Abstract
This study aimed to assess the safety profile of post-exposure prophylaxis (PEP) for rabies in pregnant women. All of the subjects received the Essen vaccination regimen. Systemic and local reactions were monitored within 72 hours following the immunization, and the subjects were followed until six months after delivery. No moderate or severe adverse effects occurred in any subject following the vaccination. Among the 72 subjects in this follow-up study, four had voluntary abortions, one subject had an accidental miscarriage, and the remaining 67 subjects delivered babies vaginally or by caesarean section. All of the infants exhibited normal development.The purified Vero cell rabies vaccine and the purified chick embryo cell vaccine were both safe for the PEP of pregnant women and did not interfere with the development of the fetuses or infants. Education is needed in China to stop pregnancy terminations due to concerns about rabies vaccination risk.
Keywords: post-exposure prophylaxis, pregnancy, rabies vaccine, vaccine safety, China
Introduction
Rabies has a mortality rate of nearly 100%. China remains a high-risk environment for rabies. During the period from 2006 to 2010, the numbers of deaths due to rabies nationwide were 3,293, 3,303, 2,466, 2,213 and 2,048, respectively (Annual Notifiable Infectious Diseases Report, by Ministry of Health of the People’s Republic of China, from 2006 to 2010). During the same time period, the numbers of cases reported in Guangdong province were 387, 334, 319, 330 and 301(Annual Notifiable Infectious Diseases Report, by Department of Health of Guangdong Province, from 2006 to 2010). The high prevalence of rabies poses a great degree of concern. Following exposure to rabies, proper wound care, immunization with a rabies vaccine, and the injection of anti-rabies immunoglobulin (RIG) can maximize the effectiveness of rabies prophylaxis3 For people exposed to rabies, post-exposure prophylaxis (PEP) consists of wound cleansing, rabies vaccination and passive immunization with rabies immune globulin (RIG) for Category III exposures.3 Due to their exposure to potential or known rabid animals, most urban residents are aware of PEP. In China, at least 12–15 million people receive PEP annually,4 of which there are 0.1 million in Guangzhou, a city with a population of 12.70 million in Guangdong province in Southern China. Pregnant patients visiting clinics for rabies PEP are common, however, there are no published data on how many pregnant women receive PEP annually, which is a topic that deserves further investigation.
The human rabies vaccines available in China are the imported purified chick embryo cell vaccine (PCECV), manufactured by Novartis (Rabipur®), the purified Vero cell rabies vaccine (PVRV), manufactured by Sanofi Pasteur (Verorab®), and several other domestic PVRVs. There are several published studies on rabies PEP safety in pregnancy overseas,6-8,21-23 but rabies PEP safety has not been reported in China. Because almost no one animal medical institution to carry out professional dog rabies risk assessment project in China, so considering the uncertain potential rabies risk and the fatal disease of rabies, PEP usually have to be received to anyone including pregnant women .In this study, we observed and followed up pregnant women receiving rabies PEP to analyze the safety of PEP for pregnant women and to provide scientific evidence for the use of rabies PEP in China.
