Abstract
This study describes an outbreak of varicella, in a small town in the region of Puglia, Southern Italy, in the period between February–March 2011. This outbreak presented the opportunity to assess varicella vaccine effectiveness and its determinants. The outbreak occurred in a small community in Puglia; parents of the children attending the schools of the community were contacted by telephone and information was gathered on current disease and varicella history. Varicella vaccination history was verified through the immunization registry of the Local Health Unit. Before the outbreak, immunization coverage was 86.6% of children attending preschool and 51.9% of children attending elementary school. In day care center where the outbreak was happened, the attack rate in vaccinated individuals was 32.1% and 80% in susceptible unvaccinated individuals. VE is therefore estimated as 59.9% (95% CI = 48.3–69.8). In the elementary school the VE can be calculated as 69.2% (95% CI = 50.5–88.1), since the attack rate in unvaccinated children was of 23.1% and in vaccinated of 7.1. The time between vaccination and the onset of the epidemic appears higher in children with a vaccine failure. The results of this study highlight the need for a reflection on the desirability of adopting a shorter schedule in Italy, with a minimum 1 mo interval between MMRV doses.
Keywords: varicella, varicella vaccine, breakthrough, universal mass vaccination, interval between doses
Introduction
The live varicella attenuated vaccine was introduced to the world in 1974.1 Today, two live vaccines for attenuated varicella zoster virus are available for the prevention of varicella. Since 2006, two measles-mumps-rubella-varicella (MMRV) combination vaccines have been available in many countries. Previous studies have shown that the MMRV vaccines are as immunogenic and well tolerated as separate measles-mumps-rubella (MMR) and varicella vaccinations.2-4 However, MMRV is associated with an increased risk for febrile seizures after the first dose vaccination of young children.5
In 1995, the American Academy of Pediatrics recommended that the varicella vaccine be added to the childhood immunization schedule.6 In Europe, varicella is listed as a routine childhood vaccination in four countries. In Germany and Greece it is part of the childhood immunizations program, with doses given at 15–23 mo and 12–18 mo of age respectively. Spain offers the vaccine at 10–14 y without a history of disease and in Austria varicella vaccination is recommended only for those, aged 7–16 y with no previous history of varicella or who have negative serology results for varicella. Cyprus, France and Slovenia recommend varicella to risk groups.7
In Italy, local recommendations differ and varicella is offered routinely in some areas - 33% (Basilicata, Calabria, Puglia, Sicilia, Veneto) of regions have adopted a universal vaccination program. The varicella vaccine is given in two doses: children should be given the first shot at the age of 13–15 mo and the second at the age of 5–6 y. In the other regions, the varicella vaccination is offered free of charge to all eligible people at risk of complications because of chronic diseases.8
Following approval of the license for the varicella vaccine in 1995, many investigations into the effectiveness of the vaccine have been published. Studies post-license have assessed varicella vaccine effectiveness (VE) in child care, school, household, and community settings, commonly during outbreak investigations. The breakthrough varicella infection rates these studies have reported range from 4% to 68% and VE for one dose was between 20% and 88% of outbreak investigations in the USA, Israel and Germany.9-14 Most investigations have found vaccine effectiveness of 80–85%. A review of US studies published in 2008 showed that one dose of varicella vaccine was 84.5% effective in preventing all varicella and 100% effective in preventing severe varicella.15 The efficacy for 2 doses was significantly higher than for a single dose varicella vaccine.16
It has yet to be established whether the second dose should be administered as close to the first as possible (within 4–6 weeks) in order to complete protection from (partially) primary vaccine failure or at the age of 5 or 6 y, for more effective long-term protection.11
In 2006, the region of Puglia introduced a universal mass vaccination (UMV) against the varicella disease; Varilrix (GlaxoSmithKline Biologicals) and Varivax (Merck) vaccines have been used, depending to the district. The program involved administering just a single dose vaccine to children aged between 12–24 mo. Since 2010, two doses strategy has been employed with the first dose of vaccine administered at the age of 13–15 mo, and the second at the age of 5–6 y. A catch-up strategy for susceptible adolescents has been adopted. Since the adoption of two doses strategy, all Puglian vaccination services used Priorix-Tetra™ (MMRV-GlaxoSmithKline Biologicals).
