Abstract
Objective
To evaluate the effects of letrozole on ovarian size and steroidogenesis in vivo, as well as on proliferation and steroidogenesis of theca-interstitial cells alone and in coculture with granulosa cells using an in vitro model.
Design
In vivo and in vitro studies.
Setting
Research laboratory.
Animal(s)
Immature Sprague-Dawley female rats.
Intervention(s)
In vivo effects of letrozole were studied in intact rats receiving either letrozole (90-day continuous-release SC pellets, 400 µg/d) or placebo pellets (control group). In in vitro experiments, theca cells were cultured alone or in coculture with granulosa cells in the absence or presence of letrozole.
Main Outcome Measure(s)
Deoxyribonucleic acid synthesis was determined by thymidine incorporation assay; steroidogenesis by mass spectrometry; and steroidogenic enzyme messenger RNA (mRNA) expression by polymerase chain reaction.
Result(s)
In vivo, letrozole induced an increase in ovarian size compared with the control group and also induced a profound increase of androgen, LH levels, and Cyp17a1 mRNA expression. Conversely, a decrease in Star, Cyp11a1, and Hsd3b1 transcripts was observed in letrozole-exposed rats. In vitro, letrozole did not alter either theca cell proliferation or Cyp17a1 mRNA expression. Similarly, letrozole did not affect Cyp17a1 transcripts in granulosa-theca cocultures.
Conclusion(s)
These findings suggest that letrozole exerts potent, but indirect, effect on growth of rat ovary and dramatically increases androgen levels and Cyp17a1 mRNA expression, the key enzyme regulating the androgen biosynthesis pathway. The present findings reveal novel mechanisms of action of letrozole in the rat ovary.
Keywords: CYP17A1, letrozole, ovarian theca-interstitial cells, proliferation, steroidogenesis
Aromatase is a cytochrome P450 enzyme responsible for the rate-limiting step in estrogen biosynthesis, catalyzing the conversion of C19 steroids, androstenedione (A), and T into C18 steroids, estrone, and E2, respectively (1). Aromatase, encoded by CYP19, is widely distributed in several cells, including the ovarian granulosa cell, the placental syncytiotrophoblast, and the testicular Leydig cells, as well as various extraglandular sites such as brain, fat, and skin (2). Letrozole is a potentandhighly specific nonsteroidal aromatase inhibitor that competitively binds to the heme of the cytochrome P450 subunit, leading to a near-complete blockade of the aromatization in peripheral tissues without exerting effects on other steroidogenic pathways (3).
Since its approval as first-line therapy for hormone receptor–positive, metastatic breast cancer in postmenopausal women in 1997 (4), letrozole has emerged as a promising therapeutic agent to ameliorate certain gynecologic disorders, expanding its indications for use in premenopausal women. Recent studies have demonstrated the ovulation-inducing capacity of letrozole (5, 6), showing potential advantages over clomiphene citrate, including the development of a monofollicular response and the lack of adverse effects on either the endometrium or the cervicalmucus (7, 8). In addition, a letrozole-induced hypoestrogenic effect may play a role in the treatment of some estrogen-dependent diseases in premenopausal women, such as endometriosis and uterine fibroids (9, 10).
However, little is known about the potential effect of letrozole on the regulation of ovarian growth and steroidogenesis. Manneras et al. (11) demonstrated an increase in ovarian size and number of cystic follicles together with the cainterstitial cell hyperplasia in letrozole-exposed adult female rats, meeting the morphologic criteria for polycystic ovary syndrome. In contrast, Kafali et al. (12) did not detect any changes in ovarian weight after letrozole treatment. Furthermore, the role of letrozole in the regulation of the key genes involved in steroidogenesis needs to be evaluated. Immunohistochemical studies have demonstrated an increase in the expression of the androgen receptor, steroidogenic acute regulatory protein (Star), and 3-β-hydroxysteroid dehydrogenase (Hsd3b1) and a decrease in estrogen receptor β in letrozole-induced polycystic ovaries of rats (13). However, it is not known whether letrozole affects expression of the genes involved in the ovarian androgen biosynthesis pathway, such as Cyp17a1. Furthermore, it is not known whether altered ovarian growth and androgen production are due to local–intraovarian effects of letrozole, such as reduced aromatization of androgens on the ovarian level, or due to altered hypothalamo–pituitary function resulting in altered release of LH.
In view of these considerations, the present study was designed to evaluate the potential effect of letrozole on ovarian growth and steroidogenesis using both in vivo and in vitro rodent models.
MATERIALS AND METHODS
Animals
Three Wistar dams, each with 10 female pups, were obtained from Charles River Laboratories. Pups were raised with the lactating dam (not the biological mother of all the pups) until 21 days of age and then housed two per cage under controlled conditions (21–24°C, 55%–65% humidity, 12-hour light/12-hour dark cycle). Rats were fed standard commercial food and tap water ad libitum. At 21 days of age, rats were randomly divided into two experimental groups (control [n = 14] and letrozole [n = 14]) and implanted SC with 70-day continuous-release pellets (Innovative Research of America) containing 28 mg of letrozole (daily dose, 400 µg) (Novartis Pharma) or placebo. The dose of letrozole was chosen according to a previous study (12). The control group received identical pellets lacking the bioactive molecule. The animals were anesthetized using isoflurane before the SC insertion of the pellet. Rats were weighed every week from 21 days of age. Estrous cycle stage was determined microscopically with Giemsa staining of the predominant cell type in vaginal smears obtained daily from the ninth week of age to the end of the experiment, considering cycles with duration of 4 to 5 days to be regular (14, 15). The study was concluded after 10 weeks of exposure to letrozole, when the rats were 13 weeks of age. All treatments and procedures were carried out in accord with accepted standards of human animal care as outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and a protocol approved by the Institutional Animal Care and Use Committee at the University of California, Davis.
Tissue Sampling
After 10 weeks the rats were killed in diestrous phase by intra-cardiac perfusion of 0.9% saline under anesthesia using ketamine and xylazine (75/5 mg/kg, IP); trunk blood was collected, and plasma was stored at −20°C until assayed. The heart, liver, pancreas, adrenals, spleen, kidneys, uterus, and ovaries were excised. One half ovary from each animal was frozen and stored at −80°C for subsequent use.