Results
Baseline characteristics
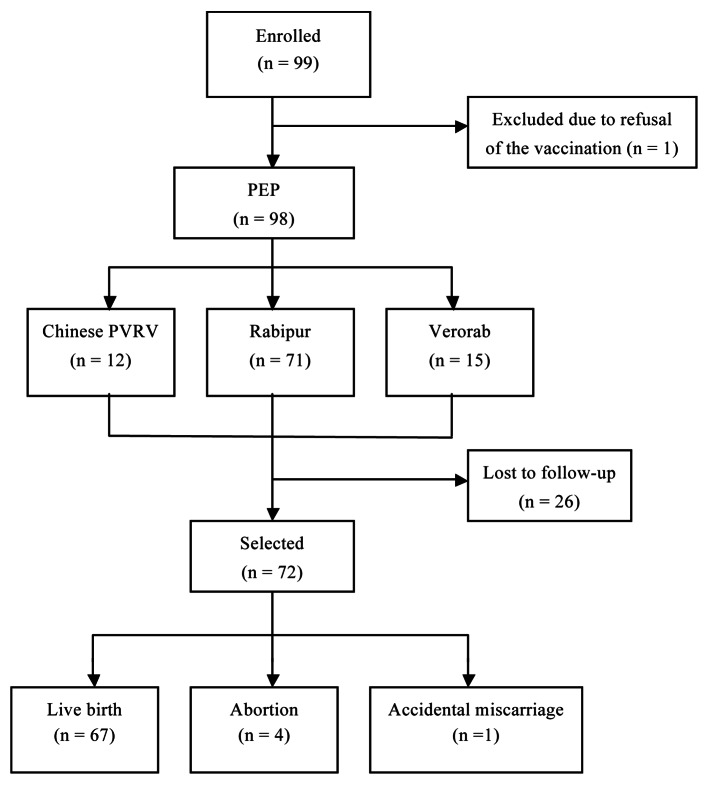
During the study period, 99 pregnant women aged 19 to 40 y visited the clinic for PEP, with an occupation distribution of 54 company staff, 34 housewives, 6 teachers, 3 medical staff and 2 farmers. Two subjects had prior miscarriages, and the rest of the subjects were experiencing their first pregnancy. One pregnant woman at the 10th week of gestation worried about the possible effects on the fetus, ignored the doctor's advice, decided not to receive PEP, whereas the remainder of the subjects volunteered to receive the standard immunization schedule. The entire telephone follow-up was completed for 72 subjects, 26 subjects were lost to follow-up due to failure to establish telephone contact after the expected date of delivery and the childbirth outcomes for these women are therefore unknown (Fig. 1).
Figure 1. Study flowchart
Animal exposure and wounds
Most wounds were inflicted by dogs (65/98, 66.33%), followed by cats (33/98, 33.67%). The wounds were located on the legs (55/98, 56.12%), arms(30/98, 30.61%), and fingers(6/98,6.12%). Among the 39 subjects with category III exposure who received PEP, none of them consented to receive RIG, regardless of the physicians’recommendation (Table 1). No rabies cases were reported for any of the subjects or babies.
Table 1. Animal exposure of pregnant women receiving PEP (n = 98).
| Gestational | Age | n | Animal exposure | Wound location | Category | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| week |
Mean ± SD |
Dog |
|
Cat |
arms |
fingers |
legs |
Trunk |
Head/face |
Multiple |
II |
III |
| ≤ 4 |
26.1 ± 3.4 |
14 |
10 |
4 |
4 |
2 |
6 |
1 |
|
1 |
9 |
5 |
| 5–8 |
26.8 ± 4.5 |
11 |
10 |
1 |
2 |
0 |
9 |
|
|
|
8 |
3 |
| 9–12 |
29.3 ± 4.5 |
5 |
4 |
1 |
2 |
0 |
3 |
|
|
|
4 |
1 |
| 13–16 |
28.2 ± 4.5 |
8 |
4 |
4 |
2 |
1 |
5 |
|
|
|
6 |
2 |
| 17–20 |
26.2 ± 3.5 |
13 |
10 |
3 |
3 |
0 |
8 |
1 |
|
1 |
8 |
5 |
| 21–24 |
26.6 ± 4.4 |
9 |
6 |
3 |
3 |
2 |
4 |
|
|
|
5 |
4 |
| 25–28 |
27.3 ± 5.9 |
9 |
6 |
3 |
4 |
0 |
5 |
|
|
|
6 |
3 |
| 29–32 |
26.8 ± 3.3 |
15 |
5 |
10 |
5 |
1 |
7 |
1 |
1 |
|
5 |
10 |
| 33–36 |
25.7 ± 4.6 |
9 |
5 |
4 |
4 |
0 |
4 |
|
|
1 |
6 |
3 |
| 37–40 |
26.2 ± 2.4 |
5 |
5 |
0 |
1 |
0 |
4 |
|
|
|
2 |
3 |
| Total | 26.7 ± 3.9 | 98 | 65 | 33 | 30 | 6 | 55 | 3 | 1 | 3 | 59 | 39 |
Safety profile of vaccine and immunization compliance
No immediate reaction occurred in any subject following immunization. At 72 h after vaccination, mild pain at the injection site occurred in 23 subjects, fatigue in 18, headache in 4, erythema in 2, and mild fever in 2. None of these adverse effects required medical intervention (Table 2). There were no significant differences between the age groups (χ2 = 0.355, p = 0.701). No moderate or severe adverse effects occurred during the study period. Of the 98 enrolled patients, 63 completed the entire vaccination program, and the other 35 patients only took 1 to 4 doses (Table 3).