This study describes an outbreak of varicella, in a small town in the region of Puglia, Southern Italy, in the period between February–March 2011. This outbreak presented the opportunity to assess varicella vaccine effectiveness and its determinants.
Results
The investigation involved 568 (77.6%) of the 732 children attending school in the town; of these, 358 attended elementary school and 210 attended preschool. 164 children either could not be reached or their parents refused to participate in the survey.
Before the epidemic outbreak, 241 children had contracted varicella (42.4%); more precisely 62.8% (n = 225/358) of elementary school children and 7.6% (n = 16/210) of preschool children (chi-square = 162.3, p < 0.0001).
72.5% (n = 237) of susceptible children studied (n = 327) had been administered one dose of the varicella vaccine. Dividing children by type of school, immunization coverage was 86.6% (n = 168/194) of children attending preschool and 51.9% (n = 69/133) of children attending elementary school (chi-square = 47.7, p < 0.0001). Four children, two of whom attended elementary school and two preschool, had contracted chickenpox despite having been vaccinated. The theoretical pattern of susceptibility therefore was 16.5% (n = 94/568) with no statistically significant differences between preschools (13.3%, n = 28/210) and elementary schools (18.4%, n = 66/358; chi-square = 2.5, p = 0114).
No children had received two doses of varicella vaccine in the community where the investigation took place. Table 1 shows vaccination coverage, the pattern of immunity in natural infection and the theoretical patterns of susceptibility per school.
Table 1. Vaccination coverage, pattern of immunity in natural infection and the theoretical patterns of susceptibility per school.
| School | Registered children | N. Children Investigated | Vaccination Coverage for one dose (%) | N. Children with Contracted Varicella (%) | N. Susceptible Children (%) |
|---|---|---|---|---|---|
| Elementary 1 |
358 |
232 |
21.1 |
60.8 |
18.5 |
| Elementary 2 |
162 |
126 |
15.9 |
66.7 |
18.2 |
| Day care center 1 |
56 |
42 |
78.6 |
9.5 |
14.3 |
| Day care center 2 |
81 |
62 |
79 |
6.5 |
14.5 |
| Day care center 3 |
52 |
45 |
80 |
11.1 |
11.1 |
| Day care center 4 |
40 |
27 |
81.5 |
7.4 |
11.1 |
| Day care center 5 | 35 | 34 | 82.4 | 2.9 | 14.7 |
The index case was a healthy 4 y-old girl, vaccinated in January 2009, who attended Day Care Center 5. On February 1 2011 she developed a macupapular rash (with fewer than 50 lesions), without fever or other systemic symptoms. The source of the infection for the index case could not be identified. In the period between February–March 2011, there were 12 other second cases of varicella at Day Care Centre 5, of which 8 involved children vaccinated between 2007 and 2009. During March, there were registered three cases of varicella in the Elementary School 1; one of these was the brother of a case of the Day Care Center 5 and the other were close contact of him. None was immunized against varicella. Another case of chickenpox was reported in the Elementary School 2; he was the brother of a case of the Day Care Center 5 and he received one dose of vaccine in 2007.
