (+)-methamphetamine conjugate vaccines. J Med Chem. 2011;54:5221–8. doi: 10.1021/jm2004943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreno AY, Mayorov AV, Janda KD. Impact of distinct chemical structures for the development of a methamphetamine vaccine. J Am Chem Soc. 2011;133:6587–95. doi: 10.1021/ja108807j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li QQ, Luo YX, Sun CY, Xue YX, Zhu WL, Shi HS, et al. A morphine/heroin vaccine with new hapten design attenuates behavioral effects in rats. J Neurochem. 2011;119:1271–81. doi: 10.1111/j.1471-4159.2011.07502.x. [DOI] [PubMed] [Google Scholar]
- 19.Haney M, Kosten TR. Therapeutic vaccines for substance dependence. Expert Rev Vaccines. 2004;3:11–8. doi: 10.1586/14760584.3.1.11. [DOI] [PubMed] [Google Scholar]
- 20.Esson L, Leeder SR. The millennium development goals and tobacco control: an opportunity for global partnership. Geneva: World Health Organization (WHO): 2004. [Google Scholar]
- 21.Tobacco Free Initiative (TFI). Why is tobacco a public health priority? World Health Organization (WHO): 2005 [http://www.who.int/tobacco/health_priority/en (accessed on March 15, 2012)] [Google Scholar]
- 22.Rose JE, Mukhin AG, Lokitz SJ, Turkington TG, Herskovic J, Behm FM, et al. Kinetics of brain nicotine accumulation in dependent and nondependent smokers assessed with PET and cigarettes containing 11C-nicotine. Proc Natl Acad Sci U S A. 2010;107:5190–5. doi: 10.1073/pnas.0909184107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houtsmuller EJ, Henningfield JE, Stitzer ML. Subjective effects of the nicotine lozenge: assessment of abuse liability. Psychopharmacology (Berl) 2003;167:20–7. doi: 10.1007/s00213-002-1361-2. [DOI] [PubMed] [Google Scholar]
- 24.Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362:2295–303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polosa R, Benowitz NL. Treatment of nicotine addiction: present therapeutic options and pipeline developments. Trends Pharmacol Sci. 2011;32:281–9. doi: 10.1016/j.tips.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frishman WH. Smoking cessation pharmacotherapy. Ther Adv Cardiovasc Dis. 2009;3:287–308. doi: 10.1177/1753944709335754. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. 2004 Surgeon General’s Report – The health consequences of smoking. [http://www.cdc.gov/tobacco/data_statistics/sgr/2004/index.htm (accessed on March 15, 2012)] [PubMed]
- 28.Rose JE. Disrupting nicotine reinforcement: from cigarette to brain. Ann N Y Acad Sci. 2008;1141:233–56. doi: 10.1196/annals.1441.019. [DOI] [PubMed] [Google Scholar]
- 29.Kosten T, Owens SM. Immunotherapy for the treatment of drug abuse. Pharmacol Ther. 2005;108:76–85. doi: 10.1016/j.pharmthera.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Orson FM, Kinsey BM, Singh RAK, Wu Y, Gardner T, Kosten TR. Substance abuse vaccines. Ann N Y Acad Sci. 2008;1141:257–69. doi: 10.1196/annals.1441.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Escobar-Chávez JJ, Domínguez-Delgado CL, Rodríguez-Cruz IM. Targeting nicotine addiction: the possibility of a therapeutic vaccine. Drug Des Devel Ther. 2011;5:211–24. doi: 10.2147/DDDT.S10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cerny EH, Cerny T. Vaccines against nicotine. Hum Vaccin. 2009;5:200–5. doi: 10.4161/hv.5.4.7310. [DOI] [PubMed] [Google Scholar]
- 33.de Villiers SHL, Lindblom N, Kalayanov G, Gordon S, Baraznenok I, Malmerfelt A, et al. Nicotine hapten structure, antibody selectivity and effect relationships: results from a nicotine vaccine screening procedure. Vaccine. 2010;28:2161–8. doi: 10.1016/j.vaccine.2009.12.051. [DOI] [PubMed] [Google Scholar]
- 34.Keyler DE, Dufek MB, Calvin AD, Bramwell TJ, LeSage MG, Raphael DE, et al. Reduced nicotine distribution from mother to fetal brain in rats vaccinated against nicotine: time course and influence of nicotine dosing regimen. Biochem Pharmacol. 2005;69:1385–95. doi: 10.1016/j.bcp.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 35.LeSage MG, Keyler DE, Hieda Y, Collins G, Burroughs D, Le C, et al. Effects of a nicotine conjugate vaccine on the acquisition and maintenance of nicotine self-administration in rats. Psychopharmacology (Berl) 2006;184:409–16. doi: 10.1007/s00213-005-0027-2. [DOI] [PubMed] [Google Scholar]
