Abstract
The immunogenic properties of heat shock proteins (HSPs) have prompted investigations into their application as immuno-modulatory agents. HSPs have been used as potent adjuvants in immunotherapy of cancer and infectious diseases. Some studies showed that immune activities reside within N- or C-terminal fragments of HSPs. These small fragments are sufficient to link peptides, to bind and be taken up by the receptors CD91 and scavenger receptor type A on antigen presenting cells (APCs). Thus, these mini-chaperones can be used in immunotherapy of tumors and vaccine development. The data clearly demonstrated the potential of using HSP fragments as a possible adjuvant to augment CTL response against infectious diseases. Some HSP domains have been shown to inhibit endothelial cell growth, angiogenesis or tumor growth. In this review, we describe the immuno-stimulatory activities of various mini-chaperones in development of different vaccine strategies (DNA-based vaccine and protein/peptide-based vaccines).
Keywords: heat shock protein, mini-chaperone, immuno-adjuvant, vaccine, cancer, infectious disease
Introduction
New efficient vaccines against infectious diseases are in demand. Major challenge in developing effective vaccines is to induce effective immune responses to antigen of interest with no or very limited side effects. The immunogenicity can be improved by using appropriate carriers and adjuvant molecules.1 Adjuvants can be used for various purposes including: (1) enhancement of the immunogenicity of antigen (as a DNA, peptide or protein form); (2) reduction of the quantity of antigen or the number of administration for eliciting immune responses; (3) improvement of the vaccine potency in subjects with different conditions (e.g., newborns, the elderly or immuno-compromised persons) and (4) uptake of antigen by the mucosa as an antigen delivery system. Adjuvants can be classified according to their source, mechanism of action or physicochemical properties.2
It is obvious from different studies that the currently licensed vaccine adjuvants are not sufficiently effective for the induction of potent immune responses. Currently, heat shock proteins (HSPs) have known as an efficient adjuvant in vaccine development. Heat shock proteins are highly conserved, stress-induced proteins and function as chaperones stabilizing and delivering peptides.2 Mammalian HSPs have been classified into several families according to their molecular weights: HSP100, HSP90, HSP70, HSP60 and small molecular HSPs (e.g., HSP27).3 HSPs have been involved to elicit both innate and adaptive immunity. The capacity of HSPs to bind antigenic peptides and deliver them to antigen presenting cells (APCs) is the basis of the generation of peptide-specific T lymphocyte responses both in vitro and in vivo.2 Stressful conditions (e.g., hypoxia, nutrient deprivation, etc.) induce HSP synthesis, and several types of cancer cells expose HSPs on their surface. These cells are recognized and killed by the cytotoxic T cells (CTLs). Regarding to current studies, the levels of heat shock proteins are elevated in some cancers including breast cancer and are molecular targets for novel therapies.4 HSP-peptide complexes extracted from tumors have been extensively studied in the preceding two decades, confirming to be safe and effective in treating a number of malignant disorders. However, these complexes target a range of antigens expressed in tumors of each patient, individually.5 Currently, the HSP-peptide vaccine designed by in vitro reconstitution is one of many promising immunotherapeutic strategies being evaluated in preclinical as well as clinical trials 6-10 (Fig. 1).
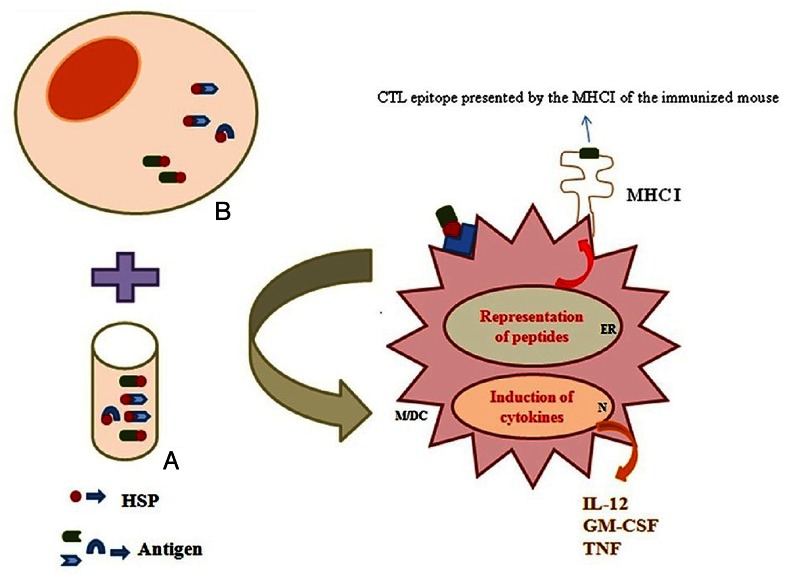
Figure 1. Heat shock proteins bind the antigenic repertoire of cancers or pathogen-infected cells. These molecules can bind to synthetic or natural peptides in vitro/ in vivo (A/B)). They deliver antigenic peptides and maturation signals to antigen-presenting cells (Macrophage/ Dendritic cell) and induce release of cytokines. DC, dendritic cell; HSP, heat shock protein; ER, endoplasmic reticulum; M, macrophage; N, nucleus.
HSPs were first observed as proteins induced in massive amounts in normal cells exposed to stresses that lead to protein denaturation. Their expanded expression in mammary carcinoma appears to be largely due to the proliferation of mal-folded mutant proteins and overexpressed oncoproteins that trigger transcription of HSP genes.4 HSPs play major roles in malignant transformation and progression mediated through their intrinsic molecular chaperone properties. These permit the emergence of new malignant traits through the facilitated accumulation of altered oncoproteins. The elevation of HSP concentrations in mammary carcinoma is at least partially dependent on heat shock transcription factor 1 (HSF1), a protein that responds to unfolded proteins and leads to HSP transcription. HSF1 activation has additional downstream activities, crucial for emergence of the breast cancer phenotype including activated cell signaling; HSP-mediated ability to evade apoptosis and an HSF1-dependent bias in transcriptional activity toward a metastatic phenotype.4
Heat shock proteins (HSPs) have a dual function depending on their intracellular or extracellular location. Intracellular HSPs (e.g., HSP70 and HSP90) have numerous cyto-protective roles described by their anti-apoptotic properties. In tumor cells, the unusual high expression of HSP70 and/or HSP90 leads to oncogenesis and resistance to chemotherapy. Regarding to these functions, the inhibition of HSPs has been applied as an efficient strategy in cancer therapy.11 In contrast, extracellular or membrane-bound HSPs are potent inducers of innate and adaptive immune systems. Most immunotherapeutical approaches based on HSPs utilize their role as a carrier for immunogenic peptides.11
Heat shock proteins have been described in vaccine development for cancer and infectious diseases in preclinical and clinical trials. HSPs chaperone antigenic peptides that are generated intra-cellular, increase presentation of antigens to effector cells and augment cellular and humoral immune responses against their associated antigens. Indeed, they act as a strong modulator, as well as potentiator of the immune system. Some studies showed that all immuno- stimulatory properties of HSPs reside within various domains of HSPs.12 These small fragments so-called “mini-chaperones” can be used in immunotherapy of tumors and vaccine development. These mini-chaperones have been described to enhance fusion vaccine potency.13 In this review, we describe immune-modulatory properties of different domains of HSPs.
