Abstract
Many mammalian species use chemosignals to coordinate reproduction by altering the physiology and behavior of both sexes. Chemosignals prime reproductive physiology so that individuals become sexually mature and active at times when mating is most probable and suppress it when it is not. Once in reproductive condition, odors produced and deposited by both males and females are used to find and select individuals for mating. The production, dissemination and appropriate responses to these cues are modulated heavily by organizational and activational effects of gonadal sex steroids and thereby intrinsically link chemical communication to the broader reproductive context. Many compounds have been identified as “pheromones” but very few have met the expectations of that term: a unitary, species-typical substance that is both necessary and sufficient for an experience-independent behavioral or physiological response. In contrast, most responses to chemosignals are dependent or heavily modulated by experience, either in adulthood or during development. Mechanistically, chemosignals are perceived by both main and accessory (vomeronasal) olfactory systems with the importance of each system tied strongly to the nature of the stimulus rather than to the response. In the central nervous system, the vast majority of responses to chemosignals are mediated by cortical and medial amygdala connections with hypothalamic and other forebrain structures. Despite the importance of chemosignals in mammals, many details of chemical communication differ even among closely related species and defy clear categorization. Although generating much research and public interest, strong evidence for the existence of a robust chemical communication among humans is lacking.
Keywords: Pheromone, Odor, Scent, Behavior, Physiology, Mammal, Olfactory, Vomeronasal, Sex, Sexual
Chemical signals or chemosignals, often termed “pheromones”, are an important and often critical means of communication for most mammalian species. Most mammals make, distribute and respond to chemosignals in many contexts, including those surrounding reproduction, parent-offspring interactions and territorial/dominance relationships (Brown and Macdonald, 1985). This review focuses on the direct links between social odors and reproduction, that is, the effects of chemosignals on reproductive physiology and behavior in male and female mammals. The voluminous literature on chemosignals and reproduction precludes discussion of chemosignal-influenced behaviors or physiological processes that are further removed from copulation. So, while territorial acquisition and defense (Gosling et al., 2001; Hurst and Beynon, 2004) and maternal behavior (Kendrick et al., 1997) are critical for reproductive success and involve chemosignals, research in these areas will not be covered. Also, a comprehensive review that covers each mammalian order is not possible here and so this review will focus on species for which there is the most information. Unfortunately, this will generally limit discussion to farm and laboratory species; the interested reader is directed to several sources that provide a more comprehensive treatment of odor-communication in mammals ((Brown and Macdonald, 1985) and relevant sections in (Hurst et al., 2008; Mason et al., 2005; Wyatt, 2003)).
Chemosignals and Pheromones
Before asking what role chemosignals play in mammalian reproduction, one must explain why the term “pheromone” is not used throughout this review. “Pheromone” was used initially to describe the conspecific chemosignals that elicit behavioral and physiological responses of insects and was defined as “substances which are secreted to the outside by an individual and received by a second individual of the same species, in which they release a specific reaction, for example, a definite behavior or a developmental process” (Karlson and Luscher, 1959; Wyatt, 2009). This idea, roughly analogous to an external hormone, was a logical extension of the classic ethological idea of a species-specific “sign stimulus” that released a biologically important, unlearned (or “innate”) and stereotyped behaviors described as “fixed action patterns” (Tinbergen, 1951). The early observations that one or, at most, a handful of chemical compounds were necessary and sufficient for eliciting various behaviors in insects suggested to researchers that similar effects would be observed in mammals (Wyatt, 2003), and that these pheromones could be divided into “releaser pheromones” that affected behavior, and “primer pheromones” that affected developmental/physiological processes.
The problems with extending the term “pheromone” to mammalian biology were recognized early by researchers who noted that mammalian reproductive behavior and physiology is not rigid but is, instead, flexible, context-dependent and modifiable by experience (Doty, 2010; Doty, 1986; McClintock, 2002), characteristics quite different from the typical conception of “pheromone”. Despite some successes in isolating specific and behaviorally active compounds (primarily in rodents), these individual substances have rarely been as effective as the full odor and, in several cases, are not species-specific (Gelez and Fabre-Nys, 2004; Ingersoll and Launay, 1986; Rasmussen et al., 1996; Zhang et al., 2008b). Indeed, many identified compounds in social odors have multiple behavioral and physiological functions (Novotny, 2003), suggesting that the releaser vs. primer pheromone distinction is not meaningful at the stimulus level. Moreover, fine distinctions made by animals on the basis of social odors (such as individuality, familiarity, kin) are unlikely to be mediated by the presence or absence of one or a very small number of unique compounds and may, instead, require processing of an odor mosaic or blend (Johnston, 2008). Consequently, in this review, the use of the term “pheromone” will be mostly avoided and the more neutral terms “chemosignal“ or “scent” or “odor” will be used in its place.
Chemosensory Systems
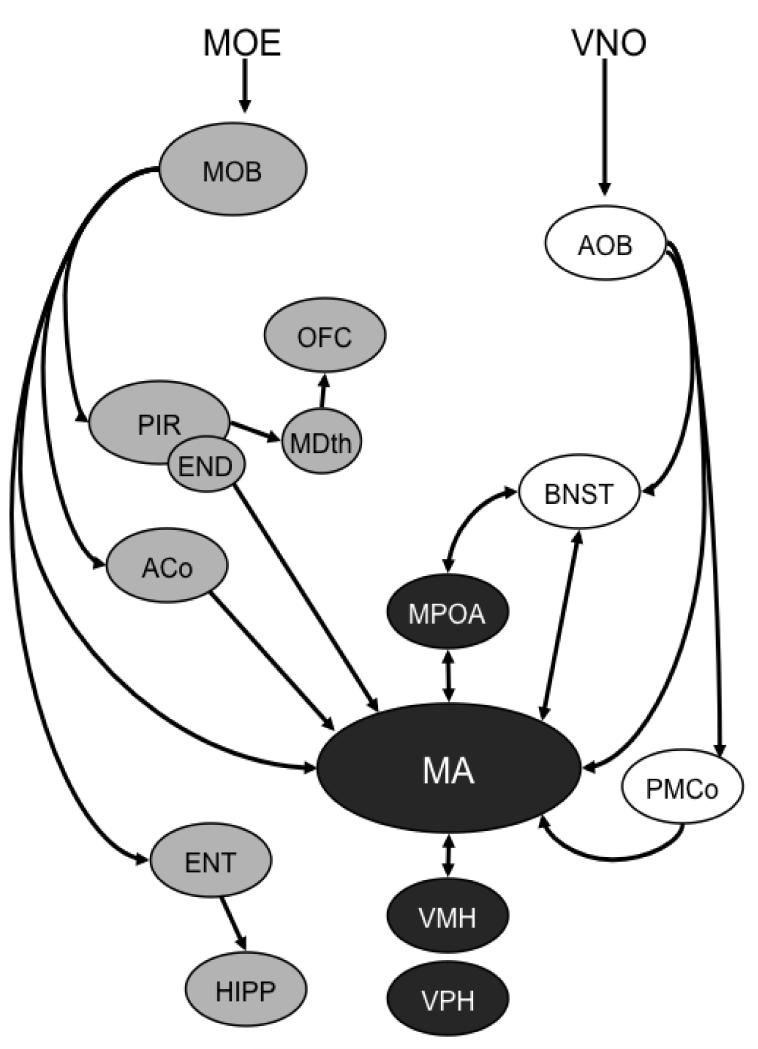
The basic anatomy and physiology of chemosignal-processing circuits is well defined and consists primarily of the main olfactory system (MOS) and the accessory olfactory system (AOS) and is reviewed in detail elsewhere (Chamero et al., 2012; Halpern and Martinez-Marcos, 2003; Zufall and Leinders-Zufall, 2007). These two systems are largely separate, having both separate sensory neuron populations in the nasal cavity (MOS: main olfactory epithelium (MOE); AOS: vomeronasal organ (VNO)), segregated representations in the nervous system (Figure 1) and responsiveness to different types of chemosignals: primarily volatile chemicals for the MOS and primarily non-volatiles molecules for the AOS. Nevertheless, complex interactions can occur between these two systems as evidenced by increased accessory olfactory bulb (AOB) activity to volatile chemosignals, an effect dependent on the integrity of the MOS and not on the VNO, (Martel and Baum, 2007) and is likely due to the main olfactory bulb (MOB)-recipient zone in MA projecting back to the AOB (Martel and Baum, 2009b).
Figure 1.
Abbreviated schematic of main olfactory (MOS; grey) and accessory olfactory or vomeronasal (AOS; white) systems along with integrative areas (black). For clarity, only unidirectional olfactory bulb connections are presented and several areas without known relevance to social odor processing are omitted. AOB, accessory olfactory bulb; ACo, anterior cortical amygdala; BNST, bed nucleus of stria terminalis; END, endopiriform nucleus; ENT, entorhinal cortex; HIPP, hippocampus; MA, medial amygdala; MDth, mediodorsal thalamus; MOB, main olfactory bulb; MOE, main olfactory epithelium; MPOA, medial preoptic area; OFC, orbitofrontal cortex; PIR, piriform cortex; PMCo, posteromedial cortical amygdala; VMH, ventromedial hypothalamus; VNO, vomeronasal organ; VPH, ventral premammilary hypothalamus.
The separation of the central AOS/MOS components is not complete as the anterior medial amygdala (MA) receives both direct AOB and MOB information (Fan and Luo, 2009; Kang et al., 2009; Mohedano-Moriano et al., 2012). Interconnections between the two systems also occur within the cortical and MA (Maras and Petrulis, 2010a; Martinez-Marcos, 2009) as well as within downstream structures (Newman, 1999). The MA and connected areas also contain many neurons that are responsive to gonadal steroids (Wood, 1997). Detailed connectional analysis of the MA, bed nucleus of the stria terminalis (posterior) (BNST), medial preoptic area (MPOA) and ventromedial hypothalamus (VMH) has revealed that within each structure, the region that receives the majority of chemosensory information is separate from the area that contains the greatest concentration of androgen (AR) and estrogen (ER) receptors, thus suggesting that steroid and chemosensory information are represented by separate and parallel systems (Newman, 1999; Wood, 1997). For example, the MA can be divided into the chemoreceptive zone that includes the anterior medial amygdala (MAa) and the hormone-sensitive posterodorsal medial amygdala (MApd). Indeed, neural activity in responses to odors in MAa and MApd appear to be different, with greater selectivity for social odors evident in MApd than in MAa (Meredith and Westberry, 2004; Samuelsen and Meredith, 2009) and that the chemosensory-induced immediate-early gene (IEG) response in MApd, BNST and MPOA requires an intact MAa, but not MApd (Maras and Petrulis, 2010b). Likewise, the BNST can be divided into a chemosensory region, the posterior intermediate BNST that receives heavy input from MAa and the hormonal region, the posteromedial BNST, which contains a large population of steroid-sensitive cells (de Vries and Sodersten, 2009; Wood and Swann, 2005). A similar separation continues within the MPOA and VMH (Wood, 1997). Each of these systems is heavily interconnected with itself but, within each anatomical element (MA, BNST, diencephalic areas), the steroid-sensitive and chemosensory sub-regions are also interconnected with one another (Coolen and Wood, 1998; Gomez and Newman, 1992) thereby providing a substrate whereby steroid hormones and chemosensory systems can interact (Been and Petrulis, 2011; Maras and Petrulis, 2010a). This distinction between hormonal and chemosensory aspects of MA, BNST and diencephalic areas is not absolute, as MAa does contain sex-steroid receptors (Wood, 1998; Wood et al., 1992) and MApd does receive indirect main olfactory input from ACo as well as limited direct and indirect (via posteromedial cortical amygdala: PMCo) AOB input (Kevetter and Winans, 1981a, b).
In general, perception of social chemosignals elicits increased IEG expression, an indirect measure of neural activity (Morgan and Curran, 1989), in neurons within most parts of the AOS and MOS of both sexes including the olfactory bulbs; the greatest sex-differences are often observed in the MPOA and ventral hypothalamic regions (Baum and Kelliher, 2008). The MOB/AOB of males and female show similar levels of IEG response to same- or opposite-sex chemosignals in mice (Halem et al., 1999), hamsters (Fiber and Swann, 1996), ferrets (Kelliher et al., 1998) and rats (Bressler and Baum, 1996) whereas IEG responses to chemosignals in MPOA are sexually-dimorphic yet quite variable across species and stimulus. For example, IEG response of MPOA to female chemosignals is only seen in male hamsters (Fiber and Swann, 1996) and female ferrets (Kelliher et al., 1998) but are similar in male and female mice (Brock et al., 2012; Pierman et al., 2008). Conversely, MPOA responses to male odors are strongly sexually-differentiated in mice: females show much higher IEG expression than males (Halem et al., 1999; Pierman et al., 2008).
The response of different brain areas to chemosignals is altered by gonadal steroid action, but whether these responses are organized developmentally or are due to activational effects of steroids varies between brain regions. In mice, the chemosensory-IEG response in males (primarily in diencephalic structures and BNST) appears due to organizational effects of androgens and not estrogens (Bodo and Rissman, 2007, 2008; Pierman et al., 2008; Wersinger and Rissman, 2000) whereas IEG responses in females may develop due to early estrogen action (Brock et al., 2012). This is in contrast to male rats that do require estrogenic activity during early development for sex-differentiated IEG response to male odors but, interestingly, not for responses to female odors (Bakker et al., 1996a). Sex differences in responses of MA to female odors and, in some species the BNST, may be primarily due to adult circulating levels of testosterone as these sex differences are eliminated by testosterone (T) treatment in gonadectomized hamsters (Fiber and Swann, 1996) and rats (Bressler and Baum, 1996; Paredes et al., 1998) and are unaffected by early aromatase knockdown (Pierman et al., 2008) or AR disruption (Bodo and Rissman, 2007) in mice. Indeed, the known sex difference in MA size and morphology is due primarily to circulating effects of steroids (Cooke, 2006).
AOS and MOS responses to chemosignals depend on a functioning VNO or main olfactory epithelium (MOE) but this sensitivity interacts with the socio-sexual experience of the animal. VNO removal in sexually-naïve male mice and hamsters eliminated or greatly reduced IEG expression in MA/BNST/VMH/MPOA in response to female chemosignals (Fernandez-Fewell and Meredith, 1994, 1998; Pankevich et al., 2006; Samuelsen and Meredith, 2009). In contrast, the IEG response to female chemosignals in MA/BNST/MPOA of sexually-experienced male hamsters is dependent on the MOE and less so on the VNO (Fewell and Meredith, 2002; Swann et al., 2001) or both systems in sexually-experienced male rats (Dhungel et al., 2011a). For example, although VNO removal eliminates AOB IEG responses and reduces MApd responses to female chemosignals, IEG expression in MAa/BNST/MPOA is not reduced post-surgery (Fewell and Meredith, 2002). This suggests that conditioning between volatile chemosignals and non-volatile cues (via VNO activity) and other sexual stimuli during copulation is sufficient to allow the MOS to drive chemosignal-based neural activity. Indeed, male rats exposed to non-social odors that have been conditioned during copulation show IEG responses different from those that they show toward chemosignals from estrous females and primarily activate MOS and reward-related structures, such as the nucleus accumbens (Kippin et al., 2003). Similarly, IEG responses in the MPOA or nucleus accumbens to sexual chemosignals only occur in sexually experienced male and female rats (Hosokawa and Chiba, 2005, 2007).
Chemosignals and Physiology: Females
Reproductive Facilitation
Puberty Acceleration (Vandenbergh Effect)
The presence of male chemosignals prior to reproductive maturity accelerates the onset of puberty (first day of estrus) in juvenile female house mice (Vandenbergh, 1969), meadow voles (Baddaloo and Clulow, 1981), Siberian hamsters (Reasner and Johnston, 1988), prairie voles (Carter et al., 1980), pine voles (Lepri and Vandenbergh, 1986) and sheep (Knight et al., 1975). It is, however, the tactile interactions between male and female in addition to male urinary cues that lead to the greatest acceleration of puberty (Bronson and Maruniak, 1975). In mice, this pubertal acceleration occurs in response to soiled bedding or urine produced by reproductively active, dominant male mice (Vandenbergh, 1969) and this chemosignal would appear to be androgen-dependent as T injections restore activity to a castrated male’s or even to a diestrous female’s urine. However, urine from singly housed pregnant, lactating or estrous adult female mice also accelerates puberty in juvenile female mice (Drickamer and Hoover, 1979) as will exposure to male rat urine (Colby and Vandenberg, 1974) indicating that scent from any reproductively-active individual may be effective in accelerating puberty.
The puberty-accelerating chemosignals in male mouse urine were initially thought to be the protein fraction (Vandenbergh et al., 1975) but it now apparent that these major urinary proteins (MUPs) bind to several low molecular weight compounds that accelerate puberty (Novotny et al., 1999b). These MUPs may normally protect these molecules from oxidation and act as a reservoir for the gradual release of volatile chemosignals and possibly aid in the transport and interaction of volatiles to chemosensory structures rather than acting directly as chemosignals (Hurst and Beynon, 2004; Novotny et al., 1999b; Utsumi et al., 1999) but see (Mucignat-Caretta et al., 1995). These volatile compounds have been identified as 2-sec-butyl dihydrothiazole (SBT), 3, 4-dehydro-exo-brevicomin (DHB), 6-hydroxy-6-methyl-3-heptanone (HMH) and two farnesenes (Novotny et al., 1999a; Novotny et al., 1999b). Other urinary compounds such as isobutylamine and isoamylamine may also accelerate puberty (Nishimura et al., 1989). The occurrence of these volatile chemosignals matches the conditions under which urine will accelerate puberty; they are only found in the urine of dominant adult male mice and are T-dependent (Novotny et al., 1999a). Similarly, MUPs are more abundant in male urine and show some dependence on circulating androgen levels (Armstrong et al., 2005). The puberty-acceleration substances found in the urine of estrous, pregnant or lactating females partially overlap with those found in male urine (Jemiolo et al., 1989).
Puberty-accelerating chemosignals increase the release of luteinizing hormone (LH) from the pituitary in juvenile female mice within thirty minutes of exposure (Bronson and Desjardins, 1974), which, through increases in gonadal estrogen secretion, induces puberty (Sisk and Foster, 2004). This effect is eliminated by removing the olfactory bulbs (OBX) (Zarrow et al., 1970) or damaging the AOS (Kaneko et al., 1980; Lomas and Keverne, 1982). Indeed, puberty-accelerating chemosignals alter activity in VNO receptor neurons with both high specificity and high sensitivity (Leinders-Zufall et al., 2000) with different chemosignals activating different VNO receptor populations (Boschat et al., 2002; Brennan et al., 1999). Although the MOE can detect HMH (Trinh and Storm, 2003), females with MOE damage still accelerate their puberty in response to male urine but, curiously, not to urine from pregnant or lactating females (Drickamer, 1986). Both the MOS and AOS, via MA, provide input to the hypothalamic gonadotropin-releasing hormone (GnRH) cells that control release of luteinizing hormone (LH). MA neurons pre-synaptic to GnRH cells show increased IEG activity in response to male urine and α-farnesene suggesting that these amygdala efferents mediate the effects of male chemosignals on puberty acceleration (Boehm et al., 2005; Yoon et al., 2005).
Based on the short life-span of wild mice, any acceleration of reproductive physiology in the presence of breeding opportunity, such as indicated by cues from opposite-sex individuals, would seem to be an adaptive response. This is not necessarily the case, however, as early pubertal onset induced by males or male urine reduces survivorship and leads to fewer litters with fewer offspring per litter in wild female mice (Drickamer, 1988). Although puberty-accelerating chemosignals are present in wild male mice (Massey and Vandenbergh, 1981) and do increase population density (Drickamer and Mikesic, 1990), it appears that female reproductive success increases by avoiding pubertal acceleration under natural conditions.
Ovulation Induction (Whitten Effect; (Whitten et al., 1968))
Similar to the puberty acceleration by male cues, the presence of chemosignals from adult males can also promote ovulation in anovulatory group-housed mice (Marsden and Bronson, 1964) or rats (Johns et al., 1978) through increases in LH and decreases in prolactin (PRL) secretion (Keverne and de la Riva, 1982). This pro-ovulatory “male effect” of chemosignals also occurs in induced-ovulator species such as prairie voles (Carter et al., 1980) and gray short-tailed opossum (Fadem, 1987) as well as in anestrous domestic sheep and goats (Gelez and Fabre-Nys, 2004). Although not directly comparable to species in which ovulation is triggered by male chemosignals, there is evidence in humans of a modest advancement of LH surges in women following prolonged exposure to male axillary scent (Preti et al., 2003). Normally only reproductively active males generate the chemosignals that induce ovulation and this ability depends on T in mice (Bronson and Whitten, 1968), Siberian hamsters (Dodge et al., 2002), prairie voles (Carter et al., 1980) and goats (Iwata et al., 2000). However, it should be noted, that in other species, such as pine voles, exposure to males themselves, rather than their chemosignals, is required for reproductive activation (Solomon et al., 1996) indicating substantial species-differences in the requirement of chemosignals for ovulation induction.
In mice, volatile estrous-accelerating chemosignals overlap with those that promote early puberty (Bronson and Whitten, 1968; Jemiolo et al., 1986). Similarly, volatile chemosignals also drive LH/ovulation in goats and sheep (Gelez and Fabre-Nys, 2004) and appears to be a blend of 1, 2-hexanedecanediol, 1, 2-octanedecanediol and fatty acids in sheep (Cohen-Tannoudji et al., 1994) and substances derived from 4-ethyl octanoic acid in goats (Murata et al., 2009). However, not all estrous-accelerating chemosignals are volatile; direct contact is required for reproductive activation in female prairie voles (Carter et al., 1980). Experience with the male may modulate the response in ewe’s response to rams: the number of females experiencing LH responses to male odors was greater in sexually experienced ewes than in virgin ewes. Moreover, the LH responses can become conditioned to neutral odorants following pairing with a male (Gelez et al, 2004a).