Table 2. Adverse reactions to rabies PEP following vaccination in pregnant women (n = 98).
| Adverse effects | Chinese PVRV | Rabipur | Verorab | N |
|---|---|---|---|---|
| Total doses |
12 × |
71 × |
15 × |
490 |
| Local |
|
|
|
|
| Pain |
3 |
16 |
4 |
23 (4.69%) |
| Erythema |
1 |
1 |
0 |
2 (0.41%) |
| Systemic |
|
|
|
|
| Fever |
0 |
2 |
0 |
2 (0.41%) |
| Headache |
1 |
1 |
2 |
4 (0.82%) |
| Fatigue |
2 |
13 |
3 |
18 (3.67%) |
| Total (%) | 7 (11.67%) | 33 (9.30%) | 9 (12.0%) | 49 (10.0%) |
Table 3. PEP compliance in pregnant women (n = 98).
| Vaccine | Gestational week | One dose | Two doses | Three doses | Four doses | Five doses | Total |
|---|---|---|---|---|---|---|---|
| Chinese PVRV |
|
|
|
|
|
|
|
| |
≤ 4 |
0 |
0 |
0 |
2 |
2 |
4 |
| |
5–8 |
0 |
0 |
0 |
1 |
2 |
3 |
| |
17–20 |
0 |
0 |
0 |
1 |
2 |
3 |
| |
21–24 |
0 |
0 |
0 |
0 |
1 |
1 |
| |
29–32 |
0 |
0 |
0 |
0 |
1 |
1 |
| |
Subtotal |
0 |
0 |
0 |
4 |
8 |
12 |
| Rabipur |
|
|
|
|
|
|
|
| |
≤ 4 |
1 |
1 |
0 |
0 |
4 |
6 |
| |
5–8 |
0 |
0 |
0 |
0 |
6 |
6 |
| |
9–12 |
1 |
0 |
0 |
1 |
2 |
4 |
| |
13–16 |
1 |
0 |
0 |
0 |
5 |
6 |
| |
17–20 |
2 |
0 |
1 |
0 |
6 |
9 |
| |
21–24 |
0 |
0 |
1 |
0 |
6 |
7 |
| |
25–28 |
0 |
1 |
1 |
0 |
7 |
9 |
| |
29–32 |
1 |
3 |
1 |
2 |
5 |
12 |
| |
33–36 |
1 |
0 |
1 |
1 |
5 |
8 |
| |
37–40 |
0 |
0 |
2 |
1 |
1 |
4 |
| |
Subtotal |
7 |
5 |
7 |
5 |
47 |
71 |
| Verorab |
|
|
|
|
|
|
|
| |
≤ 4 |
1 |
1 |
0 |
0 |
4 |
6 |
| |
5–8 |
0 |
0 |
0 |
1 |
2 |
3 |
| |
17–20 |
0 |
0 |
0 |
1 |
1 |
2 |
| |
29–32 |
1 |
0 |
0 |
0 |
1 |
2 |
| |
33–36 |
0 |
0 |
1 |
0 |
0 |
1 |
| |
37–40 |
1 |
0 |
0 |
0 |
0 |
1 |
| |
Subtotal |
3 |
1 |
1 |
2 |
8 |
15 |
| Total | 10 | 6 | 8 | 11 | 63 | 98 |
None of the subjects elected to take RIG despite the important information and recommendations provided by the physicians.
Pregnancy, labor, neonatal and infant outcomes
We continued to collect 72 patients’ information during the follow-up study. During pregnancy, the patients remained generally healthy and free from any pregnancy-associated complications during the study period. No miscarriages, stillbirths, or fetal malformations were reported. During the follow-up interviews, four pregnancies were terminated by voluntary abortions during the 1st week, 4th week, 5th week and 17th week and after having received 1–3 doses of the rabies vaccine. The abortions were performed without the consent of our outpatient doctor's professional opinion, without any physical discomfort, nor did any of Ultrasonographic examination. The only reason is in that they have been advised by family and friends, and most of them think that the rabies vaccines would take effects on fetal development. An accidental miscarriage occurred in one subject at the 21st gestational week. The remaining 67 subjects delivered vaginally (n = 48) or by caesarean section (n = 19). One subject gave birth prematurely because of premature rupture of membranes at the 33rd gestational week. The gestational terms of other 66 pregnancies were ≥ 37 weeks (Table 4). The cesarean section rates between the groups revealed no significant difference. Caesarean section was primarily indicated for cephalopelvic disproportion (Table 5). All of the newborns, including 40 males and 27 females, exhibited normal development. The 66 newborns weighed 3257 ± 450 g, in accordance with the average weight of newborns in China.12 One low-weight premature infant was 1,600 g at birth and nursed to 5,660 g, a normal weight, at four months of age during the follow-up study. One newborn had physiological jaundice, and one baby had complicated mycoplasma pneumonia but recovered after treatment (Table 3). At the 4-mo follow-up, all of the infants were healthy, had developed appropriately, and were being fed an additive diet. One baby had never received the vaccinations of the national immunization program, not due to health problems but to safety concerns of the parents.