Chaperone Molecules as the Efficient Vaccines
Immunization with purified gp96 and heat shock protein 70 bound to synthetic or natural peptides (as in vitro/ in vivo generation) has been shown to induce peptide-specific cytotoxic T lymphocytes (CTL).14,15 The peptides chaperoned by heat shock proteins are taken up by APCs, migrated to the endoplasmic reticulum (ER), loaded on MHC I and transported as MHC I- peptide complexes to the cell surface as shown in Figure 1.16 Different vaccination strategies have already been employed by HSP molecules.17 These methods contain (1) the use of purified HSP-peptides from infected tissues; (2) DNA vaccination by antigen/HSP covalent linkage or non-covalent co-administration; (3) protein vaccination using HSP or its fragments as an adjuvant; d) prime-boost vaccination using HSP or its fragments (e.g., DNA/ protein).17
Generally, the immuno-modulatory functions of HSPs are based on two intrinsic properties: (1) HSPs are potent adjuvants due to their ability to activate dendritic cells (DCs); (2) HSPs can bind directly to their receptors on DCs to load HSP-associated peptides on MHC molecules.6 The existence of heat-shock protein (HSP) receptors on antigen-presenting cells (APCs) was hypothesized in 1994. The first receptor, CD91 or LRP, was identified in 2000. Six putative HSP receptors have been identified including CD40, LOX-1, CD36, TLR-2, TLR-4 and SR-A.18,19
Several in vitro studies have revealed that independent of their bound peptides, Gp96, HSP90 and HSP70 are able to induce macrophages to produce the pro-inflammatory cytokines such as interleukin (IL)-1β, TNF-α, IL-12 and GM-CSF as well as C-C chemokines such as MCP-1, MIP-1 and RANTES.8 Furthermore, HSPs induce DC maturation as determined by their upregulation of MHC class II and the co-stimulatory molecules CD86 (B7–2) and CD40 and promote their accumulation in draining lymph node in vivo. Interaction of Gp96 with murine DC or HSP70 with human monocytes also triggers the translocation of NF-κB, a key signaling transduction pathway in immune responses.8
The HSP-peptide complex can elicit a potent specific cellular adaptive immune response that depends on its ability to chaperone a large variety of peptides. The reported data have indicated that APCs (e.g., peritoneal macrophages and DC) internalize purified or artificially reconstituted HSP-peptide complexes by receptors-mediated endocytosis such as CD91 and/or LOX-1.8,9,20
With the help of signaling receptors such as CD14 and TLR-2/4, the antigenic peptides carried by HSP are then channeled into the endogenous presenting pathway and presented to the surface of APCs and recognized by CD8+T cells as a peptide-MHC class I complex.8,9,21 The cross-presentation of peptides chaperoned by HSP are approximately 200–400 more efficient than loading antigenic peptides directly to class MHCI onto live cells. Interestingly, this process is not observed in B-cells or fibroblasts. Then, potent cytotoxic T lymphocyte response toward the target cell is primed. The unique ability of the HSP-peptide complex to prime a robust T cell response from minute amounts of chaperoned antigenic peptides is thought to result mainly from the specific interaction of HSPs with APCs.8
Autoimmune responses are a possible concern for using HSP70 family. Indeed, anti- HSP60, HSP65 22-24 and HSP70 25,26 immunities have been revealed in some autoimmune diseases. Some characteristics of HSP70 families including a high degree of sequence homology between different species and intrinsic immunogenicity have suggested that inappropriate immune reactivity to HSP70 might lead to pro-inflammatory responses and subsequently the development of autoimmune disease.27 The investigators have shown that the degree of homology varies between different regions of HSP70 especially C-terminal domain.28 Thus, the C-terminal domain of HSP70, with a lower degree of homology, may represent an advantage in vaccine design to avoid possible autoimmune reactions.28 Indeed, the determination of the effective domain of HSP70 rather than the native HSP70 as an adjuvant is a vital issue in vaccine design against various disorders.
In addition, some scientists believe that heat shock proteins alone are not efficient for vaccine development in clinical trials. Therefore, they tried to potentiate their functions. For instance, the researchers have shown that a B-class CpG ODN (BW006) as a TLR9 agonist, could enhance anti-tumor activity of the recombinant HSP65-Her2 fusion protein, accompanied with improved HSP65-Her2 specific Th1 response in mice bearing Her2 (+) B16 melanoma.29
Mini-Chaperones are Potent Immuno-Adjuvants in Vaccine Development
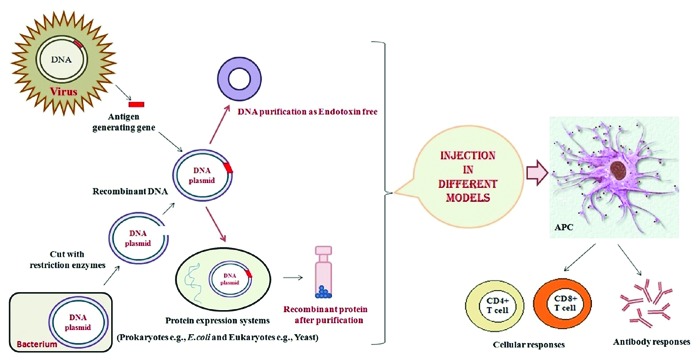
Several studies indicated that immune activities of HSPs reside within their N- or C-terminal fragments known as mini-chaperones. These potent regions of HSPs are involved in development of various vaccines including DNA-based vaccine and protein/peptide-based vaccine (Fig. 2).
Figure 2. Production of recombinant vaccines: The recombinant vaccines such as DNA and protein -based vaccines combined with the potent immunological stimulants (Adjuvants, delivery systems and …) are designed to stimulate host immune system to recognize and destroy infectious/cancer cells.
The mini-chaperone is a useful practical tool for the refolding of proteins in vitro.30 For example, the fragments surrounding the apical domain of GroEL, called mini-chaperones, facilitate the refolding of several proteins in vitro without requiring GroES, ATP or the cage-like structure of multimeric GroEL.31 These minimal constructs of heat shock proteins (e.g., Gp96 or HSP70) can augment peptide presentation in culture and induce antigen-specific CTL in naive mice only because it loads APCs with the relevant peptide. For example, the N-terminal of Gp96 (sequence 1–355 aa) is the immunologically sufficient module of Gp96 and this ‘mini-chaperone’ can be used in immunotherapy of tumors and vaccine development. Indeed, the N-terminal fragment binds to DCs and macrophages via the same CD91 and SR-A receptors previously reported to mediate binding of the full-length protein (Gp96).12 Herein, we will describe the use of these efficient regions in numerous immunotherapies.
DNA-Based Vaccines
DNA vaccines represent an attractive approach to therapy of infectious diseases as well as cancer, autoimmune diseases and allergy because of its ability to generate antigen-specific immunity (Fig. 2). However, their potency has been insufficient to elicit the efficient protective immunity in human clinical trials. Several strategies have been applied to enhance the potency of DNA vaccine including the use of various adjuvants e.g., heat shock proteins.32 We describe some recent advances in upgrading the efficiency of DNA vaccines using different fragments of HSPs (N-/C-terminal) in animal models.
