Abstract
Appalachia is a geographic region with existing cancer disparities, yet little is known about its burden of HPV-related cancers outside of cervical cancer. We assessed the burden of HPV-related cancers in three Appalachian states and made comparisons to non-Appalachian regions. We examined 1996–2008 cancer registry data for Ohio, Kentucky, West Virginia and the Surveillance, Epidemiology and End Results (SEER) 9 program. For each gender, we calculated age-adjusted incidence rates per 100,000 population for each HPV-related cancer type (cervical, vaginal, vulvar, penile, anal and oral cavity and pharyngeal cancers) and all HPV-related cancers combined. Incidence rates among females for all HPV-related cancers combined were higher in Appalachian Kentucky [24.6 (95% CI: 23.5–25.7)], West Virginia [22.8 (95% CI: 22.0–23.6)] and Appalachian Ohio [21.9 (95% CI: 21.0–22.8)] than SEER 9 [18.8 (95% CI: 18.6–19.0)]. Similar disparities were found among females when examining cervical and vulvar cancers separately. Among males, Appalachian [21.3 (95% CI: 20.2–22.4)] and non-Appalachian [21.9 (95% CI: 21.2–22.7)] Kentucky had higher incidence rates for all HPV-related cancers combined than SEER 9 [18.3 (95% CI: 18.1–18.6)]. The incidence rate of all HPV-related cancers combined was higher among males from Appalachian Ohio compared with those from non-Appalachian Ohio [17.6 (95% CI: 16.8–18.5) vs. 16.3 (95% CI: 16.0–16.6)]. Our study suggests that HPV-related cancer disparities exist in Appalachia beyond the known high cervical cancer incidence rates. These results have important public health implications by beginning to demonstrate the potential impact that widespread HPV vaccination could have in Appalachia.
Keywords: Human papillomavirus, HPV, Cancer, Appalachia, Disparities
Introduction
Human papillomavirus (HPV) infection is an extremely common sexually transmitted infection (STI) among both females and males. About 43% of females have a current anogenital HPV infection,1 and most studies have shown that at least 20% of asymptomatic males have an anogenital infection.2 Furthermore, about 10% of males and 4% of females have a current oral HPV infection.3 HPV concordance levels between sexual partners are high,4 suggesting transmission between partners is common. Persistent infection with oncogenic HPV types (mainly types 16 and 18) can lead to cancers of the cervix, vagina, vulva, penis, anus and oral cavity and pharynx.5-7 HPV infection is associated with almost all cervical and anal cancers (over 90%) and lower percentages of these other cancers.6,7 It is estimated that HPV causes over 19,000 cancers each year in the US.6,8 The economic burden of these cancers is substantial, with an annual cost of about $400 million.9
When assessing the burden of HPV-related cancers in the US, it may be particularly important to examine high-risk geographic regions, such as Appalachia. The Appalachian region of the U.S. ranges from New York to Mississippi and consists of more than 400 counties in 13 states. Appalachian residents tend to have lower levels of education, higher levels of poverty and poorer health and health behaviors compared with the rest of the US10,11 Important to HPV infection, risky sexual behavior and tobacco use may be more common among residents of Appalachia.11-13 Risky sexual behavior (e.g., higher number of sexual partners) increases the risk of HPV infection,1,14 while smoking may decrease the clearance of HPV infections.15
Cancer in Appalachia is a major public health concern, with previous research showing higher incidence rates for all cancer sites combined among Appalachian males and females (568.7 and 415.1 cases per 100,000, respectively) than non-Appalachian males and females (539.4 and 398.6 cases per 100,000, respectively).16 In terms of HPV-related cancers, Appalachia also appears to suffer from an excessive burden. Appalachia has a higher cervical cancer incidence rate compared with non-Appalachian US, with some Appalachian areas having among the highest cervical cancer incidence rates in the country.16-18 Males in Appalachia have a higher incidence rate of oral cavity and pharyngeal cancer compared with non-Appalachian males (16.1 vs. 15.3 cases per 100,000), though rates are identical among females (5.9 cases per 100,000 in both Appalachia and non-Appalachia).16 Comparisons within states containing Appalachian counties indicate that incidence rates for cervical and oral cavity and pharyngeal cancers are often higher in the Appalachian portions of these states.19,20
Despite these existing data on HPV-related cancers in Appalachia, there are some important epidemiological gaps in the literature. Research has not examined the incidence of vaginal, vulvar, penile and anal cancers in Appalachia. More importantly, no study to our knowledge has assessed the total burden of HPV-related cancers (i.e., combining data for all HPV-related cancers) in Appalachia and how it compares to non-Appalachian regions. The current study uses cancer registry data from three Appalachian states and the Surveillance, Epidemiology and End Results (SEER) Program to address these gaps and examine HPV-related cancers among both genders from this high-risk geographic region. In doing so, we compare Appalachian regions to non-Appalachian regions within the same states and to national data. Results not only help clarify existing disparities involving HPV-related cancers in Appalachia but also begin to demonstrate the potential impact that widespread HPV vaccination could have in this geographic region.