Species differences are present in the sensory systems that mediate estrous-acceleration or induction. In female rats (Johns et al., 1978), opossums (Jackson and Harder, 1996), and prairie voles (Lepri and Wysocki, 1987) AOS damage prevents or reduces induction of estrus. In contrast, VNO removal does not block onset of mating in meadow voles (Meek et al., 1994) or male-chemosignal induction of LH release in sheep (Cohen-Tannoudji et al., 1989). In ewes, damaging the MOE impairs LH secretion in response to male odors implicating the MOS in this response (Gelez and Fabre-Nys, 2004). Indeed, inactivation of anterior cortical amygdala, but not the MA, eliminates the endocrine response to male odors in ewes (Gelez et al., 2004). In female rats, the connections from MA and BNST to hypothalamus, via the ventral premammilary hypothalamus (VPH) is the critical pathway for stimulatory effects of male odors on female ovarian cyclicity. Stimulation of either MA or BNST advances LH surge in proestrous females and increases LH in estrogen-treated OVX female rats (Beltramino and Taleisnik, 1978, 1980) and lesions of VPH block these effects (Beltramino and Taleisnik, 1985). In ruminants, electrophysiological evidence has demonstrated that male chemosignals directly increase GnRH cell activity in the ventral forebrain of females (Okamura et al., 2010).
Even though chemosignals are sufficient to trigger LH release, they are not necessary in sheep as OBX has no effect on LH released by exposure to the male itself (Cohen-Tannoudji et al., 1986) showing that non-chemosensory cues can elicit LH release (Bakker and Baum, 2000) as they do in ferrets (Bakker et al., 2001). Indeed, non-chemosensory cues, such as genital stimulation, synergize with chemosensory ones for a maximal LH response in ruminants and are required to maintain elevated LH long enough for ovulation to occur (Delgadillo et al., 2009).
Ovarian Synchrony
In addition to the well-studied phenomenon of male-induced ovarian synchrony, several reports suggest that mammals show chemosignal-induced synchronization of their ovulatory cycles. An early study presented evidence that cohabitating female humans synchronize their menstrual cycles over time and postulated that this was mediated by chemosignals (McClintock, 1971). Similarly, housing female rats together or sharing airborne cues reportedly increased the number of animals showing estrous cycle synchrony (McClintock, 1984; McClintock and Adler, 1978). Computational modeling suggested that synchrony could be brought about by two chemosignals: a phase-advancing signal produced by follicular stage females and a phase-delay signal produced by ovulatory females (Schank and McClintock, 1992). Such an effect was reported for humans (Stern and McClintock, 1998) and has been inferred in earlier studies reporting synchrony in humans (Weller and Weller, 1993). However, other studies or re-analysis of previous studies, have found no evidence of cycle synchrony in humans (Schank, 2001b; Strassmann, 1999; Whitten, 1999; Wilson, 1987, 1992), non-human primates (chimpanzee: (Matsumoto-Oda et al., 2007); Mandrill: (Setchell et al., 2011); macaque (Furtbauer et al., 2011)), rats (Schank, 2001a) or hamsters (Schank, 2000). It might be that cycle synchrony occurs only in very restricted and contextually-specific circumstances (McClintock, 2002) but, on balance, the evidence suggests that ovarian synchrony does not exist as a biologically meaningful phenomenon (Schank, 2001b, 2006).
Reproductive Suppression
Puberty Delay
The onset of puberty can be delayed by several days in single-sex, group-housed female mice (Colby and Vandenberg, 1974) and pine voles (Lepri and Vandenbergh, 1986; Rissman and Johnston, 1985) exposed to urine from group-housed females. Unlike puberty-acceleration, this delay of sexual maturation is the same as that produced by group-housing itself (Drickamer, 1977) indicating that social odors are the critical social factor retarding puberty in mice. Of note, bladder urine from single-housed females delayed sexual maturation in juvenile female mice (McIntosh and Drickamer, 1977) unless treated with urethral tissue homogenates from singly-housed female mice, indicating that single-housed females normally produce peri-genital substances that suppress puberty-delay chemosignals. These chemosignals are produced by reproductively-active, juvenile and ovariectomized (OVX) group-housed females (Drickamer, 1977; Drickamer et al., 1978) suggesting little involvement of the female reproductive system in production of the chemosignal. In contrast, adrenalectomy eliminated production of the puberty-delay chemosignal (Drickamer and McIntosh, 1980); this adrenal-dependent chemosignal was identified as 2,5-dimethylpyrazine (DMP) (Jemiolo and Novotny, 1994) and appears to be as potent as the urine itself (Novotny et al., 1986).
Damage to the MOE did not impair puberty-delay in response to group-housed female urine (Drickamer, 1986). Although not directly tested, the VNO is likely required for response to the puberty-delay chemosignal, as DMP increased cellular activity in VNO receptor neurons (Sam et al., 2001). Although the central mechanisms underlying the puberty-delay phenomenon have not been identified, they may work through central and basolateral amygdala (BLA) connections with the anterior BNST and lateral hypothalamus: stimulation of BLA and anterior BNST inhibited LH surges and ovulation in female rats (Beltramino and Taleisnik, 1978, 1980).
The fact that puberty-inhibition is caused by group-housing in laboratory settings suggests that delay of reproduction might exist under natural conditions of high population density and increases in resource competition. Indeed, urine from females in dense wild populations delayed puberty in juvenile female laboratory mice whereas urine from stable, less dense populations did not (Massey and Vandenbergh, 1980). Similarly, application of urine from group-housed females to mice living in large outdoor enclosures resulted in reduced population densities (Drickamer and Mikesic, 1990). Taken together, chemosignal-induced delay of reproductive maturity is likely to occur under natural conditions.
Inhibition of Ovarian Cyclicity (Lee-Boot Effect: (Van Der Lee and Boot, 1955, 1956)
Exposure to group-housed female odors can also suppress estrous cyclicity in mice (Champlin, 1971) via adrenal gland activity (Ma et al., 1998) similar to their ability to delay puberty. Indeed, DMP, the same adrenal-sensitive chemosignal that delays puberty, also suppresses estrus in female mice (Ma et al., 1998). Estrous suppression by odors from grouped-housed female mice depends on a VNO-mediated increase in PRL secretion (Reynolds and Keverne, 1979).
Pregnancy Blockade (Bruce Effect: (Bruce, 1959))
Recently inseminated female mice exposed to novel males show a high rate of pregnancy failure and early resumption of estrous cycles, but not if the male is the one that mated with the female (Bruce, 1969). This selective pregnancy block is mediated by chemosensory cues as urine from novel males alone can disrupt pregnancy (Dominic, 1966). The ability of male urine to induce pregnancy disruption depends on T; urine from gonadectomized or juvenile male mice does not block pregnancy whereas T-replacement, even in females, restores the ability of urine to disrupt pregnancy (Dominic, 1965). Male urine is as effective as the presence of a male in disrupting pregnancy in mice (Dominic, 1966), pine voles (Schadler, 1981) and prairie voles (Smale, 1988), but not for field or meadow voles (Milligan, 1976). Even in mice, the genetic strain of subjects and those of the chemosignal donors as well as the specifics of experimental testing conditions determine the strength of chemosignal-specific effects (de Catanzaro et al., 1999; deCatanzaro et al., 1995).
The constituents of male mouse urine that induce pregnancy disruption are currently unknown but do appear to require contact (deCatanzaro et al., 1995). More recently the low molecular weight (LMW), but not the high molecular weight (HMW), fraction of unfamiliar male’s urine was found to block pregnancy in some females suggesting that the individually-specific information is carried by volatile components of urine (Peele et al., 2003). Nevertheless, the LMW fraction of unfamiliar male urine was not as effective as un-fractionated urine in blocking pregnancy unless recombined with the HMW component. This may indicate that volatile signals may bind to MUPs for transport to, and interaction with, chemosensory receptors. Similarly, volatiles might also bind to major histocompatibility complex (MHC) gene peptides that are known to contribute to odor individuality (Spehr et al., 2006; Yamazaki et al., 1983) and activate the VNO (Leinders-Zufall et al., 2004). However, MHC peptides are not normally present in mouse urine casting doubt on whether these components normally assist or mediate pregnancy block (He et al., 2008). The volatile components of an individual’s odor profile that allow a female to recognize familiar or unfamiliar males are unknown but do not involve the volatile male mouse chemosignals SBT, DHB or farnesenes (Brennan et al., 1999; Zacharias et al., 2000).
In addition to the hypotheses that MHC and/or MUP-related chemosignals mediate the selective male-induced pregnancy blockade, there is evidence that unconjugated estrogens secreted in male urine may function as pregnancy disruptors (as well as puberty accelerants) in mice (deCatanzaro, 2011). Small increases in estrogens, via systemic injections or by topical/intranasal contact, prevent embryo implantation in mice (deCatanzaro et al., 2001). Reducing estrogen levels in males, via aromatase inhibition and low phytoestrogen diet, reduces the ability of these novel males to disrupt pregnancy (Beaton and deCatanzaro, 2005) whereas increasing estrogen levels in castrated males reinstates their ability to prevent implantation (Thorpe and deCatanzaro, 2012). Nasal exposure to urine from males treated with radioactive estrogens results in substantial distribution of labeled estrogens in brain and uterus of treated females (Guzzo et al., 2010); similar effects are observed in females interacting with male or female conspecifics treated with radioactive estrogens (Guzzo et al., 2013). Novel males exposed to females modestly increase estrogen levels (close to levels needed to disrupt pregnancy) in their urine, an effect not seen in sires (familiar mates) housed near their female mates (deCatanzaro et al., 2006). As in most of these experiments, pregnant females are exposed to more than just chemosignals from the hormone-injected males and so hormone-induced behavioral interactions or other, non-chemosensory cues may determine if pregnancy is terminated or preserved in these cases. In fact, sires housed next to females are reported to be less vigorous in interacting with their mates compared to novel males that actively interact and scent mark near pregnant females (deCatanzaro, 2011). Moreover, behavioral interactions between the sire and inseminated female may mitigate her normal interest in novel male’s odor and thereby offer a behavioral mechanism for the ability of sires to protect their mates pregnancy from novel-male pregnancy disruption (deCatanzaro and Murji, 2004). Unfortunately, this line of work does not readily explain why just urine from sires and novel males can have such different effects on pregnancy maintenance in other experiments (Brennan, 2009).
Pregnancy disruption via urine from unfamiliar males results from of a VNO-mediated neuroendocrine response (Brennan, 2009; Brennan and Keverne, 1997). Removal of the VNO, but not MOE, prevents the urine of unfamiliar males from disrupting early pregnancy (Lloyd-Thomas and Keverne, 1982). However, new evidence suggests that inhibition of MOS processing, via dopamine action in the MOB, may prevent chemosignal-induced disruption of late pregnancy (Serguera et al., 2008). Normally, perception of male urinary chemosignals by the VNO, and subsequently MA, increases LH secretion as well as decreasing PRL secretion by the anterior pituitary (Li et al., 1990; Marchlewska-Koj and Jemiolo, 1978). Decreasing PRL in unmated females, along with increased LH secretion, accelerates puberty and induces estrus. In a mated females, however, this chemosignal-induced drop in PRL eliminates support for the corpora lutea, depresseing progesterone and thereby preventing embryo implantation (Bellringer et al., 1980). Indeed, urine from unfamiliar males only disrupts pregnancy if given during the twice daily, post-mating, elevations of PRL (Rosser et al., 1989). To prevent the mating male’s urine from disrupting his own mate’s pregnancy, the female forms a memory of the stud male that effectively disrupts AOB output to MA in response to his urinary cues (Brennan et al., 1990). The AOB itself is the site of this suppressive memory of the stud odor and may require release of norepinephrine, induced by mating, that ultimately restructures reciprocal interactions between projection and interneurons in AOB (Brennan, 2009; Brennan and Keverne, 1997). Increased dopaminergic activity in the MOB also impairs olfactory sensitivity during late pregnancy and may serve to limit behavioral investigation and processing of strange males’ odors (Serguera et al., 2008). Indeed, recently-mated female mice often avoid contact with male odors and thereby prevent pregnancy disruption (Becker and Hurst, 2009).
Prevention of pregnancy blockade by the sire would seem to benefit both the stud male and the dam. Nevertheless, attempts at demonstrating male-induced pregnancy termination in the field have produced equivocal results. An initial study using outside enclosures reported that prairie voles do show evidence of pregnancy block in more natural conditions (Heske and Nelson, 1984). However, in larger enclosures that resemble the actual size of vole home ranges, rapid turnover of males by trapping and introducing new males does not lead to population-level changes in pregnancy in grey-tailed voles (de la Maza et al., 1999) and only minor effects in prairie voles (Mahady and Wolff, 2002). More recently, pregnancy blockade in response to novel males has been documented in a wild population of gelada monkeys (Roberts et al., 2012a) indicating that this phenomenon may be more widespread among mammals than previously thought. Unfortunately, the relative contribution of chemosignals to pregnancy termination in the wild is unknown.
Chemosignals and Physiology: Males
Reproductive Development
Unlike the body of knowledge concerning chemosignal effects on female reproductive physiology, there is substantially less information about similar effects in males (Koyama, 2004). What is known suggests that development and function of male reproductive physiology is also sensitive to chemosignal exposure. For example, the development of puberty in male mice is delayed by exposure to male urine, grouped-female urine and DMP, a puberty-delay chemosignal derived from urine of grouped females (Jemiolo and Novotny, 1994). In California voles, chemosignals from mothers suppress their son’s pubertal onset more strongly than odors from their father (Rissman and Johnston, 1985). However, exposure to unrelated, non-grouped females does not advance puberty in male mice (Maruniak et al., 1978).
Sperm Allocation
Chemosignals can alter male gonadal physiology in adulthood. For example, dominant, but not subordinate, male mice show increased sperm density when housed with female bedding suggesting that exposure to female chemosignals increases spermatogenesis (Koyama and Kamimura, 2000). Sperm allocation is also adaptively regulated in scent contexts that signal increased sperm competition: more sperm is ejaculated by meadow voles during copulation in the presence of another male’s scent (Delbarco-Trillo and Ferkin, 2004). This effect is due to chemosignal-induced increase in movement of sperm to the vas deferens prior to copulation (Delbarco-Trillo and Ferkin, 2007) and is context-specific as it does not occur in response to low-quality (underfed) males (Vaughn et al., 2008).
Hormone Release
Post-pubertal ((Schulz et al., 2009)) male mice (Macrides et al., 1975), Syrian hamsters (Macrides et al., 1974; Richardson et al., 2004), Siberian hamsters (Anand et al., 2004), rats (Bonilla-Jaime et al., 2006), common marmosets (Ziegler et al., 2005) and macaques (Cerda-Molina et al., 2006) release LH within 15-30 minutes, followed by a dramatic peak in circulating androgens, in response to conspecific female odors,. A similar effect occurs in humans: exposure to chemosignals from peri-ovulatory women modestly reduces a decrease in testosterone in male humans normally observed during laboratory testing (Miller and Maner, 2010). This surge is subject to contextual cues in experimental animals. For example, this response rapidly habituates to repeated presentations of the same female’s chemosignals and is increased by presentation of cues from novel females (Coquelin and Bronson, 1979), indicating that female novelty strongly modulates this response. In addition, the LH/androgen increase in marmosets is modulated by parental status, as the effects are not observed in paternal males (Ziegler et al., 2005).
This hormone surge to female chemosignals can be elicited by both sexually naïve and sexually-experienced male Syrian hamsters (Pfeiffer and Johnston, 1994) and mice (Maruniak and Bronson, 1976) but not by sexually naïve rats (Bonilla-Jaime et al., 2006) indicating significant species differences in their unconditioned physiological responses. Nevertheless, LH/androgen increases to the presentation of the entire female survive combined AOS and MOS lesions in sexually-experienced male Syrian hamsters (Pfeiffer and Johnston, 1992, 1994) indicating that, even in species with seemingly unconditioned initial responses to odors, hormonal surges can become conditioned to non-chemosignal copulatory cues. Indeed, pairing of neutral odor stimuli with mating causes male rats to produce LH/androgen surges in response to the previously neutral odor (Graham and Desjardins, 1980).
Surprisingly, the reproductive state of the female has minimal impact on her ability to produce chemosignals that induce LH/androgen surges (Johnston and Bronson, 1982). The identity of the female chemosignals eliciting LH/androgen surges are not known but, in mice, appear to be LMW molecules that may be bound to MUPs (Singer et al., 1988).
VNO removal eliminates or reduces LH/androgen surges in both sexually experienced and naïve males in response to female chemosignals, but not to the female herself; damage to MOE is without effect (Coquelin et al., 1984; Pfeiffer and Johnston, 1994; Wysocki et al., 1983). Combined lesions of VNO and MOE do eliminate surges in response to females themselves in sexually-naïve Syrian hamsters, but not sexually-experienced males, indicating that chemosensory function is required for LH/androgen response prior to the animal’s initial copulatory experience (Pfeiffer and Johnston, 1994). The neural mechanisms underlying this response to female chemosignals has not been delineated but it does not appear to require a functional estrogen receptor alpha (Wersinger and Rissman, 2000).
The function of the reflexive LH/androgen surge has remained elusive but evidence suggests that it may facilitate male copulatory behavior by reducing anxiety or by altering penile reflexes (Nyby, 2008).
Chemosignals and Behavior: Females
Attraction and Investigation
Post-pubertal female Syrian hamsters (Johnston, 1979), rats (Carr et al., 1965), mice (Hurst, 1990), meadow voles (Ferkin and Zucker, 1991) and ferrets (Kelliher and Baum, 2002) are preferentially attracted to the chemosignals of reproductive male conspecifics, independent of sexual experience. This should not imply that learning plays no role in female sexual attraction. Indeed, initial female attraction to male odors in mice, but not Syrian hamsters (Maras and Petrulis, 2008a), may develop through a conditioning process that requires direct, post-natal, contact with male odors (Martinez-Garcia et al., 2009). That is, female mice without exposure to adult male chemosignals since weaning do not prefer male volatiles until they have contacted non-volatiles in male urine; although this effect interacts with degree of sexual receptivity: chemically-naïve females in full estrus do show a preference for male volatile cues (DiBenedictis et al., 2012; Martel and Baum, 2009a). This conditioned response to volatile chemosignals can be very specific: females contacting one male’s urine will develop a preference for volatiles from that individual male only (Ramm et al., 2008). Importantly, this unconditioned, contact-based chemosignal has been identified: a male-specific and invariant MUP protein dubbed “Darcin” (Roberts et al., 2010). Darcin is both necessary and sufficient for driving the initial, experience-independent, preference for approaching a male-scented area and thereby conditions future approach to urinary volatiles that co-occur with it. Indeed, Darcin provides the chemical substrate for the ability of female mice to remember and prefer areas previously associated with males, but that no longer contain scent marks (Roberts et al., 2012b). Learning about mate scents also occurs in adulthood as female rats do form a conditioned preference for chemosignals associated with prior copulation (Coria-Avila et al., 2005).
Given that female copulatory behavior is usually tightly linked to changes in ovarian steroids (Pfaff et al., 2008), it is reasonable to suspect that female interest and attraction to male cues is also similarly linked to hormone condition. However, significant species differences exist in the hormone sensitivity of female attraction to male chemosignals. Female Syrian hamsters are attracted to male chemosignals across their entire estrous cycle (not just during estrus) and during lactation but not during pregnancy (Eidson et al., 2007; Johnston, 1979). Female meadow voles are attracted to male scents across pregnancy and also during lactation (Ferkin and Johnston, 1995). Indeed, female mice and Syrian hamsters continue to display preferences for male odors following gonadectomy (Eidson et al., 2007; Moncho-Bogani et al., 2004). In contrast, preference for male chemosignals in rats (Xiao et al., 2004), ferrets (Woodley and Baum, 2003) and meadow voles (Ferkin et al., 1991) decreased following ovariectomy are reinstated by estradiol (E) or T injections. Development of female preference for male chemosignals may require early action of estrogens: eliminating aromatase action pre/post-natally in female mice impairs female preference for male scents and this deficit can be rescued by pre-pubertal E injections (Brock et al., 2011).
Female preference for investigating male conspecifics is strongly modulated by chemosensory indicators of male quality such as high androgen levels (Kempenaers et al., 2008) that can be mediated by both production of androgen-sensitive chemosignals and by androgen-sensitive scent marking behavior (Thiessen and Rice, 1976). For example, gonadectomy decreased attractiveness of male scents in meadow voles and was reversed by gonadal steroid treatment (Ferkin and Johnston, 1993). Attractiveness of male scents is also modulated by other cues of reproductive fitness such as social status, diet, and pathogen load. For example, female house mice prefer scents of dominant males to those of subordinates (Mossman and Drickamer, 1996), and meadow voles prefer chemosignals of males fed on high protein diets, a normally scarce and valuable resource (Ferkin et al., 1997). Female mice also decrease their preference for chemosignals from males that are sub-clinically infected with parasites (Kavaliers and Colwell, 1995).
Breeding in several species may also biased by choosing mates that differ from oneself at the MHC locus, a cluster of genes critical for self/non-self immune recognition, as a means of avoiding inbreeding and/or increasing genetic diversity (Penn et al., 2002). Although female mice discriminate between the volatile chemosignals of males differing only at the MHC locus (Singer et al., 1997), they do not prefer chemosignals from dissimilar MHC males (Ehman and Scott, 2001). Moreover, MUP variation, rather than that of MHC, in wild-type male mice appears to regulate female preference in this species (Cheetham et al., 2007; Thom et al., 2008).
In addition to factors altering the intrinsic attractiveness of male chemosignals, female interest is also modulated by the spatial pattern of scent-deposition indicative of a territory owner (Gosling et al., 2001). Female mice and Syrian hamsters prefer scents from males they have previously encountered and those that have counter-marked a competitor’s scent (Hurst and Beynon, 2004; Johnston, 2008).