Ovarian Morphology
One ovary from each animal was fixed in 10% formalin, embedded in paraffin wax, and then sectioned serially at 5-µm thickness. Sequential sections were mounted and stained by the hematoxylin and eosin procedure. The specimens were evaluated under ×400 magnification.
Total RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction
Total RNA was isolated from the ovarian specimen and from theca-interstitial cells using the MagMAX-96 Total RNA Isolation Kit (Applied Biosystems) and the King Fisher robot (Thermo Scientific). Reverse transcription of total RNA to complementary DNA (cDNA) was performed using the High Capacity cDNA Reverse Transcription Kit for reverse transcription–polymerase chain reaction (RT-PCR) (Applied Biosystems). The PCR assays were set up in 28-µL volumes, consisting of 5 µL cDNA, 4.5 µL forward and 4.5 µL reverse 900n Mprimers, and 14 µL of 2× SYBR Green PCR Master Mix (Applied Biosystems).
Quantitative real-time PCR reactions were performed in triplicate using the ABI 7300 Real-Time PCR System (Applied Biosystems). Separate cDNA dilutions were included in each PCR run to generate standard curves. Data were analyzed using SDS 1.4 software (Applied Biosystems). The relative amount of target messenger RNA (mRNA) was expressed as a ratio normalized to hypoxanthine phosphoribosyltransferase (Hprt). The primer sequences were as follows: rat Star forward (5′-GCC TGA GCA AAG CGG TGT C-3′) and reverse (5′-CTG GCG AAC TCT ATC TGG GTC TGT-3′); rat Cyp11a1 forward (5′-GCT GGA AGG TGT AGC TCA GG-3′) and reverse (5′-CAC TGG TGT GGA ACA TCT GG-3′); rat Hsd3b1 forward (5′-CCA GAA ACC AAG GAG GAA T-3′) and reverse (5′-CCA GAA ACC AAG GAG GAA T-3′); rat Cyp17a1 forward (5′-ACT GAG GGT ATC GTG GAT GC-3′) and reverse (5′-CCG TCA GGC TGG AGA TAG AC-3′); and rat Hprt forward (5′-TTG TTG GAT ATG CCC TTG ACT-3′) and reverse (5′-CCG CTG TCT TTT AGG CTT TG-3′).
Sample Preparation and Processing for Quantification of Steroids
Androstenedione and Twere obtained from Steraloids, whereas testosterone-d3 was obtained from Cerillient. Acetonitrile and methanol were high-performance liquid chromatography (HPLC) grade and obtained from Burdick and Jackson. Acetone, isopropanol, and ammoniumhydroxide were Optima grade and obtained from Fisher. Formic acid was American Chemical Society (ACS) grade and obtained from EMD.
Each sample was directly assayed; the following extraction procedure was applied to each specimen. Each sample aliquot (300 µL) was placed in a 2.0-mL autosampler vial and spiked with 150 µL of internal standard solution (i.e., androsteneione-d7 and testosterone-d3). Detection and quantitation of all analytes was accomplished using selective reaction monitoring.
Mass Spectrometry
We developed a novel turbulent flow chromatography (TFC) HPLC–tandem mass spectrometry (MS/MS) method that allowed the simultaneous detection of A and T. It consists of a high-pressure liquid chromatography instrument configuration multiplexing Thermo Aria TLX-2 TFC (two loading pumps and two eluting pumps; Shimadzu LC-10AD) system and an autosampler outfitted with a 300-position Peltier tray, coupled to a Thermo Scientific TSQ Vantage triple quadrupole mass spectrometer, equipped with a heated electro-spray ionization source. The instrument was controlled using Aria software (version 1.6.1). A Thermo Cyclone P extraction column (0.5 × 50 mm, 60-µm particle size) was used for online sample extraction of diluted serum, and HPLC separation was carried out by a 2.1 × 100-mm, 3-µm particle size ACE C18 column protected by a reverse-phase guard cartridge (Mac-Mod) contained within a Hot Pocket column heater (Thermo Scientific).
Precursor and product ions for each target analyte were chosen for selective reaction monitoring transitions, and the related parameters for the different analytes were isolated by HPLC separation according to the following mobile-phase gradient: solvent A, water containing 0.1% formic acid; B, methanol; C, acetonitrile/isopropyl alcohol/acetone 60/30/10; D, water/acetonitrile (98/2 vol/vol) with 0.1% ammonium hydroxide.
Detection and quantification used select reaction monitoring LC-MS/MS transitions of initial precursor ions for A and T mass to charge ratio (m/z) 287.2, 291.4, and 315.2, respectively. The responses for the major product ions for each of the analytes were plotted, and peaks at the proper retention time were integrated using LCQuan (Thermo Scientific). This software was used to generate calibration curves and quantitate the analytes in all samples. The concentrations of A and T in each sample (e.g., calibrators, quality control, and unknowns) were determined by an internal standard method using the peak area ratio and linear regression analysis. The responses for A and T were linear and gave correlation coefficients (R2) of 0.99 or better.
Rat LH Determination
Determination of serum level of rat LH was performed by the Center for Research in Reproduction, School of Medicine, University of Virginia using LH sandwich assay using monoclonal antibodies against bovine LH (no. 581B7) and against the human LH-β subunit (no. 5303; Medix Biochemica) (16, 17). Reference LH (rat) was provided by Dr. A. F. Parlow (National Hormone and Peptide Program).
In Vitro Experiments
In experiments evaluating possible direct actions of letrozole on ovarian theca-interstitial cells, ovaries were obtained from intact young rats not treated with letrozole, and theca-interstitial cells were purified as described previously (18, 19). Theca-interstitial cells were incubated in 24-well fibronectin-coated plates at a density of 400,000 cells per well for steroido-genesis and in 96-well fibronectin-coated plates at a density of 35,000 cells per well for proliferation assay. The cultures were carried out for 48 hours at 37°C in an atmosphere of 5% CO2 humidified air in serum-free McCoy's 5A culture medium supplemented with 1% antibiotic/antimycotic mix, 0.1% bovine serum albumin, and 2 mM l-glutamine. The cells were incubated in the absence (control) or in the presence of letrozole (0.1–1 µM). The concentrations of this compound were selected on the basis of previous studies (20, 21). All cultures were carried out in the presence of ovine LH (5 ng/mL). All the above chemicals were purchased from Sigma Chemical except for letrozole, which was purchased from Novartis Pharma, and LH, which was obtained from the National Hormone and Pituitary Program at the Harbor-UCLA Medical Center.