Table 4. Pregnancy, labor and newborn profiles in pregnant women with complete follow-up data (n = 72).
| Chinese PVRV | Rabipur | Verorab | N (%) | |
|---|---|---|---|---|
| Delivery |
|
|
|
|
| Vaginal |
9 |
33 |
6 |
48(71.64%) |
| Caesarean section |
2 |
14 |
3 |
19(28.36%) |
| Abortion |
1 |
2 |
1 |
4 |
| Accidental miscarriage |
0 |
1 |
0 |
1 |
| Miscarriage |
0 |
0 |
0 |
0 |
| ≥ 37th gestation wk (n) |
11 |
46 |
9 |
66(98.51%) |
| ≥ 37th gestation wk (n) |
0 |
1 |
0 |
1(1.49%) |
| mean ± SD (d) |
275.7 ± 8.4 |
274.1 ± 9.8 |
270 ± 8.3 |
|
| Range (d) |
265–288 |
266–290 |
267–285 |
|
| Sex |
|
|
|
|
| Male |
7 |
27 |
6 |
40(59.7%) |
| Female |
4 |
20 |
3 |
27(40.3%) |
| Birth weight (cm) |
|
|
|
|
| Male (mean ± SD) |
3167 ± 44 |
3235 ± 99 |
3410 ± 36 |
3257 ± 50 |
| Range |
2900–4000 |
2115–4250 |
3100–3900 |
2115–4250 |
| Female (mean ± SD) |
3433 ± 01 |
3156 ± 01 |
2833 ± 04 |
3150 ± 12 |
| Range |
2900–4000 |
1600–4300 |
2250–3750 |
1600–4300 |
| Birth height (g) |
|
|
|
|
| Male (mean ± SD) |
50.0 ± 0.0 |
50.8 ± 0.5 |
50.8 ± 0.8 |
50.7 ± 0.4 |
| Range |
49–51 |
49–54 |
50–52 |
49–54 |
| Female (mean ± SD) |
50.7 ± 0.1 |
50.1 ± 0.7 |
48.7 ± 0.3 |
50.0 ± 0.5 |
| Range |
49–53 |
42–53 |
46–50 |
42–53 |
| Feeding at 4 mo |
|
|
|
|
| Breast milk |
8 |
25 |
8 |
41 |
| Mixed |
3 |
5 |
0 |
8 |
| Artificial |
0 |
17 |
1 |
18 |
| Additive diet | 11 | 47 | 9 | 67 |
Table 5. Gynecological indications of cesarean section in pregnant women receiving PEP (n = 19).
| Indication | N | % | |
|---|---|---|---|
| 1 |
Cephalopelvic disproportion |
5 |
26.32 |
| 2 |
Macrosomia |
4 |
21.05 |
| 3 |
Nuchal cord |
2 |
10.53 |
| 4 |
Breech delivery |
2 |
10.53 |
| 5 |
Premature rupture of the membrane |
2 |
10.53 |
| 6 |
Advanced age |
2 |
10.53 |
| 7 |
Previous C-section |
1 |
5.26 |
| 8 |
Social factors |
1 |
5.26 |
| Total | 19 | 100 |
No case of pregnancy abnormalities, childbirth or fetal abnormalities, or death were reported from the 26 subjects lost to follow-up.