Treating urine of castrated males with the mouse chemosignals SBT and DHB (that also accelerate puberty and ovarian function), hexadecanol and hexadecyl acetate and (methylthio)methanethiol increase the attractiveness of this stimulus to females (Jemiolo et al., 1985; Lin et al., 2005; Zhang et al., 2008a). However, these volatile molecules are ineffective or less effective in water implying that other constituents of urine, such as MUPs, are important for maximal attraction. In addition, α- and β-farnesene, compounds that also accelerate puberty and ovarian function, are attractive to females at physiologically relevant levels but only in sexually-experienced females (Jemiolo et al., 1991).
Female preference for male chemosignals requires both the AOS and MOS although the relative contribution of these two systems varies with the nature of the stimulus. VNO removal or prevention of access of stimuli to the VNO in female Syrian hamsters (Petrulis et al., 1999), mice (Keller et al., 2006c), pigs (Dorries et al., 1997) and ferrets (Woodley et al., 2004) did not impair their preference for volatile male chemosignals. Attraction to distant male chemosignals is, instead, dependent on the MOS; impairing MOE eliminated preference for volatile male scents in female mice (Keller et al., 2006c) and ferrets (Kelliher and Baum, 2001). In contrast, female attraction to non-volatile components of male scents was reduced by AOS damage in mice (Keller et al., 2006c), Syrian hamsters (Petrulis et al., 1999), ferrets (Woodley et al., 2004), opossums (Zuri and Halpern, 2005) and rats (Beltramino and Taleisnik, 1983). It appears that the AOS is not critical for attraction of females to distal male chemosignals but is required for close and persistent investigation of male scents and for the development of conditioned preference for male volatiles in mice (Martinez-Ricos et al., 2008).
More centrally, female preference for male chemosignals were eliminated by MA damage in female Syrian hamsters (Petrulis and Johnston, 1999), mice (DiBenedictis et al., 2012) and rats (Kondo and Sakuma, 2005) but does not appear to require processing by the BNST (Martinez and Petrulis, 2011), as it does in males (see below; (Been and Petrulis, 2010a)). The nature of further diencephalic processing is dependent on the species investigated. Specifically, lesions of the VMH, but not the MPOA, reduced the preference of female ferrets for investigating anesthetized males (Robarts and Baum, 2007) whereas MPOA lesions eliminated preference for male volatile scents in female rats (Xiao et al., 2005) and hamsters (Martinez and Petrulis, 2013). Other forebrain structures receiving chemosensory information, such as entorhinal cortex (ENT) and orbitofrontal cortex (OFC) are not required for female attraction to male chemosignals (Petrulis et al., 1998; Petrulis et al., 2000).
The neurochemical systems underlying female chemoinvestigatory interest in males also show significant species-differences. Attraction to male scents (vs. clean bedding) by female mice is either independent of, or suppressed by, dopaminergic and opiate neural systems (Lanuza et al., 2008). For example, dopaminergic lesions of either the ventral tegmental area (Martinez-Hernandez et al., 2006) or the medial shell of the nucleus accumbens and olfactory tubercle (Martinez-Hernandez et al., 2012) did not impair investigation of male scents nor did systemic treatment with dopamine and opiate antagonists (Agustin-Pavon et al., 2007, 2008). In contrast, sexual conditioning of artificial odors in female rats requires opiate and dopaminergic activity (Coria-Avila et al., 2008a; Coria-Avila et al., 2008b). Although oxytocin and vasopression have been implicated in social odor learning (Wacker and Ludwig, 2012), blocking oxytocin receptors in BNST or MPOA did not alter female preference for male scents (Martinez et al., 2010b).
In humans, women are reported to be able to discriminate sex-specific body odors (Russell, 1976) but this effect may be due to known intensity differences between male and female odors. For example, strong body odors from women are labeled as “male” and both sexes rated male odors as less pleasant and more intense than female odors (Doty et al., 1978). Nevertheless, several studies have suggested that androgens (androstenol, androstenone, androstadienone: ANDs) act as putative human male “pheromones” in that they may increase ratings of attractiveness of people’s photographs (Cowley et al., 1977), prevent a decrease in positive mood in laboratory settings for both men and women (Jacob and McClintock, 2000) and influence measures of physiological arousal in women (Jacob et al., 2001). However, the lack of a sex difference in behavioral response to ANDs (Jacob and McClintock, 2000; Pause, 2004), difficulties in replicating effects in more naturalistic settings (Black and Biron, 1982; Saxton et al., 2008), the highly variable concentrations of ANDs on the human body (Nixon et al., 1988) as well as the fact that significant number of people are selectively anosmic to these odors (Whissell-Buechy and Amoore, 1973) militates against assigning ANDs a unique status as sex-attractant chemosignals. Indeed, human mood, cognition and physiology can be altered by non-social odors (Graham et al., 2000; Kiecolt-Glaser et al., 2008; Moss et al., 2003). Similarly, initial reports (using PET scanning) of ANDs inducing sex-specific activity in the hypothalamus of women (Savic et al., 2001) have not been replicated using fMRI and physiologically relevant levels of ANDs (Burke et al., 2012).
Proceptive or Solicitation Behavior (Beach, 1976)
Scent Marking
Female mice (Rich and Hurst, 1999), Syrian hamsters (Johnston, 1977), rabbits (Gonzalez-Mariscal et al., 1990), ferrets (Chang et al., 2000) and rats (Birke, 1984) scent-mark in response to male scents and change their marking across reproductive states to advertise impending sexual receptivity. The best-studied case of female reproductive marking is vaginal marking by Syrian hamsters, a stereotyped behavior that deposits a sexually-attractive vaginal secretion on the substrate (Been and Petrulis, 2008; Been et al., 2012). Vaginal marking peaks on the night prior to behavioral receptivity but disappears during sexual receptivity, consistent with its role in attracting widely-dispersed males for mating (Gattermann et al., 2001; Johnston, 1977). This substantial rise in vaginal marking during behavioral proestrus appears is mediated by rising E levels, and the rapid switch between vaginal marking and receptive behavior is most likely due to the subsequent rise in progesterone (Lisk and Nachtigall, 1988). The striking hormonally-mediated changes in vaginal marking across the estrous cycle appear to be regulated by the MPOA and VMH: E implants in these areas reinstated vaginal marking in OVX females (Takahashi et al., 1985). However, the VMH may not be critical for vaginal marking as lesions of this structure did not eliminate the behavior (Floody, 2002). The role of the MPOA is more complex as large, fiber-damaging lesions of the MPOA do disrupt vaginal marking (Malsbury et al., 1977) but smaller, excitotoxic lesions limited to the MPOA do not (Martinez and Petrulis, 2013). This suggests that additional areas damaged in the earlier study, such as the posterior BNST, are more important for regulating vaginal marking than the MPOA proper. Indeed, lesions confined to the posterior BNST impair vaginal marking (Martinez and Petrulis, 2011).
Vaginal marking by female Syrian hamsters is increased by exposure to male conspecific scents, specifically those from sexually-dimorphic flank glands (Petrulis and Johnston, 1997), and inhibited by female chemosignals (Johnston, 1977; Johnston and Brenner, 1982) with most marking directed toward scents of unrelated males (Heth et al., 1998). Preferential marking toward male odors requires early experience with scents from male siblings (Maras and Petrulis, 2008a) similar to the development of odor preference in female mice (Moncho-Bogani et al., 2002). Not surprisingly, OBX greatly reduces vaginal marking toward males (Kairys et al., 1980). The MOS is the primary chemoreceptive system driving vaginal marking as VNO removal has minimal effect on vaginal marking whereas MOE destruction reduces the behavior (Johnston, 1992; Petrulis et al., 1999). MOS connections with the MA appear to control vaginal marking responses to odors as MA lesions dramatically reduced vaginal marking to both male and female odors (Petrulis and Johnston, 1999). However, MA-lesioned females still marked more in response to male odors than to female odors and continued to show cyclic variation in their vaginal marking, indicating that MA is not critical for hormonal control of marking or differential marking responses. In contrast, the BNST, via non-MA connections, is critical for differential marking to male odors (Martinez and Petrulis, 2011). This effect appears due to oxytocin action in the BNST as oxytocin antagonist injections into BNST also prevented preferential marking toward male odors (Martinez et al., 2010a). Other MOS structures such as ENT and OFC do not mediate vaginal marking (Petrulis et al., 1998; Petrulis et al., 2000).
Vocalizations
Female rats (White et al., 1991) and hamsters (Floody et al., 1977) in behavioral estrus (Floody et al., 1979; Matochik et al., 1992) often produce ultrasonic vocalizations (USV) in response to males or their odors and this response likely facilitates short-range localization of mates (Pfaff et al., 2008). The USV response to male scents requires chemosensory input as OBX in females greatly reduced USVs toward males (Kairys et al., 1980) and removal of either VNO or MOE also substantially reduced USVs toward male odors (Johnston, 1992). The neural pathway controlling this response involves the MA (Kirn and Floody, 1985) and its connections to MPOA (Floody, 1989) with an inhibitory system originating in the lateral septum (Floody, 1993; Imondi and Floody, 1998; Kirn and Floody, 1985).
Copulatory Behavior
Historically, chemosensory cues have been thought to have only minor effects on female sexual receptivity (Pfaff, 1980). This perspective was, in large part, due to minimal (or even facilitatory; (Williams et al., 1992)) effects of OBX on lordosis, the female receptive posture in rats (Kelche and Aron, 1984) and hamsters (Carter, 1973). This perspective requires revision as male chemosignals do facilitate lordosis in pigs (Melrose et al., 1971; Reed et al., 1974), rats (Rajendren and Moss, 1994), musk shrews (Rissman, 1989) and prairie voles (Curtis et al., 2001). Moreover, chemosignals play a critical role in female receptivity in mice (Keller et al., 2006a; Keller et al., 2006c). In contrast, chemosignals do not play an obligatory role in human reproduction as congenitally anosmic humans showed no direct impairments in reproductive parameters (e.g. number of children)(Croy et al., 2012).
The identity of the relevant chemosignals that facilitate or control receptivity has been identified for mice and for pigs. An identified peptide, ESP1, found in male mouse tear-ducts specifically increases receptivity, but not precopulatory behavior, in females (Haga et al., 2010). In swine, application of ANDs from boar saliva and other fluids increased a sow’s receptive response to back pressure (Melrose et al., 1971; Reed et al., 1974).
The precise neural mechanisms underlying odor effects on receptivity are species-specific. The chemosignal-facilitated standing response in sows survived surgery that prevents chemosignal access to the VNO and therefore suggests that the MOS is likely to be the responsible system (Dorries et al., 1997). In mice, however, removal of either the VNO or MOE strongly impaired female copulatory behavior (Keller et al., 2006a; Keller et al., 2006c) and the identified male-derived peptide, ESP1, works through a VNO-dependent mechanism (Haga et al., 2010). Similarly, VNO removal reduced pair bond formation in female prairie voles, independent of its effects on ovarian activation (Curtis et al., 2001), and eliminated the increase in sexual receptivity observed with repeated matings in female rats (Rajendren and Moss, 1994). This latter effect in female rats depends on an intact AOB (Dudley and Moss, 1994) and appears to result from interactions of the MA (Rajendren and Moss, 1993) with the VMH (Rajendren et al., 1991).
Chemosignals and Behavior: Males
Attraction and Investigation
Adult males of numerous mammalian species approach female scent and engage in prolonged investigation of these odors as part of their precopulatory behavior (Beauchamp and Beruter, 1973; Brown, 1978; Johnston, 1974). Males use the arrangement, spatial patterning and freshness of female scent marks to guide their search toward nearby mating partners (Johnston, 2008) and toward areas associated with female odors (Pankevich et al., 2006) or where the presence of estrous females are anticipated (Ferkin et al., 2008) even in the face of predator cues (Kavaliers et al., 2001). There is species variation in the optimal stimulus that elicits male investigation. In species like the Syrian hamster, in which females are dispersed and scent mark to signal impending receptivity, males are equally attracted to vaginal secretion from females across reproductive conditions (Johnston, 1974; Kwan and Johnston, 1980; Macrides et al., 1984b) and independent of sexual experience (Ballard and Wood, 2007). In other species, such as rats, where interactions with females are more common, males are more attracted to odors from estrous females than from diestrous females (Lydell and Doty, 1972). This attraction to estrous odors is dependent on sexual experience in male rats, mice and dogs (Doty and Dunbar, 1974; Hayashi and Kimura, 1974; Stern, 1970), but see (Agmo, 2003); which suggests that aspects of odor preference are learned during adulthood. In support of this idea, male mice and Syrian hamsters can be conditioned to reduce investigation of female odors by pairing the scents with gastrointestinal distress; however, this conditioning is rapidly extinguished by social experience (Johnston and Zahorik, 1975; Kay and Nyby, 1992).
Although many compounds have been identified from females of several species (Zhang et al., 2007; Zhang et al., 2005; Zhang et al., 2008b) there are very few cases in which actual attractant chemosignals have been isolated. One prominent case is the identification of (Z)-7-dodecenyl acetate as the major attractant compound found in urine of estrous female Asian elephants (Rasmussen et al., 1996). Another well-known example is dimethyl disulfide (DMDS) that was hypothesized to be the key attractant chemosignal within hamster vaginal secretion. DMDS is more attractive than several other volatile components within the secretion and is half as attractive as the entire secretion when tested in an animal’s home cage (O’Connell et al., 1978; Singer et al., 1976). However, when presented in a neutral arena, DMDS is much less effective than volatiles from vaginal secretion in attracting male hamsters (Petrulis and Johnston, 1995). Moreover, unlike vaginal secretion, no sex-difference in investigation was evident toward DMDS and attraction to it does not depend on gonadal hormones. Thus, in a context more similar to how male hamsters might find vaginal secretion, DMDS by itself does not meet the definition of a sex-attractant chemosignal.
The extent to which gonadal steroids are involved in the development of attraction to female odors is species-specific and complex. In mice, conflicting data exist as to whether masculinization of odor preference is due to action of AR or ER-alpha. On one hand, post-natal injections of DHT (a non-aromatizable androgen), but not E, masculinized female investigation of female odors (Bodo and Rissman, 2008). Moreover, male mice lacking a functional AR did not show a male-typical preference for female odors (Bodo and Rissman, 2007) whereas males lacking aromatase still displayed increased investigation of female odor over male odor (Aste et al., 2003). In contrast, others have reported that both ER alpha and aromatase knock-out mice showed elimination of male-typical odor preferences (Bakker et al., 2002; Wersinger and Rissman, 2000). Unlike mice, the masculinization of odor preference in rats appears entirely due to the greater levels of circulating T in males. Testosterone treatment of both male and female rats increased interest in female scents and was unaffected by pre/post-natal disruption of androgen and estrogen action (Bakker et al., 1996b; Dominguez-Salazar et al., 2002). Golden hamsters occupy an intermediate position between mice and rats in the degree of hormone-mediated organization of male-typical odor preference. Pre-pubertal male hamsters (normally showing low preference for female scents) display adult levels of attraction to female odors when treated with T (Johnston and Coplin, 1979; Meek et al., 1997). However, testosterone treatment of OVX females in adulthood increases their attraction to female odors to only half that of T-treated adult males (Fiber and Swann, 1996), indicating some role for organizational effects of hormones in hamster chemoinvestigation.
In adulthood, increased circulating levels of testosterone activate attraction to female chemosignals as castration eliminated this attraction and was reversed by T-treatment (Ballard and Wood, 2007; Gregory et al., 1975; Stern, 1970) or by the combination of its estrogenic and androgenic metabolites (Powers et al., 1985; Steel and Hutchison, 1986). Treatment with estrogens or non-aromatizable androgens alone is sufficient to increase attraction to female scents in ferrets (Woodley and Baum, 2003) but are either insufficient or sub-optimal for restoring normal odor preference in male rats (Xiao et al., 2004), hamsters (Powers et al., 1985; Steel and Hutchison, 1986), meadow voles (Ferkin and Gorman, 1992) and mice (Bean et al., 1986).
Preference for female odors is eliminated by OBX in male Syrian hamsters (Murphy and Schneider, 1970) and rats (Edwards et al., 1990) but the relative contribution of the AOS and MOS to odor preference depends on the type of access animals have to the scent. If only volatile chemosignals are available, removal of access to MOE, but not to the VNO, impairs opposite-sex odor attraction in male Syrian hamsters (O’Connell and Meredith, 1984; Powers and Winans, 1973), mice (Pankevich et al., 2004) rats (Dhungel et al., 2011a) and ferrets (Kelliher and Baum, 2001). If direct contact is allowed, damage to either system can decrease attraction to vaginal secretion in sexually inexperienced male Syrian hamsters (Pfeiffer and Johnston, 1994; Powers et al., 1979) and mice (Pankevich et al., 2004) but not in sexually experienced Syrian hamsters (O’Connell and Meredith, 1984). Similarly, VNO removal in sexually inexperienced guinea pigs leads to rapid extinction of urine investigative behavior (Beauchamp et al., 1982).
More centrally, lesions of either the MAa or MApd, but not PMCo (Maras and Petrulis, 2008b) eliminated preferential investigation of female odors in sexually-naïve male Syrian hamsters, albeit in different ways (Maras and Petrulis, 2006). MAa damage dramatically increased investigation of both male and female odors whereas males with MApd lesions show reduced investigation only toward female odors. This suggests that the chemosensitive MAa functions to evaluate the significance of social stimuli whereas the hormone-sensitive MApd normally generates undifferentiated attraction to social odors. Similar reductions in preference for female scents occur after MApd lesions (Dhungel et al., 2011b) or after AR blockade of MApd (but not MAa) in sexually-experienced male rats (Hosokawa and Chiba, 2010).
Outputs from the MA to the BNST are critical for driving odor attraction to volatile chemosignals in hamsters as BNST lesions eliminated preference for volatile female odors (Been and Petrulis, 2010a) and functional disconnection of MA and BNST (but not MPOA) also eliminated odor preference (Been and Petrulis, 2012). High levels of investigation of non-volatile (contacted) chemosignals are also dependent on the integrity of the MA (Lehman et al., 1980) and the BNST in hamsters and rats (Been and Petrulis, 2010a; Edwards et al., 1996; Powers et al., 1987) but, surprisingly, this is not due to interactions between MA and BNST: functional disconnections of these two structures did not impair odor preference if contact was allowed (Been and Petrulis, 2012). This suggests that the direct AOB projections to BNST may drive contact-based preference in the absence of the MA.
Chemosensory inputs to the MPOA may also be important for attraction to female chemosignals. Lesions that include MPOA reverse the normal preference for volatile female odors in sexually-naïve ferrets (Alekseyenko et al., 2007; Hurtazo and Paredes, 2005) and similar lesions eliminate contact-based preference for estrous female chemosignals (vs. anestrous female) or volatile female odors (vs. male odors) in sexually-experienced rats (Dhungel et al., 2011b; Hurtazo and Paredes, 2005; Hurtazo et al., 2008). In all of these cases, however, the ventral or caudal-most part of the BNST was also ablated and consequently the deficits in preference may be due primarily to ventral/caudal BNST damage and not to MPOA damage per se. Indeed, small lesions limited to the MPOA eliminated preference for volatile female odors only in sexually-naïve hamsters unless damage extended into ventral BNST; contact-based preference was intact in both MPOA and MPOA+ ventral BNST lesioned males (Been and Petrulis, 2010b). Clearly, more research is required that examines the role of the caudal/ventral BNST in attraction and preference for opposite-sex odors as this structure is known to be critical for copulatory behavior in rats (Finn and Yahr, 2005) and gerbils (Yahr and Gregory, 1993). Other structures may also be involved in male attraction toward female odors. For example, in Syrian hamsters OFC lesions eliminated preference for volatile female odor over male odors (Sapolsky and Eichenbaum, 1980) and lesions to the ENT impaired the Coolidge effect in hamsters; that is they no longer preferred to investigate novel females over familiar mates (Petrulis and Eichenbaum, 2003).
In humans, males showed a very modest ability to discriminate chemosignals from ovulatory and non-ovulatory women but did not judge these odors as either attractive or unattractive (Gildersleeve et al., 2012). Human males also appear to have a modest reduction in self-reports of sexual arousal, testosterone levels and brain responses to visual erotica when exposed to odors from human female tears (Gelstein et al., 2011) indicating a possible role for chemosignals in human sexual responding. Based on its presence on the skin surface (Wysocki and Preti, 2004) and its purported ability to alter activity in the putative human male VNO (Monti-Bloch and Grosser, 1991), EST (estratetraenol) has been considered as a candidate human pheromone produced by women. Exposure to EST decreased positive mood in men (Jacob and McClintock, 2000) as well as increasing their sympathetic tone (Jacob et al., 2001) but neither of these effects have been replicated (Bensafi et al., 2003). EST has also been reported to increase activity in the hypothalamus of men, but not women, using PET scans (Savic et al., 2001), but no sex difference in hypothalamic response was found using fMRI and with physiological concentrations of EST (Burke et al., 2012).
Vocalizations
Male rats (Geyer and Barfield, 1978), mice (Nyby et al., 1977b) hamsters (Johnston and Kwan, 1984) and guinea pigs (Eisthen et al., 1987) produce USVs in response to female, but not male, odors. Production of USVs in mice are eliminated by gonadectomy and restored, and even induced in females, by T or its metabolites (Bean et al., 1986; Matochik and Barfield, 1991; Nyby et al., 1977a). A rapidly oxidizing component of female urine is necessary to induce USV in sexually-naïve male mice (without rapid habituation of the response) and supports conditioned USV response to previously neutral stimuli (Sipos et al., 1992). Inhibition of USVs to chemosignals also can be conditioned by pairing urine exposure with gastric distress although this effect is easily extinguished (Kay and Nyby, 1992).
Removal of olfactory bulbs in males eliminates USVs to female odors and similar effects are also observed after VNO removal, especially in sexually-naïve animals (Bean, 1982; Eisthen et al., 1987; Wysocki et al., 1982) as well as after MOE damage (Sipos et al., 1995). Both VNO and MOE signals may ultimately work through steroid-sensitive regions in MPOA to trigger USVs, as T or E implants in MPOA restore USVs in castrated male mice (Nyby et al., 1992). However, MPOA lesions in male mice did not eliminate USVs to females (Bean et al., 1981) and so the precise nature of this circuitry remains to be explored.