Cell Proliferation Assays
To evaluate possible effects of letrozole on proliferation of cells, the purified theca-interstitial cells were incubated for 48 hours in 96-well fibronectin-coated plates at a density of 35,000 cells/well in the absence (control) or in the presence of letrozole (0.1–1 µM) and supplemented with LH (5 ng/ mL). The extent of DNA synthesis was determined through a thymidine incorporation assay. Radiolabeled [3H] thymidine (1 µCi per well) was added to the cells 24 hours before the culture was stopped. Subsequently, the cells were harvested with a multiwell cell harvester (PHD Harvester, Model 290; Cambridge Technology), and radioactivity was measured in a liquid scintillation counter (Wallac 1409; PerkinElmer).
The total number of viable cells was estimated with the use of a CellTiter-Blue Cell Viability Assay (Promega). This assay involves the conversion of resazurin to resorufin by metabolically active cells, resulting in the generation of a fluorescent product at the excitation wavelength 544 nm and the emission wavelength 590 nm that is proportional to the number of viable cells. Fluorescence was determined with the use of a microplate reader (Fluostar Omega; BMG). To validate the assay, a standard curve with a known number of cells was generated, and a linear correlation was verified (r2 = 0.99, P<.001). Each experiment was repeated three times (eight replicates per experiment).
Granulosa-Theca Cell Coculture Experiment
Twenty-four-well fibronectin-coated plates with transwell inserts (6.5-mm diameter) with 8.0-µm pore size polycarbonate membrane (Transwell Permeable Supports; Corning Life Sciences) were used for granulosa-theca cell coculture experiments. The membranes of precooled inserts were coated using 40 µL of Matrigel (ECM gel, growth factor reduced, without phenol red, from Engelberth-Holm-Swarm mouse sarcoma; Sigma-Aldrich) diluted to a final protein concentration of 0.3 mg/mL with cold McCoy's media. Matrigel was used to mimic basement membrane layer and to facilitate communication of theca and granulosa compartments.
The granulosa cells were transferred to the transwell inserts (200,000 cells per transwell insert), and the theca cells (200,000 cells per well) were placed in the lower chambers of the wells. Subsequently, the cells were cultured for 48 hours without (control) or with letrozole (0.1–1 µM). Luteinizing hormone (5 ng/mL) and FSH (30 ng/ml) were added to all cell cultures. After 48 hours media were collected for steroids evaluation, and RNA isolation and cDNA synthesis were conducted separately for granulosa and theca cells. Determination of expression of Cyp17a1 was performed as described above by quantitative real-time PCR using Hprt as a reference gene. Each experiment was repeated three times.
Statistical Analysis
Statistical analysis was performed using JMP 9.0 software (SAS). Data are presented as the mean ± SEM. Means were compared by Student's t test. When appropriate, data were logarithmically transformed. A value of P<.05 was considered statistically significant.
RESULTS
In Vivo Experiment
Effect of letrozole on rat body weight
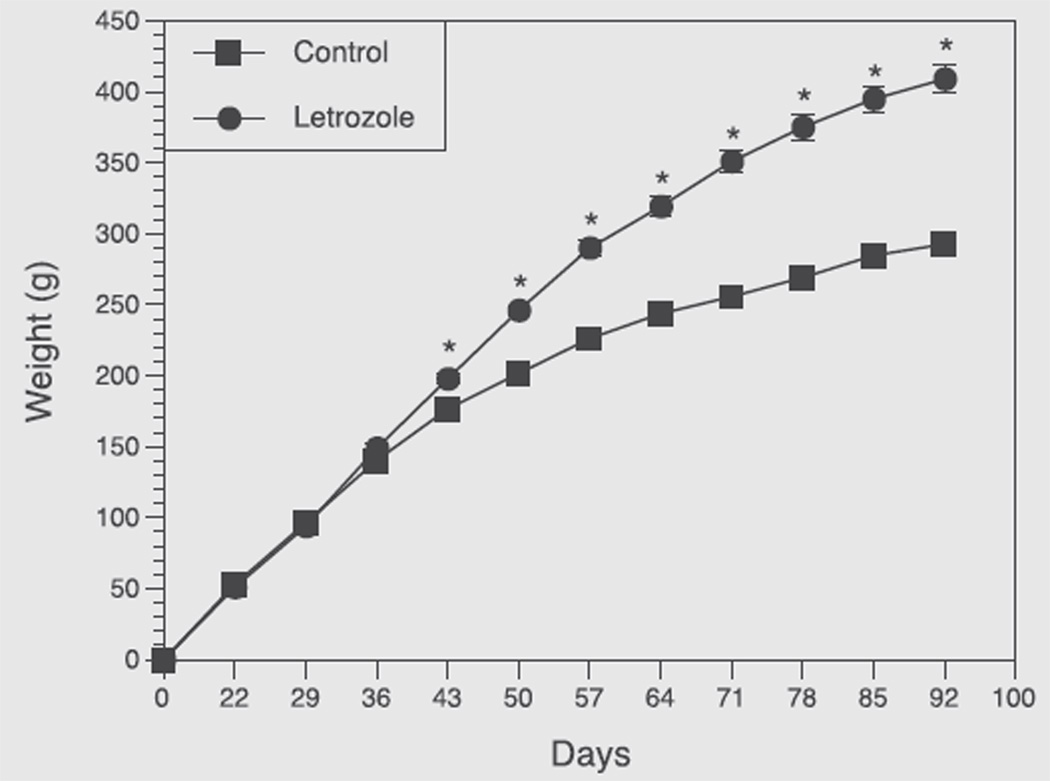
After 3 weeks of treatment, letrozole-treated rats had gained more weight than the control rats (P<.001), and this difference progressively increased until the end of the study (Fig. 1).
FIGURE 1.
Growth curves of rats exposed to placebo (control, n = 14) or letrozole (n = 14) from birth to 92nd day of age. Pellets were implanted at 21 days of age. Each point represents mean ± SEM. *P<.001 vs. control.