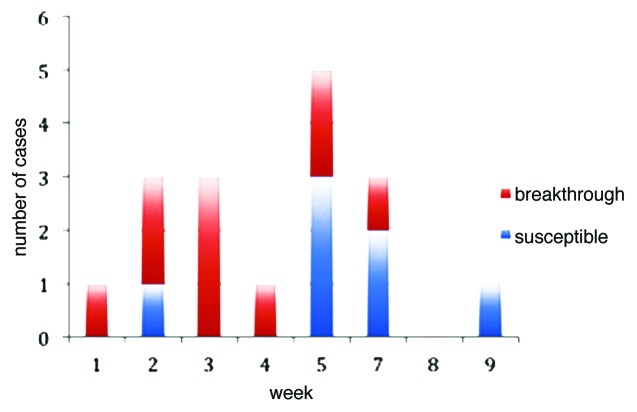
In our investigation, we did not detected children with varicella vaccinated 14–42 days earlier the onset of the rash. Figure 1 shows the distribution of cases by week, with the first week of February indicated as a Week 1. The average age of cases was 5.2 ± 1.4 y; seven (41.2%) of the 17 reported cases were female. Sixteen children (94.1%) were absent from school because of illness, the average days of absence was 8 ± 3.6 (range = 0–15) days; no one was hospitalized. 34 children from Day Care Center 5 were analyzed, 28 of which were vaccinated and 1 had contracted the disease before the epidemic. Therefore, there were 5 susceptible children in the school. During the outbreak there were 13 cases (AR = 38.2%), including 9 in vaccinated individuals. The attack rate in vaccinated subjects was 32.1% and 80% in susceptible unvaccinated individuals. Vaccine effectiveness (VE) is therefore estimated as 59.9% (95% CI = 48.3–69.8). In the elementary school the VE can be calculated as 69.2% (95% CI = 50.5–88.1), since the attack rate in unvaccinated children was of 23.1% (3/13) and in vaccinated of 7.1 (1/14).
Figure 1. Reported cases of varicella according to vaccination status.
All breakthrough cases were mild, and therefore the effectiveness of one dose of vaccine against moderate to severe varicella was 100%. Referring solely to classes where cases of varicella have been confirmed, the average time elapsed between vaccination and exposure is higher in breakthrough cases (987.4 ± 213.8 d) compared with cases of vaccinated individuals who did not develop disease (684.6 ± 435.7 d; t = 2.10, p = 0.02). No statistically significant differences were observed in the distribution of asthma, allergies, eczema, chronic disease and episodes of hospitalization among vaccinated breakthrough cases and vaccinated non-breakthrough cases (see Table 2). The logistic regression model was used to evaluate the time between vaccination and exposure, age, asthma, eczema, allergies, chronic illness and previous hospitalization and setting where the outbreak happened (elementary or day care); while the factors investigated did not appear to increase the risk of breakthrough infection
Table 2. Proportion of vaccinated subjects with asthma, allergies, eczema, chronic illness and episodes of hospitalization, per breakthrough diagnosis. Puglia, 2011.
| Breakthrough cases (n = 14) | Non breakthrough vaccinated individuals (n = 221) |
Chi-square | p | |
|---|---|---|---|---|
| Asthma |
2 (14.3%) |
24 (10.8%) |
3.09 |
0.078 |
| Eczema |
1 (7.1%) |
25 (11.3%) |
0.21 |
0.642 |
| Allergies |
1 (7.1%) |
75 (33.9%) |
0.061 |
0.938 |
| Chronic illness |
1 (7.1%) |
25 (11.3%) |
0.21 |
0.646 |
| Hospitalization within previous year | - | 40 (18.1%) | 1.09 | 0.29 |
Discussion
Our study documents an outbreak of varicella exchanged between two groups with a theoretical pattern of susceptibility below 20%. This attributable fraction was found in the group of primary school children due to the high proportion of subjects who had contracted natural infection before the of a vaccination strategy. While in the other group (Day Care Center children) this was due to high vaccination coverage, despite this being below optimum percentage (about 80%), for one dose of varicella vaccine.
We observed an important difference in the VE estimated in the two settings (Day care and elementary school). This may result from the different intensity of exposure (much different attack rates among both vaccinated and unvaccinated in the two settings), and the setting of exposure acts as a confounding variable (the setting is associated with both exposure (vaccination coverage) and outcome (risk of getting disease).
The time between vaccination and the onset of the epidemic is higher in the breakthrough and the spread of the epidemic seems to favor the setting where the vaccine coverage is higher, the same pattern of susceptibility theory, specifically in relation to low vaccine effectiveness.