Copulatory behavior
Male investigation of the female ano-genital area is a common feature of mammalian sexual behavior and usually precedes the actual copulatory sequence (Dewsbury, 1975). Consequently, manipulations that eliminate chemosensory processing primarily impair the initiation of copulatory behavior rather than the copulatory sequence itself (Devor and Murphy, 1973). The absolute requirement for chemosignal input differs between species and also with sexual experience. For example, OBX eliminates copulatory behavior in Syrian hamsters (Murphy and Schneider, 1970) and mice (Rowe and Edwards, 1972) independent of their sexual experience whereas in rats and guinea pigs OBX produces more variable or contextually-specific deficits of copulation and these can be mitigated by prior sexual experience (Beauchamp et al., 1977; Edwards et al., 1990; Edwards et al., 1996). In sexually experienced Syrian hamsters, female vaginal secretions are sufficient for eliciting mounting in about half of the behavioral tests when applied to the ano-genital area of anesthetized males (Johnston, 1975; Murphy, 1973). Although volatiles from vaginal secretions can induce low levels of mounting, the mounting stimulus is primarily in the non-volatile, HMW fraction (O’Connell and Meredith, 1984; Singer et al., 1984). The major active component of this fraction was identified as a protein (“Aphrodisin”) that is produced within the vaginal tract (Briand et al., 2004). Like MUPs, Aphrodisin normally binds a number of water-insoluble volatile ligands and may act as carrier molecule; the pure protein does not promote mounting (Singer and Macrides, 1990). Although sufficient for mounting of surrogate females, vaginal secretion is not necessary for copulatory behavior in sexually-experienced male hamsters (Johnston, 1986) and aversive conditioning of it does not greatly impair copulation (Johnston et al., 1978).
In addition to chemoinvestigation of the female, male rats can also experience penile erections in response to volatile chemosignals from urine/feces from females (Kondo et al., 1999; Sachs et al., 1994) or even to fecal samples from estrous female horses and foxes (Rampin et al., 2006). This non-contact erection (NCE) response does not require sexual experience (Sachs, 1997) but does requires systemic androgens, but not estrogens (Manzo et al., 1999).
Sexual responses to non-social odors can be conditioned in rats such that males will ejaculate first, and copulate more frequently with, females scented with neutral odors previously paired with estrous females (Kippin and Pfaus, 2001; Kippin et al., 2001). It is not clear if this capacity is widespread within rodents, as attempts to condition hamster copulatory behavior to artificial odors have failed (Macrides et al., 1984a).
Both the AOS and MOS are important for regulating the initiation of copulatory behavior of male rodents but the relative importance of each system varies across species. Although damage to the MOE does not impair copulatory behavior in Syrian hamsters (Powers and Winans, 1973), it eliminates copulation in mice, independent of experience (Keller et al., 2006b; Mandiyan et al., 2005) and greatly reduces NCEs by rats in response to volatile odors from conspecific females (Kondo et al., 1999). In contrast, VNO damage eliminates copulation in 20-30% of male hamsters (Powers and Winans, 1975) but has less pronounced effects (or even no effect: sexually-naïve mice (Pankevich et al., 2004)) in male rats and mice (Clancy et al., 1984; Saito and Moltz, 1986) and no effect in guinea pigs (Beauchamp et al., 1982). The absence of copulation in a sub-population of VNO-lesioned male hamsters is not observed if animals have prior sexual experience (Meredith, 1986) or if they have had contact with vaginal secretions (Westberry and Meredith, 2003). In sexually-naïve hamsters, central injections of GnRH rescued copulatory deficits induced by VNO removal, suggesting that activation of neural GnRH pathways is part of the mechanism underling the initial, VNO-based, control of copulation in this species (Meredith and Fernandez-Fewell, 1994). In mice, functional disorganization of the VNO induces high levels of mounting toward male conspecifics (Leypold et al., 2002; Stowers et al., 2002) but this effect was not observed following VNO removal, suggesting that impairment of VNO function throughout early life may be more disruptive than adult VNO removal (Pankevich et al., 2004). These species differences may be due, in part, to the nature of the chemosensory stimuli that facilitate copulation in different species. For example, in Syrian hamsters, the pro-copulatory aspects of vaginal secretion are largely non-volatile and may therefore depend more on the VNO (Halpern and Martinez-Marcos, 2003; Zufall and Leinders-Zufall, 2007), whereas in mice the vaginal chemosignals that promote mating may be primarily volatile (Hayashi and Kimura, 1974).
More centrally, lesions of MA, especially the MAa, eliminate copulatory behavior and greatly reduce ano-genital investigation by male hamsters (Lehman et al., 1980). In rats, damage to MApd, and to a lesser extent MAa, impairs copulation (McGregor and Herbert, 1992) and non-contact erections to volatile odors from females (Kondo and Sachs, 2002; Kondo et al., 1998). The BNST may be a key structure for processing female cues during copulation as large lesions of BNST (Powers et al., 1987) impaired ano-genital investigation in hamsters and reduced NCEs in male rats in response to female odors (Liu et al., 1997). However, lesions restricted to the BNST (Been and Petrulis, 2010a) and functional disconnection of MA and BNST (Been and Petrulis, 2012) had minimal effects on copulation itself. In contrast, functional disconnections between MA and MPOA eliminated copulation in hamsters (Been and Petrulis, 2012), rats (Kondo and Arai, 1995) and gerbils (Heeb and Yahr, 2000), demonstrating the existence of overlapping, yet separate, neural systems mediating the initial approach toward females (MA-BNST) and initiation of copulation (MA-MPOA). Steroidal effects on chemoinvestigatory behavior during mating are also mediated by MA and MPOA/BNST, as T or E implants in either of these areas reinstated ano-genital investigation and copulation in sexually experienced, castrated Syrian hamsters (Wood, 1996; Wood and Newman, 1995). Similarly, implants of androgens and, to a lesser degree, estrogens in MApd maintained NCEs in rats (Bialy & Sachs, 2002).
Conclusions
It is clear that social odors play a substantial and oftentimes obligatory role in mammalian reproduction. These chemosignals coordinate reproductive physiology and behavior and to adapt these responses to current environmental conditions. For example, chemosensory signals accelerate or decelerate maturation and adult function of the hypothalamic-pituitary-gonadal (HPG) axis of recipients such that increased HPG functioning occurs to the appearance of mating partners, and decreases in HPG function occur in reproductively sub-optimal conditions. Similarly, perception of scents from opposite-sex conspecifics leads reproductively active adults to initiate behaviors that increase the likelihood of contacting mates, such as chemosensory investigation, scent marking and vocalizations. Even after contact occurs, the initiation of copulatory behavior in both sexes may still depend on chemosensory cues, perhaps because these cues allow a more detailed evaluation of the mating partner. The coordination of reproductive physiology and behavior by social odors is most evident by the observation that gonadal steroids not only regulate odor-guided reproductive behavior but also regulate the production (in some cases acting as precursors), release and deposition of the social odors themselves.
It is also clear that the simple formulation of “VNO = pheromone detector” is inaccurate as has been pointed out previously (Baxi et al., 2006; Stowers and Marton, 2005). The role of the AOS and MOS in behavioral or physiological processes primarily depends on whether the relevant chemosignals are volatile or non-volatile, and this may vary from species to species. However, even this conclusion must be tempered with the knowledge that complex anatomical interactions exist between MOS and AOS in rodents (Martel and Baum, 2007, 2009b) and findings that VNO receptor proteins are found in the MOE of ungulates (Ohara et al., 2009). Despite these differences and complexities at the receptor and olfactory bulb level, the central neural systems involved in transforming chemosignal perception into physiological or behavioral action appear to be fundamentally similar across species. In almost all cases where it has been investigated, the cortico-medial amygdala (MA, ACo, PLCo, PMCo) is indispensible for chemosensory-driven behavioral and physiological responses and is a key site of hormone-chemosignal interaction. Through its connections with the BNST, MPOA and hypothalamus, the MA implements a biased behavioral (BNST: attraction; MPOA: copulation) and physiological response (hypothalamus: prolactin and LH release) to opposite-sex conspecifics. However, important functional sex differences exist within this framework (e.g. the role of BNST and VMH in sexual attraction) and require further investigation.
The role of learning and memory in the behavioral and physiological responses to opposite-sex conspecifics is generally under-appreciated by the non-specialist. Many of the reproductive effects reviewed above are clearly subject to learning in adulthood as evidenced by their dependency on sexual experience or prior contact with chemosignals as well as by their ability to be conditioned to previously neutral odors. Although beyond the scope of this review, the appropriate guidance of chemosensory-guided social behaviors are also profoundly dependent on learning about chemosignals during early post-natal development or even pre-natally (Nowak et al., 2000). For example, cross-fostering young animals to heterospecific parents reverses their preferences for conspecifics (Huck and Banks, 1980; Quadagno and Banks, 1970) and removing social odors from parents during development alters the future behavior of offspring (Blum et al., 1975). Even the most basic orientation responses to odors develop during nursing (Alberts and Brunjes, 1978) and prenatal life (Robinson and Smotherman, 1992).
This dependence on learning and context undercuts the most fundamental idea of a pheromone; that is, that responses to it are “instinctual” and therefore not learned. Similarly, the fact that most chemosignals altering mammalian behavior and/or physiology are complex mixtures often lacking species-specificity, rather than being potent and essential singular compounds, further erodes the utility of the term “pheromone”. Because of these concerns, it is perhaps wise to restrict the term to molecules that have met each and every criterion of a pheromone, as used in the classical ethological sense. With this in mind, the protein chemosignal Darcin, that guides initial approach of female mice to male scent marks and drives learning of volatile constituents of male urine (Roberts et al., 2012b; Roberts et al., 2010) as well as ESP1, the peptide in male mouse tear-ducts that stimulates female receptivity, are perhaps the only reproduction-related mammalian pheromones identified thus far.
Lastly, it should be clear that chemosignals are neither necessary nor sufficient for human reproduction nor do they have a privileged place in directing human social behavior. Positive findings of human chemical communication, when not based on flawed analysis (see (Winman, 2004) examination of putative pheromone effects on human sexual behavior (Cutler et al., 1998; McCoy and Pitino, 2002)), are often inconsistent, and at best, demonstrate modest effects on human behavior and physiology. This should not be surprising given our heavy reliance on visual and auditory cues for social behavior and on the massive expansion of the visual and auditory cortex compared to the olfactory system. Indeed, adult humans do not appear to have a functioning AOS and the human MA may be more responsive to auditory and visual social cues (Ball et al., 2009; Bzdok et al., 2012; Davis et al., 2010; Fruhholz and Grandjean, 2012; Goossens et al., 2009). The putative human VNO is not an elaborated structure as it is in many mammals and does not appear to contain receptor neurons, nor is there evidence that a VNO nerve or AOB exist in humans; (Bhatnagar et al., 2002; Meredith, 2001; Smith et al., 2002; Trotier et al., 2000). Moreover, congenitally anosmic humans have no apparent difficulty in reproducing successfully (Croy et al., 2012). So, although adult humans do produce odors and can discriminate between different categories of individuals, such as males and females or kin and non-kin, there is no evidence that adult social behavior is altered by any of these odors (Wysocki and Preti, 2004). The existing evidence suggests that, unlike many other mammals, social odors play only a minor role in human reproductive physiology and behavior.
Highlights.
All aspects of chemical communication are influenced by sex and hormone status
Very few chemosignals qualify as “pheromones”
Most responses to chemosignals are dependent on context and learning
Chemosignal processing is mediated by the medial amygdala and its connections
Species-differences in all aspects of chemical communication are evident
Acknowledgements
This work was supported by NIH grant MH072930 to A. Petrulis, and in part by the Center for Behavioral Neuroscience under the STC program of the NSF, under agreement IBN 9876754.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agmo A. Unconditioned sexual incentive motivation in the male Norway rat (Rattus norvegicus) J Comp Psychol. 2003;117:3–14. doi: 10.1037/0735-7036.117.1.3. [DOI] [PubMed] [Google Scholar]
- Agustin-Pavon C, Martinez-Ricos J, Martinez-Garcia F, Lanuza E. Effects of dopaminergic drugs on innate pheromone-mediated reward in female mice: a new case of dopamine-independent “liking.”. Behav Neurosci. 2007;121:920–932. doi: 10.1037/0735-7044.121.5.920. [DOI] [PubMed] [Google Scholar]
- Agustin-Pavon C, Martinez-Ricos J, Martinez-Garcia F, Lanuza E. Sex versus sweet: opposite effects of opioid drugs on the reward of sucrose and sexual pheromones. Behav Neurosci. 2008;122:416–425. doi: 10.1037/0735-7044.122.2.416. [DOI] [PubMed] [Google Scholar]
- Alberts JR, Brunjes PC. Ontogeny of thermal and olfactory determinants of huddling in the rat. Journal of comparative and physiological psychology. 1978;92:897–906. doi: 10.1037/h0077533. [DOI] [PubMed] [Google Scholar]
- Alekseyenko OV, Waters P, Zhou H, Baum MJ. Bilateral damage to the sexually dimorphic medial preoptic area/anterior hypothalamus of male ferrets causes a female-typical preference for and a hypothalamic Fos response to male body odors. Physiol Behav. 2007;90:438–449. doi: 10.1016/j.physbeh.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand S, Turek FW, Horton TH. Chemosensory stimulation of luteinizing hormone secretion in male Siberian hamsters (Phodopus sungorus) Biol Reprod. 2004;70:1033–1040. doi: 10.1095/biolreprod.103.019380. [DOI] [PubMed] [Google Scholar]
- Armstrong SD, Robertson DH, Cheetham SA, Hurst JL, Beynon RJ. Structural and functional differences in isoforms of mouse major urinary proteins: a male-specific protein that preferentially binds a male pheromone. Biochem J. 2005;391:343–350. doi: 10.1042/BJ20050404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aste N, Honda S, Harada N. Forebrain Fos responses to reproductively related chemosensory cues in aromatase knockout mice. Brain Res Bull. 2003;60:191–200. doi: 10.1016/s0361-9230(03)00035-2. [DOI] [PubMed] [Google Scholar]
- Baddaloo EGY, Clulow FV. Effects of the male on growth, sexual maturation, and ovulation of young female meadow voles, Microtus pennsylvanicus. Canadian Journal of Zoology. 1981;59:415–421. [Google Scholar]
- Bakker J, Baum MJ. Neuroendocrine regulation of GnRH release in induced ovulators. Front Neuroendocrinol. 2000;21:220–262. doi: 10.1006/frne.2000.0198. [DOI] [PubMed] [Google Scholar]
- Bakker J, Baum MJ, Slob AK. Neonatal inhibition of brain estrogen synthesis alters adult neural Fos responses to mating and pheromonal stimulation in the male rat. Neuroscience. 1996a;74:251–260. doi: 10.1016/0306-4522(96)00096-6. [DOI] [PubMed] [Google Scholar]
- Bakker J, Honda S, Harada N, Balthazart J. Sexual partner preference requires a functional aromatase (cyp19) gene in male mice. Horm Behav. 2002;42:158–171. doi: 10.1006/hbeh.2002.1805. [DOI] [PubMed] [Google Scholar]
- Bakker J, Kelliher KR, Baum MJ. Mating induces gonadotropin-releasing hormone neuronal activation in anosmic female ferrets. Biol Reprod. 2001;64:1100–1105. doi: 10.1095/biolreprod64.4.1100. [DOI] [PubMed] [Google Scholar]
- Bakker J, Van Ophemert J, Slob AK. Sexual differentiation of odor and partner preference in the rat. Physiol Behav. 1996b;60:489–494. doi: 10.1016/s0031-9384(96)80023-0. [DOI] [PubMed] [Google Scholar]
- Ball T, Derix J, Wentlandt J, Wieckhorst B, Speck O, Schulze-Bonhage A, Mutschler I. Anatomical specificity of functional amygdala imaging of responses to stimuli with positive and negative emotional valence. J Neurosci Methods. 2009;180:57–70. doi: 10.1016/j.jneumeth.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Ballard CL, Wood RI. Partner preference in male hamsters: steroids, sexual experience and chemosensory cues. Physiol Behav. 2007;91:1–8. doi: 10.1016/j.physbeh.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum MJ, Kelliher KR. Complementary Roles of the Main and Accessory Olfactory Systems in Mammalian Mate Recognition. Annu Rev Physiol. 2008 doi: 10.1146/annurev.physiol.010908.163137. [DOI] [PubMed] [Google Scholar]
- Baxi KN, Dorries KM, Eisthen HL. Is the vomeronasal system really specialized for detecting pheromones? Trends Neurosci. 2006;29:1–7. doi: 10.1016/j.tins.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Beach FA. Sexual attractivity, proceptivity, and receptivity in female mammals. Horm Behav. 1976;7:105–138. doi: 10.1016/0018-506x(76)90008-8. [DOI] [PubMed] [Google Scholar]
- Bean NJ. Olfactory and vomeronasal mediation of ultrasonic vocalizations in male mice. Physiol Behav. 1982;28:31–37. doi: 10.1016/0031-9384(82)90097-x. [DOI] [PubMed] [Google Scholar]
- Bean NJ, Nyby J, Kerchner M, Dahinden Z. Hormonal regulation of chemosignal-stimulated precopulatory behaviors in male housemice (Mus musculus) Horm Behav. 1986;20:390–404. doi: 10.1016/0018-506x(86)90002-4. [DOI] [PubMed] [Google Scholar]
- Bean NY, Nunez AA, Conner R. Effects of medial preoptic lesions on male mouse ultrasonic vocalizations and copulatory behavior. Brain Res Bull. 1981;6:109–112. doi: 10.1016/s0361-9230(81)80033-0. [DOI] [PubMed] [Google Scholar]
- Beaton EA, deCatanzaro D. Novel males’ capacity to disrupt early pregnancy in mice (Mus musculus) is attenuated via a chronic reduction of males’ urinary 17beta-estradiol. Psychoneuroendocrinology. 2005;30:688–697. doi: 10.1016/j.psyneuen.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Beauchamp GK, Beruter J. Source and stability of attractive components in guinea pig (Cavia porcellus) urine. Behav Biol. 1973;9:43–47. doi: 10.1016/s0091-6773(73)80167-1. [DOI] [PubMed] [Google Scholar]
- Beauchamp GK, Magnus JG, Shmunes NT, Durham T. Effects of olfactory bulbectomy on social behavior of male guinea pigs (Cavia procellus) J Comp Physiol Psychol. 1977;91:336–346. doi: 10.1037/h0077327. [DOI] [PubMed] [Google Scholar]
- Beauchamp GK, Martin IG, Wysocki CJ, Wellington JL. Chemoinvestigatory and sexual behavior of male guinea pigs following vomeronasal organ removal. Physiol Behav. 1982;29:329–336. doi: 10.1016/0031-9384(82)90022-1. [DOI] [PubMed] [Google Scholar]
- Becker SD, Hurst JL. Female behaviour plays a critical role in controlling murine pregnancy block. Proceedings of the Royal Society B: Biological Sciences. 2009;276:1723–1729. doi: 10.1098/rspb.2008.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been L, Petrulis A. The Neurobiology of Sexual Solicitation: Vaginal Marking in Female Syrian Hamsters (Mesocricetus auratus) Chemical Signals in Vertebrates. 2008;11:231–239. [Google Scholar]