Effect of letrozole on estrous cyclicity and organ weight
Most of control rats had a normal 4-day estrous cycle. However, all letrozole-treated rats were entirely acyclic, as reflected by the presence of leukocytes in the vaginal smears, which is the predominant cell type of the diestrous phase, consistent with “pseudo-diestrous.” Ovarian weight was greater (179.1 ± 9.463 mg vs. 115 ± 5.19 mg; P<.001) in the letrozole group than in controls. Similarly, letrozole induced a significant increase in other organ weights, such as heart, liver, spleen, and kidney. Interestingly, adrenal gland and uterus were the only organs that had significantly lower weight in animals receiving letrozole treatment (Table 1).
TABLE 1.
Effect of letrozole on estrous cycle and organ weight.
| Parameter | Control (n = 14) |
Letrozole (n = 14) |
P value |
|---|---|---|---|
| Vaginal diestrous days (% of total) | 63.69 ± 4.51 | 100 | <.0001 |
| Heart (mg) | 1,021 ± 34.37 | 1,250 ± 30.46 | <.0001 |
| Adrenal (mg) | 88.3 ± 4.067 | 67.4 ± 1.91 | <.0001 |
| Liver (g) | 10.81 ± 0.433 | 15.76 ± 0.432 | <.0001 |
| Ovary (mg) | 115 ± 5.19 | 179.1 ± 9.463 | <.0001 |
| Uterus (mg) | 197.3 ± 8.06 | 110.1 ± 13.36 | <.0001 |
| Spleen (mg) | 846.6 ± 34.61 | 1,070 ± 29.6 | <.0001 |
| Kidney (g) | 2.412 ± 0.083 | 3.282 ± 0.091 | <.0001 |
Values represent mean ± SEM. P<.05 is significantly different.
Effect of letrozole on ovarian morphology
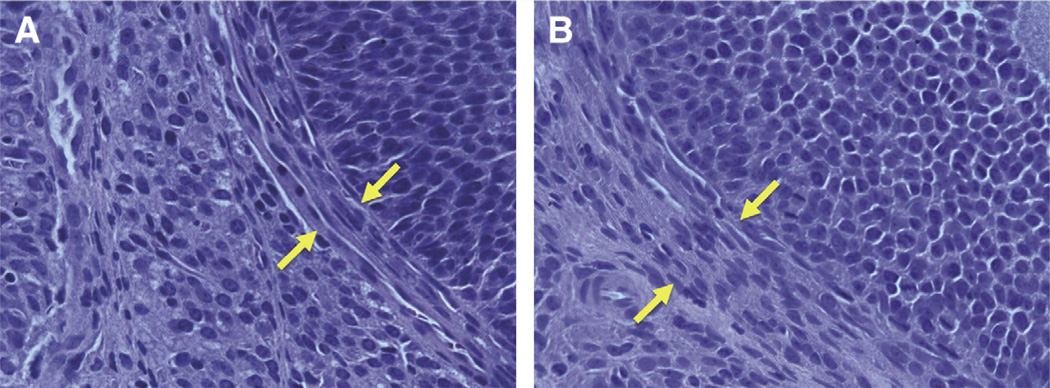
To determine the effects of letrozole on ovarian morphology, the ovaries were sectioned and stained with hematoxylin and eosin and finally evaluated under a conventional microscope. As demonstrated in Figure 2follicles of rats treated with letrozole had markedly greater thickness of theca-interstitial cells than in the control group.
FIGURE 2.
Ovarian morphology of (A) control rats and (B) letrozole-treated rats. Ovarian sections were stained with hematoxylin and eosin and analyzed under a conventional microscope (original magnification, ×400). Theca-interstitial cell layers are indicated by yellow arrows.
Effect of letrozole on hormone concentrations
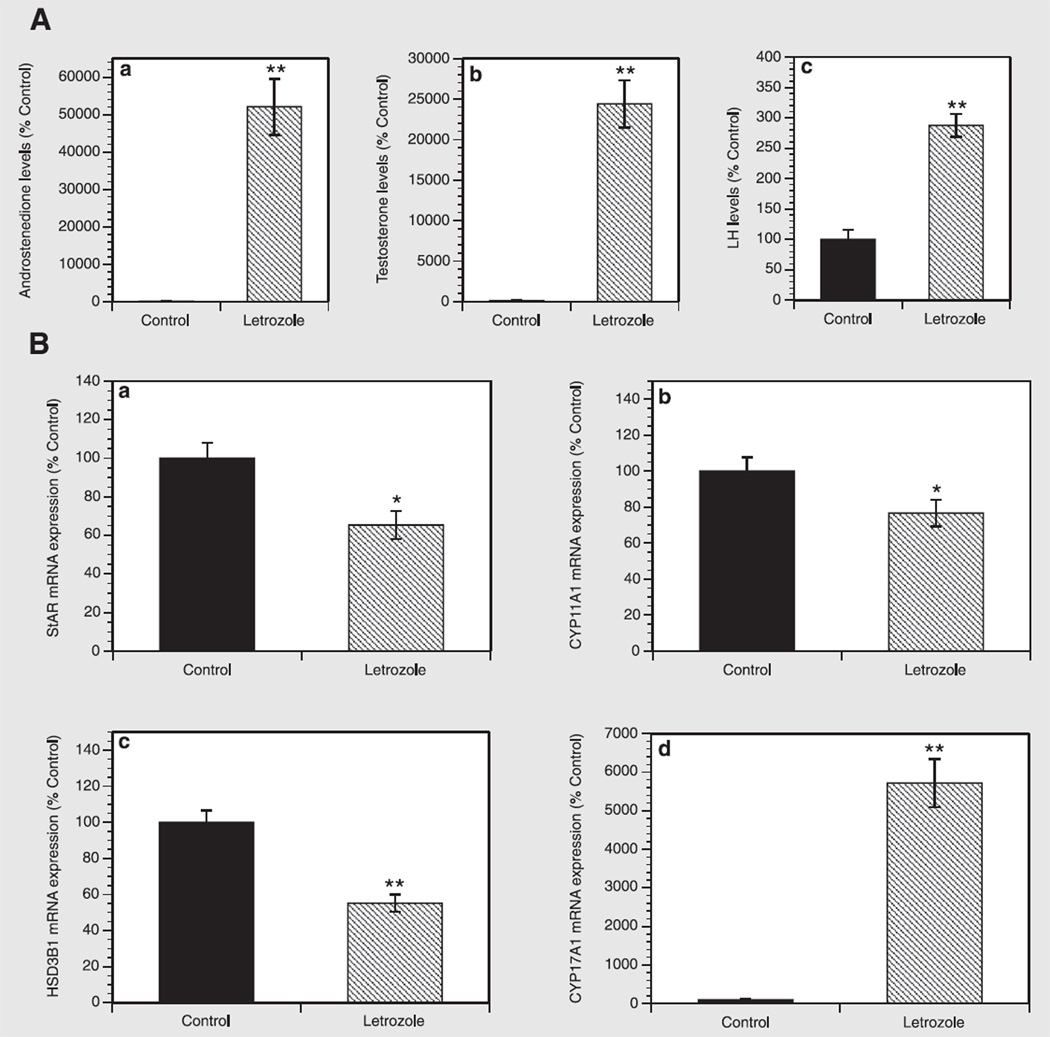
To evaluate the effects of letrozole on individual hormones, the levels of A and T were evaluated in plasma samples using liquid chromatography–mass spectrometry, and LH concentration was measured in serum by a sensitive two-site sandwich immunoassay. As shown in Figure 3A, letrozole dramatically increased androgen levels, inducing a 521- and 244-fold increase (both at P<.001) in both A and T levels, respectively. Similarly, letrozole induced a 2.8-fold increase (P<.001) in LH concentrations compared with the control group.