Heat shock protein 70 (HSP70) has been shown to be an excellent candidate, capable of cross-priming tumor-associated antigen (TAA) by APCs leading to a robust T-cell response. Mycobacterium tuberculosis HSP70 has been shown to act as an adjuvant when co-administered with peptide/ protein antigens.33
The effects of two truncated HSP70 molecules, N-terminal domain (HSP70 1–360, amino acids 1–360) and C-terminal domain (HSP70 359–610, amino acids 359–610) of mycobacterial HSP70 have been evaluated on the potency of antigen-specific immunity generated by a chronic hepatitis B virus (HBV) DNA vaccination. The data showed that only the HSP70 359–610-fused HBV DNA vaccination resulted in a significant increase in hepatitis B surface antigen (HBsAg)-specific humoral response.33 HSP70 359–610-fused DNA vaccine not only enhanced HBsAg-specific cytotoxic lymphocytes (CTL) responses but also did not induce anti-HSP70 antibody. In addition, HSP70 359–610 mediated T helper (Th) cell balance toward Th1 pathway. In a HBV transgenic mouse model, the HSP70 359–610 fusion vaccine facilitated clearance of circulating HBsAg and downregulation of HBV replication. These results suggested that C-terminal domain of mycobacterial HSP70 molecule could be a potent candidate to induce the adjuvant effect in HBV DNA vaccination in contrast with the full length HSP70 molecule.33 In this line, it was shown that the C-terminal fragment of mHSP70 acts as a carrier in mice when fused to the malarial antigen EB200 (HSP70-EB200) and considerably induces the efficient immune responses.34 In other studies, the effects of HSP70 359–610 on foot and mouth virus (FMDV) and Japanese encephalitis virus (JEV), individually indicated that this fragment of HSP70 markedly enhances both the humoral and cell-mediated immune responses in mice.35,36
Currently, the researchers have evaluated the immune effects of a fusion DNA vaccine encoding mycobacterial HSP70 and MPT51, a major secreted protein of Mycobacterium tuberculosis. The data indicated that mice immunized with fusion HSP70-MPT51 DNA produce a higher amount of IFN-γ than mice immunized with MPT51 DNA alone.37 In continuation, the domains of HSP70 responsible for its enhancing effect [i.e., the N-terminal ATPase domain (NT- HSP70) and the C-terminal peptide-binding domain (CT-HSP70)] were identified. The fusion DNA vaccine encoding the CT-HSP70 and MPT51 induced a higher MPT51-specific IFN-γ production by CD4+ T cells than the vaccine encoding MPT51 alone or MPT51- NT-HSP70. Similar results were obtained by immunization with the fusion proteins. These results suggested that the DNA vaccine encoding a chimeric antigen molecule fused with mHSP70, especially with its C-terminal domain, can induce a stronger antigen-specific Th1 response than antigen DNA alone.37
HSP70 fused to downstream of Her2/neu as DNA vaccine has been shown to be efficient against Her2-expressing tumors. Following this investigation, the researchers have examined if N-terminally fusion of Her2/neu to HSP70 can improve efficiency of Her2/neu DNA vaccine. Surprisingly, HSP70 fusion to N-terminal of rat Her2/neu led to tumor progression. These results propose that fusion direction of biologic adjuvant as well as different domains are important issues when Her2/neu is used.38
Briefly, microbial HSP70 contains three functionally distinct domains: an N-terminal 44 kDa ATPase domain (amino acids 1–358), followed by an 18 kDa peptide-binding domain (amino acids 359–494) and a C-terminal 10 kDa domain (amino acids 495–609). Immunological functions of different HSP70 domains in stimulating antigen presenting cells have not been fully defined. Most studies have indicated that the C-terminal fragment as well as peptide binding domain (i.e., amino acids 359–610) stimulate the generation of CC chemokines, IL-12, TNF-α, NO and maturation of dendritic cells (DCs). In addition, this fragment can act as an adjuvant to elicit the immune responses.39
The glycoprotein 96 (Gp96/GRP94) is an important member of the HSP90 family located in the endoplasmic reticulum (ER). This protein possesses the N-terminal fragment with signal peptide of 21 amino acids which is cleaved co-translationally and the C-terminal fragment containing an ER-retention sequence, KDEL. This chaperone has been proved to increase peptide presentation to T cells. It can link antigenic peptides, bind to receptors on APCs, activate these cells and after internalization, transfer the peptides to MHC class I for T cell activation.40 Adjuvant activity of Gp96 with some viral, bacterial and tumor antigens has been reported in DNA vaccination. Previously, our group studied the immunity of HPV16 E7 along with Gp96 as an adjuvant in C57BL/6 mice model.41 Human papillomaviruses, especially type 16 (HPV16) is an important cause in more than 99% of cervical cancers. E7 is the major oncoprotein produced in cervical cancer-associated HPV16. Evaluation of cellular immune responses demonstrated that DNA vaccination using co-administration of E7 and Gp96 induces Th1 response. Moreover, co- delivery of naked DNA E7 + Gp96 plasmid was immunologically more effective than E7 alone.41
Several studies showed that the N- or C-terminal fragments of gp96 as mini-chaperones are better choice for immunization.12 The studies have indicated that co-administration of Gp96 + Her2/neu DNA vaccines led to the decreased CD4+ CD25+ Foxp3+ naturally occurring regulatory T cells (Tregs) at the tumor site and increased IFN-γ/ IL-4 level.42 The data demonstrated a bi-phasic pattern of tumor size in which partial inhibition of tumor growth initially occurred when the tumor was small, but finally its preventive effect was reversed by increasing tumor size.43 The other study also indicated that HSP110/Her2 vaccine was not effective by the end of tumor growth similar to this observation.43 There are two possible reasons: (1) failure of the immune system at the final stages due to antigen loss and tumor escape. It has been reported that IFN-γ could cause tumor escape from anti-tumor immune responses 44,45; (2) a kinetic in regulatory burden and tumors may weaken the immune system to facilitate disease progression.46
With regard to previous studies indicating potent adjuvant activity of the full length Gp96, the immune responses of two DNA vaccines containing C-terminal fragment of gp96 fused to the C-terminal end of transmembrane and extracellular domain (TM+ECD) of rat Her2/neu as well as N-terminally fusion of gp96 C-terminal domain to TM+ECD were evaluated in tumor mice model. The results showed that adjuvant activity of C-terminal domain is enhanced when fused N-terminally to TM+ECD of rat Her2/neu. Therefore, the adjuvant activity of C-terminal domain of gp96 toward Her2/neu is fusion direction-dependent.47 Moreover, pHer2/CT was able to increase IFN-γ/IL-4 secretion and CD8+ cell proliferation at the tumor site along with an increase in the percentage of specific cell lyses (CTL activity). Decline in Tregs was observed at both spleen and tumor site of pHer2/CT-vaccinated mice, in comparison with pCT/Her2-vaccinated group. All these properties indicated the potent adjuvant activities of C-terminal domain of gp96 when fused down-stream of Her2/neu and the significance of fusion direction in DNA vaccine. It seems that conformational changes and different ubiquitination pattern in pHer2/CT may increase its efficiency in comparison with pCT/Her2.47 It is likely that N-terminally fusion of Her2/neu to C-terminal domain of gp96 might have caused conformational change or steric hindrance, led to the decrease of Her/neu DNA vaccine potency. On the other hand, different fusion direction might have induced different pattern of ubiquitination on the product of each construct (i.e., pCT/Her2 and/or pHer2/CT).48