- Been LE, Bauman JM, Petrulis A, Chang YH. X-ray kinematics analysis of vaginal scent marking in female Syrian hamsters (Mesocricetus auratus) Physiol Behav. 2012;105:1021–1027. doi: 10.1016/j.physbeh.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been LE, Petrulis A. Lesions of the posterior bed nucleus of the stria terminalis eliminate opposite-sex odor preference and delay copulation in male Syrian hamsters: role of odor volatility and sexual experience. Eur J Neurosci. 2010a;32:483–493. doi: 10.1111/j.1460-9568.2010.07277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been LE, Petrulis A. The role of the medial preoptic area in appetitive and consummatory reproductive behaviors depends on sexual experience and odor volatility in male Syrian hamsters. Neuroscience. 2010b;170:1120–1132. doi: 10.1016/j.neuroscience.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been LE, Petrulis A. Chemosensory and hormone information are relayed directly between the medial amygdala, posterior bed nucleus of the stria terminalis, and medial preoptic area in male Syrian hamsters. Horm Behav. 2011;59:536–548. doi: 10.1016/j.yhbeh.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been LE, Petrulis A. Dissociated functional pathways for appetitive and consummatory reproductive behaviors in male Syrian hamsters. Horm Behav. 2012;61:204–211. doi: 10.1016/j.yhbeh.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellringer JF, Pratt HP, Keverne EB. Involvement of the vomeronasal organ and prolactin in pheromonal induction of delayed implantation in mice. J Reprod Fertil. 1980;59:223–228. doi: 10.1530/jrf.0.0590223. [DOI] [PubMed] [Google Scholar]
- Beltramino C, Taleisnik S. Facilitatory and inhibitory effects of electrochemical stimulation of the amygdala on the release of luteinizing hormone. Brain Res. 1978;144:95–107. doi: 10.1016/0006-8993(78)90437-7. [DOI] [PubMed] [Google Scholar]
- Beltramino C, Taleisnik S. Dual action of electrochemical stimulation of the bed nucleus of the stria terminalis on the release of LH. Neuroendocrinology. 1980;30:238–242. doi: 10.1159/000123007. [DOI] [PubMed] [Google Scholar]
- Beltramino C, Taleisnik S. Release of LH in the female rat by olfactory stimuli. Effect of the removal of the vomeronasal organs or lesioning of the accessory olfactory bulbs. Neuroendocrinology. 1983;36:53–58. doi: 10.1159/000123436. [DOI] [PubMed] [Google Scholar]
- Beltramino C, Taleisnik S. Ventral premammillary nuclei mediate pheromonal-induced LH release stimuli in the rat. Neuroendocrinology. 1985;41:119–124. doi: 10.1159/000124164. [DOI] [PubMed] [Google Scholar]
- Bensafi M, Brown WM, Tsutsui T, Mainland JD, Johnson BN, Bremner EA, Young N, Mauss I, Ray B, Gross J, Richards J, Stappen I, Levenson RW, Sobel N. Sex-steroid derived compounds induce sex-specific effects on autonomic nervous system function in humans. Behav Neurosci. 2003;117:1125–1134. doi: 10.1037/0735-7044.117.6.1125. [DOI] [PubMed] [Google Scholar]
- Bhatnagar KP, Smith TD, Winstead W. The human vomeronasal organ: part IV. Incidence, topography, endoscopy, and ultrastructure of the nasopalatine recess, nasopalatine fossa, and vomeronasal organ. Am J Rhinol. 2002;16:343–350. [PubMed] [Google Scholar]
- Birke LI. Effects of estradiol and progesterone on scent-marking behavior of female rats. Horm Behav. 1984;18:95–98. doi: 10.1016/0018-506x(84)90054-0. [DOI] [PubMed] [Google Scholar]
- Black SL, Biron C. Androstenol as a human pheromone: no effect on perceived physical attractiveness. Behav Neural Biol. 1982;34:326–330. doi: 10.1016/s0163-1047(82)91711-3. [DOI] [PubMed] [Google Scholar]
- Blum SL, Balsiger D, Ricci JS, Spiegel DK. Effects of early exposure to ventral gland odor on physical and behavioral development and adult social behavior in mongolian gerbils. Journal of comparative and physiological psychology. 1975;89:1210. doi: 10.1037/h0077174. [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF. Androgen receptor is essential for sexual differentiation of responses to olfactory cues in mice. Eur J Neurosci. 2007;25:2182–2190. doi: 10.1111/j.1460-9568.2007.05484.x. [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF. The androgen receptor is selectively involved in organization of sexually dimorphic social behaviors in mice. Endocrinology. 2008;149:4142–4150. doi: 10.1210/en.2008-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm U, Zou Z, Buck LB. Feedback loops link odor and pheromone signaling with reproduction. Cell. 2005;123:683–695. doi: 10.1016/j.cell.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Bonilla-Jaime H, Vazquez-Palacios G, Arteaga-Silva M, Retana-Marquez S. Hormonal responses to different sexually related conditions in male rats. Horm Behav. 2006;49:376–382. doi: 10.1016/j.yhbeh.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Boschat C, Pelofi C, Randin O, Roppolo D, Luscher C, Broillet MC, Rodriguez I. Pheromone detection mediated by a V1r vomeronasal receptor. Nat Neurosci. 2002;5:1261–1262. doi: 10.1038/nn978. [DOI] [PubMed] [Google Scholar]
- Brennan P, Kaba H, Keverne EB. Olfactory recognition: a simple memory system. Science. 1990;250:1223–1226. doi: 10.1126/science.2147078. [DOI] [PubMed] [Google Scholar]
- Brennan PA. Outstanding issues surrounding vomeronasal mechanisms of pregnancy block and individual recognition in mice. Behav Brain Res. 2009;200:287–294. doi: 10.1016/j.bbr.2008.10.045. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Keverne EB. Neural mechanisms of mammalian olfactory learning. Prog Neurobiol. 1997;51:457–481. doi: 10.1016/s0301-0082(96)00069-x. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Schellinck HM, Keverne EB. Patterns of expression of the immediate-early gene egr-1 in the accessory olfactory bulb of female mice exposed to pheromonal constituents of male urine. Neuroscience. 1999;90:1463–1470. doi: 10.1016/s0306-4522(98)00556-9. [DOI] [PubMed] [Google Scholar]
- Bressler SC, Baum MJ. Sex comparison of neuronal Fos immunoreactivity in the rat vomeronasal projection circuit after chemosensory stimulation. Neuroscience. 1996;71:1063–1072. doi: 10.1016/0306-4522(95)00493-9. [DOI] [PubMed] [Google Scholar]
- Briand L, Trotier D, Pernollet JC. Aphrodisin, an aphrodisiac lipocalin secreted in hamster vaginal secretions. Peptides. 2004;25:1545–1552. doi: 10.1016/j.peptides.2003.10.026. [DOI] [PubMed] [Google Scholar]
- Brock O, Baum MJ, Bakker J. The development of female sexual behavior requires prepubertal estradiol. J Neurosci. 2011;31:5574–5578. doi: 10.1523/JNEUROSCI.0209-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock O, Keller M, Douhard Q, Bakker J. Female mice deficient in alpha-fetoprotein show female-typical neural responses to conspecific-derived pheromones. PLoS ONE. 2012;7:e39204. doi: 10.1371/journal.pone.0039204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson FH, Desjardins C. Relationships between scent marking by male mice and the pheromone-induced secretion of the gonadotropic and ovarian hormones that accompany puberty in female mice. Adv Behav Biol. 1974;11:157–178. doi: 10.1007/978-1-4684-3069-1_7. [DOI] [PubMed] [Google Scholar]
- Bronson FH, Maruniak JA. Male-induced puberty in female mice: evidence for a synergistic action of social cues. Biol Reprod. 1975;13:94–98. doi: 10.1095/biolreprod13.1.94. [DOI] [PubMed] [Google Scholar]
- Bronson FH, Whitten WK. Oestrus-accelerating pheromone of mice: assay, androgen-dependency and presence in bladder urine. J Reprod Fertil. 1968;15:131–134. doi: 10.1530/jrf.0.0150131. [DOI] [PubMed] [Google Scholar]
- Brown RE. Hormonal control of odor preferences and urine-marking in male and female rats. Physiol Behav. 1978;20:21–24. doi: 10.1016/0031-9384(78)90197-x. [DOI] [PubMed] [Google Scholar]
- Brown RE, Macdonald DW. Social odours in mammals. Clarendon Press; Oxford [Oxfordshire]; New York: 1985. [Google Scholar]
- Bruce HM. An exteroceptive block to pregnancy in the mouse. Nature. 1959;184:105. doi: 10.1038/184105a0. [DOI] [PubMed] [Google Scholar]
- Bruce HM. Pheromones and behavior in mice. Acta Neurol Psychiatr Belg. 1969;69:529–538. [PubMed] [Google Scholar]
- Burke SM, Veltman DJ, Gerber J, Hummel T, Bakker J. Heterosexual men and women both show a hypothalamic response to the chemo-signal androstadienone. PLoS ONE. 2012;7:e40993. doi: 10.1371/journal.pone.0040993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Laird AR, Zilles K, Fox PT, Eickhoff SB. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr WJ, Loeb LS, Dissinger ML. Responses of Rats to Sex Odors. J Comp Physiol Psychol. 1965;59:370–377. doi: 10.1037/h0022036. [DOI] [PubMed] [Google Scholar]
- Carter CS. Olfaction and sexual receptivity in the female golden hamster. Physiol Behav. 1973;10:47–51. doi: 10.1016/0031-9384(73)90084-x. [DOI] [PubMed] [Google Scholar]
- Carter CS, Getz LL, Gavish L, McDermott JL, Arnold P. Male-related pheromones and the activation of female reproduction in the prairie vole (Microtus ochrogaster) Biol Reprod. 1980;23:1038–1045. doi: 10.1095/biolreprod23.5.1038. [DOI] [PubMed] [Google Scholar]
- Cerda-Molina AL, Hernández-López L, Chavira R, Cárdenas M, Paez-Ponce D, Cervantes-De la Luz H, Mondragón-Ceballos R. Endocrine changes in male stumptailed macaques (Macaca arctoides) as a response to odor stimulation with vaginal secretions. Hormones and Behavior. 2006;49:81–87. doi: 10.1016/j.yhbeh.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Chamero P, Leinders-Zufall T, Zufall F. From genes to social communication: molecular sensing by the vomeronasal organ. Trends in neurosciences. 2012;35:597–606. doi: 10.1016/j.tins.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Champlin AK. Suppression of oestrus in grouped mice: the effects of various densities and the possible nature of the stimulus. J Reprod Fertil. 1971;27:233–241. doi: 10.1530/jrf.0.0270233. [DOI] [PubMed] [Google Scholar]
- Chang YM, Kelliher KR, Baum MJ. Steroidal modulation of scent investigation and marking behaviors in male and female ferrets (Mustela putorius furo) J Comp Psychol. 2000;114:401–407. doi: 10.1037/0735-7036.114.4.401. [DOI] [PubMed] [Google Scholar]
- Cheetham SA, Thom MD, Jury F, Ollier WE, Beynon RJ, Hurst JL. The genetic basis of individual-recognition signals in the mouse. Curr Biol. 2007;17:1771–1777. doi: 10.1016/j.cub.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Clancy AN, Coquelin A, Macrides F, Gorski RA, Noble EP. Sexual behavior and aggression in male mice: involvement of the vomeronasal system. J Neurosci. 1984;4:2222–2229. doi: 10.1523/JNEUROSCI.04-09-02222.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Tannoudji J, Einhorn J, Signoret JP. Ram sexual pheromone: first approach of chemical identification. Physiol Behav. 1994;56:955–961. doi: 10.1016/0031-9384(94)90329-8. [DOI] [PubMed] [Google Scholar]
- Cohen-Tannoudji J, Lavenet C, Locatelli A, Tillet Y, Signoret JP. Non-involvement of the accessory olfactory system in the LH response of anoestrous ewes to male odour. J Reprod Fertil. 1989;86:135–144. doi: 10.1530/jrf.0.0860135. [DOI] [PubMed] [Google Scholar]
- Cohen-Tannoudji J, Locatelli A, Signoret JP. Non-pheromonal stimulation by the male of LH release in the anoestrous ewe. Physiol Behav. 1986;36:921–924. doi: 10.1016/0031-9384(86)90453-1. [DOI] [PubMed] [Google Scholar]
- Colby DR, Vandenberg JG. Regulatory effects of urinary pheromones on puberty in the mouse. Biol Reprod. 1974;11:268–279. doi: 10.1095/biolreprod11.3.268. [DOI] [PubMed] [Google Scholar]
- Cooke BM. Steroid-dependent plasticity in the medial amygdala. Neuroscience. 2006;138:997–1005. doi: 10.1016/j.neuroscience.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. J Comp Neurol. 1998;399:189–209. doi: 10.1002/(sici)1096-9861(19980921)399:2<189::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Coquelin A, Bronson FH. Release of luteinizing hormone in male mice during exposure to females: habituation of the response. Science. 1979;206:1099–1101. doi: 10.1126/science.573924. [DOI] [PubMed] [Google Scholar]
- Coquelin A, Clancy AN, Macrides F, Noble EP, Gorski RA. Pheromonally induced release of luteinizing hormone in male mice: involvement of the vomeronasal system. J Neurosci. 1984;4:2230–2236. doi: 10.1523/JNEUROSCI.04-09-02230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coria-Avila GA, Gavrila AM, Boulard B, Charron N, Stanley G, Pfaus JG. Neurochemical basis of conditioned partner preference in the female rat: II. Disruption by flupenthixol. Behav Neurosci. 2008a;122:396–406. doi: 10.1037/0735-7044.122.2.396. [DOI] [PubMed] [Google Scholar]
- Coria-Avila GA, Ouimet AJ, Pacheco P, Manzo J, Pfaus JG. Olfactory conditioned partner preference in the female rat. Behav Neurosci. 2005;119:716–725. doi: 10.1037/0735-7044.119.3.716. [DOI] [PubMed] [Google Scholar]
- Coria-Avila GA, Solomon CE, Vargas EB, Lemme I, Ryan R, Menard S, Gavrila AM, Pfaus JG. Neurochemical basis of conditioned partner preference in the female rat: I. Disruption by naloxone. Behav Neurosci. 2008b;122:385–395. doi: 10.1037/0735-7044.122.2.385. [DOI] [PubMed] [Google Scholar]
- Cowley JJ, Johnson AL, Brooksbank BW. The effect of two odorous compounds on performance in an assessment-of-people test. Psychoneuroendocrinology. 1977;2:159–172. doi: 10.1016/0306-4530(77)90021-x. [DOI] [PubMed] [Google Scholar]
- Croy I, Negoias S, Novakova L, Landis BN, Hummel T. Learning about the functions of the olfactory system from people without a sense of smell. PLoS ONE. 2012;7:e33365. doi: 10.1371/journal.pone.0033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis JT, Liu Y, Wang Z. Lesions of the vomeronasal organ disrupt mating-induced pair bonding in female prairie voles (Microtus ochrogaster) Brain Res. 2001;901:167–174. doi: 10.1016/s0006-8993(01)02343-5. [DOI] [PubMed] [Google Scholar]
- Cutler WB, Friedmann E, McCoy NL. Pheromonal influences on sociosexual behavior in men. Arch Sex Behav. 1998;27:1–13. doi: 10.1023/a:1018637907321. [DOI] [PubMed] [Google Scholar]
- Davis FC, Johnstone T, Mazzulla EC, Oler JA, Whalen PJ. Regional response differences across the human amygdaloid complex during social conditioning. Cereb Cortex. 2010;20:612–621. doi: 10.1093/cercor/bhp126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Catanzaro D, Muir C, Sullivan C, Boissy A. Pheromones and novel male-induced pregnancy disruptions in mice: exposure to conspecifics is necessary for urine alone to induce an effect. Physiol Behav. 1999;66:153–157. doi: 10.1016/s0031-9384(98)00277-7. [DOI] [PubMed] [Google Scholar]
- de la Maza HM, Wolff JO, Lindsey A. Exposure to strange adults does not cause pregnancy disruption or infanticide in the gray-tailed vole. Behavioral Ecology and Sociobiology. 1999;45:107–113. [Google Scholar]
- de Vries GJ, Sodersten P. Sex differences in the brain: the relation between structure and function. Horm Behav. 2009;55:589–596. doi: 10.1016/j.yhbeh.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deCatanzaro D. Blastocyst implantation is vulnerable to stress-induced rises in endogenous estrogens and also to excretions of estrogens by proximate males. J Reprod Immunol. 2011;90:14–20. doi: 10.1016/j.jri.2011.04.005. [DOI] [PubMed] [Google Scholar]
- deCatanzaro D, Baptista MA, Spironello-Vella E. Administration of minute quantities of 17beta-estradiol on the nasal area terminates early pregnancy in inseminated female mice. Pharmacol Biochem Behav. 2001;69:503–509. doi: 10.1016/s0091-3057(01)00554-8. [DOI] [PubMed] [Google Scholar]
- deCatanzaro D, Beaton EA, Khan A, Vella E. Urinary oestradiol and testosterone levels from novel male mice approach values sufficient to disrupt early pregnancy in nearby inseminated females. Reproduction. 2006;132:309–317. doi: 10.1530/rep.1.00965. [DOI] [PubMed] [Google Scholar]
- deCatanzaro D, Murji T. Inseminated female mice (Mus musculus) investigate rather than avoid novel males that disrupt pregnancy, but sires protect pregnancy. J Comp Psychol. 2004;118:251–257. doi: 10.1037/0735-7036.118.3.251. [DOI] [PubMed] [Google Scholar]
- deCatanzaro D, Wyngaarden P, Griffiths J, Ham M, Hancox J, Brain D. Interactions of contact, odor cues, and androgens in strange-male-induced early pregnancy disruptions in mice (Mus musculus) J Comp Psychol. 1995;109:115–122. doi: 10.1037/0735-7036.109.2.115. [DOI] [PubMed] [Google Scholar]
- Delbarco-Trillo J, Ferkin MH. Male mammals respond to a risk of sperm competition conveyed by odours of conspecific males. Nature. 2004;431:446–449. doi: 10.1038/nature02845. [DOI] [PubMed] [Google Scholar]
- Delbarco-Trillo J, Ferkin MH. Increased sperm numbers in the vas deferens of meadow voles, Microtus pennsylvanicus, in response to odors of conspecific males. Behavioral Ecology and Sociobiology. 2007;61:1759–1764. [Google Scholar]
- Delgadillo JA, Gelez H, Ungerfeld R, Hawken PAR, Martin GB. The ’male effect’ in sheep and goats—Revisiting the dogmas. Behavioural Brain Research. 2009;200:304–314. doi: 10.1016/j.bbr.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Devor M, Murphy MR. The effect of peripheral olfactory blockade on the social behavior of the male golden hamster. Behav Biol. 1973;9:31–42. doi: 10.1016/s0091-6773(73)80166-x. [DOI] [PubMed] [Google Scholar]
- Dewsbury DA. Diversity and adaptation in rodent copulatory behavior. Science. 1975;190:947–954. doi: 10.1126/science.1188377. [DOI] [PubMed] [Google Scholar]
- Dhungel S, Masaoka M, Rai D, Kondo Y, Sakuma Y. Both olfactory epithelial and vomeronasal inputs are essential for activation of the medial amygdala and preoptic neurons of male rats. Neuroscience. 2011a;199:225–234. doi: 10.1016/j.neuroscience.2011.09.051. [DOI] [PubMed] [Google Scholar]
- Dhungel S, Urakawa S, Kondo Y, Sakuma Y. Olfactory preference in the male rat depends on multiple chemosensory inputs converging on the preoptic area. Horm Behav. 2011b;59:193–199. doi: 10.1016/j.yhbeh.2010.11.011. [DOI] [PubMed] [Google Scholar]
- DiBenedictis BT, Ingraham KL, Baum MJ, Cherry JA. Disruption of urinary odor preference and lordosis behavior in female mice given lesions of the medial amygdala. Physiol Behav. 2012;105:554–559. doi: 10.1016/j.physbeh.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge JC, Kristal MB, Badura LL. Male-induced estrus synchronization in the female Siberian hamster (Phodopus sungorus sungorus) Physiol Behav. 2002;77:227–231. doi: 10.1016/s0031-9384(02)00851-x. [DOI] [PubMed] [Google Scholar]
- Dominguez-Salazar E, Portillo W, Baum MJ, Bakker J, Paredes RG. Effect of prenatal androgen receptor antagonist or aromatase inhibitor on sexual behavior, partner preference and neuronal Fos responses to estrous female odors in the rat accessory olfactory system. Physiol Behav. 2002;75:337–346. doi: 10.1016/s0031-9384(01)00674-6. [DOI] [PubMed] [Google Scholar]
- Dominic CJ. The origin of the pheromones causing pregnancy block in mice. J Reprod Fertil. 1965;10:469–472. doi: 10.1530/jrf.0.0100469. [DOI] [PubMed] [Google Scholar]
- Dominic CJ. Observations on the reproductive pheromones of mice. I. Source. J Reprod Fertil. 1966;11:407–414. doi: 10.1530/jrf.0.0110407. [DOI] [PubMed] [Google Scholar]
- Dorries KM, Adkins-Regan E, Halpern BP. Sensitivity and behavioral responses to the pheromone androstenone are not mediated by the vomeronasal organ in domestic pigs. Brain Behav Evol. 1997;49:53–62. doi: 10.1159/000112981. [DOI] [PubMed] [Google Scholar]
- Doty R. The great pheromone myth: mammalian pheromones, audiomones, visuomones and snarks. John Hopkins University Press; Baltimore: 2010. [Google Scholar]
- Doty RL. Odor-guided behavior in mammals. Experientia. 1986;42:257–271. doi: 10.1007/BF01942506. [DOI] [PubMed] [Google Scholar]
- Doty RL, Dunbar I. Attraction of beagles to conspecific urine, vaginal and anal sac secretion odors. Physiol Behav. 1974;12:825–833. doi: 10.1016/0031-9384(74)90020-1. [DOI] [PubMed] [Google Scholar]
- Doty RL, Orndorff MM, Leyden J, Kligman A. Communication of gender from human axillary odors: relationship to perceived intensity and hedonicity. Behavioral biology. 1978;23:373–380. doi: 10.1016/s0091-6773(78)91393-7. [DOI] [PubMed] [Google Scholar]
- Drickamer LC. Delay of sexual maturation in female house mice by exposure to grouped females or urine from grouped females. J Reprod Fertil. 1977;51:77–81. doi: 10.1530/jrf.0.0510077. [DOI] [PubMed] [Google Scholar]
- Drickamer LC. Peripheral anosmia affects puberty-influencing chemosignals in mice: donors and recipients. Physiol Behav. 1986;37:741–746. doi: 10.1016/0031-9384(86)90179-4. [DOI] [PubMed] [Google Scholar]
- Drickamer LC. Long-term effects of accelerated or delayed sexual maturation on reproductive output in wild female house mice (Mus musculus) J Reprod Fertil. 1988;83:439–445. doi: 10.1530/jrf.0.0830439. [DOI] [PubMed] [Google Scholar]