FIGURE 3.
(A) Effect of letrozole on (a) A, (b) T, and (c) LH levels in plasma and serum samples. Androgen levels were determined using liquid chromatography–mass spectrometry. Luteinizing hormone levels were measured in serum by a sensitive two-site sandwich immunoassay. Results are presented as a percentage of control. Each bar represents the mean ± SEM. **P<.001 vs. control. (B) Effect of letrozole on mRNA expression of (a) Star, (b) Cyp11a1, (c) Hsd3b1, and (d) Cyp17a1 in rat ovaries. Total cellular RNA was isolated, and mRNA expression was determined using quantitative real-time PCR reactions and normalized to Hprt mRNA levels. Results are presented as a percentage of control. Each bar represents the mean ± SEM. *P<.05 vs. control; **P<.001 vs. control.
Effect of letrozole on mRNA expression of steroidogenic enzymes
To investigate whether letrozole affects the expression of the key genes involved in the regulation of steroidogenesis, quantitative real-time PCR was performed in ovarian tissue specimens. As presented in Figure 3B, letrozole induced a significant decrease in Star, Cyp11a1, and Hsd3b1 mRNA expression, respectively, by 35% (P<.05), 23% (P<.05), and 45% (P<.001) compared with controls. Surprisingly, letrozole exerted a 57-fold increase (P<.001) in Cyp17a1 transcripts over the control group.
In Vitro Experiment
Effect of letrozole on theca cell proliferation
To determine whether letrozole affects DNA synthesis, rat theca cells were cultured for 48 hours in the absence or presence of increasing doses of letrozole (0.1–1 µM). The exposure of theca cells to letrozole did not alter cell proliferation at any of the tested concentrations (data not shown).
Effect of letrozole on theca cell steroidogenesis
To evaluate the effect of letrozole on Cyp17a1 mRNA expression, quantitative RT-PCR reactions were performed. Letrozole concentrations ranging from 0.1 to 1 µM did not affect Cyp17a1 mRNA levels (data not shown).
Effect of letrozole in theca-granulosa cocultures
To evaluate whether the effect of letrozole in the rat ovary is mediated by reduction of aromatization and/or loss of estrogen-induced intraovarian negative feedback loop on theca cell steroidogenesis via modulation of Cyp17a1 expression, mRNA levels of this gene and concentrations of P, A, androsterone, and E2 were evaluated in cocultures of theca-interstitial and granulosa cells. Letrozole had no effect on either Cyp17a1 mRNA expression or steroid production in theca-granulosa cocultures (data not shown).
DISCUSSION
The present study has demonstrated that administration of letrozole in intact female rats [1] increases ovarian size and thickness of the theca-interstitial cell layer; [2] reduces Star, Cyp11a1, and Hsd3b1 mRNA levels; [3] dramatically upregulates androgen production and Cyp17a1 mRNA expression; and [4] increases LH levels; but [5] letrozole in cultures of rat theca-interstitial cells has no direct effect on cell proliferation or Cyp17a1 mRNA expression; and [6] letrozole does not exert any significant effect on Cyp17a1 mRNA expression or steroid production in granulosa-theca cocultures.
Letrozole-treated rats showed a significant increase in organ weights, such as heart, kidney, liver, ovary, and spleen compared with the control group, whereas a lower weight was observed for adrenals and uterus. It is likely that letrozole may alter organ weight by inhibiting the aromatization of androgens and hence increasing the level of androgens and decreasing the level of estrogens. Consequently, it leads to anabolic/proliferative effects on androgen-sensitive tissues and the opposite effect on estrogen-dependent organs. Indeed, androgenic stimulation has been previously shown to account for cardiomyocyte and hepatocyte proliferation (22, 23), marked renal hypertrophy (23), and theca-interstitial cell hyperplasia (11). Conversely, the inhibitory effect of letrozole on weight of the uterus is in agreement with previous studies, whereby letrozole treatment decreased the fibroid and polyp size, owing to letrozole-induced decrease in estrogen production (24). However, the underlying mechanism by which letrozole decreases adrenal size is still unknown, although a potential androgen receptor–dependent inhibitory effect of T might be involved, as described by others (25).
Despite the growing evidence that letrozole plays a major role in the treatment of several estrogen-dependent gynecologic disorders, little is known about its effects on ovarian physiology. Letrozole-induced increases in rat ovarian size as well as theca-interstitial cell hyperplasia have been demonstrated during in vivo studies evaluating the role of letrozole in inducing both ovarian and metabolic features of polycystic ovary syndrome (11, 26). However, other authors did not observe any changes in ovarian weight when administering letrozole to rats, which was likely due to a short exposure time to lower letrozole doses together with oral administration (12). In the present study, ovarian weight and thickness of the theca-interstitial cell layer were significantly higher in letrozole-exposed rats than in controls. We hypothesize that this effect on ovarian growth may be mediated by either direct action on the ovary or via action at the level of the hypothalamo–pituitary axis.