A study has shown the adjuvant activity of both domains of gp96 toward Her2/neu, as DNA vaccine in a Her2/neu-positive breast cancer model. Immunological test indicated that treatment with Her2/neu fused to N-terminal domain of gp96 led to significantly lower survival rates, higher IFN-γ secretion and induced infiltration of CD4+/CD8+ cells to the tumor site. But, it could not induce CTL activity, did not decrease regulatory T cell percentage at the tumor site, and finally led to tumor progression in comparison with the groups vaccinated with pCT/Her2 or pHer2. Therefore, N-terminal domain of gp96 did not have adjuvant activity toward Her2/neu. According to the obtained results, immune-stimulatory properties of different gp96 domains may be affected by the antigen of interest.49 Even, the reports on the adjuvant activity of C-terminal domain of gp96 are controversial, depending on the antigen.50,51 The data showed that the construct containing the C-terminal of gp96 fused with Her2/neu, but not the co-administration of the two separated constructs, decreased CD4+CD25+foxp3+ regulatory T cells at the tumor site and enhanced CTL activity as well as IFN-γ secretion. Therefore, the C-terminal of gp96 can be used as molecular adjuvant along with other tumor/ bacterial/viral antigens for improving vaccine potency.52
Furthermore, our research group applied two strategies including linkage of immune- stimulatory molecules (N-terminal of gp96), accompanied with PEI600-Tat as non-viral gene delivery system to evaluate DNA vaccine efficacy against HPV infections. It was found that immunization with E7-NT (gp96) fusion gene led to increase IFN-γ level as compared with E7 alone. This fusion showed considerable protective potency in tumor mice model. In addition, delivery of E7-NT (gp96) with PEI600-Tat was further protective against E7-expressing tumors. These data indicated that fusion of NT (gp96) to E7 delivered by PEI600-Tat can enhance the potency of HPV DNA vaccines.53 In continuation, we generated DNA construct encoding HPV16 E7 linked to C-terminal of gp96 and evaluated immune responses after delivering with electroporation in C57BL/6 tumor mice model. Immunization with E7-CT (gp96) fusion gene led to increase IFN-γ level and protective efficacy as compared with E7 or E7- NT (gp96) DNA against E7-expressing tumors (Immunology Letters 2012, in press). Thus, the importance of different domains of gp96 is still open to discussion.
Protein/Peptide-Based Vaccine
Protein-based vaccines are capable of generating CD8+ T cell responses in vaccinated animals and humans as well as humoral immune responses (Fig. 2). Therapeutic protein vaccination has been limited by insufficient antigen-specific immune responses. Thus, development of novel strategies that enhance protein vaccine potency is important for generation of effective cancer immuno-therapies. Among these strategies, HSPs especially CRT, HSP70 and Gp96 have been known to act as potent immuno-adjuvant to enhance antigen-specific tumor immunity.
Calreticulin (CRT) binds to mis-folded proteins and prevents their export from the ER to the Golgi apparatus. Calreticulin is expressed in many cancer cells and plays a role to promote APCs e.g., macrophages to overcome tumor cells. Herein, one question is why the cells are not destroyed by CRT. The reason is the presence of signal CD47 blocking CRT. Therefore, antibodies blocking CD47 might be useful as a cancer treatment. For instance, in mice models of myeloid leukemia and non-Hodgkin’s lymphoma, anti-CD47 antibody was effective in killing tumor cells while normal cells were unaffected.54 The studies have shown that the purified recombinant NH2-terminal domain of calreticulin (amino acids 1–180 named vasostatin) inhibits the proliferation of endothelial cells and also angiogenesis in vivo. Vasostatin is a small, soluble and stable molecule that is easy to produce and deliver in comparison with other inhibitors of angiogenesis. When vasostatin inoculated into athymic mice, it significantly reduced growth of human Burkitt lymphoma and human colon carcinoma.55
Previous studies have indicated that N-terminal of CRT (NT-CRT) or C-terminal half of HSP70 (CT-HSP) linked with HPV16 E7 is capable of inducing potent antigen-specific CTL activity in animal models. NT-CRT and CT-HSP synergistically exhibited significant increases in E7-specific CD8+ T cell responses and notable antitumor effects against E7-expressing tumors. Indeed, the recombinant NT-CRT/E7/CT-HSP fusion protein as a novel therapeutic vaccine generated potent anti-tumor immunity and anti-angiogenesis.56
In viral systems, the identification of serological markers would facilitate the diagnosis of virus-related diseases. The changes in HSP levels have been shown in some diseases including parasite, virus and tumor model systems. However, the quantity of immune response to gp96 has been rarely studied. In our previous study, the recombinant (r) proteins of E7, NT (gp96) and CT (gp96) were expressed in E.coli and seroreactivities of patients with invasive cervical cancer against these recombinant proteins were examined as diagnostic markers. The aim of the present investigation was to determine and search for serologic markers in cervical cancer patients associated with HPV. Our findings indicated that patients with an anti-HPV16 E7 response had a high reactivity to rNT (gp96) or rCT (gp96), but there was no seroreactivity in control sera. Moreover, there was not any significant difference in seroreactivities between normal and patients in adenocarcinoma cases. The results indicated that patients with high antibody response to HPV16 E7 had significant seroreactivity to CT-gp96 fragment as well. Indeed, the fragments of gp96 especially CT (gp96) besides viral oncogenic protein could be used to screen the patients suffering from squamous cell carcinoma (SCC).57
Regarding to previous studies, the N-terminal of gp96 as an immuno-adjuvant can induce effective immune responses against clinical disorders, especially cancers. Our group showed the preventive efficacy of recombinant HPV16 E7-NT (gp96) fusion protein as compared with HPV 16 E7 protein after challenging with cancerous TC-1 cell line. The data demonstrated that vaccination with fused E7-NT (gp96) protein induces high IFN-γ response and delays the tumor occurrence and growth in comparison with E7 protein alone. These results suggest that protein vaccination with adjuvant-free E7-NT (gp96) protein could direct the immune responses toward Th1 immunity. Furthermore, the linkage of NT (gp96) to E7 could increase protective anti-tumor immunity.58
Lipophosphoglycan 3 (LPG3), the Leishmania homologous with Gp96, is involved in assembly of LPG as the most abundant macromolecule on the surface of Leishmania promastigotes. Our group have tested LPG3 as a vaccine candidate in two various strategies, DNA/DNA and prime-boost (DNA/protein), against Leishmania major infection in BALB/c mice model. We showed that LPG3 and its N-terminal fragment (rNT-LPG3) are highly immunogenic in BALB/c mice and can stimulate the production of both IgG1 and IgG2a.59
However, the level of antibody response in DNA/protein vaccination was higher as compared with DNA vaccination. In addition, the generation of IFN-γ in mice immunized with DNA/DNA and DNA/protein regimens was significantly higher in comparison with control groups. Indeed, prime-boost vaccination indicated higher ratio of IFN-γ/IL-5, suggesting a shift toward a Th1 response.59 However, further investigations are recommended on the usage of LPG3 co-delivery with candidate antigens for vaccine development against leishmaniasis.