- Drickamer LC, Hoover JE. Effects of urine from pregnant and lactating female house mice on sexual maturation of juvenile females. Dev Psychobiol. 1979;12:545–551. doi: 10.1002/dev.420120604. [DOI] [PubMed] [Google Scholar]
- Drickamer LC, McIntosh TK. Effects of adrenalectomy on the presence of a maturation-delaying pheromone in the urine of female mice. Horm Behav. 1980;14:146–152. doi: 10.1016/0018-506x(80)90006-9. [DOI] [PubMed] [Google Scholar]
- Drickamer LC, McIntosh TK, Rose EA. Effects of ovariectomy on the presence of a maturation-delaying pheromone in the urine of female mice. Horm Behav. 1978;11:131–137. doi: 10.1016/0018-506x(78)90064-8. [DOI] [PubMed] [Google Scholar]
- Drickamer LC, Mikesic DG. Urinary chemosignals, reproduction, and population size for house mice (Mus domesticus) living in field enclosures. Journal of Chemical Ecology. 1990;16:2955–2968. doi: 10.1007/BF00979487. [DOI] [PubMed] [Google Scholar]
- Dudley CA, Moss RL. Lesions of the accessory olfactory bulb decrease lordotic responsiveness and reduce mating-induced c-fos expression in the accessory olfactory system. Brain Res. 1994;642:29–37. doi: 10.1016/0006-8993(94)90902-4. [DOI] [PubMed] [Google Scholar]
- Edwards DA, Griffis KT, Tardivel C. Olfactory bulb removal: effects on sexual behavior and partner-preference in male rats. Physiol Behav. 1990;48:447–450. doi: 10.1016/0031-9384(90)90342-2. [DOI] [PubMed] [Google Scholar]
- Edwards DA, Walter B, Liang P. Hypothalamic and olfactory control of sexual behavior and partner preference in male rats. Physiol Behav. 1996;60:1347–1354. doi: 10.1016/s0031-9384(96)00260-0. [DOI] [PubMed] [Google Scholar]
- Ehman KD, Scott ME. Urinary odour preferences of MHC congenic female mice, Mus domesticus: implications for kin recognition and detection of parasitized males. Animal Behaviour. 2001;62:781–789. [Google Scholar]
- Eidson LN, Maras PM, Epperson E, Petrulis A. Female hamster preference for odors is not regulated by circulating gonadal hormones. Physiol Behav. 2007;91:134–141. doi: 10.1016/j.physbeh.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisthen HL, Wysocki CJ, Beauchamp GK. Behavioral responses of male guinea pigs to conspecific chemical signals following neonatal vomeronasal organ removal. Physiol Behav. 1987;41:445–449. doi: 10.1016/0031-9384(87)90079-5. [DOI] [PubMed] [Google Scholar]
- Fadem BH. Activation of estrus by pheromones in a marsupial: stimulus control and endocrine factors. Biol Reprod. 1987;36:328–332. doi: 10.1095/biolreprod36.2.328. [DOI] [PubMed] [Google Scholar]
- Fan S, Luo M. The organization of feedback projections in a pathway important for processing pheromonal signals. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.03.065. [DOI] [PubMed] [Google Scholar]
- Ferkin M, Johnston R. Effects of pregnancy, lactation and postpartum oestrus on odour signals and the attraction to odours in female meadow voles,< em> Microtus pennsylvanicus</em>. Animal Behaviour. 1995 [Google Scholar]
- Ferkin MH, Combs A, delBarco-Trillo J, Pierce AA, Franklin S. Meadow voles, Microtus pennsylvanicus, have the capacity to recall the “what”, “where”, and “when” of a single past event. Anim Cogn. 2008;11:147–159. doi: 10.1007/s10071-007-0101-8. [DOI] [PubMed] [Google Scholar]
- Ferkin MH, Gorman MR. Photoperiod and gonadal hormones influence odor preferences of the male meadow vole, Microtus pennsylvanicus. Physiol Behav. 1992;51:1087–1091. doi: 10.1016/0031-9384(92)90098-m. [DOI] [PubMed] [Google Scholar]
- Ferkin MH, Gorman MR, Zucker I. Ovarian hormones influence odor cues emitted by female meadow voles, Microtus pennsylvanicus. Horm Behav. 1991;25:572–581. doi: 10.1016/0018-506x(91)90022-a. [DOI] [PubMed] [Google Scholar]
- Ferkin MH, Johnston RE. Roles of gonadal hormones in control of five sexually attractive odors of meadow voles (Microtus pennsylvanicus) Horm Behav. 1993;27:523–538. doi: 10.1006/hbeh.1993.1038. [DOI] [PubMed] [Google Scholar]
- Ferkin MH, Sorokin ES, Johnston RE, Lee CJ. Attractiveness of scents varies with protein content of the diet in meadow voles. Animal Behaviour. 1997;53:133–141. [Google Scholar]
- Ferkin MH, Zucker I. Seasonal control of odour preferences of meadow voles (Microtus pennsylvanicus) by photoperiod and ovarian hormones. J Reprod Fertil. 1991;92:433–441. doi: 10.1530/jrf.0.0920433. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fewell GD, Meredith M. c-fos expression in vomeronasal pathways of mated or pheromone-stimulated male golden hamsters: contributions from vomeronasal sensory input and expression related to mating performance. J Neurosci. 1994;14:3643–3654. doi: 10.1523/JNEUROSCI.14-06-03643.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Fewell GD, Meredith M. Olfactory contribution to Fos expression during mating in inexperienced male hamsters. Chem Senses. 1998;23:257–267. doi: 10.1093/chemse/23.3.257. [DOI] [PubMed] [Google Scholar]
- Fewell GD, Meredith M. Experience facilitates vomeronasal and olfactory influence on Fos expression in medial preoptic area during pheromone exposure or mating in male hamsters. Brain Res. 2002;941:91–106. doi: 10.1016/s0006-8993(02)02613-6. [DOI] [PubMed] [Google Scholar]
- Fiber JM, Swann JM. Testosterone differentially influences sex-specific pheromone-stimulated Fos expression in limbic regions of Syrian hamsters. Horm Behav. 1996;30:455–473. doi: 10.1006/hbeh.1996.0050. [DOI] [PubMed] [Google Scholar]
- Finn PD, Yahr P. Projection from the ventral bed nucleus of the stria terminalis to the retrorubral field in rats and the effects of cells in these areas on mating in male rats versus gerbils. Horm Behav. 2005;47:123–138. doi: 10.1016/j.yhbeh.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Floody OR. Dissociation of hypothalamic effects on ultrasound production and copulation. Physiol Behav. 1989;46:299–307. doi: 10.1016/0031-9384(89)90271-0. [DOI] [PubMed] [Google Scholar]
- Floody OR. Cuts between the septum and preoptic area increase ultrasound production, lordosis, and body weight in female hamsters. Physiol Behav. 1993;54:383–392. doi: 10.1016/0031-9384(93)90127-2. [DOI] [PubMed] [Google Scholar]
- Floody OR. Time course of VMN lesion effects on lordosis and proceptive behavior in female hamsters. Horm Behav. 2002;41:366–376. doi: 10.1006/hbeh.2002.1776. [DOI] [PubMed] [Google Scholar]
- Floody OR, Merkle DA, Cahill TJ, Shopp GM., Jr. Gonadal hormones stimulate ultrasound production by female hamsters. Horm Behav. 1979;12:172–184. doi: 10.1016/0018-506x(79)90019-9. [DOI] [PubMed] [Google Scholar]
- Floody OR, Pfaff DW, Lewis CD. Communication among hamsters by high-frequency acoustic signals: II. Determinants of calling by females and males. Journal of comparative and physiological psychology. 1977;91:807. [Google Scholar]
- Fruhholz S, Grandjean D. Amygdala subregions differentially respond and rapidly adapt to threatening voices. Cortex. 2012 doi: 10.1016/j.cortex.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Furtbauer I, Mundry R, Heistermann M, Schulke O, Ostner J. You mate, I mate: macaque females synchronize sex not cycles. PLoS ONE. 2011;6:e26144. doi: 10.1371/journal.pone.0026144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattermann R, Fritzsche P, Neumann K, Al-Hussein I, Kayser A, Abiad M, Yakti R. Notes on the current distribution and the ecology of wild golden hamsters (Mesocricetus auratus) Journal of Zoology. 2001;254:359–365. [Google Scholar]
- Gelez H, Archer E, Chesneau D, Magallon T, Fabre-Nys C. Inactivation of the olfactory amygdala prevents the endocrine response to male odour in anoestrus ewes. Eur J Neurosci. 2004;19:1581–1590. doi: 10.1111/j.1460-9568.2004.03261.x. [DOI] [PubMed] [Google Scholar]
- Gelez H, Fabre-Nys C. The “male effect” in sheep and goats: a review of the respective roles of the two olfactory systems. Horm Behav. 2004;46:257–271. doi: 10.1016/j.yhbeh.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Gelstein S, Yeshurun Y, Rozenkrantz L, Shushan S, Frumin I, Roth Y, Sobel N. Human tears contain a chemosignal. Science. 2011;331:226–230. doi: 10.1126/science.1198331. [DOI] [PubMed] [Google Scholar]
- Geyer LA, Barfield RJ. Influence of gonadal hormones and sexual behavior on ultrasonic vocalization in rats: I. Treatment of females. J Comp Physiol Psychol. 1978;92:438–446. doi: 10.1037/h0077480. [DOI] [PubMed] [Google Scholar]
- Gildersleeve KA, Haselton MG, Larson CM, Pillsworth EG. Body odor attractiveness as a cue of impending ovulation in women: Evidence from a study using hormone-confirmed ovulation. Hormones and Behavior. 2012;61:157–166. doi: 10.1016/j.yhbeh.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Gomez DM, Newman SW. Differential projections of the anterior and posterior regions of the medial amygdaloid nucleus in the Syrian hamster. J Comp Neurol. 1992;317:195–218. doi: 10.1002/cne.903170208. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal G, Melo AI, Zavala A, Beyer C. Variations in chin-marking behavior of New Zealand female rabbits throughout the whole reproductive cycle. Physiol Behav. 1990;48:361–365. doi: 10.1016/0031-9384(90)90328-2. [DOI] [PubMed] [Google Scholar]
- Goossens L, Kukolja J, Onur OA, Fink GR, Maier W, Griez E, Schruers K, Hurlemann R. Selective processing of social stimuli in the superficial amygdala. Hum Brain Mapp. 2009;30:3332–3338. doi: 10.1002/hbm.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosling LM, Roberts SC, Peter JB, Slater JSRCTS, Timothy JR. Scent-marking by male mammals: Cheat-proof signals to competitors and mates, Advances in the Study of Behavior. Academic. 2001;Press:169–217. [Google Scholar]
- Graham CA, Janssen E, Sanders SA. Effects of fragrance on female sexual arousal and mood across the menstrual cycle. Psychophysiology. 2000;37:76–84. [PubMed] [Google Scholar]
- Graham JM, Desjardins C. Classical conditioning: induction of luteinizing hormone and testosterone secretion in anticipation of sexual activity. Science. 1980;210:1039–1041. doi: 10.1126/science.7434016. [DOI] [PubMed] [Google Scholar]
- Gregory E, Engel K, Pfaff D. Male hamster preference for odors of female hamster vaginal discharges: studies of experiential and hormonal determinants. J Comp Physiol Psychol. 1975;89:442–446. doi: 10.1037/h0077043. [DOI] [PubMed] [Google Scholar]
- Guzzo AC, Berger RG, deCatanzaro D. Excretion and binding of tritium-labelled oestradiol in mice (Mus musculus): implications for the Bruce effect. Reproduction. 2010;139:255–263. doi: 10.1530/REP-09-0382. [DOI] [PubMed] [Google Scholar]
- Guzzo AC, Pollock TJ, Decatanzaro D. Transfer of [3H]estradiol-17beta and [3H]progesterone from conspecifics to cohabiting female mice. J Endocrinol. 2013 doi: 10.1530/JOE-12-0279. [DOI] [PubMed] [Google Scholar]
- Haga S, Hattori T, Sato T, Sato K, Matsuda S, Kobayakawa R, Sakano H, Yoshihara Y, Kikusui T, Touhara K. The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature. 2010;466:118–122. doi: 10.1038/nature09142. [DOI] [PubMed] [Google Scholar]
- Halem HA, Cherry JA, Baum MJ. Vomeronasal neuroepithelium and forebrain Fos responses to male pheromones in male and female mice. J Neurobiol. 1999;39:249–263. [PubMed] [Google Scholar]
- Halpern M, Martinez-Marcos A. Structure and function of the vomeronasal system: an update. Prog Neurobiol. 2003;70:245–318. doi: 10.1016/s0301-0082(03)00103-5. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Kimura T. Sex-attractant emitted by female mice. Physiol Behav. 1974;13:563–567. doi: 10.1016/0031-9384(74)90287-x. [DOI] [PubMed] [Google Scholar]
- He J, Ma L, Kim S, Nakai J, Yu CR. Encoding gender and individual information in the mouse vomeronasal organ. Science. 2008;320:535–538. doi: 10.1126/science.1154476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeb MM, Yahr P. Cell-body lesions of the posterodorsal preoptic nucleus or posterodorsal medial amygdala, but not the parvicellular subparafascicular thalamus, disrupt mating in male gerbils. Physiol Behav. 2000;68:317–331. doi: 10.1016/s0031-9384(99)00182-1. [DOI] [PubMed] [Google Scholar]
- Heske EJ, Nelson RJ. Pregnancy interruption in Microtus ochrogaster: laboratory artifact or field phenomenon? Biol Reprod. 1984;31:97–103. doi: 10.1095/biolreprod31.1.97. [DOI] [PubMed] [Google Scholar]
- Heth G, Todrank J, Johnston RE. Kin recognition in golden hamsters: evidence for phenotype matching. Anim Behav. 1998;56:409–417. doi: 10.1006/anbe.1998.0747. [DOI] [PubMed] [Google Scholar]
- Hosokawa N, Chiba A. Effects of sexual experience on conspecific odor preference and estrous odor-induced activation of the vomeronasal projection pathway and the nucleus accumbens in male rats. Brain Res. 2005;1066:101–108. doi: 10.1016/j.brainres.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Hosokawa N, Chiba A. Effects of sexual experience on conspecific odor preference and male odor-induced activation of the vomeronasal projection pathway and the nucleus accumbens in female rats. Brain Res. 2007;1175:66–75. doi: 10.1016/j.brainres.2007.07.071. [DOI] [PubMed] [Google Scholar]
- Hosokawa N, Chiba A. Androgen receptor blockade in the posterodorsal medial amygdala impairs sexual odor preference in male rats. Horm Behav. 2010;58:493–500. doi: 10.1016/j.yhbeh.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Huck UW, Banks EM. The effects of cross-fostering on the behaviour of two species of North American lemmings, dicrostonyx groenlandicus and lemmus trimucronatus: II. Sexual behaviour. Animal Behaviour. 1980;28:1053–1062. [Google Scholar]
- Hurst JL. Urine marking in populations of wild house mice Mus domesticus Rutty. III. Communication between the sexes. Animal Behaviour. 1990;40:233–243. [Google Scholar]
- Hurst JL, Beynon RJ. Scent wars: the chemobiology of competitive signalling in mice. Bioessays. 2004;26:1288–1298. doi: 10.1002/bies.20147. [DOI] [PubMed] [Google Scholar]
- Hurst JL, Beynon RJ, Roberts SC, Wyatt TD. Chemical Signals in Vertebrates. . Springer; New York, NY: 2008. p. 11. [Google Scholar]
- Hurtazo HA, Paredes RG. Olfactory preference and Fos expression in the accessory olfactory system of male rats with bilateral lesions of the medial preoptic area/anterior hypothalamus. Neuroscience. 2005;135:1035–1044. doi: 10.1016/j.neuroscience.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Hurtazo HA, Paredes RG, Agmo A. Inactivation of the medial preoptic area/anterior hypothalamus by lidocaine reduces male sexual behavior and sexual incentive motivation in male rats. Neuroscience. 2008;152:331–337. doi: 10.1016/j.neuroscience.2007.10.063. [DOI] [PubMed] [Google Scholar]
- Imondi RL, Floody OR. Separation of septal influences on lordosis, ultrasound production, and body weight. Physiol Behav. 1998;63:481–488. doi: 10.1016/s0031-9384(97)00483-6. [DOI] [PubMed] [Google Scholar]
- Ingersoll DW, Launay J. Murine aggression induced by a boar chemosignal: a stimulus presentation dependency. Physiol Behav. 1986;36:263–269. doi: 10.1016/0031-9384(86)90014-4. [DOI] [PubMed] [Google Scholar]
- Iwata E, Wakabayashi Y, Kakuma Y, Kikusui T, Takeuchi Y, Mori Y. Testosterone-dependent primer pheromone production in the sebaceous gland of male goat. Biol Reprod. 2000;62:806–810. doi: 10.1095/biolreprod62.3.806. [DOI] [PubMed] [Google Scholar]
- Jackson LM, Harder JD. Vomeronasal organ removal blocks pheromonal induction of estrus in gray short-tailed opossums (Monodelphis domestica) Biol Reprod. 1996;54:506–512. doi: 10.1095/biolreprod54.2.506. [DOI] [PubMed] [Google Scholar]
- Jacob S, Hayreh DJ, McClintock MK. Context-dependent effects of steroid chemosignals on human physiology and mood. Physiol Behav. 2001;74:15–27. doi: 10.1016/s0031-9384(01)00537-6. [DOI] [PubMed] [Google Scholar]
- Jacob S, McClintock MK. Psychological state and mood effects of steroidal chemosignals in women and men. Horm Behav. 2000;37:57–78. doi: 10.1006/hbeh.1999.1559. [DOI] [PubMed] [Google Scholar]
- Jemiolo B, Alberts J, Sochinski-Wiggins S, Harvey S, Novotny M. Behavioural and endocrine responses of female mice to synthetic analogues of volatile compounds in male urine. Animal Behaviour. 1985;33:1114–1118. [Google Scholar]
- Jemiolo B, Andreolini F, Xie TM, Wiesler D, Novotny M. Puberty-affecting synthetic analogs of urinary chemosignals in the house mouse, Mus domesticus. Physiol Behav. 1989;46:293–298. doi: 10.1016/0031-9384(89)90270-9. [DOI] [PubMed] [Google Scholar]
- Jemiolo B, Harvey S, Novotny M. Promotion of the Whitten effect in female mice by synthetic analogs of male urinary constituents. Proc Natl Acad Sci U S A. 1986;83:4576–4579. doi: 10.1073/pnas.83.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemiolo B, Novotny M. Inhibition of sexual maturation in juvenile female and male mice by a chemosignal of female origin. Physiol Behav. 1994;55:519–522. doi: 10.1016/0031-9384(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Jemiolo B, Xie TM, Novotny M. Socio-sexual olfactory preference in female mice: attractiveness of synthetic chemosignals. Physiol Behav. 1991;50:1119–1122. doi: 10.1016/0031-9384(91)90570-e. [DOI] [PubMed] [Google Scholar]
- Johns MA, Feder HH, Komisaruk BR, Mayer AD. Urine-induced reflex ovulation in anovulatory rats may be a vomeronasal effect. Nature. 1978;272:446–448. doi: 10.1038/272446a0. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Sexual attraction function of golden hamster vaginal secretion. Behav Biol. 1974;12:111–117. doi: 10.1016/s0091-6773(74)91101-8. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Sexual excitation function of hamster vaginal secretion. Anim Learn Behav. 1975;3:161–166. [PubMed] [Google Scholar]
- Johnston RE. The causation of two scent-marking behaviour patterns in female hamsters (Mesocricetus auratus) Anim Behav. 1977;25:317–327. doi: 10.1016/0003-3472(77)90007-0. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Olfactory preferences, scent marking, and “proceptivity” in female hamsters. Horm Behav. 1979;13:21–39. doi: 10.1016/0018-506x(79)90032-1. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Effects of female odors on the sexual behavior of male hamsters. Behav Neural Biol. 1986;46:168–188. doi: 10.1016/s0163-1047(86)90654-0. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Vomeronasal and/or olfactory mediation of ultrasonic calling and scent marking by female golden hamsters. Physiol Behav. 1992;51:437–448. doi: 10.1016/0031-9384(92)90163-v. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Individual Odors and Social Communication: Individual Recognition, Kin Recognition, and Scent Over-Marking. In: Jane Brockmann H, John TJRMNKEW-ECB, M C, editors. Advances in the Study of Behavior. Academic Press; 2008. pp. 439–505. [Google Scholar]
- Johnston RE, Brenner D. Species-specificity of scent marking in hamsters. Behavioral and Neural Biology. 1982;35:46–55. [Google Scholar]
- Johnston RE, Bronson F. Endocrine control of female mouse odors that elicit luteinizing hormone surges and attraction in males. Biol Reprod. 1982;27:1174–1180. doi: 10.1095/biolreprod27.5.1174. [DOI] [PubMed] [Google Scholar]
- Johnston RE, Coplin B. Development of responses to vaginal secretion and other substances in golden hamsters. Behavioral and Neural Biology. 1979;25:473–489. doi: 10.1016/s0163-1047(79)90242-5. [DOI] [PubMed] [Google Scholar]
- Johnston RE, Kwan M. Vaginal scent marking: effects on ultrasonic calling and attraction of male golden hamsters. Behav Neural Biol. 1984;42:158–168. doi: 10.1016/s0163-1047(84)91007-0. [DOI] [PubMed] [Google Scholar]
- Johnston RE, Zahorik DM. Taste aversions to sexual attractants. Science. 1975;189:893–894. doi: 10.1126/science.1154027. [DOI] [PubMed] [Google Scholar]
- Johnston RE, Zahorik DM, Immler K, Zakon H. Alterations of male sexual behavior by learned aversions to hamster vaginal secretion. J Comp Physiol Psychol. 1978;92:85–93. doi: 10.1037/h0077428. [DOI] [PubMed] [Google Scholar]
- Kairys DJ, Magalhaes H, Floody OR. Olfactory bulbectomy depresses ultrasound production and scent marking by female hamsters. Physiol Behav. 1980;25:143–146. doi: 10.1016/0031-9384(80)90195-x. [DOI] [PubMed] [Google Scholar]
- Kaneko N, Debski EA, Wilson MC, Whitten WK. Puberty acceleration in mice. II. Evidence that the vomeronasal organ isa receptor for the primer pheromone in male mouse urine. Biol Reprod. 1980;22:873–878. doi: 10.1095/biolreprod22.4.873. [DOI] [PubMed] [Google Scholar]