A direct letrozole-induced proliferative effect has been reported in cultures of human endometrium (27). However, the potential mechanisms by which letrozole could exert its anabolic effect on theca-interstitial cells, and thus ovarian size, are not known. Bajetta and colleagues have shown that daily administration of letrozole at doses of 0.5 or 2.5 mg increases insulin-like growth factor 1 (IGF-1) levels in postmenopausal women with advanced breast cancer, and these findings have been confirmed by recent studies using letrozole and another aromatase inhibitor, anastrozole (28, 29). In light of these findings, one may speculate that letrozole-induced proliferative effect might be mediated through up-regulation of growth factors belonging to the IGF family. Indeed, mRNA encoding both IGF-1 and IGF-1 receptor has been detected in human (30, 31), bovine (32), and rat theca cells (33, 34), suggesting that letrozole might stimulate expression and synthesis of IGF-1 and thus may be inducing growth of theca-interstitial cells. Although the mediation of letrozole effects by IGF-1 cannot be excluded, the lack of a letrozole-induced stimulatory effect on theca-interstitial cell proliferation in vitro indicates that a direct effect of letrozole on growth of the theca-interstitial cell compartment is unlikely. However, one cannot exclude the possibility that such effects may occur upon longer exposure to letrozole. Indeed, Manneras et al. (11) demonstrated the presence of theca hyperplasia in the ovaries of letrozole-treated rats after 11–13 weeks of drug administration. In our in vivo experiment, a letrozole-induced increase in body weight was observed only after more than 40 days of letrozole treatment; it is therefore possible that letrozole may also induce delayed but direct effects on growth of tissues, including ovaries.
Alternatively, the letrozole-induced growth-inducing effect on the theca-interstitial cell compartment and ovarian morphology may be due to indirect mechanisms. First, a letrozole-induced increase of intraovarian androgens may affect follicle development and ovarian growth. Several studies have shown the presence of androgen receptor in theca cells of human (35), primates (36), and rats (37). Thus, it is conceivable that letrozole-induced overproduction of androgens might induce androgen receptor–mediated trophic effects on theca cell proliferation. In a model closer to humans, intact monkeys treated with androgens had increased numbers of preantral and small antral follicles as androgens stimulated theca and granulosa cell proliferation and inhibited apoptosis (36). Second, reduction of estrogen production due to letrozole-induced aromatase inhibition could enhance LH secretion by releasing a negative feedback of estrogens on the hypothalamus and pituitary (12), serving as a trophic stimulus to theca-interstitial cell growth that leads to overproduction of androgens. Indeed, letrozole treatment induced an increase in LH levels in our in vivo experiment, suggesting that a letrozole-induced increase in ovarian growth may be due to stimulation of the hypothalamic–pituitary axis. Thus, letrozole-induced androgen excess via inhibition of aromatase together with an increase in LH secretion due to letrozole-induced release of the negative feedback on the hypothalamic–pituitary axis might induce a trophic action on theca cell proliferation.
The present in vivo study showed that letrozole increased androgen production and reduced the mRNA expression of several genes involved in steroidogenesis except for Cyp17a1, which was greatly enhanced in letrozole-exposed rats compared with controls. We speculate that an androgen-dependent feedback inhibition on steroidogenic enzyme expression within the ovary may account for the above-mentioned decrease in mRNA expression. Indeed, an androgen-induced negative feedback on Star mRNA expression has been previously shown in rat Leydig cells (38), indicating that androgens may regulate steroidogenesis at the crucial, rate-limiting step of cholesterol transfer to the inner mitochondrial membrane. In addition, Star expression has been shown to be upregulated by estrogen (39), suggesting that the decreased estrogen output in letrozole-exposed rats may account for letrozole-induced decrease in Star mRNA expression. Thus, these findings suggest that steroid hormone-induced feedback on steroidogenic enzymes may lead to auto/paracrine mechanisms through which steroid hormones can regulate their own production in ovarian tissue.
In the ovary, androgens are synthesized in LH-stimulated theca cells and play an important role in preovulatory follicular development and maintenance of oocyte viability (40). The Cyp17a1 gene is the rate-limiting step in androgen biosynthesis, encoding the microsomal cytochrome P450c17α, which is a single enzyme with dual activity: 17α-hydroxylase and 17,20-lyase. In the present study we demonstrated a profound up-regulation of both androgen and LH levels together with an increase in Cyp17a1 mRNA expression in letrozole-exposed rats. Previously it has been shown that granulosa cell-derived estrogens modulate theca cell steroidogenesis via a short negative-feedback loop within the rodent follicle (41). Thus, we speculate that the letrozole-induced increase in androgen levels may be due not only to the direct effect of letrozole on the blockade of aromatization but also to a release of the negative-feedback loop of estrogens on theca cell steroidogenesis. Indeed, in vitro rodent follicle cultures have shown that estrogen receptor-α mediates an intraovarian negative feedback loop on theca cell steroidogenesis via modulation of Cyp17a1 mRNA expression (42). However, the lack of effect of letrozole on Cyp17a1 mRNA expression and on steroid production in granulosa-theca cocultures does not support the idea of the estrogen-induced intraovarian negative feedback loop on theca cell steroidogenesis. Therefore, the present findings suggest that it is unlikely that a letrozole-induced decrease of estrogen levels might stimulate theca cell androgen production by abrogating the negative feedback of estrogen on Cyp17a1 mRNA expression in a paracrine manner.
Although the ovary represents the major source of androgens, other endocrine tissues are equipped with steroidogenic enzymes to produce androgens and thus might contribute to the letrozole-induced increase in androgen production observed in our in vivo study. For example, Manneras et al. (11) demonstrated that letrozole-treated rats showed increased weights of the main adipose tissue depots. Adipose tissue has been shown to possess the enzymatic capability to produce androgens, including 17α-hydroxylase activity (43). Therefore, a contribution of adipose tissue in letrozole-induced increase in androgen production cannot be ruled out.
In conclusion, the present study provides new insights into the effects of letrozole on ovarian growth and steroidogenesis using in vivo and in vitro rat models. Letrozole stimulates ovarian growth and greatly up-regulates androgen production and Cyp17a1 mRNA expression, the key gene regulating the androgen biosynthesis pathway, and the effect is likely related to increased LH level. It is important to note that these effects were observed after long-term exposure to letrozole and that the above conclusions should not be extrapolated to short-term and intermittent exposure, such as that occurring in women undergoing ovulation induction.
Acknowledgments
This study was supported by a grant (R01-HD050656) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (to A.J.D.).