Identification and selection of immuno-dominant antigens of Mycobacterium tuberculosis (MTB), capable of efficiently inducing a protective immune response is the ultimate goal of TB vaccine development studies. The potential of mHSP70 to function as adjuvants when fused to or co-delivered with protein antigens, make them attractive vaccine candidates. Recently, a novel M. tuberculosis fusion protein has been designed including MTB ESAT-6 (early secreted antigenic target-6 kDa), as a potent immunogenic protein, linked to C-terminus of MTB HSP70 (HSP70 359–610), as an adjuvant. The results of this study showed the efficient induction of specific immune responses in mice model indicating the ability of fusion protein, as a potential tuberculosis vaccine candidate.60 The studies showed that fusing mycobacterial HSP70 to HIV-1 gag p24 ,61,62 influenza A M2-protein (M2e) ,63,64 HPV16 E7 65 and synthetic malarial antigen (NANP) ,66 enhanced the immunogenicity of the antigens and hindered the need for adjuvant. For instance, mice immunized with a kinetoplasmid membrane protein-11 (KMP11) covalently fused to HSP70 from Trypanosoma cruzi elicited a CTL response against the Jurkat-A2/Kb cells expressing the KMP11 protein.67 Moreover, chimeric proteins formed by antigens coupled to the C-terminal fragment of HSP70 from M. tuberculosis 68,69 and N-terminal fragment of HSP70 and HSP83 from Leishmania infantum ,70 induced humoral and cell mediated immune responses to the coupled antigens.
In another study by our group, mice immunization was primed with Leishmania HSP70 DNA and boosted with recombinant (r) HSP70 protein (or its different fragments) mixed with Montanide 720. The data showed the highest immunogenicity with the complete open-reading frame of HSP70 (amino acids 221–604) and rCT-HSP70 (amino acids 491–604), but not rNT- HSP70 (amino acids 221–291) as a booster regimen.71
The humoral immune responses against the different truncated forms of HSP70 suggested a mixed IgG1/IgG2a response in vivo. Furthermore, the comparative studies in human patient samples indicated that sera from active cutaneous and visceral leishmaniasis patients were reactive to all three forms of HSP70. This study demonstrated the potential of HSP70 in stimulating humoral responses in humans and mice.71 The similar results were obtained in vaccine design against HPV infection. In prime-boost immunization strategies, C57BL/6 mice were immunized with the complete open-reading frame of E7 and Gp96 as DNA and then boosted with recombinant E7, NT and CT proteins mixed with Montanide 720 in different formulations. The humoral immune responses against rE7 and the different truncated forms of rGp96 suggested a mixed IgG1/IgG2a response in vivo.41
Most studies on the immune effect of gp96 were focused on knowing whether the recombinant N-terminal fragment of gp96 (NT-gp96, amino acid 22–355) expressed in E. coli can stimulate the immune system similar to native gp96 isolated from livers of normal BALB/c mice.72 Thus, in an experiment, mice groups received one of the following regimens subcutaneously including native gp96; NT-gp96; HBsAg; HBsAg + gp96; HBsAg + NT-gp96; HBsAg + incomplete Freud’s adjuvant and HBsAg + NT-gp96 (95°C heated for 30 min). The results demonstrated that native gp96 or NT-gp96 greatly improved humoral immune response induced by HBsAg, but failed to increase the CTL response. These data demonstrated the potential of gp96 or its N-terminal fragment as a possible adjuvant to augment humoral immune response against HBV infection.72
Peptide vaccination is a simple and safe approach in worldwide. For peptide preparation, protein fragments of any length obtain from chemical synthesis. One obstacle of using short peptides is the limitation of the intervention to defined members of the population that carry the MHC antigens which the peptides bind to it (MHC restriction). This limitation can be improved by using a physical or chemical mixture of short peptides or to extend the size of the peptide fragment in order to cover the MHC antigens of the entire population.73 The mycobacterial HSP70 covalently fused to ovalbumin (OVA)-derived fragments has been shown to generate MHC class I-restricted CTL responses. Moreover, five different CTL epitopes, including peptides derived from Plasmodium yoelii circumsporozoite protein, tumor antigens, HY antigen and OVA, were genetically fused to either the N- or C-terminus of murine Hsc70 and expressed in E. coli.74
Vaccination with all five fusion proteins and also bone marrow-derived dendritic cells pulsed with Hsc70 fusion proteins elicited peptide-specific CTL responses.74
In another study, to evaluate the adjuvant effect of gp96, mice were co-immunized with gp96 or its fragments and human HLA-A11-restricted 9-mer peptide (YVNVNMGLK) of hepatitis B virus (HBV) core antigen. The results demonstrated the potential of using gp96 or its N-terminal fragment of gp96, but not the C-terminal fragment of gp96, as a possible adjuvant to enhance CTL response against hepatitis B virus infection (HBV) infection and hepatocellular carcinoma (HCC).75 Increasing amounts of the immunizing peptide resulted in dose-dependent increases in the CTL response when gp96 or its N-terminal fragment was used as the adjuvant, indicating that their adjuvant effects were peptide concentration dependent. One explanation is that the increased amount of peptide may form more HSP-peptide complex to obtain sufficient cross-presentation. Additionally, HSPs might induce an innate immune response to produce an immunological environment optimal for adequate peptide quantities to be cross-presented.75
Future and Alternative Directions
One of the main topics in vaccine development is the use of new adjuvants to improve the antigen presentation and elicit the protective immune response. The structural domains of immuno-chaperones show the potential of generating effective immune responses against different clinical disorders as an area of recent studies. Comparable regions of these immuno-chaperones (N-/C-terminal fragments of HSPs) may have qualitatively different immunological effects in vaccine design. For instance, the peptide binding activity of HSP70 (i.e., the C-terminal domain of HSP70) may have a dual role of eliciting chaperokine effects as well as adaptive immune responses. In addition, the N-/C-terminal domain of gp96 has modest antitumor activity and seems to induce innate immunity via activation of APCs and generation of cytokines by CD4+ T cells. Therefore, these mini-chaperones have been suggested as the efficient immuno-adjuvants in fusion form with antigen. However, type and dose of antigen, method of injection, and properties of HSP domains affect immune responses in animal model. With our increasing insight of HSP structure and function, it will result for efficient vaccine development in clinical trial.
Perspectives: Myths and Facts
Molecular chaperones are crucial for the protection of our own life against proteotoxic stress. Chaperones are one of the most abundant proteins in our cells, and may help to maintain the structure of the cytoplasm in eukaryotes. Recent developments in the creation of several clinical methods which utilize the role of chaperones in peptide presentation make this field an exciting area for future clinical studies. Heat shock proteins (HSPs) are proposed as natural adjuvants that they can stimulate the innate and adaptive immune responses against infectious diseases and cancer. Thus, HSPs or different domains of HSPs have been known as a promising tumor vaccine candidate in animal models. However, heat shock protein (HSP)-based tumor vaccines have not yet succeeded in the clinical trials, involving the necessity to be formulated with appropriate delivery systems to enhance their immunogenicity.