Results
HPV-Related Cancers Among Females
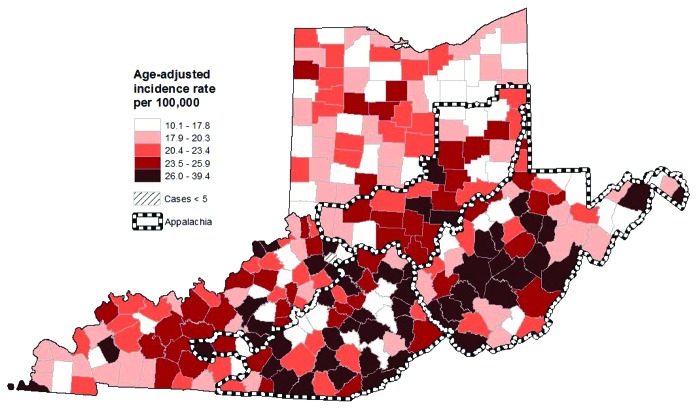
Among females, incidence rates (expressed per 100,000 population) for all HPV-related cancers combined were 22.8 [95% confidence interval (CI): 22.2–23.3; 6,663 cases] for Kentucky, 22.8 (95% CI: 22.0–23.6; 3,200 cases) for West Virginia, 19.0 (95% CI: 18.7–19.3; 15,813 cases) for Ohio and 18.8 (95% CI: 18.6–19.0; 35,243 cases) for SEER 9 (Table 1). Rates for all HPV-related cancers combined were higher in Appalachian Kentucky [24.6 (95% CI: 23.5–25.7) vs. 22.0 (95% CI: 21.4–22.7)] and Appalachian Ohio [21.9 (95% CI: 21.0–22.8) vs. 18.6 (95% CI: 18.3–18.9)] compared with the non-Appalachian regions of these states. The incidence rate for West Virginia was similar to those for Appalachian Kentucky and Appalachian Ohio. Rates for the Appalachian portions of all three states were higher than the SEER 9 rate. Within Appalachia, county-level rates were particularly high in the southern portion of Appalachian Ohio, central and southern West Virginia and several clusters in Appalachian Kentucky (Fig. 1).
Table 1. Incidence of HPV-related cancers among females, 1996–2008 (all races).
| All HPV-Relateda | Cervix | Vulva | Anus | Vagina | Oral Cavity/Pharynx | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Cases |
IR (95% CI) |
Cases |
IR (95% CI) |
Cases |
IR (95% CI) |
Cases |
IR (95% CI) |
Cases |
IR (95% CI) |
Cases |
IR (95% CI) |
| US (SEER 9) |
35,243 |
18.8 (18.6-19.0) |
14,093 |
7.7 (7.6-7.8) |
4,547 |
2.4 (2.3-2.4) |
2,982 |
1.6 (1.5-1.6) |
1,334 |
0.7 (0.7-0.7) |
12,287 |
6.5 (6.4-6.6) |
| Kentucky |
6,663 |
22.8 (22.2-23.3) |
3,002 |
10.7 (10.3-11.0) |
896 |
3.0 (2.8-3.2) |
588 |
2.0 (1.8-2.1) |
257 |
0.8 (0.7-0.9) |
1,920 |
6.3 (6.1-6.6) |
| Appalachia |
2,031 |
24.6 (23.5-25.7) |
982 |
12.4 (11.6-13.2) |
308 |
3.6 (3.2-4.0) |
157 |
1.8 (1.6-2.1) |
73 |
0.9 (0.7-1.1) |
511 |
5.9 (5.4-6.4) |
| Non-Appalachia |
4,632 |
22.0 (21.4-22.7) |
2,020 |
10.0 (9.5-10.4) |
588 |
2.7 (2.5-2.9) |
431 |
2.0 (1.8-2.2) |
184 |
0.8 (0.7-1.0) |
1,409 |
6.5 (6.2-6.8) |
| Ohio |
15,813 |
19.0 (18.7-19.3) |
6,646 |
8.4 (8.2-8.7) |
2,204 |
2.5 (2.4-2.6) |
1,351 |
1.6 (1.5-1.7) |
607 |
0.7 (0.6-0.7) |
5,005 |
5.8 (5.6-5.9) |
| Appalachia |
2,307 |
21.9 (21.0-22.8) |
1,080 |
10.9 (10.2-11.6) |
332 |
2.9 (2.6-3.3) |
205 |
1.9 (1.6-2.2) |
90 |
0.8 (0.6-1.0) |
600 |
5.4 (5.0-5.8) |
| Non-Appalachia |
13,506 |
18.6 (18.3-18.9) |
5,566 |
8.1 (7.9-8.3) |
1,872 |
2.4 (2.3-2.5) |
1,146 |
1.5 (1.5-1.6) |
517 |
0.7 (0.6-0.7) |
4,405 |
5.8 (5.7-6.0) |
| West Virginiab | 3,200 | 22.8 (22.0-23.6) | 1,429 | 11.1 (10.5-11.7) | 442 | 2.9 (2.6-3.2) | 308 | 2.1 (1.9-2.3) | 132 | 0.9 (0.7-1.0) | 889 | 5.9 (5.5-6.3) |
HPV, human papillomavirus; IR, incidence rate; CI, confidence interval; SEER, Surveillance, Epidemiology and End Results. All rates are expressed per 100,000 population and are age-adjusted to the 2000 U.S. standard population. aIncludes cervical, vulvar, anal, vaginal and oral cavity/pharyngeal cancers. bThe entire state of West Virginia is in the Appalachian region.