The force of infection also has an important role in the spread of outbreak. In our epidemic, there was not much exposure in the elementary schools, and there were fewer opportunities for becoming a case compared with day-care 5 where there was a lot of virus circulating (13 of 17 cases).
The transition from the one-dose varicella vaccination schedule, introduced in Puglia in 2006, to a two-dose schedule, planned from 2010, created a particular epidemiological pattern across the region. In the primary school there is a low vaccination coverage and a lot of clusters of susceptible children, because of the increase of the age of infection. Many children, in the transition time, are going to receive the second dose of varicella vaccine at 12–13 y, because a mop-up strategy has not been planned. In this field, outbreaks could occur and vaccine failures could be frequent. This scenario will recur in other nations, especially if you take into account that vaccination against varicella is under consideration as part of pediatric immunization programs in an increasing number of countries. Moreover, in many countries where a second dose of varicella vaccine is administered, it is given at 4–6 y of age or 10–12 y of age.17 Therefore, our study captures the epidemiological framework that could recur in other countries over the coming years.
The main limitation of the study is the lack of a diagnostic examination of the chickenpox; in fact the study is based on what has been reported by parents, which is due to laboratory based confirmation of varicella being very sporadic and to activities supporting molecular diagnostics of epidemiological surveillance not having been initiated.
In last years, outbreaks of varicella have been reported in settings with high 1 dose varicella coverage. In these outbreaks, overall attack rates have ranged from 11% to 17% (40% in certain classes),18-20 higher than rates calculated in our study. In our survey, measured VE was lower than other studies, and ranged from 20% to 89%.9,12,14,15,21,22 These differences are mostly attributable to peculiarities of the environments considered.
The National Vaccination Prevention Plan 2012−2014 reveals that in some Italian regions the introduction of universal vaccination against varicella are already in place, and European-wide evaluation studies on the potential impact of these strategies are underway. As yet, under the Plan the introduction of universal vaccination against varicella in all Regions has been postponed to 2015, when results will be available from the evaluation studies and monitoring data from the pilot vaccination programs. These programs have adopted a two-dose vaccination schedule, with the MMRV vaccine, providing the first dose in 13th−15th month and the second dose at 5–6 y. This schedule may have benefits in terms of compliance because of a later second dose fits with current vaccination schedules.
The results of this study highlight the need for a reflection on the desirability of adopting a shorter schedule in Italy, with a minimum 1 mo interval between MMRV doses. A shorter schedule would maximize the benefits of a second dose, including addressing the possible increased likelihood for breakthrough cases with time since the first dose. After two doses of the MMRV administered vaccine with a 4 week interval, the immunogenic response was found to be adequate without any safety or reactogenicity issues.17 A 2 dose strategy is predicted to reduce varicella and zoster cases by about 90% and 10%, respectively, over 80 y22 and might also be necessary to reduce the risk of breakthrough infection.
To date, two studies (including a clinical trial) showed that a two-dose regimen was significantly more effective than a single injection for protection against varicella,16,23 while one study of outbreaks in elementary school children with low 2 dose coverage found that the vaccine effectiveness of 1 and 2 doses were similar.24 More studies across a large population are needed in order to evaluate the effectiveness of the 2 dose vaccine and it is important to monitor the two-dose coverage among eligible age groups to guide future policy and intervention design.
Materials and Methods
In February 2011, a pediatrician from a small Apulian community of about 8,000 people—940 of whom were under the age of 14—highlighted seven cases of varicella in children under 10 y (5 of which involved vaccinated children) to the Regional Observatory Unit (Osservatorio Epidemiologico Regionale). An epidemiological investigation followed.
The investigation subsequent to the outbreak detected at the end of February involved cases which had already been reported and ones that arose subsequently, and were recorded following notification from local doctors. The investigation was conducted by the authors.
In the first phase of the investigation a list of preschools and elementary schools in the town was compiled. Within the town there was one state school which was divided into five complexes, of which, two housed elementary schools and three preschools. The school principals were contacted and a list of children enrolled at the schools was requested, as were parents’ telephone numbers.