Discussion
This study provides domestic clinical data of the PEP safety in pregnancy to assist physicians in providing guidance to patients. The immunization of pregnant women has been widely accepted in western countries. The Centers for Disease Control and Prevention (CDC) recommended in 1999 that HBsAb-negative pregnant women be immunized against hepatitis B virus to minimize the risk of hepatitis B infection.12 In 2003, CDC also suggested that all pregnant women beyond the 14th gestational week be immunized against influenza in seasons of high prevalence to minimize the effects of influenza infection.4 Additionally, pregnant women beyond the 14th gestational week are advised to be immunized with the 23-valent pneumococcal vaccine (PPV-23) to confer immune protection to themselves and their newborns.19 The safety of the above vaccines has been extensively documented.1,5,17,24 Prior studies about the safety of rabies PEP in pregnancy had been performed since 1990s. The consensus from all of these studies is that rabies PEP is safe during pregnancy.5-10 Supawat Chutivongse et al. reported a study of rabies PEP safety in 202 Thai pregnant women. The studies reported by Chutivongse S. et al., Sudarshan M.K. and Figueroa DR have also demonstrated the safety of PEP in pregnant women. Sudarshan M.K. reported the safety of pregnant woman in India receiving both the rabies vaccine and RIG for treatment. In our study, no moderate or severe adverse effects occurred in any pregnant woman following PEP. Individual subjects presented mild adverse effects such as pain, erythema, fever, headache and fatigue, which were mild symptoms not requiring medication and did not cause long-term physical or mental effects on the patients. The adverse effects were not more severe than results from other recent studies from general populations.14,19
In this study, although our physicians demonstrated positive positions toward rabies PEP during pregnancy, most subjects sought further information from obstetricians, friends and relatives. As a result, four subjects terminated their pregnancy after one to three doses of rabies vaccination, one subject refused to take the PEP treatment and 35 subjects interrupted the PEP treatment. Subjects receiving PEP suffered pressure from family and society. We also found that one infant at four months of age had not received vaccines from the national immunization program because of the parents’concerns about vaccine safety.
One of the shortcomings of this study is that none of the subjects in our study consented to receive RIG together with the rabies vaccine. In China, especially in urban areas, most exposures are low risk for developing rabies. In fact, RIG usage has always been an issue that concerns physicians in rabies immunization clinics. According to the Standard of Preventive Treatment for Rabies Exposure published by China Health Ministry, RIG usage is necessary in cases of category III exposure. However, most low grade exposures are treated with only a rabies vaccine, and only rare hydrophobic cases were reported even without taking RIG in the city. Therefore, in case of exposure to household pets, most physicians would not strongly persuade patients to take RIG, except in rural areas. From the point of most patients, the financial expense of RIG is rather high, and the RIG treatment is considered unnecessary, as their relatives and friends have not likely not taken RIG for PEP treatments. Our clinic is located in the center of Guangzhou, where most patients visiting for rabies PEP are exposed to household pets. One reason for not taking RIG is that they believe it is unnecessary. Another reason is that patients are reluctant to pay for the high expense of the treatment, which includes 30 to 50 dollars for the rabies vaccine and 190 to 320 dollars for the RIG per patient. In addition, physicians must consider the probability that the animals are rabid. In China, most rabies cases are due to exposured to rural rabid dogs.11,20 In an epidemiological study conducted from 2004–2006, the RIG use rate found to be 2.6% of PEP treatments with no rabies cases.9 Failure cases were reported when PEP was administered without RIG in rural areas, where there is a high prevalence of rabies. Among the 18 rabies cases reported in Guangzhou from 1997–2005, two unsuccessful PEP treatments did not receive RIG when the wounds were located on the face.15 Pregnant women in this study did not consent to receiving RIG, which is also observed in most urban patients. In “The Standard of Preventive Treatment for Rabies Exposure (2009 Edition),” the Chinese Ministry of Health defined the phrase “exposure” as bites or scratches, licks on broken skin and contamination of mucous membranes with saliva from licks by a suspected rabid animal. After exposure, almost no one animal health agencies provide service to identify whether the animal is a rabid dog/cat or not. Regardless of the likelihood of infection, PEP should be administered in compliance with the standard procedures. If a patient declines RIG, documents should be signed by the patients to prevent legal recourse against the physicians. Effective measures must be discussed for the accurate assessment of rabies exposure to prevent unnecessary PEP treatments.
Because the pregnant women’s personal information collection procedure is incomplete, only rely on telephone follow-up, thus results in effective follow-up rate are low, which might lead to loss of information.
It is noted that this study provides the information on the China-produced vaccines safety observation data in pregnant women.