- 36.Sanderson SD, Cheruku SR, Padmanilayam MP, Vennerstrom JL, Thiele GM, Palmatier MI, et al. Immunization to nicotine with a peptide-based vaccine composed of a conformationally biased agonist of C5a as a molecular adjuvant. Int Immunopharmacol. 2003;3:137–46. doi: 10.1016/S1567-5769(02)00260-6. [DOI] [PubMed] [Google Scholar]
- 37.Keyler DE, Hieda Y, St Peter J, Pentel PR. Altered disposition of repeated nicotine doses in rats immunized against nicotine. Nicotine Tob Res. 1999;1:241–9. doi: 10.1080/14622299050011361. [DOI] [PubMed] [Google Scholar]
- 38.Pravetoni M, Keyler DE, Pidaparthi RR, Carroll FI, Runyon SP, Murtaugh MP, et al. Structurally distinct nicotine immunogens elicit antibodies with non-overlapping specificities. Biochem Pharmacol. 2012;83:543–50. doi: 10.1016/j.bcp.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selecta Biosciences: Recent news. Selecta Biosciences Initiates Phase 1 Clinical Study of SEL-068, a First-in-Class Synthetic Nicotine Vaccine for Smoking Cessation and Relapse Prevention [http://www.selectabio.com/news/recent-news/Selecta-Biosciences-Initiates-Phase-1-Clinical-Study-of-SEL-068.cfm (accessed on January 14, 2012)]
- 40.Cornish KE, Harris AC, LeSage MG, Keyler DE, Burroughs D, Earley C, et al. Combined active and passive immunization against nicotine: minimizing monoclonal antibody requirements using a target antibody concentration strategy. Int Immunopharmacol. 2011;11:1809–15. doi: 10.1016/j.intimp.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malin DH, Moon WD, Goyarzu P, Magallanes N, Blair MB, Alexander MR, et al. Passive immunization against nicotine attenuates somatic nicotine withdrawal syndrome in the rat. Nicotine Tob Res. 2010;12:438–44. doi: 10.1093/ntr/ntq021. [DOI] [PubMed] [Google Scholar]
- 42.Raupach T, Hoogsteder PHJ, Onno van Schayck CP. Nicotine vaccines to assist with smoking cessation: current status of research. Drugs. 2012;72:e1–16. doi: 10.2165/11599900-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hasman A, Holm S. Nicotine conjugate vaccine: is there a right to a smoking future? J Med Ethics. 2004;30:344–5. doi: 10.1136/jme.2002.001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kinsey BM, Jackson DC, Orson FM. Anti-drug vaccines to treat substance abuse. Immunol Cell Biol. 2009;87:309–14. doi: 10.1038/icb.2009.17. [DOI] [PubMed] [Google Scholar]
- 45.Keyler DE, Roiko SA, Earley CA, Murtaugh MP, Pentel PR. Enhanced immunogenicity of a bivalent nicotine vaccine. Int Immunopharmacol. 2008;8:1589–94. doi: 10.1016/j.intimp.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hieda Y, Keyler DE, Ennifar S, Fattom A, Pentel PR. Vaccination against nicotine during continued nicotine administration in rats: immunogenicity of the vaccine and effects on nicotine distribution to brain. Int J Immunopharmacol. 2000;22:809–19. doi: 10.1016/S0192-0561(00)00042-4. [DOI] [PubMed] [Google Scholar]
- 47.Satoskar SD, Keyler DE, LeSage MG, Raphael DE, Ross CA, Pentel PR. Tissue-dependent effects of immunization with a nicotine conjugate vaccine on the distribution of nicotine in rats. Int Immunopharmacol. 2003;3:957–70. doi: 10.1016/S1567-5769(03)00094-8. [DOI] [PubMed] [Google Scholar]
- 48.Pravetoni M, Keyler DE, Raleigh MD, Harris AC, Lesage MG, Mattson CK, et al. Vaccination against nicotine alters the distribution of nicotine delivered via cigarette smoke inhalation to rats. Biochem Pharmacol. 2011;81:1164–70. doi: 10.1016/j.bcp.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hatsukami DK, Jorenby DE, Gonzales D, Rigotti NA, Glover ED, Oncken CA, et al. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther. 2011;89:392–9. doi: 10.1038/clpt.2010.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagena EJ, de Vos A, Horwith G, van Schayck CP. The immunogenicity and safety of a nicotine vaccine in smokers and nonsmokers: results of a randomized, placebo-controlled phase 1/2 trial. Nicotine Tob Res. 2008;10:213–8. doi: 10.1080/14622200701704921. [DOI] [PubMed] [Google Scholar]
- 51.Cytos Biotechnology. CYT002 Nic Qb: a novel vaccine for nicotine addiction. Zurich: Cytos Biotechnology; 2012. [http://www.cytos.com/userfiles/file/Cytos_Press_E_091015.pdf (accessed on January 15, 2012)] [Google Scholar]
- 52.Roiko SA, Harris AC, Keyler DE, Lesage MG, Zhang Y, Pentel PR. Combined active and passive immunization enhances the efficacy of immunotherapy against nicotine in rats. J Pharmacol Exp Ther. 2008;325:985–93. doi: 10.1124/jpet.107.135111. [DOI] [PubMed] [Google Scholar]