Acknowledgments
The authors are grateful to Farnaz Zahedifard, Elham Mohit and Amin Daemi (Molecular Immunology and Vaccine Research Lab, Pasteur Institute of Iran) for experimental works.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/22248
References
- 1.Ebrahimi SM, Tebianian M. Role of mycobacterial heat shock protein 70 (mHSP70) as genetic vaccine adjuvants. World Applied Sciences Journal. 2011;14:1569–75. [Google Scholar]
- 2.Javid B, MacAry PA, Lehner PJ. Structure and function: heat shock proteins and adaptive immunity. J Immunol. 2007;179:2035–40. doi: 10.4049/jimmunol.179.4.2035. [DOI] [PubMed] [Google Scholar]
- 3.Kim LS, Kim JH. Heat shock protein as molecular targets for breast cancer therapeutics. J Breast Cancer. 2011;14:167–74. doi: 10.4048/jbc.2011.14.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calderwood SK, Gong J. Molecular chaperones in mammary cancer growth and breast tumor therapy. J Cell Biochem. 2012;113:1096–103. doi: 10.1002/jcb.23461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murshid A, Gong J, Stevenson MA, Calderwood SK. Heat shock proteins and cancer vaccines: developments in the past decade and chaperoning in the decade to come. Expert Rev Vaccines. 2011;10:1553–68. doi: 10.1586/erv.11.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z. In vitro reconstitution of heat shock protein-peptide complexes for generating peptide-specific vaccines against cancers and infectious diseases. Methods. 2004;32:25–8. doi: 10.1016/S1046-2023(03)00183-X. [DOI] [PubMed] [Google Scholar]
- 7.Blachere NE, Li Z, Chandawarkar RY, Suto R, Jaikaria NS, Basu S, et al. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med. 1997;186:1315–22. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang HH, Mao CY, Teng LS, Cao J. Recent advances in heat shock protein-based cancer vaccines. Hepatobiliary Pancreat Dis Int. 2006;5:22–7. [PubMed] [Google Scholar]
- 9.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- 10.Takakura Y, Takemoto S, Nishikawa M. Hsp-based tumor vaccines: state-of-the-art and future directions. Curr Opin Mol Ther. 2007;9:385–91. [PubMed] [Google Scholar]
- 11.Joly AL, Wettstein G, Mignot G, Ghiringhelli F, Garrido C. Dual role of heat shock proteins as regulators of apoptosis and innate immunity. J Innate Immun. 2010;2:238–47. doi: 10.1159/000296508. [DOI] [PubMed] [Google Scholar]
- 12.Biswas C, Sriram U, Ciric B, Ostrovsky O, Gallucci S, Argon Y. The N-terminal fragment of GRP94 is sufficient for peptide presentation via professional antigen-presenting cells. Int Immunol. 2006;18:1147–57. doi: 10.1093/intimm/dxl049. [DOI] [PubMed] [Google Scholar]
- 13.Bolhassani A, Mohit E, Rafati S. Different spectra of therapeutic vaccine development against HPV infections. Hum Vaccin. 2009;5:671–89. doi: 10.4161/hv.5.10.9370. [DOI] [PubMed] [Google Scholar]
- 14.Udono H, Yamano T, Kawabata Y, Ueda M, Yui K. Generation of cytotoxic T lymphocytes by MHC class I ligands fused to heat shock cognate protein 70. Int Immunol. 2001;13:1233–42. doi: 10.1093/intimm/13.10.1233. [DOI] [PubMed] [Google Scholar]
- 15.Ramirez SR, Singh-Jasuja H, Warger T, Braedel-Ruoff S, Hilf N, Wiemann K, et al. Glycoprotein 96-activated dendritic cells induce a CD8-biased T cell response. Cell Stress Chaperones. 2005;10:221–9. doi: 10.1379/CSC-117R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callahan MK, Garg M, Srivastava PK. Heat-shock protein 90 associates with N-terminal extended peptides and is required for direct and indirect antigen presentation. Proc Natl Acad Sci USA. 2008;105:1662–7. doi: 10.1073/pnas.0711365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolhassani A, Rafati S. Heat-shock proteins as powerful weapons in vaccine development. Expert Rev Vaccines. 2008;7:1185–99. doi: 10.1586/14760584.7.8.1185. [DOI] [PubMed] [Google Scholar]
- 18.Binder RJ, Vatner R, Srivastava P. The heat-shock protein receptors: some answers and more questions. Tissue Antigens. 2004;64:442–51. doi: 10.1111/j.1399-0039.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 19.Figueiredo C, Wittmann M, Wang D, Dressel R, Seltsam A, Blasczyk R, et al. Heat shock protein 70 (HSP70) induces cytotoxicity of T-helper cells. Blood. 2009;113:3008–16. doi: 10.1182/blood-2008-06-162727. [DOI] [PubMed] [Google Scholar]
- 20.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–13. doi: 10.1016/S1074-7613(01)00111-X. [DOI] [PubMed] [Google Scholar]
- 21.Asea A. Heat shock proteins and toll-like receptors. Handb Exp Pharmacol. 2008;183:111–27. doi: 10.1007/978-3-540-72167-3_6. [DOI] [PubMed] [Google Scholar]
- 22.Mandal K, Foteinos G, Jahangiri M, Xu Q. Role of antiheat shock protein 60 autoantibodies in atherosclerosis. Lupus. 2005;14:742–6. doi: 10.1191/0961203305lu2212oa. [DOI] [PubMed] [Google Scholar]
- 23.Wick G, Knoflach M, Xu Q. Autoimmune and inflammatory mechanisms in atherosclerosis. Annu Rev Immunol. 2004;22:361–403. doi: 10.1146/annurev.immunol.22.012703.104644. [DOI] [PubMed] [Google Scholar]
- 24.van Halteren AG, Roep BO, Gregori S, Cooke A, van Eden W, Kraal G, et al. Cross-reactive mycobacterial and self hsp60 epitope recognition in I-A(g7) expressing NOD, NOD-asp and Biozzi AB/H mice. J Autoimmun. 2002;18:139–47. doi: 10.1006/jaut.2001.0578. [DOI] [PubMed] [Google Scholar]
- 25.Abulafia-Lapid R, Gillis D, Yosef O, Atlan H, Cohen IR. T cells and autoantibodies to human HSP70 in type 1 diabetes in children. J Autoimmun. 2003;20:313–21. doi: 10.1016/S0896-8411(03)00038-6. [DOI] [PubMed] [Google Scholar]
- 26.Salvetti M, Ristori G, Buttinelli C, Fiori P, Falcone M, Britton W, et al. The immune response to mycobacterial 70-kDa heat shock proteins frequently involves autoreactive T cells and is quantitatively disregulated in multiple sclerosis. J Neuroimmunol. 1996;65:143–53. doi: 10.1016/0165-5728(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 27.Todryk SM, Gough MJ, Pockley AG. Facets of heat shock protein 70 show immunotherapeutic potential. Immunology. 2003;110:1–9. doi: 10.1046/j.1365-2567.2003.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunt C, Morimoto RI. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci USA. 1985;82:6455–9. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan Y, Cao Z, Yang M, Li H, Wei H, Fu Y, et al. A CpG oligodeoxynucleotide potentiates the anti-tumor effect of HSP65-Her2 fusion protein against Her2 positive B16 melanoma in mice. Int Immunopharmacol. 2012;12:402–7. doi: 10.1016/j.intimp.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Wang QH, Buckle AM, Fersht AR. From mini-chaperone to GroEL. J Mol Biol. 2000;304:837–81. [Google Scholar]