Figure 1. Incidence of HPV-related cancers by county among females from Ohio, West Virginia and Kentucky (1996–2008; all races). HPV-related cancers include cervical, vulvar, anal, vaginal and oral cavity/pharyngeal cancers. All rates are age-adjusted to the 2000 U.S. standard population.
When examining HPV-related cancers individually, we found a similar pattern of results for cervical and vulvar cancers (Table 1). Appalachian Kentucky [cervical: 12.4 (95% CI: 11.6–13.2); vulvar: 3.6 (95% CI: 3.2–4.0)], Appalachian Ohio [cervical: 10.9 (95% CI: 10.2–11.6); vulvar: 2.9 (95% CI: 2.6–3.3)] and West Virginia [cervical: 11.1 (95% CI: 10.5–11.7); vulvar: 2.9 (95% CI: 2.6–3.2)] all had higher incidence rates for these two cancer types compared with SEER 9 [cervical: 7.7 (95% CI: 7.6–7.8); vulvar: 2.4 (95% CI: 2.3–2.4)]. Within states, Appalachian Kentucky and Ohio had higher incidence rates for both cervical and vulvar cancers compared with the non-Appalachian portions of these states. West Virginia [2.1 (95% CI: 1.9–2.3)] and non-Appalachian Kentucky [2.0 (95% CI: 1.8–2.2)] had higher anal cancer incidence rates compared with SEER 9 [1.6 (95% CI: 1.5–1.6)]. Vaginal cancer incidence rates were similar across all geographic areas. The SEER 9 incidence rate for oral cavity and pharyngeal cancer [6.5 (95% CI: 6.4–6.6)] was higher than rates for Appalachian Ohio [5.4 (95% CI: 5.0–5.8)], non-Appalachian Ohio [5.8 (95% CI: 5.7–6.0)] and West Virginia [5.9 (95% CI: 5.5–6.3)].
HPV-Related Cancers Among Males
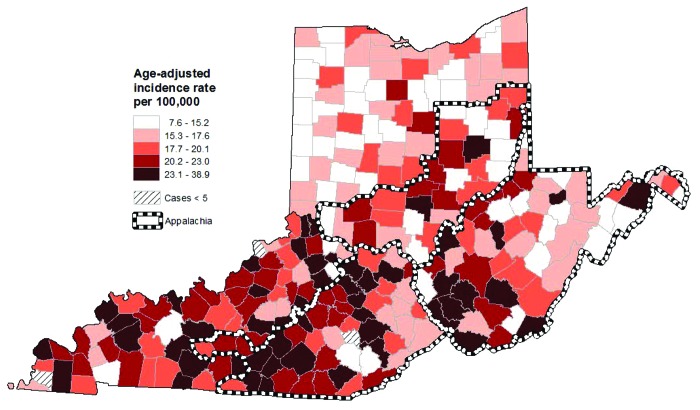
Among males, incidence rates for all HPV-related cancers combined were 21.7 (95% CI: 21.2–22.3; 5,436 cases) for Kentucky, 16.5 (95% CI: 16.2–16.8; 11,638 cases) for Ohio, 19.2 (95% CI: 18.4–19.9; 2,418 cases) for West Virginia and 18.3 (95% CI: 18.1–18.6; 28,946 cases) for SEER 9 (Table 2). Appalachian Ohio had a higher incidence rate for all HPV-related cancers combined compared with non-Appalachian Ohio [17.6 (95% CI: 16.8–18.5) vs. 16.3 (95% CI: 16.0–16.6)], though rates for Appalachian and non-Appalachian Kentucky were similar [21.3 (95% CI: 20.2–22.4) vs. 21.9 (95% CI: 21.2–22.7)]. The SEER 9 rate was comparable to Appalachian Ohio and West Virginia but lower than both Appalachian and non-Appalachian Kentucky. Within Appalachia, the northern and western parts of Appalachian Kentucky had especially high rates of all HPV-related cancers combined among males (Fig. 2). The highest incidence rates within West Virginia were again seen in the central and southern parts of the state, while only a few counties in Appalachian Ohio had high incidence rates among males.