- 53.Raupach T, van Schayck CP. Pharmacotherapy for smoking cessation: current advances and research topics. CNS Drugs. 2011;25:371–82. doi: 10.2165/11590620-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 54.Leader AE, Lerman C, Cappella JN. Nicotine vaccines: will smokers take a shot at quitting? Nicotine Tob Res. 2010;12:390–7. doi: 10.1093/ntr/ntq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carrozzi L, Pistelli F, Viegi G. Pharmacotherapy for smoking cessation. Ther Adv Respir Dis. 2008;2:301–17. doi: 10.1177/1753465808096136. [DOI] [PubMed] [Google Scholar]
- 56.Hall WD. Will nicotine genetics and a nicotine vaccine prevent cigarette smoking and smoking-related diseases? PLoS Med. 2005;2:e266–, quiz e351. doi: 10.1371/journal.pmed.0020266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maurer P, Jennings GT, Willers J, Rohner F, Lindman Y, Roubicek K, et al. A therapeutic vaccine for nicotine dependence: preclinical efficacy, and Phase I safety and immunogenicity. Eur J Immunol. 2005;35:2031–40. doi: 10.1002/eji.200526285. [DOI] [PubMed] [Google Scholar]
- 58.Cornuz J, Zwahlen S, Jungi WF, Osterwalder J, Klingler K, van Melle G, et al. A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS One. 2008;3:e2547. doi: 10.1371/journal.pone.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Successful Results from Second Clinical Trial of Anti-Smoking Vaccine, TA-NIC; 2004 [http://www.globenewswire.com/newsroom/news.html?d=60776 (accessed on January 15, 2012)]
- 60.Xenova Group plc: Anti-Smoking Vaccine TA-NIC Preliminary 12 Month Clinical Trial Results [press release]. Berkshire, UK: Xenowa Group; 2005 [http://www.globenewswire.com/newsroom/news.html?d=73733 (accessed on January 15, 2012)] [Google Scholar]
- 61.ClinicalTrials.gov [http://www.clinicaltrials.gov/ct2/show/NCT00633321?term=TA-NIC&rank=1 (accessed on January 14, 2012)]
- 62.Nabi Biopharmaceuticals: Clinical Trials. NicVAX® (Nicotine Conjugate Vaccine) [http://www.nabi.com/pipeline/clinicaltrials.php (accessed on November 11, 2012)]
- 63.Hatsukami DK, Rennard S, Jorenby D, Fiore M, Koopmeiners J, de Vos A, et al. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clin Pharmacol Ther. 2005;78:456–67. doi: 10.1016/j.clpt.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 64.Nabi Biopharmaceuticals: News Release. Nabi Biopharmaceuticals Announces Results of Second NicVAX(R) Phase III Clinical Trial [http://phx.corporate-ir.net/phoenix.zhtml?c=100445&p=irol-newsArticle&ID=1626882&highlight (accessed on January 14, 2012)]
- 65.The Local. Sweden’s News in English. Swedish anti-smoking vaccine to undergo tests [http://www.thelocal.se/11392/20080428/ (accessed on March 15, 2012)]
- 66.Oliver JL, Pashmi G, Barnett P, Mettens P, Biemans R, Monteyne P, et al. Development of an anti-cotinine vaccine to potentiate nicotine-based smoking cessation strategies. Vaccine. 2007;25:7354–62. doi: 10.1016/j.vaccine.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 67.Bevins RA, Wilkinson JL, Sanderson SD. Vaccines to combat smoking. Expert Opin Biol Ther. 2008;8:379–83. doi: 10.1517/14712598.8.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Le Houezec J. Why a nicotine vaccine? Clin Pharmacol Ther. 2005;78:453–5. doi: 10.1016/j.clpt.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 69.Fahim RE, Kessler PD, Fuller SA, Kalnik MW. Nicotine vaccines. CNS Neurol Disord Drug Targets. 2011;10:905–15. doi: 10.2174/187152711799219343. [DOI] [PubMed] [Google Scholar]
- 70.Keyler DE, Shoeman D, LeSage MG, Calvin AD, Pentel PR. Maternal vaccination against nicotine reduces nicotine distribution to fetal brain in rats. J Pharmacol Exp Ther. 2003;305:587–92. doi: 10.1124/jpet.102.046805. [DOI] [PubMed] [Google Scholar]
- 71.Kuehn BM. Could a novel vaccine help smokers quit? JAMA. 2005;294:891–2. doi: 10.1001/jama.294.8.891. [DOI] [PubMed] [Google Scholar]
- 72.LeSage MG, Keyler DE, Pentel PR. Current status of immunologic approaches to treating tobacco dependence: vaccines and nicotine-specific antibodies. AAPS J. 2006;8:E65–75. doi: 10.1208/aapsj080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gartner CE, Barendregt JJ, Wallace A, Hall WD. Would vaccination against nicotine be a cost-effective way to prevent smoking uptake in adolescents? Addiction. 2012;107:801–9. doi: 10.1111/j.1360-0443.2011.03718.x. [DOI] [PubMed] [Google Scholar]