Footnotes
I.O. has nothing to disclose. A.S. has nothing to disclose. J.A.V. has nothing to disclose. A.B.C. has nothing to disclose. D.H.W. has nothing to disclose. E.S.-V. has nothing to disclose. S.D.S. has nothing to disclose. A.J.D. has nothing to disclose.
REFERENCES
- 1.Simpson ER. Role of aromatase in sex steroid action. J Mol Endocrinol. 2000;25:149–156. doi: 10.1677/jme.0.0250149. [DOI] [PubMed] [Google Scholar]
- 2.Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994;15:342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- 3.Bhatnagar AS. The discovery and mechanism of action of letrozole. Breast Cancer Res Treat. 2007;105(Suppl 1):7–17. doi: 10.1007/s10549-007-9696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dombernowsky P, Smith I, Falkson G, Leonard R, Panasci L, Bellmunt J, et al. Letrozole, a new oral aromatase inhibitor for advanced breast cancer: double-blind randomized trial showing a dose effect and improved efficacy and tolerability compared with megestrol acetate. J Clin Oncol. 1998;16:453–461. doi: 10.1200/JCO.1998.16.2.453. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Velasco JA, Moreno L, Pacheco A, Guillen A, Duque L, Requena A, et al. The aromatase inhibitor letrozole increases the concentration of intraovarian androgens and improves in vitro fertilization outcome in low responder patients: a pilot study. Fertil Steril. 2005;84:82–87. doi: 10.1016/j.fertnstert.2005.01.117. [DOI] [PubMed] [Google Scholar]
- 6.Papanikolaou EG, Polyzos NP, Humaidan P, Pados G, Bosch E, Tournaye H, et al. Aromatase inhibitors in stimulated IVF cycles. Reprod Biol Endocrinol. 2011;9:85. doi: 10.1186/1477-7827-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamath MS, George K. Letrozole or clomiphene citrate as first line for anovulatory infertility: a debate. Reprod Biol Endocrinol. 2011;9:86. doi: 10.1186/1477-7827-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He D, Jiang F. Meta-analysis of letrozole versus clomiphene citrate in polycystic ovary syndrome. Reprod Biomed Online. 2011;23:91–96. doi: 10.1016/j.rbmo.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Attar E, Bulun SE. Aromatase inhibitors: the next generation of therapeutics for endometriosis? Fertil Steril. 2006;85:1307–1318. doi: 10.1016/j.fertnstert.2005.09.064. [DOI] [PubMed] [Google Scholar]
- 10.Han M, Kim JY, Park JE, Kim JM, Lee KS. Effects of letrozole on proliferation and apoptosis in cultured leiomyoma cells treated with prostaglandin E(2) Eur J Obstet Gynecol Reprod Biol. 2008;138:83–88. doi: 10.1016/j.ejogrb.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 11.Manneras L, Cajander S, Holmang A, Seleskovic Z, Lystig T, Lonn M, et al. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology. 2007;148:3781–3791. doi: 10.1210/en.2007-0168. [DOI] [PubMed] [Google Scholar]
- 12.Kafali H, Iriadam M, Ozardali I, Demir N. Letrozole-induced polycystic ovaries in the rat: a new model for cystic ovarian disease. Arch Med Res. 2004;35:103–108. doi: 10.1016/j.arcmed.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Zurvarra FM, Salvetti NR, Mason JI, Velazquez MM, Alfaro NS, Ortega HH. Disruption in the expression and immunolocalisation of steroid receptors and steroidogenic enzymes in letrozole-induced polycystic ovaries in rat. Reprod Fertil Dev. 2009;21:827–839. doi: 10.1071/RD09026. [DOI] [PubMed] [Google Scholar]
- 14.Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- 15.Hubscher CH, Brooks DL, Johnson JR. A quantitative method for assessing stages of the rat estrous cycle. Biotech Histochem. 2005;80:79–87. doi: 10.1080/10520290500138422. [DOI] [PubMed] [Google Scholar]
- 16.Fallest PC, Trader GL, Darrow JM, Shupnik MA. Regulation of rat luteinizing hormone beta gene expression in transgenic mice by steroids and a gonadotropin- releasing hormone antagonist. Biol Reprod. 1995;53:103–109. doi: 10.1095/biolreprod53.1.103. [DOI] [PubMed] [Google Scholar]
- 17.Haavisto AM, Pettersson K, Bergendahl M, Perheentupa A, Roser JF, Huhtaniemi I. A supersensitive immunofluorometric assay for rat luteinizing hormone. Endocrinology. 1993;132:1687–1691. doi: 10.1210/endo.132.4.8462469. [DOI] [PubMed] [Google Scholar]
- 18.Magoffin DA, Erickson GF. Purification of ovarian theca-interstitial cells by density gradient centrifugation. Endocrinology. 1988;122:2345–2347. doi: 10.1210/endo-122-5-2345. [DOI] [PubMed] [Google Scholar]
- 19.Duleba AJ, Spaczynski RZ, Olive DL, Behrman HR. Effects of insulin and insulin-like growth factors on proliferation of rat ovarian theca-interstitial cells. Biol Reprod. 1997;56:891–897. doi: 10.1095/biolreprod56.4.891. [DOI] [PubMed] [Google Scholar]
- 20.Bonelli MA, Fumarola C, Alfieri RR, La Monica S, Cavazzoni A, Galetti M, et al. Synergistic activity of letrozole and sorafenib on breast cancer cells. Breast Cancer Res Treat. 2010;124:79–88. doi: 10.1007/s10549-009-0714-5. [DOI] [PubMed] [Google Scholar]
- 21.Lu WJ, Bies R, Kamden LK, Desta Z, Flockhart DA. Methadone: a substrate and mechanism-based inhibitor of CYP19 (aromatase) Drug Metab Dispos. 2010;38:1308–1313. doi: 10.1124/dmd.110.032474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsumoto T, Takagi H, Mori M. Androgen dependency of hepatocarcino-genesis in TGFalpha transgenic mice. Liver. 2000;20:228–233. doi: 10.1034/j.1600-0676.2000.020003228.x. [DOI] [PubMed] [Google Scholar]
- 23.Blantz RC, Peterson OW, Blantz ER, Wilson CB. Sexual differences in glomerular ultrafiltration: effect of androgen administration in ovariectomized rats. Endocrinology. 1988;122:767–773. doi: 10.1210/endo-122-3-767. [DOI] [PubMed] [Google Scholar]