Table 2. Incidence of HPV-related cancers among males, 1996–2008 (all races).
| All HPV-Relateda | Penis | Anus | Oral Cavity / Pharynx | |||||
|---|---|---|---|---|---|---|---|---|
| |
Cases |
IR (95% CI) |
Cases |
IR (95% CI) |
Cases |
IR (95% CI) |
Cases |
IR (95% CI) |
| US (SEER 9) |
28,946 |
18.3 (18.1–18.6) |
1,164 |
0.8 (0.8–0.9) |
2,148 |
1.3 (1.3–1.4) |
25,634 |
16.2 (16.0–16.4) |
| Kentucky |
5,436 |
21.7 (21.2–22.3) |
305 |
1.3 (1.1–1.4) |
296 |
1.2 (1.1–1.3) |
4,835 |
19.3 (18.7–19.8) |
| Appalachia |
1,563 |
21.3 (20.2–22.4) |
124 |
1.7 (1.4–2.0) |
82 |
1.1 (0.9–1.4) |
1,357 |
18.4 (17.4–19.4) |
| Non-Appalachia |
3,873 |
21.9 (21.2–22.7) |
181 |
1.1 (1.0–1.3) |
214 |
1.2 (1.0–1.4) |
3,478 |
19.6 (19.0–20.3) |
| Ohio |
11,638 |
16.5 (16.2–16.8) |
533 |
0.8 (0.7–0.9) |
766 |
1.1 (1.0–1.2) |
10,339 |
14.6 (14.3–14.9) |
| Appalachia |
1,663 |
17.6 (16.8–18.5) |
100 |
1.1 (0.9–1.3) |
100 |
1.1 (0.9–1.3) |
1,463 |
15.5 (14.7–16.3) |
| Non-Appalachia |
9,975 |
16.3 (16.0–16.6) |
433 |
0.7 (0.7–0.8) |
666 |
1.1 (1.0–1.2) |
8,876 |
14.5 (14.2–14.8) |
| West Virginiab | 2,418 | 19.2 (18.4–19.9) | 144 | 1.2 (1.0–1.4) | 148 | 1.2 (1.0–1.4) | 2,126 | 16.8 (16.1–17.5) |
HPV, human papillomavirus; IR, incidence rate; CI, confidence interval; SEER, Surveillance, Epidemiology and End Results. All rates are expressed per 100,000 population and are age-adjusted to the 2000 U.S. standard population. aIncludes penile, anal and oral cavity/pharyngeal cancers. bThe entire state of West Virginia is in the Appalachian region.
Figure 2. Incidence of HPV-related cancers by county among males from Ohio, West Virginia and Kentucky (1996–2008; all races). HPV-related cancers include penile, anal and oral cavity/pharyngeal cancers. All rates are age-adjusted to the 2000 U.S. standard population.
When examining HPV-related cancers individually, the Appalachian regions of both Kentucky [1.7 (95% CI: 1.4–2.0) vs. 1.1 (95% CI: 1.0–1.3)] and Ohio [1.1 (95% CI: 0.9–1.3) vs. 0.7 (95% CI: 0.7–0.8)] had higher penile cancer incidences rates than the non-Appalachian regions (Table 2). The SEER 9 rate for penile cancer was 0.8 (95% CI: 0.8–0.9), which was lower than both Appalachian Kentucky and West Virginia [1.2 (95% CI: 1.0–1.4)]. Anal cancer incidence rates did not differ greatly across geographic areas. There were also no within state differences for oral cavity and pharyngeal cancer incidence in Kentucky or Ohio. However, both Appalachian and non-Appalachian Kentucky [18.4 (95% CI: 17.4–19.4) and 19.6 (95% CI: 19.0–20.3), respectively] had higher incidence rates for oral cavity and pharyngeal cancer compared with SEER 9 [16.2 (95% CI: 16.0–16.4)].
Discussion
Persistent infection with oncogenic HPV types can lead to cancers of the cervix, vagina, vulva, penis, anus and oral cavity and pharynx.5-7 Appalachia is a geographic region with many existing cancer disparities, including cervical cancer.16,17 However, very little is known about the burden of other HPV-related cancers in Appalachia or the total burden of HPV-related cancers in this region. We used cancer registry data from three Appalachian states and the SEER 9 Program to compare Appalachian regions to non-Appalachian regions of the same states and to national data. To our knowledge, this study provides the most comprehensive examination to date on HPV-related cancers in the Appalachian region.