In this study, the control group without PEP treatment was not used to compare the results from pregnant women receiving PEP. Because we could not afford the expenses of prenatal care or designate a maternity hospital for the subjects, they gave birth in different hospitals and a control group could not be established based on delivery patterns. Local published information on the delivery patterns of pregnant women could be used. The high rate of cesarean section is a major public health concern in China and reached 60% in some areas, which is well beyond the rate of 15% recommended by WHO.3,16 The rate of cesarean section among our study participants was 28.36%, lower than those recently reported in this area (40.18% and 56.7%)25,26 and in other domestic areas(65%).10,18The most common indication of cesarean section was cephalopelvic disproportion, in accordance with a previous report,12 which was not attributed to the vaccination. Although 53% of the pregnant women were at less than 20 gestational weeks along in their pregnancy, and thus highly prone to teratogenesis, all of the newborns developed and grew normally, and their birth weights and heights were within the normal ranges for Chinese newborns. In our study the gender ratio of 40 male vs. 27 female babies is imbalance, although there are some problems about infant ratio imbalance as a result of various social factors in recent years in China,27 we did not do any baby gender selection intervention, just because the sample size is too small to explain the relation between vaccination and gender.
This study demonstrated that the rabies vaccines had favorable safety profiles across all three trimesters. Further health education is needed to eliminate the perceived safety concerns over the use of rabies vaccines for pregnant women and to avoid unnecessary abortions following PEP and the subsequent injury and suffering. Rabies PEP should be maximized to effectively prevent the occurrence of rabies.
Materials and Methods
Subjects
The protocol for surveillance was approved by the ethics committee of Guangzhou Center for Disease Control and Prevention (GZCDC), and informed consent was obtained from all subjects recruited to receive PEP. GZCDC runs a designated rabies immunization clinicwith a daily volume of more than 100 vaccinations. The immunizations studied herein were provided by qualified nurses, and the safety observation and phone-based follow-up were performed by experienced physicians. Using a cluster sampling protocol, pregnant women who visited our clinic for rabies PEP because of dog or cat wounds were enrolled between January and June, 2009. Ninety-nine pregnant women were enrolled, aged 19–38 y and in the 4–37th gestational week. Prior to the PEP, all of the wounds were appropriately treated in accordance with the WHO post-exposure management guidelines. The level of exposure was graded as follows: Category (1) contact with or breeding animals, with intact skin licked by the animals; Category (2) bare skin slightly bitten or scratched by the animals without inducing bleeding; Category (3) single or multiple penetrating skin wound(s) from bites or scratches, a skin wound that was licked by an animal, or mucosal contamination with animal body fluids. The management of grade III wound exposure requires wound care and injections of a rabies vaccine and RIG. Prior to the PEP, pregnant women were advised of the type, action, contraindications, adverse effects, and precautions related to the vaccine or RIG and signed informed consent forms.
Vaccines and immunizations
The vaccines and RIG applied in this study were products with testing certification by the National Institutes for Food and Drug Control, and all have had more than 8 y of clinic experience. The PVRV was derived from the rabies Pasteur PV-2061 strain and was manufactured by Chengda Biotechnology. The Rabipur® purified chick embryo cell vaccine (PCECV) was purchased from Novartis Behring. The Verorab® PVRV was purchased from Sanofi Pasteur. The potency of each vaccine was > 2.5 IU per dose. The human RIG was manufactured by Shuang Lin Biotechnology. PEP is not administered free in China and the patients have to pay the PEP expense. Physicians provided advice from a professional standpoint. Most patients took into consideration the cost, product origins and so on to make a final decision about choosing a rabies vaccine and taking RIG or not. The storage of the vaccines or RIG consisted of a standard cold chain, and multiple batches were used in this study over the six-month study period. The Essen regimen was used for all of the subjects involved, one dose was administered in the deltoid muscle at D0, D3, D7, D14 and D28. In the cases of category III exposure, physicians recommended pregnant women to receive RIG according to the WHO guidelines,3 but the final decisions were made by the subjects.
Safety observation
Prior to each vaccination, body temperature was measured. Immediate reactions within 30 minnutes after the vaccination and local or systemic reactions during the first 72 h post-immunization were monitored and evaluated in accordance with the “Clinical Study Guidelines for Adverse Effects Evaluation of Prophylactic Vaccine,” (published by China State Food and Drug Administration, 2005). Fevers were graded as follows: mild (37.1–37.5°), moderate (37.6–39.0°), and severe (> 39°). The severity of the adverse effects was graded as follows: mild, transient (< 48 h) discomfort requiring no medical care; moderate, restricting daily activities requiring no or minimal medical intervention; severe, seriously restricting daily activities requiring routine care, medical care or even hospitalization; life-threatening, extremely restricting daily activities, requiring specific care, medical treatment and hospitalization.