- 24.Morales L, Timmerman D, Neven P, Konstantinovic ML, Carbonez A, Van Huffel S, et al. Third generation aromatase inhibitors may prevent endometrial growth and reverse tamoxifen-induced uterine changes in postmenopausal breast cancer patients. Ann Oncol. 2005;16:70–74. doi: 10.1093/annonc/mdi021. [DOI] [PubMed] [Google Scholar]
- 25.Stalvey JR. Inhibition of 3beta-hydroxysteroid dehydrogenase-isomerase in mouse adrenal cells: a direct effect of testosterone. Steroids. 2002;67:721–731. doi: 10.1016/s0039-128x(02)00023-5. [DOI] [PubMed] [Google Scholar]
- 26.Maharjan R, Nagar PS, Nampoothiri L. Effect of Aloe barbadensis Mill. formulation on Letrozole induced polycystic ovarian syndrome rat model. J Ayurveda Integr Med. 2010;1:273–279. doi: 10.4103/0975-9476.74090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khazaei M, Montaseri A, Casper RF. Letrozole stimulates the growth of human endometrial explants cultured in three-dimensional fibrin matrix. Fertil Steril. 2009;91:2172–2176. doi: 10.1016/j.fertnstert.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 28.Cigler T, Tu D, Yaffe MJ, Findlay B, Verma S, Johnston D, et al. A randomized, placebo-controlled trial (NCIC CTGMAP1) examining the effects of letrozole on mammographic breast density and other end organs in postmenopausal women. Breast Cancer Res Treat. 2010;120:427–435. doi: 10.1007/s10549-009-0662-0. [DOI] [PubMed] [Google Scholar]
- 29.Ferrari L, Martinetti A, Zilembo N, Pozzi P, Buzzoni R, La Torre I, et al. Short-term effects of anastrozole treatment on insulin-like growth factor system in postmenopausal advanced breast cancer patients. J Steroid Biochem Mol Biol. 2002;80:411–418. doi: 10.1016/s0960-0760(02)00040-7. [DOI] [PubMed] [Google Scholar]
- 30.Bergh C, Carlsson B, Olsson JH, Selleskog U, Hillensjo T. Regulation of androgen production in cultured human thecal cells by insulin-like growth factor I and insulin. Fertil Steril. 1993;59:323–331. doi: 10.1016/s0015-0282(16)55675-1. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez ER, Hurwitz A, Vera A, Pellicer A, Adashi EY, LeRoith D, et al. Expression of the genes encoding the insulin-like growth factors and their receptors in the human ovary. J Clin Endocrinol Metab. 1992;74:419–425. doi: 10.1210/jcem.74.2.1309838. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong DG, Gutierrez CG, Baxter G, Glazyrin AL, Mann GE, Woad KJ, et al. Expression of mRNA encoding IGF-I, IGF-II and type 1 IGF receptor in bovine ovarian follicles. J Endocrinol. 2000;165:101–113. doi: 10.1677/joe.0.1650101. [DOI] [PubMed] [Google Scholar]
- 33.Yan Z, Lee GY, Anderson E. Influence of dehydroepiandrosterone on the expression of insulin-like growth factor-1 during cystogenesis in polycystic rat ovaries and in cultured rat granulosa cells. Biol Reprod. 1997;57:1509–1516. doi: 10.1095/biolreprod57.6.1509. [DOI] [PubMed] [Google Scholar]
- 34.Kwintkiewicz J, Spaczynski RZ, Foyouzi N, Pehlivan T, Duleba AJ. Insulin and oxidative stress modulate proliferation of rat ovarian theca-interstitial cells through diverse signal transduction pathways. Biol Reprod. 2006;74:1034–1040. doi: 10.1095/biolreprod.105.049908. [DOI] [PubMed] [Google Scholar]
- 35.Horie K, Takakura K, Imai K, Liao S, Mori T. Immunohistochemical localization of androgen receptor in the human endometrium, decidua, placenta and pathological conditions of the endometrium. Hum Reprod. 1992;7:1461–1466. doi: 10.1093/oxfordjournals.humrep.a137595. [DOI] [PubMed] [Google Scholar]
- 36.Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;101:2622–2629. doi: 10.1172/JCI2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirai M, Hirata S, Osada T, Hagihara K, Kato J. Androgen receptor mRNA in the rat ovary and uterus. J Steroid Biochem Mol Biol. 1994;49:1–7. doi: 10.1016/0960-0760(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 38.Houk CP, Pearson EJ, Martinelle N, Donahoe PK, Teixeira J. Feedback inhibition of steroidogenic acute regulatory protein expression in vitro and in vivo by androgens. Endocrinology. 2004;145:1269–1275. doi: 10.1210/en.2003-1046. [DOI] [PubMed] [Google Scholar]
- 39.Townson DH, Wang XJ, Keyes PL, Kostyo JL, Stocco DM. Expression of the steroidogenic acute regulatory protein in the corpus luteum of the rabbit: dependence upon the luteotropic hormone, estradiol-17 beta. Biol Reprod. 1996;55:868–874. doi: 10.1095/biolreprod55.4.868. [DOI] [PubMed] [Google Scholar]
- 40.Hillier SG. Intrafollicular paracrine function of ovarian androgen. J Steroid Biochem. 1987;27:351–357. doi: 10.1016/0022-4731(87)90327-x. [DOI] [PubMed] [Google Scholar]
- 41.Erickson GF, Magoffin DA, Dyer CA, Hofeditz C. The ovarian androgen producing cells: a review of structure/function relationships. Endocr Rev. 1985;6:371–399. doi: 10.1210/edrv-6-3-371. [DOI] [PubMed] [Google Scholar]
- 42.Taniguchi F, Couse JF, Rodriguez KF, Emmen JM, Poirier D, Korach KS. Estrogen receptor-alpha mediates an intraovarian negative feedback loop on thecal cell steroidogenesis via modulation of Cyp17a1 (cytochrome P450, steroid 17alpha-hydroxylase/17,20 lyase) expression. FASEB J. 2007;21:586–595. doi: 10.1096/fj.06-6681com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puche C, Jose M, Cabero A, Meseguer A. Expression and enzymatic activity of the P450c17 gene in human adipose tissue. Eur J Endocrinol. 2002;146:223–229. doi: 10.1530/eje.0.1460223. [DOI] [PubMed] [Google Scholar]