We found several HPV-related cancer disparities among females from Appalachia. Our results not only reaffirm that Appalachia has high cervical cancer incidence rates17-20 but also suggest that HPV-related cancer disparities among Appalachian females exist beyond those for cervical cancer. Appalachian females consistently had higher incidence rates of vulvar cancer compared with non-Appalachian females. The incidence of anal cancer among females was also generally higher in Appalachian areas. Further, the burden of all HPV-related cancers combined was higher in Appalachian regions compared with both non-Appalachian regions of the same states and national rates. Appalachian regions had age-adjusted incidence rates of at least 21.9 cases per 100,000 population for all HPV-related cancers combined among females, similar to the national incidence rates for some of the more common cancers among females in the U.S. (e.g., thyroid cancer and melanoma of the skin21). Thus, a substantial burden of cancer among Appalachian women is likely associated with HPV infection.
The higher rates of HPV-related cancers among Appalachian females are probably attributable to a variety of factors. Risky sexual behavior may be more common among Appalachian females,22 increasing their risk of HPV infection.1 The proportion of adults who are current smokers in West Virginia (26.8%), Kentucky (24.8%) and Ohio (22.5%) is higher than the national median (17.3%),23 and evidence suggests smoking rates are higher in the Appalachian portions of Kentucky and Ohio compared with the non-Appalachian portions.24,25 Smoking is a risk factor for HPV-related cancers26 and may decrease the clearance of existing HPV infections.15 Lastly, cervical cancer screening, which can detect treatable precancerous lesions, is less common among Appalachian women compared with non-Appalachian women.27,28 It is important that continued efforts are made to educate Appalachian women about HPV transmission and HPV-related cancer prevention.
HPV-related cancer disparities were not as apparent among Appalachian males, though some differences were found. Penile cancer incidence rates tended to be higher among Appalachian males, but penile cancer is relatively rare in the US26 The burden of all HPV-related cancers combined among males was higher in Kentucky (regardless of Appalachian status) compared with the SEER 9 rate, while rates for Appalachian Ohio and West Virginia were comparable to the national rate. Incidence rates for all HPV-related cancers combined among males ranged from 17.6 to 21.3 cases per 100,000 population for the Appalachian regions examined, which are similar to the incidence rate for melanoma of the skin among U.S. males.21 Most of the HPV-related cancer burden among males was attributable to oral cavity and pharyngeal cancers, many of which may not be positive for HPV infection.6,7
Our results have important public health implications regarding HPV vaccination. Mainly, findings suggest that Appalachia can benefit greatly from HPV vaccination, a potentially important tool for preventing cancers and reducing disparities in this region. Two HPV vaccines are currently available and recommended in the US: quadrivalent HPV vaccine against types 6, 11, 16 and 18 (available for males and females ages 9–26) and bivalent HPV vaccine against types 16 and 18 (available for females ages 9–26).29,30 Quadrivalent HPV vaccine is currently approved for the prevention of cervical, vulvar, vaginal and anal cancers,31 while the bivalent vaccine is approved for cervical cancer prevention.32 HPV vaccine first became available for females in 2006 and for males in 2009 in the U.S. National estimates indicate about half (53%) of adolescent females in the U.S. have received at least one dose of HPV vaccine (i.e., vaccine initiation), and only 35% have received the recommended three doses (i.e., vaccine completion).33 HPV vaccine uptake among adolescent males is much lower, with vaccine initiation at less than 10%.33,34 HPV vaccine is readily available in Appalachia and acceptable to most residents,35-39 though data on vaccine uptake in the region are sparse. Recent data show that of the 13 states containing Appalachian counties, 12 have lower statewide estimates of HPV vaccine initiation and completion among females compared with national estimates.33 Estimates are not available for the Appalachian areas within each state, so research is needed to examine HPV vaccination among both females and males throughout Appalachia.
Although our study provides important insight into the burden of HPV-related cancers and the potential impact of HPV vaccine in Appalachia, it is important to mention that data on the HPV infection status of cancers were not available in our study since cancer registries typically do not capture this information. Thus, it was not possible to examine HPV-positive and HPV-negative cancers separately. It is estimated that HPV infection is associated with 96% of cervical cancers, 93% of anal cancers, 64% of vaginal cancers, 51% of vulvar cancers, 36% of penile cancers and up to 63% of some oral cancers (e.g., oropharyngeal cancers).6,7 Furthermore, the proportion of cancers that are associated with HPV types 16 and 18 (types prevented by both HPV vaccines) ranges from 31% of penile cancers up to 87% of anal cancers.6 Future research that is able to establish the HPV infection status of cancers is needed to fully understand the burden of HPV-related cancers in Appalachia and the impact that widespread HPV vaccination can have in this region.