Varicella vaccination history was verified through the immunization registry of the Local Health Unit. Parents of the children attending the schools were contacted, and a formal request of informed consent was made for participation in the study, conducted using a standardized questionnaire. From March 2010, parents who agreed to participate in the study were contacted by telephone and information was gathered on: current disease, varicella history, medications and the history of conditions including asthma, allergies, eczema, chronic disease and hospitalization in the previous 12 mo. The recall for varicella or breakthrough, diagnosed by pediatrician, can be considered to be 100%.25
Case definition
A case of natural varicella was defined as an illness involving a pruritic, maculopapulovesicular rash with no other apparent cause, in the period January 1, 2011 through to March 31, 2011, in a child attending one of the schools in the town, who had not received varicella vaccine or who had been vaccinated less than 14 d before the onset of rash.
Breakthrough disease was defined as varicella disease in a child who had been vaccinated 42 d or more before the onset of rash. Illness was classified as mild (fewer than 50 lesions without complications), or moderate-severe (more than 50 lesions or the occurrence of any serious complications, such as varicella pneumonitis, encephalitis, fever for five days, hospitalization or death). A child who had attended the schools during this period and did not show signs of the disease was considered as a “non-case” patient.
Children were considered to have asthma, allergies or eczema if they had a reported history of asthma, allergies or eczema and were being treated with any medication for these illnesses. Parents were also asked if the child had other chronic illness or had been admitted to hospital in the previous 12 mo.
Outbreak control measures
Varicella case patients were excluded from school until lesions crusted or faded. A letter was sent to children’s homes informing parents of the outbreak and recommending vaccination to susceptible students and varicella case contacts.
Statistical analyzes
Vaccination coverage at the start of the outbreak was defined as the proportion of those persons who had received the varicella vaccine from among susceptible investigated student (including those with a history of disease).
Vaccine effectiveness is measured by calculating the incidence rates (attack rates) of disease among vaccinated and unvaccinated persons, and determining the percentage reduction in the incidence rate of disease among vaccinated persons compared with unvaccinated persons. The formula used was: VE = [(ARU-ARV)X 100]/ARU, where VE = vaccine effectiveness, ARU = attack rate in the unvaccinated population and ARV = attack rate in vaccinated population.26
The effectiveness of the vaccine with 95% CI has been also calculated as 1 minus the matched odds ratio.27 Persons with a previous history of varicella or vaccination less than 42 d before the start of the outbreak were excluded from the vaccine effectiveness calculation. Data were analyzed using STATA MP 11.2 software. A Student’s t-test for independent samples was used to analyze the continuous variables. A Chi-square test was used to compare proportions.
A multivariate logistic regression model was made in order to evaluate the association between vaccine failure and the time elapsed between vaccination and exposure, presence of asthma, eczema, allergies, chronic illness and hospitalizations. A p-value < 0.05 was considered significant.