- Kang N, Baum MJ, Cherry JA. A direct main olfactory bulb projection to the ’vomeronasal’ amygdala in female mice selectively responds to volatile pheromones from males. Eur J Neurosci. 2009;29:624–634. doi: 10.1111/j.1460-9568.2009.06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlson P, Luscher M. Pheromones’: a new term for a class of biologically active substances. Nature. 1959;183:55–56. doi: 10.1038/183055a0. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Choleris E, Colwell DD. Brief exposure to female odors “emboldens” male mice by reducing predator-induced behavioral and hormonal responses. Horm Behav. 2001;40:497–509. doi: 10.1006/hbeh.2001.1714. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Colwell DD. Discrimination by female mice between the odours of parasitized and non-parasitized males. Proc Biol Sci. 1995;261:31–35. doi: 10.1098/rspb.1995.0113. [DOI] [PubMed] [Google Scholar]
- Kay E, Nyby J. LiCl aversive conditioning has transitory effects on pheromonal responsiveness in male house mice (Mus domesticus) Physiol Behav. 1992;52:105–113. doi: 10.1016/0031-9384(92)90439-9. [DOI] [PubMed] [Google Scholar]
- Kelche C, Aron C. Olfactory cues and accessory olfactory bulb lesion: effects on sexual behavior in the cyclic female rat. Physiol Behav. 1984;33:45–48. doi: 10.1016/0031-9384(84)90011-8. [DOI] [PubMed] [Google Scholar]
- Keller M, Douhard Q, Baum MJ, Bakker J. Destruction of the main olfactory epithelium reduces female sexual behavior and olfactory investigation in female mice. Chem Senses. 2006a;31:315–323. doi: 10.1093/chemse/bjj035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Douhard Q, Baum MJ, Bakker J. Sexual experience does not compensate for the disruptive effects of zinc sulfate--lesioning of the main olfactory epithelium on sexual behavior in male mice. Chem Senses. 2006b;31:753–762. doi: 10.1093/chemse/bjl018. [DOI] [PubMed] [Google Scholar]
- Keller M, Pierman S, Douhard Q, Baum MJ, Bakker J. The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice. Eur J Neurosci. 2006c;23:521–530. doi: 10.1111/j.1460-9568.2005.04589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher K, Baum M. Effect of sex steroids and coital experience on ferrets’ preference for the smell, sight and sound of conspecifics. Physiol Behav. 2002;76:1–7. doi: 10.1016/s0031-9384(02)00691-1. [DOI] [PubMed] [Google Scholar]
- Kelliher KR, Baum MJ. Nares occlusion eliminates heterosexual partner selection without disrupting coitus in ferrets of both sexes. J Neurosci. 2001;21:5832–5840. doi: 10.1523/JNEUROSCI.21-15-05832.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher KR, Chang YM, Wersinger SR, Baum MJ. Sex difference and testosterone modulation of pheromone-induced NeuronalFos in the Ferret’s main olfactory bulb and hypothalamus. Biol Reprod. 1998;59:1454–1463. doi: 10.1095/biolreprod59.6.1454. [DOI] [PubMed] [Google Scholar]
- Kempenaers B, Peters A, Foerster K. Sources of individual variation in plasma testosterone levels. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363:1711–1723. doi: 10.1098/rstb.2007.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick KM, Da Costa AP, Broad KD, Ohkura S, Guevara R, Levy F, Keverne EB. Neural control of maternal behaviour and olfactory recognition of offspring. Brain Res Bull. 1997;44:383–395. doi: 10.1016/s0361-9230(97)00218-9. [DOI] [PubMed] [Google Scholar]
- Keverne EB, de la Riva C. Pheromones in mice: reciprocal interaction between the nose and brain. Nature. 1982;296:148–150. doi: 10.1038/296148a0. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. I. Efferents of the “vomeronasal amygdala”. J Comp Neurol. 1981a;197:81–98. doi: 10.1002/cne.901970107. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. II. Efferents of the “olfactory amygdala”. J Comp Neurol. 1981b;197:99–111. doi: 10.1002/cne.901970108. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Graham JE, Malarkey WB, Porter K, Lemeshow S, Glaser R. Olfactory influences on mood and autonomic, endocrine, and immune function. Psychoneuroendocrinology. 2008;33:328–339. doi: 10.1016/j.psyneuen.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin TE, Cain SW, Pfaus JG. Estrous odors and sexually conditioned neutral odors activate separate neural pathways in the male rat. Neuroscience. 2003;117:971–979. doi: 10.1016/s0306-4522(02)00972-7. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Pfaus JG. The development of olfactory conditioned ejaculatory preferences in the male rat. I. Nature of the unconditioned stimulus. Physiol Behav. 2001;73:457–469. doi: 10.1016/s0031-9384(01)00484-x. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Samaha AN, Sotiropoulos V, Pfaus JG. The development of olfactory conditioned ejaculatory preferences in the male rat. II. Parametric manipulation of conditioning session number and duration. Physiol Behav. 2001;73:471–485. doi: 10.1016/s0031-9384(01)00485-1. [DOI] [PubMed] [Google Scholar]
- Kirn J, Floody OR. Differential effects of lesions in three limbic areas on ultrasound production and lordosis by female hamsters. Behav Neurosci. 1985;99:1142–1152. doi: 10.1037//0735-7044.99.6.1142. [DOI] [PubMed] [Google Scholar]
- Knight TW, Lindsay DR, Oldham CM. Proceedings: the influence of rams on the fertility of the ewe. J Reprod Fertil. 1975;43:377–378. doi: 10.1530/jrf.0.0430377. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Arai Y. Functional association between the medial amygdala and the medial preoptic area in regulation of mating behavior in the male rat. Physiol Behav. 1995;57:69–73. doi: 10.1016/0031-9384(94)00205-j. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Sachs BD. Disparate effects of small medial amygdala lesions on noncontact erection, copulation, and partner preference. Physiol Behav. 2002;76:443–447. doi: 10.1016/s0031-9384(02)00682-0. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Sachs BD, Sakuma Y. Importance of the medial amygdala in rat penile erection evoked by remote stimuli from estrous females. Behav Brain Res. 1998;91:215–222. [PubMed] [Google Scholar]
- Kondo Y, Sakuma Y. The medial amygdala controls the coital access of female rats: a possible involvement of emotional responsiveness. Jpn J Physiol. 2005;55:345–353. doi: 10.2170/jjphysiol.RP001105. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Tomihara K, Sakuma Y. Sensory requirements for noncontact penile erection in the rat. Behav Neurosci. 1999;113:1062–1070. doi: 10.1037//0735-7044.113.5.1062. [DOI] [PubMed] [Google Scholar]
- Koyama S. Primer effects by conspecific odors in house mice: a new perspective in the study of primer effects on reproductive activities. Horm Behav. 2004;46:303–310. doi: 10.1016/j.yhbeh.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Koyama S, Kamimura S. Influence of social dominance and female odor on the sperm activity of male mice. Physiol Behav. 2000;71:415–422. doi: 10.1016/s0031-9384(00)00361-9. [DOI] [PubMed] [Google Scholar]
- Kwan M, Johnston RE. The role of vaginal secretion in hamster sexual behavior: males’ responses to normal and vaginectomized females and their odors. J Comp Physiol Psychol. 1980;94:905–913. doi: 10.1037/h0077814. [DOI] [PubMed] [Google Scholar]
- Lanuza E, Novejarque A, Martinez-Ricos J, Martinez-Hernandez J, Agustin-Pavon C, Martinez-Garcia F. Sexual pheromones and the evolution of the reward system of the brain: the chemosensory function of the amygdala. Brain Res Bull. 2008;75:460–466. doi: 10.1016/j.brainresbull.2007.10.042. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Winans SS, Powers JB. Medial nucleus of the amygdala mediates chemosensory control of male hamster sexual behavior. Science. 1980;210:557–560. doi: 10.1126/science.7423209. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Brennan P, Widmayer P, S PC, Maul-Pavicic A, Jager M, Li XH, Breer H, Zufall F, Boehm T. MHC class I peptides as chemosensory signals in the vomeronasal organ. Science. 2004;306:1033–1037. doi: 10.1126/science.1102818. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Lane AP, Puche AC, Ma W, Novotny MV, Shipley MT, Zufall F. Ultrasensitive pheromone detection by mammalian vomeronasal neurons. Nature. 2000;405:792–796. doi: 10.1038/35015572. [DOI] [PubMed] [Google Scholar]
- Lepri JJ, Vandenbergh JG. Puberty in pine voles, Microtus pinetorum, and the influence of chemosignals on female reproduction. Biol Reprod. 1986;34:370–377. doi: 10.1095/biolreprod34.2.370. [DOI] [PubMed] [Google Scholar]
- Lepri JJ, Wysocki CJ. Removal of the vomeronasal organ disrupts the activation of reproduction in female voles. Physiol Behav. 1987;40:349–355. doi: 10.1016/0031-9384(87)90058-8. [DOI] [PubMed] [Google Scholar]
- Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci U S A. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Kaba H, Saito H, Seto K. Neural mechanisms underlying the action of primer pheromones in mice. Neuroscience. 1990;36:773–778. doi: 10.1016/0306-4522(90)90019-z. [DOI] [PubMed] [Google Scholar]
- Lin DY, Zhang SZ, Block E, Katz LC. Encoding social signals in the mouse main olfactory bulb. Nature. 2005;434:470–477. doi: 10.1038/nature03414. [DOI] [PubMed] [Google Scholar]
- Lisk RD, Nachtigall MJ. Estrogen regulation of agonistic and proceptive responses in the golden hamster. Horm Behav. 1988;22:35–48. doi: 10.1016/0018-506x(88)90029-3. [DOI] [PubMed] [Google Scholar]
- Liu YC, Salamone JD, Sachs BD. Lesions in medial preoptic area and bed nucleus of stria terminalis: differential effects on copulatory behavior and noncontact erection in male rats. J Neurosci. 1997;17:5245–5253. doi: 10.1523/JNEUROSCI.17-13-05245.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Thomas A, Keverne EB. Role of the brain and accessory olfactory system in the block to pregnancy in mice. Neuroscience. 1982;7:907–913. doi: 10.1016/0306-4522(82)90051-3. [DOI] [PubMed] [Google Scholar]
- Lomas DE, Keverne EB. Role of the vomeronasal organ and prolactin in the acceleration of puberty in female mice. J Reprod Fertil. 1982;66:101–107. doi: 10.1530/jrf.0.0660101. [DOI] [PubMed] [Google Scholar]
- Lydell K, Doty RL. Male rat of odor preferences for female urine as a function of sexual experience, urine age, and urine source. Horm Behav. 1972;3:205–212. doi: 10.1016/0018-506x(72)90033-5. [DOI] [PubMed] [Google Scholar]
- Ma W, Miao Z, Novotny MV. Role of the adrenal gland and adrenal-mediated chemosignals in suppression of estrus in the house mouse: the lee-boot effect revisited. Biol Reprod. 1998;59:1317–1320. doi: 10.1095/biolreprod59.6.1317. [DOI] [PubMed] [Google Scholar]
- Macrides F, Bartke A, Dalterio S. Strange females increase plasma testosterone levels in male mice. Science. 1975;189:1104–1106. doi: 10.1126/science.1162363. [DOI] [PubMed] [Google Scholar]
- Macrides F, Bartke A, Fernandez F, D’Angelo W. Effects of exposure to vaginal odor and receptive females on plasma testosterone in the male hamster. Neuroendocrinology. 1974;15:355–364. doi: 10.1159/000122326. [DOI] [PubMed] [Google Scholar]
- Macrides F, Clancy AN, Singer AG, Agosta WC. Male hamster investigatory and copulatory responses to vaginal discharge: an attempt to impart sexual significance to an arbitrary chemosensory stimulus. Physiol Behav. 1984a;33:627–632. doi: 10.1016/0031-9384(84)90382-2. [DOI] [PubMed] [Google Scholar]
- Macrides F, Singer AG, Clancy AN, Goldman BD, Agosta WC. Male hamster investigatory and copulatory responses to vaginal discharge: relationship to the endocrine status of females. Physiol Behav. 1984b;33:633–637. doi: 10.1016/0031-9384(84)90383-4. [DOI] [PubMed] [Google Scholar]
- Mahady S, Wolff J. A field test of the Bruce effect in the monogamous prairie vole (Microtus ochrogaster) Behavioral Ecology and Sociobiology. 2002;52:31–37. [Google Scholar]
- Malsbury CW, Kow LM, Pfaff DW. Effects of medial hypothalamic lesions on the lordosis response and other behaviors in remale golden hamsters. Physiol Behav. 1977;19:223–237. doi: 10.1016/0031-9384(77)90331-6. [DOI] [PubMed] [Google Scholar]
- Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat Neurosci. 2005;8:1660–1662. doi: 10.1038/nn1589. [DOI] [PubMed] [Google Scholar]
- Manzo J, Cruz MR, Hernandez ME, Pacheco P, Sachs BD. Regulation of noncontact erection in rats by gonadal steroids. Horm Behav. 1999;35:264–270. doi: 10.1006/hbeh.1999.1519. [DOI] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. Chemosensory and steroid-responsive regions of the medial amygdala regulate distinct aspects of opposite-sex odor preference in male Syrian hamsters. Eur J Neurosci. 2006;24:3541–3552. doi: 10.1111/j.1460-9568.2006.05216.x. [DOI] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. Olfactory experience and the development of odor preference and vaginal marking in female Syrian hamsters. Physiol Behav. 2008a;94:545–551. doi: 10.1016/j.physbeh.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. The posteromedial cortical amygdala regulates copulatory behavior, but not sexual odor preference, in the male Syrian hamster (Mesocricetus auratus) Neuroscience. 2008b;156:425–435. doi: 10.1016/j.neuroscience.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. Anatomical connections between the anterior and posterodorsal sub-regions of the medial amygdala: integration of odor and hormone signals. Neuroscience. 2010a;170:610–622. doi: 10.1016/j.neuroscience.2010.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. The anterior medial amygdala transmits sexual odor information to the posterior medial amygdala and related forebrain nuclei. Eur J Neurosci. 2010b;32:469–482. doi: 10.1111/j.1460-9568.2010.07289.x. [DOI] [PubMed] [Google Scholar]
- Marchlewska-Koj A, Jemiolo Evidence for the involvement of dopaminergic neurons in the pregnancy block effect. Neuroendocrinology. 1978;26:186–192. doi: 10.1159/000122780. [DOI] [PubMed] [Google Scholar]
- Marsden HM, Bronson FH. Estrous Synchrony in Mice: Alteration by Exposure to Male Urine. Science. 1964;144:1469. doi: 10.1126/science.144.3625.1469. [DOI] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. Sexually dimorphic activation of the accessory, but not the main, olfactory bulb in mice by urinary volatiles. Eur J Neurosci. 2007;26:463–475. doi: 10.1111/j.1460-9568.2007.05651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. Adult Testosterone Treatment But Not Surgical Disruption of Vomeronasal Function Augments Male-Typical Sexual Behavior in Female Mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009a;29:7658–7666. doi: 10.1523/JNEUROSCI.1311-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. A centrifugal pathway to the mouse accessory olfactory bulb from the medial amygdala conveys gender-specific volatile pheromonal signals. Eur J Neurosci. 2009b;29:368–376. doi: 10.1111/j.1460-9568.2008.06564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LA, Albers HE, Petrulis A. Blocking oxytocin receptors inhibits vaginal marking to male odors in female Syrian hamsters. Physiol Behav. 2010a doi: 10.1016/j.physbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LA, Albers HE, Petrulis A. Blocking oxytocin receptors inhibits vaginal marking to male odors in female Syrian hamsters. Physiol Behav. 2010b;101:685–692. doi: 10.1016/j.physbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LA, Petrulis A. The bed nucleus of the stria terminalis is critical for sexual solicitation, but not for opposite-sex odor preference, in female Syrian hamsters. Horm Behav. 2011;60:651–659. doi: 10.1016/j.yhbeh.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LA, Petrulis A. The medial preoptic area is necessary for sexual odor preference, but not sexual solicitation, in female Syrian hamsters. Horm Behav. 2013 doi: 10.1016/j.yhbeh.2013.02.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrtinez-Garcia F, Martinez-Ricos J, Agustin-Pavon C, Martinez-Hernandez J, Novejarque A, Lanuza E. Refining the dual olfactory hypothesis: pheromone reward and odour experience. Behav Brain Res. 2009;200:277–286. doi: 10.1016/j.bbr.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez J, Lanuza E, Martinez-Garcia F. Selective dopaminergic lesions of the ventral tegmental area impair preference for sucrose but not for male sexual pheromones in female mice. Eur J Neurosci. 2006;24:885–893. doi: 10.1111/j.1460-9568.2006.04944.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez J, Lanuza E, Martinez-Garcia F. Lesions of the dopaminergic innervation of the nucleus accumbens medial shell delay the generation of preference for sucrose, but not of sexual pheromones. Behav Brain Res. 2012;226:538–547. doi: 10.1016/j.bbr.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Martinez-Marcos A. On the organization of olfactory and vomeronasal cortices. Prog Neurobiol. 2009;87:21–30. doi: 10.1016/j.pneurobio.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Martinez-Ricos J, Agustin-Pavon C, Lanuza E, Martinez-Garcia F. Role of the vomeronasal system in intersexual attraction in female mice. Neuroscience. 2008;153:383–395. doi: 10.1016/j.neuroscience.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Maruniak JA, Bronson FH. Gonadotropic responses of male mice to female urine. Endocrinology. 1976;99:963–969. doi: 10.1210/endo-99-4-963. [DOI] [PubMed] [Google Scholar]
- Maruniak JA, Coquelin A, Bronson FH. The release of LH in male mice in response to female urinary odors: characteristics of the response in young males. Biol Reprod. 1978;18:251–255. doi: 10.1095/biolreprod18.2.251. [DOI] [PubMed] [Google Scholar]
- Mason RT, LeMaster MP, Müller-Schwarze D. Chemical Signals in Vertebrates. Springer; Boston, MA: 2005. p. 10. [Google Scholar]
- Massey A, Vandenbergh JG. Puberty delay by a urinary cue from female house mice in feral populations. Science. 1980;209:821–822. doi: 10.1126/science.7190728. [DOI] [PubMed] [Google Scholar]
- Massey A, Vandenbergh JG. Puberty acceleration by a urinary cue from male mice in feral populations. Biol Reprod. 1981;24:523–527. doi: 10.1095/biolreprod24.3.523. [DOI] [PubMed] [Google Scholar]
- Matochik JA, Barfield RJ. Hormonal control of precopulatory sebaceous scent marking and ultrasonic mating vocalizations in male rats. Horm Behav. 1991;25:445–460. doi: 10.1016/0018-506x(91)90013-8. [DOI] [PubMed] [Google Scholar]
- Matochik JA, White NR, Barfield RJ. Variations in scent marking and ultrasonic vocalizations by Long-Evans rats across the estrous cycle. Physiol Behav. 1992;51:783–786. doi: 10.1016/0031-9384(92)90116-j. [DOI] [PubMed] [Google Scholar]
- Matsumoto-Oda A, Hamai M, Hayaki H, Hosaka K, Hunt KD, Kasuya E, Kawanaka K, Mitani JC, Takasaki H, Takahata Y. Estrus cycle asynchrony in wild female chimpanzees, Pan troglodytes schweinfurthii. Behavioral Ecology and Sociobiology. 2007;61:661–668. [Google Scholar]
- McClintock MK. Menstrual synchorony and suppression. Nature. 1971;229:244–245. doi: 10.1038/229244a0. [DOI] [PubMed] [Google Scholar]
- McClintock MK. Estrous synchrony: modulation of ovarian cycle length by female pheromones. Physiol Behav. 1984;32:701–705. doi: 10.1016/0031-9384(84)90181-1. [DOI] [PubMed] [Google Scholar]
- McClintock MK. Pheromones, odors and vasanas: the neuroendocrinology of social chemosignals in humans and animals. In: Pfaff DW, Arnold A, Cardoso A, Blake D, Newcomer S, Quimby R, editors. Hormones, Brain and Behavior. Elsevier; New York: 2002. pp. 797–870. [Google Scholar]
- McClintock MK, Adler NT. Induction of persistent estrus by airborne chemical communication among female rats. Horm Behav. 1978;11:414–418. doi: 10.1016/0018-506x(78)90041-7. [DOI] [PubMed] [Google Scholar]
- McCoy NL, Pitino L. Pheromonal influences on sociosexual behavior in young women. Physiol Behav. 2002;75:367–375. doi: 10.1016/s0031-9384(01)00675-8. [DOI] [PubMed] [Google Scholar]
- McGregor A, Herbert J. Differential effects of excitotoxic basolateral and corticomedial lesions of the amygdala on the behavioural and endocrine responses to either sexual or aggression-promoting stimuli in the male rat. Brain Res. 1992;574:9–20. doi: 10.1016/0006-8993(92)90793-9. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Drickamer LC. Excreted urine, bladder urine, and the delay of sexual maturation in female house mice. Anim Behav. 1977;25:999–1004. doi: 10.1016/0003-3472(77)90051-3. [DOI] [PubMed] [Google Scholar]
- Meek LR, Lee TM, Rogers EA, Hernandez RG. Effect of vomeronasal organ removal on behavioral estrus and mating latency in female meadow voles (Microtus pennsylvanicus) Biol Reprod. 1994;51:400–404. doi: 10.1095/biolreprod51.3.400. [DOI] [PubMed] [Google Scholar]