Follow-up interview
A case document was maintained for each subject to record the patient’s information, including name, age, height, body mass, contact phone number, pregnancy progress and expected date of delivery. The PEP conditions were also documented, including animal exposure, site of the wound and the exposure category. The subjects were contacted by telephone in a prospective manner for follow-up. A self-administered questionnaire was used, including a labor/delivery profile, the date of child birth, the body mass and height of the baby, the feeding type within 4 m and the general wellbeing of the mother and baby.
Statistical analysis
The SPSS statistical software package (version 11.5, SPSS, Inc.) was used for the statistical analyses. A chi-square test was used for comparison of the adverse reaction rate and the cesarean section rate. For all analysis, P values not more than 0.05 were regarded as significant. (Author, please cite refs. 2 and 13)
Acknowledgments
This work is supported by grants from the Science and Information Technology of Guangzhou (2012J5100005) and Guangdong Provincial Department of Science and Technology (2011B050300001). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/22377
References
- 1.Ahluwalia IB, Singleton JA, Jamieson DJ, Rasmussen SA, Harrison L. Seasonal influenza vaccine coverage among pregnant women: pregnancy risk assessment monitoring system. J Womens Health (Larchmt) 2011;20:649–51. doi: 10.1089/jwh.2011.2794. [DOI] [PubMed] [Google Scholar]
- 2.Ashwathnarayana DH, Madhusudana SN, Sampath G, Sathpathy DM, Mankeshwar R, Ravish HH, et al. A comparative study on the safety and immunogenicity of Purified duck embryo vaccine [corrected] (PDEV, Vaxirab) with purified chick embryo cell vaccine (PCEC, Rabipur) and purifed vero cell rabies vaccine (PVRV, Verorab) Vaccine. 2009;28:148–51. doi: 10.1016/j.vaccine.2009.09.090. [DOI] [PubMed] [Google Scholar]
- 3.WHO Expert Consultation on rabies. World Health Organ Tech Rep Ser 2005;931:1–88. back cover. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention CDC American College of Obstetricians and Gynecologists ACOG: Vaccination during pregnancy. EPI Newsl. 1997;19:6. [Google Scholar]
- 5.Chaithongwongwatthana S, Yamasmit W, Limpongsanurak S, Lumbiganon P, Desimone JA, Baxter J, et al. Pneumococcal vaccination during pregnancy for preventing infant infection. Cochrane Database Syst Rev. 2006:D4903. doi: 10.1002/14651858.CD004903.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Chutivongse S, Wilde H. Postexposure rabies vaccination during pregnancy: experience with 21 patients. Vaccine. 1989;7:546–8. doi: 10.1016/0264-410X(89)90280-6. [DOI] [PubMed] [Google Scholar]
- 7.Chutivongse S, Wilde H, Benjavongkulchai M, Chomchey P, Punthawong S. Postexposure rabies vaccination during pregnancy: effect on 202 women and their infants. Clin Infect Dis. 1995;20:818–20. doi: 10.1093/clinids/20.4.818. [DOI] [PubMed] [Google Scholar]
- 8.Figueroa Damián R, Ortiz Ibarra FJ, Arredondo García JL. [Post-exposure antirabies prophylaxis in pregnant women] Ginecol Obstet Mex. 1994;62:13–6. [PubMed] [Google Scholar]
- 9.Huang G, Cao Q, Liu X, Zhang D, Wen S. Epidemiological Study of Rabies in Guangzhou between 2004 and 2006. J Trop Med. 2007;7:492–4. [Google Scholar]
- 10.Han W, Song J, Liu A, Huo K, Xu F, Cui S, et al. Trends in live births in the past 20 years in Zhengzhou, China. Acta Obstet Gynecol Scand. 2011;90:332–7. doi: 10.1111/j.1600-0412.2010.01065.x. [DOI] [PubMed] [Google Scholar]
- 11.He JF, Kang M, Li LH. [Exposure to human rabies and the related risk factors in Guangdong] 2009;30:532–3. [PubMed] [Google Scholar]