- 31.Chatellier J, Hill F, Lund PA, Fersht AR. In vivo activities of GroEL minichaperones. Proc Natl Acad Sci USA. 1998;95:9861–6. doi: 10.1073/pnas.95.17.9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolhassani A, Rafati S. DNA immunization as an efficient strategy for vaccination. Avicenna Journal of Medical Biotechnology. 2009;1:71–88. [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Yang X, Li L, Liu H, Liu J. A truncated C-terminal fragment of Mycobacterium tuberculosis HSP70 gene enhanced potency of HBV DNA vaccine. Vaccine. 2006;24:3321–31. doi: 10.1016/j.vaccine.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Qazi KR, Wikman M, Vasconcelos NM, Berzins K, Ståhl S, Fernández C. Enhancement of DNA vaccine potency by linkage of Plasmodium falciparum malarial antigen gene fused with a fragment of HSP70 gene. Vaccine. 2005;23:1114–25. doi: 10.1016/j.vaccine.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 35.Chunxia S, Duan X, Wang X, Wang C, Cao R, Zhou B, et al. Heterologous expression of FMDV immunodominant epitopes and HSP70 in P. pastoris and the subsequent immune response in mice. Veterinary Microbiol. 2006;124:256–263. doi: 10.1016/j.vetmic.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 36.Ge FF, Qiu YF, Gao XF, Yang YW, Chen PY. Fusion expression of major antigenic segment of JEV E protein-hsp70 and the identification of domain acting as adjuvant in hsp70. Vet Immunol Immunopathol. 2006;113:288–96. doi: 10.1016/j.vetimm.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Uto T, Tsujimura K, Uchijima M, Seto S, Nagata T, Suda T, et al. A novel vaccine strategy to induce mycobacterial antigen-specific Th1 responses by utilizing the C-terminal domain of heat shock protein 70. FEMS Immunol Med Microbiol. 2011;61:189–96. doi: 10.1111/j.1574-695X.2010.00762.x. [DOI] [PubMed] [Google Scholar]
- 38.Pakravan N, Soudi S, Hassan ZM. N-terminally fusion of Her2/neu to HSP70 decreases efficiency of Her2/neu DNA vaccine. Cell Stress Chaperones. 2010;15:631–8. doi: 10.1007/s12192-010-0175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lehner T, Wang Y, Whittall T, McGowan E, Kelly CG, Singh M. Functional domains of HSP70 stimulate generation of cytokines and chemokines, maturation of dendritic cells and adjuvanticity. Biochem Soc Trans. 2004;32:629–32. doi: 10.1042/BST0320629. [DOI] [PubMed] [Google Scholar]
- 40.Facciponte JG, Wang XY, MacDonald IJ, Park JE, Arnouk H, Grimm MJ, et al. Heat shock proteins HSP70 and GP96: structural insights. Cancer Immunol Immunother. 2006;55:339–46. doi: 10.1007/s00262-005-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bolhassani A, Zahedifard F, Taghikhani M, Rafati S. Enhanced immunogenicity of HPV16E7 accompanied by Gp96 as an adjuvant in two vaccination strategies. Vaccine. 2008;26:3362–70. doi: 10.1016/j.vaccine.2008.03.082. [DOI] [PubMed] [Google Scholar]
- 42.Pakravan N, Langroudi L, Hajimoradi M, Hassan ZM. Co-administration of GP96 and Her2/neu DNA vaccine in a Her2 breast cancer model. Cell Stress Chaperones. 2010;15:977–84. doi: 10.1007/s12192-010-0208-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manjili MH, Wang XY, Chen X, Martin T, Repasky EA, Henderson R, et al. HSP110-HER2/neu chaperone complex vaccine induces protective immunity against spontaneous mammary tumors in HER-2/neu transgenic mice. J Immunol. 2003;171:4054–61. doi: 10.4049/jimmunol.171.8.4054. [DOI] [PubMed] [Google Scholar]
- 44.He YF, Wang XH, Zhang GM, Chen HT, Zhang H, Feng ZH. Sustained low-level expression of interferon-gamma promotes tumor development: potential insights in tumor prevention and tumor immunotherapy. Cancer Immunol Immunother. 2005;54:891–7. doi: 10.1007/s00262-004-0654-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kmieciak M, Knutson KL, Dumur CI, Manjili MH. HER-2/neu antigen loss and relapse of mammary carcinoma are actively induced by T cell-mediated anti-tumor immune responses. Eur J Immunol. 2007;37:675–85. doi: 10.1002/eji.200636639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pakravan N, Hashemi SM, Hassan ZM. Adjuvant activity of GP96 C-terminal domain towards Her2/neu DNA vaccine is fusion direction-dependent. Cell Stress Chaperones. 2011;16:41–8. doi: 10.1007/s12192-010-0219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoeller D, Dikic I. Targeting the ubiquitin system in cancer therapy. Nature. 2009;458:438–44. doi: 10.1038/nature07960. [DOI] [PubMed] [Google Scholar]
- 49.Pakravan N, Hassan ZM. Comparison of adjuvant activity of N- and C-terminal domain of gp96 in a Her2-positive breast cancer model. Cell Stress Chaperones. 2011;16:449–57. doi: 10.1007/s12192-011-0258-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li H, Zhou M, Han J, Zhu X, Dong T, Gao GF, et al. Generation of murine CTL by a hepatitis B virus-specific peptide and evaluation of the adjuvant effect of heat shock protein glycoprotein 96 and its terminal fragments. J Immunol. 2005;174:195–204. doi: 10.4049/jimmunol.174.1.195. [DOI] [PubMed] [Google Scholar]
- 51.Rapp UK, Kaufmann SH. DNA vaccination with gp96-peptide fusion proteins induces protection against an intracellular bacterial pathogen. Int Immunol. 2004;16:597–605. doi: 10.1093/intimm/dxh064. [DOI] [PubMed] [Google Scholar]
- 52.Pakravan N, Soleimanjahi H, Hassan ZM. GP96 C-terminal improves Her2/neu DNA vaccine. J Gene Med. 2010;12:345–53. doi: 10.1002/jgm.1445. [DOI] [PubMed] [Google Scholar]