Our study had many strengths including the examination of all HPV-related cancers and a focus on a high-risk geographic region. We also compared incidence rates for Appalachian regions to both non-Appalachian regions of the same states and to national data. We had access to cancer registry data for only three Appalachian states, so the generalizability of our findings to other states with Appalachian counties is not known. It is possible that some cancer cases were not captured by the state cancer registries due to incomplete case ascertainment. Lastly, the prevalence of HPV infection among the general population of Appalachia is not currently known and should be examined.
We believe our study addresses important gaps in the existing literature and provides the most comprehensive examination to date on HPV-related cancers in the Appalachian region. Results suggest that HPV-related cancer disparities exist beyond those for cervical cancer among Appalachian females and are also present to a lesser extent among Appalachian males. These findings not only begin to clarify the total burden of HPV-related cancers in Appalachia but also begin to demonstrate the potential impact that widespread HPV vaccination could have on reducing disparities in this region.
Materials and Methods
We examined the burden of HPV-related cancers in Kentucky, Ohio and West Virginia. We selected these states because they are contiguously located in the Appalachian region, and the Appalachian portions of these states have all been shown to have high cervical cancer incidence rates.20 The Institutional Review Board at The Ohio State University determined this study was exempt from review.
For each state, we obtained cancer incidence data from the state cancer registries for newly diagnosed cases for years 1996–2008. The central cancer registries included the Kentucky Cancer Registry (KCR), Ohio Cancer Incidence Surveillance System (OCISS) and West Virginia Cancer Registry (WVCR). We also examined incidence data from the SEER 9 cancer registries (1996–2008) in order to provide national rates for comparison.40 The SEER 9 registries represent approximately 10% of the U.S. population and include those for Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound and Utah. The SEER Program and its data collection methodology, organizational structure, and mission have been described extensively elsewhere.40 Although the SEER Program now consists of 17 registries, we used rates from the SEER 9 registries because: (1) Kentucky is a SEER 17 cancer registry, and we did not want our comparison rates to include data from one of the Appalachian states examined in this study; and (2) the Appalachian region is mostly non-Hispanic white,10 and many of the registries added after the SEER 9 included higher percentages of minority populations.
Cancer cases were coded to the International Classification of Diseases for Oncology, third edition (ICDO-3),41 and were grouped by anatomic sites/types using the SEER Program site recode classifications.40 We examined incidence data for all HPV-related cancer types6-8: cervical, vaginal, vulvar, penile, anal and oral cavity/pharyngeal cancers. We compiled data for each HPV-related cancer type separately, and we also combined data for these cancers in order to assess the total burden of HPV-related cancers.
We used the Appalachian Regional Commission’s classification scheme to identify which counties are in the Appalachian portion of each state.42 Counties added to the Appalachian region in 2002 (Edmonson and Hart in Kentucky) were considered Appalachian for this study since they were in the Appalachian region for a majority of the data years examined. Counties added to Appalachia in late 2008 (Metcalfe, Nicholas and Robertson in Kentucky; Ashtabula, Mahoning and Trumbull in Ohio) were not considered Appalachian for this study since 2008 was the last year of data examined. Thus, Appalachian Kentucky consisted of 51 counties (out of 120 counties), Appalachian Ohio consisted of 29 counties (out of 88 counties) and all 55 West Virginian counties were classified as being in Appalachia. Appalachian Kentucky is located in the eastern part of the state, while Appalachian Ohio is located in the southern and eastern parts of the state.
Data Analysis
We used SEER*Stat version 7.0.4 to calculate cancer incidence rates, age-adjusted to the 2000 U.S. standard population using 19 age groups.43 For each gender (all races included), incidence rates were calculated for all HPV-related cancers combined and for each HPV-related cancer type separately. We calculated rates for each state (or national rates for SEER 9 data) and for the Appalachian and non-Appalachian portions of each state. All rates were expressed per 100,000 population, and we used the method of Tiwari et al. to calculate 95% CIs.44 Statistical significance was inferred by non-overlapping 95% CIs. Although this is a conservative approach in making comparisons,45 we believe it is appropriate for these analyses.
To identify areas within Appalachia that may have especially high incidence rates, we also calculated county-level age-adjusted incidence rates for all HPV-related cancers combined for Kentucky, Ohio and West Virginia. We mapped these county-level rates separately for females and males. We used ArcGIS (Environmental Systems Research Institute, Inc. ArcMap 10.0) to divide rates into quintiles and construct maps. We did not include data for counties with fewer than five cases on maps to prevent misinterpretation of rates based on a low number of cases.
Acknowledgments
Data used in this presentation were provided by the Kentucky and West Virginia Cancer Registries and the Ohio Cancer Incidence Surveillance System. This study was supported by the National Cancer Institute at the National Institutes of Health [P50CA105632, P30CA016058 and U01CA114622 (Appalachia Community Cancer Network)] and the Centers for Disease Control and Prevention (cooperative agreement number U58/CCU000768). The opinions expressed in this paper are those of the authors and do not necessarily represent those of the Centers for Disease Control and Prevention.