Glossary
Abbreviations:
- UMV
universal mass vaccination
- MMRV
measles-mumps-rubella-varicella
- MMR
measles-mumps-rubella
- VE
vaccine effectiveness
- ARU
attack rate in the unvaccinated population
- ARV
attack rate in vaccinated population
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/22373
References
- 1.Takahashi M, Otsuka T, Okuno Y, Asano Y, Yazaki T. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet. 1974;2:1288–90. doi: 10.1016/S0140-6736(74)90144-5. [DOI] [PubMed] [Google Scholar]
- 2.Schuster V, Otto W, Maurer L, Tcherepnine P, Pfletschinger U, Kindler K, et al. Immunogenicity and safety assessments after one and two doses of a refrigerator-stable tetravalent measles-mumps-rubella-varicella vaccine in healthy children during the second year of life. Pediatr Infect Dis J. 2008;27:724–30. doi: 10.1097/INF.0b013e318170bb22. [DOI] [PubMed] [Google Scholar]
- 3.Knuf M, Habermehl P, Zepp F, Mannhardt W, Kuttnig M, Muttonen P, et al. Immunogenicity and safety of two doses of tetravalent measles-mumps-rubella-varicella vaccine in healthy children. Pediatr Infect Dis J. 2006;25:12–8. doi: 10.1097/01.inf.0000195626.35239.58. [DOI] [PubMed] [Google Scholar]
- 4.Goh P, Lim FS, Han HH, Willems P. Safety and immunogenicity of early vaccination with two doses of tetravalent measles-mumps-rubella-varicella (MMRV) vaccine in healthy children from 9 months of age. Infection. 2007;35:326–33. doi: 10.1007/s15010-007-6337-z. [DOI] [PubMed] [Google Scholar]
- 5.Klein NP, Fireman B, Yih WK, Lewis E, Kulldorff M, Ray P, et al. Vaccine Safety Datalink Measles-mumps-rubella-varicella combination vaccine and the risk of febrile seizures. Pediatrics. 2010;126:e1–8. doi: 10.1542/peds.2010-0665. [DOI] [PubMed] [Google Scholar]
- 6.American Academy of Pediatrics Committee on Infectious Diseases Recommendations for the use of live attenuated varicella vaccine. Pediatrics. 1995;95:791–6. [PubMed] [Google Scholar]
- 7.Bonanni P, Breuer J, Gershon A, Gershon M, Hryniewicz W, Papaevangelou V, et al. Varicella vaccination in Europe - taking the practical approach. BMC Med. 2009;7:26. doi: 10.1186/1741-7015-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alfonsi V, D’Ancona F, Giambi C, Nacca G, Rota MC, Regional Coordinators for Infectious Diseases and Vaccinations Current immunization policies for pneumococcal, meningococcal C, varicella and rotavirus vaccinations in Italy. Health Policy. 2011;103:176–83. doi: 10.1016/j.healthpol.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Galil K, Lee B, Strine T, Carraher C, Baughman AL, Eaton M, et al. Outbreak of varicella at a day-care center despite vaccination. N Engl J Med. 2002;347:1909–15. doi: 10.1056/NEJMoa021662. [DOI] [PubMed] [Google Scholar]
- 10.Miron D, Lavi I, Kitov R, Hendler A. Vaccine effectiveness and severity of varicella among previously vaccinated children during outbreaks in day-care centers with low vaccination coverage. Pediatr Infect Dis J. 2005;24:233–6. doi: 10.1097/01.inf.0000154323.20387.82. [DOI] [PubMed] [Google Scholar]
- 11.Spackova M, Wiese-Posselt M, Dehnert M, Matysiak-Klose D, Heininger U, Siedler A. Comparative varicella vaccine effectiveness during outbreaks in day-care centres. Vaccine. 2010;28:686–91. doi: 10.1016/j.vaccine.2009.10.086. [DOI] [PubMed] [Google Scholar]
- 12.Dworkin MS, Jennings CE, Roth-Thomas J, Lang JE, Stukenberg C, Lumpkin JR. An Outbreak of Varicella among children attending preschool and elementary school in Illinois. Clin Infect Dis. 2002;35:102–4. doi: 10.1086/340868. [DOI] [PubMed] [Google Scholar]
- 13.Haddad MB, Hill MB, Pavia AT, Green CE, Jumaan AO, De AK, et al. Vaccine effectiveness during a varicella outbreak among schoolchildren: Utah, 2002-2003. Pediatrics. 2005;115:1488–93. doi: 10.1542/peds.2004-1826. [DOI] [PubMed] [Google Scholar]