- 53.Mohit E, Bolhassani A, Zahedifard F, Seyed N, Eslamifar A, Taghikhani M, et al. Immunomodulatory effects of IP-10 chemokine along with PEI600-Tat delivery system in DNA vaccination against HPV infections. Mol Immunol. 2013;53:149–60. doi: 10.1016/j.molimm.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 54.Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010;2:63ra94. doi: 10.1126/scitranslmed.3001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pike SE, Yao L, Jones KD, Cherney B, Appella E, Sakaguchi K, et al. Vasostatin, a calreticulin fragment, inhibits angiogenesis and suppresses tumor growth. J Exp Med. 1998;188:2349–56. doi: 10.1084/jem.188.12.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu B, Ye D, Song X, Zhao X, Yi L, Song J, et al. A novel therapeutic fusion protein vaccine by two different families of heat shock proteins linked with HPV16 E7 generates potent antitumor immunity and antiangiogenesis. Vaccine. 2008;26:1387–96. doi: 10.1016/j.vaccine.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 57.Bolhassani A, Zahedifard F, Taslimi Y, Taghikhani M, Nahavandian B, Rafati S. Antibody detection against HPV16 E7 & GP96 fragments as biomarkers in cervical cancer patients. Indian J Med Res. 2009;130:533–41. [PubMed] [Google Scholar]
- 58.Mohit E, Bolhassani A, Zahedifard F, Taslimi Y, Rafati S. The contribution of NT-gp96 as an adjuvant for increasing HPV16 E7-specific immunity in C57BL /6 mouse model. Scand J Immunol. 2012;75:27–37. doi: 10.1111/j.1365-3083.2011.02620.x. [DOI] [PubMed] [Google Scholar]
- 59.Abdian N, Gholami E, Zahedifard F, Safaee N, Rafati S. Evaluation of DNA/DNA and prime-boost vaccination using LPG3 against Leishmania major infection in susceptible BALB/c mice and its antigenic properties in human leishmaniasis. Exp Parasitol. 2011;127:627–36. doi: 10.1016/j.exppara.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 60.Tebianian M, Hoseini AZ, Ebrahimi SM, Memarnejadian A, Mokarram AR, Mahdavi M, et al. Cloning, expression, and immunogenicity of novel fusion protein of Mycobacterium tuberculosis based on ESAT-6 and truncated C-terminal fragment of HSP70. Biologicals. 2011;39:143–8. doi: 10.1016/j.biologicals.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 61.Suzue K, Young RA. Adjuvant-free hsp70 fusion protein system elicits humoral and cellular immune responses to HIV-1 p24. J Immunol. 1996 a;156:873–9. [PubMed] [Google Scholar]
- 62.Suzue K, Young RA. Heat shock proteins as immunological carriers and vaccines. EXS. 1996 b;77:451–65. doi: 10.1007/978-3-0348-9088-5_30. [DOI] [PubMed] [Google Scholar]
- 63.Ebrahimi SM, Tebianian M. Heterologous expression, purification and characterization of the influenza A virus M2e gene fused to Mycobacterium tuberculosis HSP70(359-610) in prokaryotic system as a fusion protein. Mol Biol Rep. 2010;37:2877–83. doi: 10.1007/s11033-009-9846-2. [DOI] [PubMed] [Google Scholar]
- 64.Ebrahimi SM, Tebianian M, Mirjalili A, Paykari H, Varshovi HR, Toghyani H, et al. Fusion and sequence analysis of the influenza A (H9N2) virus M2e and C-terminal fragment of Mycobacterium tuberculosis HSP70 (H37Rv) Archives of Razi Institute. 2009;64:71–6. [Google Scholar]
- 65.Li YL, Liu J, Liu JN, Zhang J. Immunization of protein HPV16 E7 in fusion with mouse HSP70 inhibits the growth of TC-1 cells in tumor bearing mice. Vaccine. 2011;29:5959–62. doi: 10.1016/j.vaccine.2011.06.046. [DOI] [PubMed] [Google Scholar]
- 66.Barrios C, Lussow AR, Van Embden J, Van der Zee R, Rappuoli R, Costantino P, et al. Mycobacterial heat-shock proteins as carrier molecules. II: The use of the 70-kDa mycobacterial heat-shock protein as carrier for conjugated vaccines can circumvent the need for adjuvants and Bacillus Calmette Guérin priming. Eur J Immunol. 1992;22:1365–72. doi: 10.1002/eji.1830220606. [DOI] [PubMed] [Google Scholar]
- 67.Marañón C, Thomas MC, Planelles L, López MC. The immunization of A2/K(b) transgenic mice with the KMP11-HSP70 fusion protein induces CTL response against human cells expressing the T. cruzi KMP11 antigen: identification of A2-restricted epitopes. Mol Immunol. 2001;38:279–87. doi: 10.1016/S0161-5890(01)00059-1. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y, Kelly CG, Singh M, McGowan EG, Carrara AS, Bergmeier LA, et al. Stimulation of Th1-polarizing cytokines, C-C chemokines, maturation of dendritic cells, and adjuvant function by the peptide binding fragment of heat shock protein 70. J Immunol. 2002;169:2422–9. doi: 10.4049/jimmunol.169.5.2422. [DOI] [PubMed] [Google Scholar]
- 69.Lehner T, Wang Y, Whittall T, McGowan E, Kelly CG, Singh M. Functional domains of HSP70 stimulate generation of cytokines and chemokines, maturation of dendritic cells and adjuvanticity. Biochem Soc Trans. 2004;32:629–32. doi: 10.1042/BST0320629. [DOI] [PubMed] [Google Scholar]
- 70.Rico AI, Angel SO, Alonso C, Requena JM. Immunostimulatory properties of the Leishmania infantum heat shock proteins HSP70 and HSP83. Mol Immunol. 1999;36:1131–9. doi: 10.1016/S0161-5890(99)00136-4. [DOI] [PubMed] [Google Scholar]
- 71.Rafati S, Gholami E, Hassani N, Ghaemimanesh F, Taslimi Y, Taheri T, et al. Leishmania major heat shock protein 70 (HSP70) is not protective in murine models of cutaneous leishmaniasis and stimulates strong humoral responses in cutaneous and visceral leishmaniasis patients. Vaccine. 2007;25:4159–69. doi: 10.1016/j.vaccine.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 72.Li HT, Yan JB, Li J, Zhou MH, Zhu XD, Zhang YX, et al. Enhancement of humoral immune responses to HBsAg by heat shock protein gp96 and its N-terminal fragment in mice. World J Gastroenterol. 2005;11:2858–63. doi: 10.3748/wjg.v11.i19.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Welters MJ, Kenter GG, Piersma SJ, Vloon AP, Löwik MJ, Berends-van der Meer DM, et al. Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin Cancer Res. 2008;14:178–87. doi: 10.1158/1078-0432.CCR-07-1880. [DOI] [PubMed] [Google Scholar]
- 74.Udono H, Yamano T, Kawabata Y, Ueda M, Yui K. Generation of cytotoxic T lymphocytes by MHC class I ligands fused to heat shock cognate protein 70. Int Immunol. 2001;13:1233–42. doi: 10.1093/intimm/13.10.1233. [DOI] [PubMed] [Google Scholar]
- 75.Li H, Zhou M, Han J, Zhu X, Dong T, Gao GF, et al. Generation of murine CTL by a hepatitis B virus-specific peptide and evaluation of the adjuvant effect of heat shock protein glycoprotein 96 and its terminal fragments. J Immunol. 2005;174:195–204. doi: 10.4049/jimmunol.174.1.195. [DOI] [PubMed] [Google Scholar]