Glossary
Abbreviations:
- HPV
human papillomavirus
- STI
sexually transmitted infection
- SEER
Surveillance, Epidemiology and End Results
- CI
confidence interval
- KCR
Kentucky Cancer Registry
- OCISS
Ohio Cancer Incidence Surveillance System
- WVCR
West Virginia Cancer Registry
- ICDO-3
International Classification of Diseases for Oncology, third edition
- IR
incidence rate
Disclosure of Potential Conflicts of Interest
P.L.R. and E.D.P. have received research grants from Merck Sharp and Dohme Corp., but neither has received honoraria or consulting fees from this company. These funds were not used to support this research study.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/22389
References
- 1.Hariri S, Unger ER, Sternberg M, Dunne EF, Swan D, Patel S, et al. Prevalence of genital human papillomavirus among females in the United States, the National Health And Nutrition Examination Survey, 2003-2006. J Infect Dis. 2011;204:566–73. doi: 10.1093/infdis/jir341. [DOI] [PubMed] [Google Scholar]
- 2.Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: A systematic review of the literature. J Infect Dis. 2006;194:1044–57. doi: 10.1086/507432. [DOI] [PubMed] [Google Scholar]
- 3.Gillison ML, Broutian T, Pickard RK, Tong ZY, Xiao W, Kahle L, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307:693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reiter PL, Pendergraft WF, 3rd, Brewer NT. Meta-analysis of human papillomavirus infection concordance. Cancer Epidemiol Biomarkers Prev. 2010;19:2916–31. doi: 10.1158/1055-9965.EPI-10-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006; 24 Suppl 3:S3/11-25. [DOI] [PubMed] [Google Scholar]
- 6.Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008;113(Suppl):3036–46. doi: 10.1002/cncr.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–75. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Human papillomavirus-associated cancers - United States, 2004-2008. MMWR Morb Mortal Wkly Rep. 2012;61:258–61. [PubMed] [Google Scholar]
- 9.Chaturvedi AK. Beyond cervical cancer: burden of other HPV-related cancers among men and women. J Adolesc Health. 2010;46(Suppl):S20–6. doi: 10.1016/j.jadohealth.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Pollard K, Jacobsen LA. The Appalachian region: A data overview from the 2006-2010 American Community Survey. 2012. Available at: http://www.arc.gov/assets/research_reports/PRB-DataOverview-2012.pdf
- 11.Halverson JA. An analysis of disparities in health status and access to health care in the Appalachian region. 2004. Available at: http://www.arc.gov/research/researchreportdetails.asp?REPORT_ID=82
- 12.Wewers ME, Katz M, Fickle D, Paskett ED. Risky behaviors among Ohio Appalachian adults. Prev Chronic Dis. 2006;3:A127. [PMC free article] [PubMed] [Google Scholar]
- 13.Appalachia Community Cancer Network. The Cancer Burden in Appalachia, 2009.
- 14.Giuliano AR, Lee JH, Fulp W, Villa LL, Lazcano E, Papenfuss MR, et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet. 2011;377:932–40. doi: 10.1016/S0140-6736(10)62342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giuliano AR, Sedjo RL, Roe DJ, Harri R, Baldwi S, Papenfuss MR, et al. Clearance of oncogenic human papillomavirus (HPV) infection: effect of smoking (United States) Cancer Causes Control. 2002;13:839–46. doi: 10.1023/A:1020668232219. [DOI] [PubMed] [Google Scholar]
- 16.Wingo PA, Tucker TC, Jamison PM, Martin H, McLaughlin C, Bayakly R, et al. Cancer in Appalachia, 2001-2003. Cancer. 2008;112:181–92. doi: 10.1002/cncr.23132. [DOI] [PubMed] [Google Scholar]
- 17.Lengerich EJ, Tucker TC, Powell RK, Colsher P, Lehman E, Ward AJ, et al. Cancer incidence in Kentucky, Pennsylvania, and West Virginia: disparities in Appalachia. J Rural Health. 2005;21:39–47. doi: 10.1111/j.1748-0361.2005.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 18.Horner MJ, Altekruse SF, Zou Z, Wideroff L, Katki HA, Stinchcomb DG. U.S. geographic distribution of prevaccine era cervical cancer screening, incidence, stage, and mortality. Cancer Epidemiol Biomarkers Prev. 2011;20:591–9. doi: 10.1158/1055-9965.EPI-10-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Appalachia Community Cancer Network. Addressing the Cancer Burden in Appalachian Communities, 2010.