- 12.Ingardia CJ, Kelley L, Steinfeld JD, Wax JR. Hepatitis B vaccination in pregnancy: factors influencing efficacy. Obstet Gynecol. 1999;93:983–6. doi: 10.1016/S0029-7844(98)00563-8. [DOI] [PubMed] [Google Scholar]
- 13.Lei H, Wen SW, Walker M. Determinants of caesarean delivery among women hospitalized for childbirth in a remote population in China. J Obstet Gynaecol Can. 2003;25:937–43. doi: 10.1016/s1701-2163(16)30242-0. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Huang G, Tang Q, Li J, Cao S, Fu C, et al. The immunogenicity and safety of vaccination with purified Vero cell rabies vaccine (PVRV) in China under a 2-1-1 regimen. Hum Vaccin. 2011;7:220–4. doi: 10.4161/hv.7.2.14003. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Ruo R, Ren W, Pan Z. Analysis of Rabies in Guangzhou From 1997 to 2005. J Trop Med. 2006;6:1199–200. [Google Scholar]
- 16.Lumbiganon P, Laopaiboon M, Gülmezoglu AM, Souza JP, Taneepanichskul S, Ruyan P, et al. World Health Organization Global Survey on Maternal and Perinatal Health Research Group Method of delivery and pregnancy outcomes in Asia: the WHO global survey on maternal and perinatal health 2007-08. Lancet. 2010;375:490–9. doi: 10.1016/S0140-6736(09)61870-5. [DOI] [PubMed] [Google Scholar]
- 17.Monto AS. Seasonal influenza and vaccination coverage. Vaccine. 2010;28(Suppl 4):D33–44. doi: 10.1016/j.vaccine.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 18.Qin C, Zhou M, Callaghan WM, Posner SF, Zhang J, Berg CJ, et al. Clinical indications and determinants of the rise of cesarean section in three hospitals in rural china. Matern Child Health J. 2012;16:1484–90. doi: 10.1007/s10995-011-0913-7. [DOI] [PubMed] [Google Scholar]
- 19.Shahid NS, Steinhoff MC, Hoque SS, Begum T, Thompson C, Siber GR. Serum, breast milk, and infant antibody after maternal immunisation with pneumococcal vaccine. Lancet. 1995;346:1252–7. doi: 10.1016/S0140-6736(95)91861-2. [DOI] [PubMed] [Google Scholar]
- 20.Si H, Guo ZM, Hao YT, Liu YG, Zhang DM, Rao SQ, et al. Rabies trend in China (1990-2007) and post-exposure prophylaxis in the Guangdong province. BMC Infect Dis. 2008;8:113. doi: 10.1186/1471-2334-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sudarshan MK, Giri MS, Mahendra BJ, Venkatesh GM, Sanjay TV, Narayana DH, et al. Assessing the safety of post-exposure rabies immunization in pregnancy. Hum Vaccin. 2007;3:87–9. doi: 10.4161/hv.3.3.4010. [DOI] [PubMed] [Google Scholar]
- 22.Sudarshan MK, Madhusudana SN, Mahendra BJ. Post-exposure prophylaxis with purified vero cell rabies vaccine during pregnancy--safety and immunogenicity. J Commun Dis. 1999;31:229–36. [PubMed] [Google Scholar]
- 23.Sudarshan MK, Madhusudana SN, Mahendra BJ, Ashwathnarayana DH, Jayakumary M, Gangaboriah Post exposure rabies prophylaxis with Purified Verocell Rabies Vaccine: a study of immunoresponse in pregnant women and their matched controls. Indian J Public Health. 1999;43:76–8. [PubMed] [Google Scholar]
- 24.Tamma PD, Ault KA, del Rio C, Steinhoff MC, Halsey NA, Omer SB. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol. 2009;201:547–52. doi: 10.1016/j.ajog.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 25.Xie J. Analysis of relative factors and the rate of cesarean section in the last 25 years. The Journal of Practical Medicine. 2009:3152–3154. [Google Scholar]
- 26.Yang L. Study on the trends of the rate of cesarean section in the last 8 years. Maternal and Child Health Care of China. 2005;20:2620–1. [Google Scholar]
- 27.Goodkind D. Child underreporting, fertility, and sex ratio imbalance in China. Demography. 2011;48:291–316. doi: 10.1007/s13524-010-0007-y. [DOI] [PubMed] [Google Scholar]