- 14.Lee LE, Ho H, Lorber E, Fratto J, Perkins S, Cieslak PR. Vaccine-era varicella epidemiology and vaccine effectiveness in a public elementary school population, 2002-2007. Pediatrics. 2008;121:e1548–54. doi: 10.1542/peds.2007-2031. [DOI] [PubMed] [Google Scholar]
- 15.Seward JF, Marin M, Vázquez M. Varicella vaccine effectiveness in the US vaccination program: a review. J Infect Dis. 2008;197(Suppl 2):S82–9. doi: 10.1086/522145. [DOI] [PubMed] [Google Scholar]
- 16.Kuter B, Matthews H, Shinefield H, Black S, Dennehy P, Watson B, et al. Study Group for Varivax Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatr Infect Dis J. 2004;23:132–7. doi: 10.1097/01.inf.0000109287.97518.67. [DOI] [PubMed] [Google Scholar]
- 17.Rümke HC, Loch HP, Hoppenbrouwers K, Vandermeulen C, Malfroot A, Helm K, et al. Immunogenicity and safety of a measles-mumps-rubella-varicella vaccine following a 4-week or a 12-month interval between two doses. Vaccine. 2011;29:3842–9. doi: 10.1016/j.vaccine.2011.02.067. [DOI] [PubMed] [Google Scholar]
- 18.Lopez AS, Guris D, Zimmerman L, Gladden L, Moore T, Haselow DT, et al. One dose of varicella vaccine does not prevent school outbreaks: is it time for a second dose? Pediatrics. 2006;117:e1070–7. doi: 10.1542/peds.2005-2085. [DOI] [PubMed] [Google Scholar]
- 19.Parker AA, Reynolds MA, Leung J, Anderson M, Rey A, Ortega-Sanchez IR, et al. Challenges to implementing second-dose varicella vaccination during an outbreak in the absence of a routine 2-dose vaccination requirement--Maine, 2006. J Infect Dis. 2008;197(Suppl 2):S101–7. doi: 10.1086/522134. [DOI] [PubMed] [Google Scholar]
- 20.Tugwell BD, Lee LE, Gillette H, Lorber EM, Hedberg K, Cieslak PR. Chickenpox outbreak in a highly vaccinated school population. Pediatrics. 2004;113:455–9. doi: 10.1542/peds.113.3.455. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC) National, state, and local area vaccination coverage among children aged 19-35 months--United States, 2007. MMWR Morb Mortal Wkly Rep. 2008;57:961–6. [PubMed] [Google Scholar]
- 22.van Hoek AJ, Melegaro A, Zagheni E, Edmunds WJ, Gay N. Modelling the impact of a combined varicella and zoster vaccination programme on the epidemiology of varicella zoster virus in England. Vaccine. 2011;29:2411–20. doi: 10.1016/j.vaccine.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro ED, Vazquez M, Esposito D, Holabird N, Steinberg SP, Dziura J, et al. Effectiveness of 2 doses of varicella vaccine in children. J Infect Dis. 2011;203:312–5. doi: 10.1093/infdis/jiq052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gould PL, Leung J, Scott C, Schmid DS, Deng H, Lopez A, et al. An outbreak of varicella in elementary school children with two-dose varicella vaccine recipients--Arkansas, 2006. Pediatr Infect Dis J. 2009;28:678–81. doi: 10.1097/INF.0b013e31819c1041. [DOI] [PubMed] [Google Scholar]
- 25.Celikbas A, Ergonul O, Aksaray S, Tuygun N, Esener H, Tanir G, et al. Measles, rubella, mumps, and varicella seroprevalence among health care workers in Turkey: is prevaccination screening cost-effective? Am J Infect Control. 2006;34:583–7. doi: 10.1016/j.ajic.2006.04.213. [DOI] [PubMed] [Google Scholar]
- 26.Orenstein WA, Bernier RH, Dondero TJ, Hinman AR, Marks JS, Bart KJ, et al. Field evaluation of vaccine efficacy. Bull World Health Organ. 1985;63:1055–68. [PMC free article] [PubMed] [Google Scholar]
- 27.Tang ML, Ng HK. Comment on: confidence limits for the ratio of two rates based on likelihood scores: non-iterative method. Stat Med. 2004;23:685–90, author reply 891-2. doi: 10.1002/sim.1683. [DOI] [PubMed] [Google Scholar]