- 20.Hopenhayn C, King JB, Christian A, Huang B, Christian WJ. Variability of cervical cancer rates across 5 Appalachian states, 1998-2003. Cancer. 2008;113(Suppl):2974–80. doi: 10.1002/cncr.23749. [DOI] [PubMed] [Google Scholar]
- 21.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 22.Grunbaum JA, Kann L, Kinchen S, Ross J, Hawkins J, Lowry R, et al. Youth risk behavior surveillance--United States, 2003. MMWR Surveill Summ. 2004;53:1–96. [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC). Behavioral Risk Factor Surveillance System Survey Data. Atlanta, Georgia: USA Department of Health and Human Services, Centers for Disease Control and Prevention. 2010. [Google Scholar]
- 24.Ohio Behavioral Risk Factor Surveillance System. Columbus, OH: Ohio Department of Health. 2010. [Google Scholar]
- 25.Kentucky Behavioral Risk Factor Surveillance System. 2008 annual report. Available at: http://chfs.ky.gov/NR/rdonlyres/73E49DF2-81D3-4E05-BDC3-DA348F4E7F1E/0/Kentucky2008BRFSSAnnualReport.pdf
- 26.American Cancer Society. Cancer Facts & Figures 2012. Atlanta: American Cancer Society, 2012. [Google Scholar]
- 27.Hall HI, Uhler RJ, Coughlin SS, Miller DS. Breast and cervical cancer screening among Appalachian women. Cancer Epidemiol Biomarkers Prev. 2002;11:137–42. [PubMed] [Google Scholar]
- 28.Paskett ED, McLaughlin JM, Reiter PL, Lehman AM, Rhoda DA, Katz ML, et al. Psychosocial predictors of adherence to risk-appropriate cervical cancer screening guidelines: a cross sectional study of women in Ohio Appalachia participating in the Community Awareness Resources and Education (CARE) project. Prev Med. 2010;50:74–80. doi: 10.1016/j.ypmed.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention (CDC) FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59:626–9. [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention (CDC) Recommendations on the use of quadrivalent human papillomavirus vaccine in males--Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1705–8. [PubMed] [Google Scholar]
- 31.USA Food and Drug Administration. Gardasil. 2011. Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM094042
- 32.USA Food and Drug Administration. Cervarix. 2011. Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm186957.htm
- 33.Centers for Disease Control and Prevention (CDC) National and state vaccination coverage among adolescents aged 13-17 years - United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:671–7. [PubMed] [Google Scholar]
- 34.Reiter PL, McRee AL, Kadis JA, Brewer NT. HPV vaccine and adolescent males. Vaccine. 2011;29:5595–602. doi: 10.1016/j.vaccine.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katz ML, Reiter PL, Heaner S, Ruffin MT, Post DM, Paskett ED. Acceptance of the HPV vaccine among women, parents, community leaders, and healthcare providers in Ohio Appalachia. Vaccine. 2009;27:3945–52. doi: 10.1016/j.vaccine.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katz ML, Reiter PL, Kluhsman BC, Kennedy S, Dwyer S, Schoenberg N, et al. Human papillomavirus (HPV) vaccine availability, recommendations, cost, and policies among health departments in seven Appalachian states. Vaccine. 2009;27:3195–200. doi: 10.1016/j.vaccine.2009.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christian WJ, Christian A, Hopenhayn C. Acceptance of the HPV vaccine for adolescent girls: analysis of state-added questions from the BRFSS. J Adolesc Health. 2009;44:437–45. doi: 10.1016/j.jadohealth.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Hopenhayn C, Christian A, Christian WJ, Schoenberg NE. Human papillomavirus vaccine: knowledge and attitudes in two Appalachian Kentucky counties. Cancer Causes Control. 2007;18:627–34. doi: 10.1007/s10552-007-9007-7. [DOI] [PubMed] [Google Scholar]
- 39.Oldach BR, Katz ML. Ohio Appalachia public health department personnel: Human papillomavirus (HPV) vaccine availability, and acceptance and concerns among parents of male and female adolescents. J Community Health. 2012 doi: 10.1007/s10900-012-9613-5. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Cancer Institute. Surveillance, Epidemiology and End Results. 2012. Available at: http://seer.cancer.gov/
- 41.International Classification of Diseases for Oncology. Third edition. World Health Organization. 2000. [Google Scholar]
- 42.Appalachian Regional Commission. Counties in Appalachia. 2012. Available at: http://www.arc.gov/counties
- 43.National Cancer Institute. SEER*Stat sofware version 7.0.4. 2011. Available at: http://seer.cancer.gov/seerstat/
- 44.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15:547–69. doi: 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]
- 45.Schenker N, Gentleman JF. On judging the significance of differences by examining the overlap between confidence intervals. Am Stat. 2001;55:182–6. doi: 10.1198/000313001317097960. [DOI] [Google Scholar]