- Meek LR, Romeo RD, Novak CM, Sisk CL. Actions of testosterone in prepubertal and postpubertal male hamsters: dissociation of effects on reproductive behavior and brain androgen receptor immunoreactivity. Horm Behav. 1997;31:75–88. doi: 10.1006/hbeh.1997.1371. [DOI] [PubMed] [Google Scholar]
- Melrose DR, Reed HC, Patterson RL. Androgen steroids associated with boar odour as an aid to the detection of oestrus in pig artificial insemination. Br Vet J. 1971;127:497–502. doi: 10.1016/s0007-1935(17)37337-2. [DOI] [PubMed] [Google Scholar]
- Meredith M. Vomeronasal organ removal before sexual experience impairs male hamster mating behavior. Physiol Behav. 1986;36:737–743. doi: 10.1016/0031-9384(86)90362-8. [DOI] [PubMed] [Google Scholar]
- Meredith M. Human vomeronasal organ function: a critical review of best and worst cases. Chem Senses. 2001;26:433–445. doi: 10.1093/chemse/26.4.433. [DOI] [PubMed] [Google Scholar]
- Meredith M, Fernandez-Fewell G. Vomeronasal system, LHRH, and sex behaviour. Psychoneuroendocrinology. 1994;19:657–672. doi: 10.1016/0306-4530(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Meredith M, Westberry JM. Distinctive responses in the medial amygdala to same-species and different-species pheromones. J Neurosci. 2004;24:5719–5725. doi: 10.1523/JNEUROSCI.1139-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Maner JK. Scent of a Woman: Men’s Testosterone Responses to Olfactory Ovulation Cues. Psychological science. 2010;21:276–283. doi: 10.1177/0956797609357733. [DOI] [PubMed] [Google Scholar]
- Milligan SR. Pregnancy blocking in the vole, Microtus agrestis. I. Effect of the social environment. J Reprod Fertil. 1976;46:91–95. doi: 10.1530/jrf.0.0460091. [DOI] [PubMed] [Google Scholar]
- Mohedano-Moriano A, de la Rosa-Prieto C, Saiz-Sanchez D, Ubeda-Banon I, Pro-Sistiaga P, de Moya-Pinilla M, Martinez-Marcos A. Centrifugal telencephalic afferent connections to the main and accessory olfactory bulbs. Front Neuroanat. 2012;6:19. doi: 10.3389/fnana.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncho-Bogani J, Lanuza E, Hernandez A, Novejarque A, Martinez-Garcia F. Attractive properties of sexual pheromones in mice: innate or learned? Physiol Behav. 2002;77:167–176. doi: 10.1016/s0031-9384(02)00842-9. [DOI] [PubMed] [Google Scholar]
- Moncho-Bogani J, Lanuza E, Lorente MJ, Martinez-Garcia F. Attraction to male pheromones and sexual behaviour show different regulatory mechanisms in female mice. Physiol Behav. 2004;81:427–434. doi: 10.1016/j.physbeh.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Monti-Bloch L, Grosser BI. Effect of putative pheromones on the electrical activity of the human vomeronasal organ and olfactory epithelium. J Steroid Biochem Mol Biol. 1991;39:573–582. doi: 10.1016/0960-0760(91)90255-4. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends in neurosciences. 1989;12:459–462. doi: 10.1016/0166-2236(89)90096-9. [DOI] [PubMed] [Google Scholar]
- Moss M, Cook J, Wesnes K, Duckett P. Aromas of rosemary and lavender essential oils differentially affect cognition and mood in healthy adults. Int J Neurosci. 2003;113:15–38. doi: 10.1080/00207450390161903. [DOI] [PubMed] [Google Scholar]
- Mossman CA, Drickamer LC. Odor preferences of female house mice (Mus domesticus) in seminatural enclosures. J Comp Psychol. 1996;110:131–138. doi: 10.1037/0735-7036.110.2.131. [DOI] [PubMed] [Google Scholar]
- Mucignat-Caretta C, Caretta A, Cavaggioni A. Acceleration of puberty onset in female mice by male urinary proteins. J Physiol. 1995;486(Pt 2):517–522. doi: 10.1113/jphysiol.1995.sp020830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K, Wakabayashi Y, Kitago M, Ohara H, Watanabe H, Tamogami S, Warita Y, Yamagishi K, Ichikawa M, Takeuchi Y, Okamura H, Mori Y. Modulation of gonadotrophin-releasing hormone pulse generator activity by the pheromone in small ruminants. J Neuroendocrinol. 2009;21:346–350. doi: 10.1111/j.1365-2826.2009.01836.x. [DOI] [PubMed] [Google Scholar]
- Murphy MR. Effects of female hamster vaginal discharge on the behavior of male hamsters. Behav Biol. 1973;9:367–375. doi: 10.1016/s0091-6773(73)80185-3. [DOI] [PubMed] [Google Scholar]
- Murphy MR, Schneider GE. Olfactory bulb removal eliminates mating behavior in the male golden hamster. Science. 1970;167:302–304. doi: 10.1126/science.167.3916.302. [DOI] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Utsumi K, Yuhara M, Fujitani Y, Iritani A. Identification of puberty-accelerating pheromones in male mouse urine. J Exp Zool. 1989;251:300–305. doi: 10.1002/jez.1402510306. [DOI] [PubMed] [Google Scholar]
- Nixon A, Mallet AI, Gower DB. Simultaneous quantification of five odorous steroids (16-androstenes) in the axillary hair of men. J Steroid Biochem. 1988;29:505–510. doi: 10.1016/0022-4731(88)90185-9. [DOI] [PubMed] [Google Scholar]
- Novotny M, Jemiolo B, Harvey S, Wiesler D, Marchlewska-Koj A. Adrenal-mediated endogenous metabolites inhibit puberty in female mice. Science. 1986;231:722–725. doi: 10.1126/science.3945805. [DOI] [PubMed] [Google Scholar]
- Novotny MV. Pheromones, binding proteins and receptor responses in rodents. Biochem Soc Trans. 2003;31:117–122. doi: 10.1042/bst0310117. [DOI] [PubMed] [Google Scholar]
- Novotny MV, Jemiolo B, Wiesler D, Ma W, Harvey S, Xu F, Xie TM, Carmack M. A unique urinary constituent, 6-hydroxy-6-methyl-3-heptanone, is a pheromone that accelerates puberty in female mice. Chem Biol. 1999a;6:377–383. doi: 10.1016/S1074-5521(99)80049-0. [DOI] [PubMed] [Google Scholar]
- Novotny MV, Ma W, Wiesler D, Zidek L. Positive identification of the puberty-accelerating pheromone of the house mouse: the volatile ligands associating with the major urinary protein. Proc Biol Sci. 1999b;266:2017–2022. doi: 10.1098/rspb.1999.0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak R, Porter RH, Levy F, Orgeur P, Schaal B. Role of mother-young interactions in the survival of offspring in domestic mammals. Rev Reprod. 2000;5:153–163. doi: 10.1530/ror.0.0050153. [DOI] [PubMed] [Google Scholar]
- Nyby J, Dizinno G, Whitney G. Sexual dimorphism in ultrasonic vocalizations of mice (Mus musculus): gonadal hormone regulation. J Comp Physiol Psychol. 1977a;91:1424–1431. doi: 10.1037/h0077411. [DOI] [PubMed] [Google Scholar]
- Nyby J, Matochik JA, Barfield RJ. Intracranial androgenic and estrogenic stimulation of male-typical behaviors in house mice (Mus domesticus) Horm Behav. 1992;26:24–45. doi: 10.1016/0018-506x(92)90029-u. [DOI] [PubMed] [Google Scholar]
- Nyby J, Wysocki CJ, Whitney G, Dizinno G. Pheromonal regulation of male mouse ultrasonic courtship (Mus musculus) Anim Behav. 1977b;25:333–341. doi: 10.1016/0003-3472(77)90009-4. [DOI] [PubMed] [Google Scholar]
- Nyby JG. Reflexive testosterone release: a model system for studying the nongenomic effects of testosterone upon male behavior. Front Neuroendocrinol. 2008;29:199–210. doi: 10.1016/j.yfrne.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell RJ, Meredith M. Effects of volatile and nonvolatile chemical signals on male sex behaviors mediated by the main and accessory olfactory systems. Behav Neurosci. 1984;98:1083–1093. doi: 10.1037//0735-7044.98.6.1083. [DOI] [PubMed] [Google Scholar]
- O’Connell RJ, Singer AG, Macrides F, Pfaffmann C, Agosta WC. Responses of the male golden hamster to mixtures of odorants identified from vaginal discharge. Behav Biol. 1978;24:244–255. doi: 10.1016/s0091-6773(78)93110-3. [DOI] [PubMed] [Google Scholar]
- Ohara H, Nikaido M, Date-Ito A, Mogi K, Okamura H, Okada N, Takeuchi Y, Mori Y, Hagino-Yamagishi K. Conserved repertoire of orthologous vomeronasal type 1 receptor genes in ruminant species. BMC Evolutionary Biology. 2009;9:233. doi: 10.1186/1471-2148-9-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura H, Murata K, Sakamoto K, Wakabayashi Y, Ohkura S, Takeuchi Y, Mori Y. Male Effect Pheromone Tickles the Gonadotrophin-Releasing Hormone Pulse Generator. Journal of Neuroendocrinology. 2010;22:825–832. doi: 10.1111/j.1365-2826.2010.02037.x. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Baum MJ, Cherry JA. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J Neurosci. 2004;24:9451–9457. doi: 10.1523/JNEUROSCI.2376-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankevich DE, Cherry JA, Baum MJ. Accessory olfactory neural Fos responses to a conditioned environment are blocked in male mice by vomeronasal organ removal. Physiol Behav. 2006;87:781–788. doi: 10.1016/j.physbeh.2006.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes RG, Lopez ME, Baum MJ. Testosterone augments neuronal Fos responses to estrous odors throughout the vomeronasal projection pathway of gonadectomized male and female rats. Horm Behav. 1998;33:48–57. doi: 10.1006/hbeh.1998.1435. [DOI] [PubMed] [Google Scholar]
- Pause BM. Are androgen steroids acting as pheromones in humans? Physiol Behav. 2004;83:21–29. doi: 10.1016/j.physbeh.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Peele P, Salazar I, Mimmack M, Keverne EB, Brennan PA. Low molecular weight constituents of male mouse urine mediate the pregnancy block effect and convey information about the identity of the mating male. Eur J Neurosci. 2003;18:622–628. doi: 10.1046/j.1460-9568.2003.02790.x. [DOI] [PubMed] [Google Scholar]
- Penn DJ, Damjanovich K, Potts WK. MHC heterozygosity confers a selective advantage against multiple-strain infections. Proc Natl Acad Sci U S A. 2002;99:11260–11264. doi: 10.1073/pnas.162006499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrulis A, DeSouza I, Schiller M, Johnston RE. Role of frontal cortex in social odor discrimination and scent-marking in female golden hamsters (Mesocricetus auratus) Behav Neurosci. 1998;112:199–212. doi: 10.1037//0735-7044.112.1.199. [DOI] [PubMed] [Google Scholar]
- Petrulis A, Eichenbaum H. The perirhinal-entorhinal cortex, but not the hippocampus, is critical for expression of individual recognition in the context of the Coolidge effect. Neuroscience. 2003;122:599–607. doi: 10.1016/j.neuroscience.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Petrulis A, Johnston RE. A reevaluation of dimethyl disulfide as a sex attractant in golden hamsters. Physiol Behav. 1995;57:779–784. doi: 10.1016/0031-9384(94)00332-7. [DOI] [PubMed] [Google Scholar]
- Petrulis A, Johnston RE. Causes of scent marking in female golden hamsters (Mesocricetus auratus): specific signals or classes of information? J Comp Psychol. 1997;111:25–36. doi: 10.1037/0735-7036.111.1.25. [DOI] [PubMed] [Google Scholar]
- Petrulis A, Johnston RE. Lesions centered on the medial amygdala impair scent-marking and sex-odor recognition but spare discrimination of individual odors in female golden hamsters. Behav Neurosci. 1999;113:345–357. doi: 10.1037//0735-7044.113.2.345. [DOI] [PubMed] [Google Scholar]
- Petrulis A, Peng M, Johnston RE. Effects of vomeronasal organ removal on individual odor discrimination, sex-odor preference, and scent marking by female hamsters. Physiol Behav. 1999;66:73–83. doi: 10.1016/s0031-9384(98)00259-5. [DOI] [PubMed] [Google Scholar]
- Petrulis A, Peng M, Johnston RE. The role of the hippocampal system in social odor discrimination and scent-marking in female golden hamsters (Mesocricetus auratus) Behav Neurosci. 2000;114:184–195. [PubMed] [Google Scholar]
- Pfaff DW. Estrogens and brain function: neural analysis of a hormone-controlled mammalian reporductive behavior. Springer-Verlag; New York: 1980. [Google Scholar]
- Pfaff DW, Kow LM, Loose MD, Flanagan-Cato LM. Reverse engineering the lordosis behavior circuit. Horm Behav. 2008;54:347–354. doi: 10.1016/j.yhbeh.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Pfeiffer CA, Johnston RE. Socially stimulated androgen surges in male hamsters: the roles of vaginal secretions, behavioral interactions, and housing conditions. Horm Behav. 1992;26:283–293. doi: 10.1016/0018-506x(92)90048-z. [DOI] [PubMed] [Google Scholar]
- Pfeiffer CA, Johnston RE. Hormonal and behavioral responses of male hamsters to females and female odors: roles of olfaction, the vomeronasal system, and sexual experience. Physiol Behav. 1994;55:129–138. doi: 10.1016/0031-9384(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Pierman S, Douhard Q, Bakker J. Evidence for a role of early oestrogens in the central processing of sexually relevant olfactory cues in female mice. Eur J Neurosci. 2008;27:423–431. doi: 10.1111/j.1460-9568.2007.06016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers JB, Bergondy ML, Matochik JA. Male hamster sociosexual behaviors: effects of testosterone and its metabolites. Physiol Behav. 1985;35:607–616. doi: 10.1016/0031-9384(85)90149-0. [DOI] [PubMed] [Google Scholar]
- Powers JB, Fields RB, Winans SS. Olfactory and vomeronasal system participation in male hamsters’ attraction to female vaginal secretions. Physiol Behav. 1979;22:77–84. doi: 10.1016/0031-9384(79)90407-4. [DOI] [PubMed] [Google Scholar]
- Powers JB, Newman SW, Bergondy ML. MPOA and BNST lesions in male Syrian hamsters: differential effects on copulatory and chemoinvestigatory behaviors. Behav Brain Res. 1987;23:181–195. doi: 10.1016/0166-4328(87)90019-2. [DOI] [PubMed] [Google Scholar]
- Powers JB, Winans SS. Sexual behavior in peripherally anosmic male hamsters. Physiol Behav. 1973;10:361–368. doi: 10.1016/0031-9384(73)90323-5. [DOI] [PubMed] [Google Scholar]
- Powers JB, Winans SS. Vomeronasal organ: critical role in mediating sexual behavior of the male hamster. Science. 1975;187:961–963. doi: 10.1126/science.1145182. [DOI] [PubMed] [Google Scholar]
- Preti G, Wysocki CJ, Barnhart KT, Sondheimer SJ, Leyden JJ. Male axillary extracts contain pheromones that affect pulsatile secretion of luteinizing hormone and mood in women recipients. Biol Reprod. 2003;68:2107–2113. doi: 10.1095/biolreprod.102.008268. [DOI] [PubMed] [Google Scholar]
- Quadagno DM, Banks EM. The effect of reciprocal cross fostering on the behaviour of two species of rodents, Mus musculus and Baiomys taylori ater. Animal Behaviour. 1970;18:379–390. [Google Scholar]
- Rajendren G, Dudley CA, Moss RL. Role of the ventromedial nucleus of hypothalamus in the male-induced enhancement of lordosis in female rats. Physiol Behav. 1991;50:705–710. doi: 10.1016/0031-9384(91)90006-a. [DOI] [PubMed] [Google Scholar]
- Rajendren G, Moss RL. The role of the medial nucleus of amygdala in the mating-induced enhancement of lordosis in female rats: the interaction with luteinizing hormone-releasing hormone neuronal system. Brain Res. 1993;617:81–86. doi: 10.1016/0006-8993(93)90616-u. [DOI] [PubMed] [Google Scholar]
- Rajendren GV, Moss RL. Vomeronasal organ-mediated induction of fos in the central accessory olfactory pathways in repetitively mated female rats. Brain Res Bull. 1994;34:53–59. doi: 10.1016/0361-9230(94)90186-4. [DOI] [PubMed] [Google Scholar]
- Ramm SA, Cheetham SA, Hurst JL. Encoding choosiness: female attraction requires prior physical contact with individual male scents in mice. Proc Biol Sci. 2008;275:1727–1735. doi: 10.1098/rspb.2008.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampin O, Jerome N, Briant C, Boue F, Maurin Y. Are oestrus odours species specific? Behav Brain Res. 2006;172:169–172. doi: 10.1016/j.bbr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Rasmussen LE, Lee TD, Roelofs WL, Zhang A, Daves GD., Jr. Insect pheromone in elephants. Nature. 1996;379:684. doi: 10.1038/379684a0. [DOI] [PubMed] [Google Scholar]
- Reasner DS, Johnston RE. Acceleration of reproductive development in female Djungarian hamsters by adult males. Physiol Behav. 1988;43:57–64. doi: 10.1016/0031-9384(88)90098-4. [DOI] [PubMed] [Google Scholar]
- Reed HC, Melrose DR, Patterson RL. Androgen steroids as an aid to the detection of oestrus in pig artificial insemination. Br Vet J. 1974;130:61–67. doi: 10.1016/s0007-1935(17)35991-2. [DOI] [PubMed] [Google Scholar]
- Reynolds J, Keverne EB. The accessory olfactory system and its role in the pheromonally mediated suppression of oestrus in grouped mice. J Reprod Fertil. 1979;57:31–35. doi: 10.1530/jrf.0.0570031. [DOI] [PubMed] [Google Scholar]
- Rich TJ, Hurst JL. The competing countermarks hypothesis: reliable assessment of competitive ability by potential mates. Anim Behav. 1999;58:1027–1037. doi: 10.1006/anbe.1999.1217. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Nelson AL, Ahmed EI, Parfitt DB, Romeo RD, Sisk CL. Female pheromones stimulate release of luteinizing hormone and testosterone without altering GnRH mRNA in adult male Syrian hamsters (Mesocricetus auratus) Gen Comp Endocrinol. 2004;138:211–217. doi: 10.1016/j.ygcen.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Rissman EF. Male-related chemical cues promote sexual receptivity in the female musk shrew. Behav Neural Biol. 1989;51:114–120. doi: 10.1016/s0163-1047(89)90738-3. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Johnston RE. Female reproductive development is not activated by male California voles exposed to family cues. Biol Reprod. 1985;32:352–360. doi: 10.1095/biolreprod32.2.352. [DOI] [PubMed] [Google Scholar]
- Robarts DW, Baum MJ. Ventromedial hypothalamic nucleus lesions disrupt olfactory mate recognition and receptivity in female ferrets. Horm Behav. 2007;51:104–113. doi: 10.1016/j.yhbch.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EK, Lu A, Bergman TJ, Beehner JC. A Bruce effect in wild geladas. Science (New York, NY) 2012a;335:1222–1225. doi: 10.1126/science.1213600. [DOI] [PubMed] [Google Scholar]
- Roberts SA, Davidson AJ, McLean L, Beynon RJ, Hurst JL. Pheromonal Induction of Spatial Learning in Mice. Science (NewYork, NY) 2012b;338:1462–1465. doi: 10.1126/science.1225638. [DOI] [PubMed] [Google Scholar]
- Roberts SA, Simpson DM, Armstrong SD, Davidson AJ, Robertson DH, McLean L, Beynon RJ, Hurst JL. Darcin: a male pheromone that stimulates female memory and sexual attraction to an individual male's odour. 2010;8:75. doi: 10.1186/1741-7007-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SR, Smotherman WP. The emergence of behavioral regulation during fetal development. Ann N Y Acad Sci. 1992;662:53–83. doi: 10.1111/j.1749-6632.1992.tb22854.x. [DOI] [PubMed] [Google Scholar]
- Rosser AE, Remfry CJ, Keverne EB. Restricted exposure of mice to primer pheromones coincident with prolactin surges blocks pregnancy by changing hypothalamic dopamine release. J Reprod Fertil. 1989;87:553–559. doi: 10.1530/jrf.0.0870553. [DOI] [PubMed] [Google Scholar]
- Rowe FA, Edwards DA. Olfactory bulb removal: influences on the mating behavior of male mice. Physiol Behav. 1972;8:37–41. doi: 10.1016/0031-9384(72)90127-8. [DOI] [PubMed] [Google Scholar]
- Russell MJ. Human olfactory communication. Nature. 1976;260:520–522. doi: 10.1038/260520a0. [DOI] [PubMed] [Google Scholar]
- Sachs BD. Erection evoked in male rats by airborne scent from estrous females. Physiol Behav. 1997;62:921–924. doi: 10.1016/s0031-9384(97)00307-7. [DOI] [PubMed] [Google Scholar]
- Sachs BD, Akasofu K, Citron JH, Daniels SB, Natoli JH. Noncontact stimulation from estrous females evokes penile erection in rats. Physiol Behav. 1994;55:1073–1079. doi: 10.1016/0031-9384(94)90390-5. [DOI] [PubMed] [Google Scholar]
- Saito TR, Moltz H. Copulatory behavior of sexually naive and sexually experienced male rats following removal of the vomeronasal organ. Physiol Behav. 1986;37:507–510. doi: 10.1016/0031-9384(86)90215-5. [DOI] [PubMed] [Google Scholar]
- Sam M, Vora S, Malnic B, Ma W, Novotny MV, Buck LB. Odorants may arouse instinctive behaviours. Nature. 2001;412:142. doi: 10.1038/35084137. [DOI] [PubMed] [Google Scholar]
- Samuelsen CL, Meredith M. Categorization of biologically relevant chemical signals in the medial amygdala. Brain Res. 2009;1263:33–42. doi: 10.1016/j.brainres.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Eichenbaum H. Thalamocortical mechanisms in odor-guided behavior. II. Effects of lesions of the mediodorsal thalamic nucleus and frontal cortex on odor preferences and sexual behavior in the hamster. Brain Behav Evol. 1980;17:276–290. doi: 10.1159/000121804. [DOI] [PubMed] [Google Scholar]
- Savic I, Berglund H, Gulyas B, Roland P. Smelling of odorous sex hormone-like compounds causes sex-differentiated hypothalamic activations in humans. Neuron. 2001;31:661–668. doi: 10.1016/s0896-6273(01)00390-7. [DOI] [PubMed] [Google Scholar]
- Saxton TK, Lyndon A, Little AC, Roberts SC. Evidence that androstadienone, a putative human chemosignal, modulates women’s attributions of men’s attractiveness. Horm Behav. 2008;54:597–601. doi: 10.1016/j.yhbeh.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Schadler MH. Postimplantation abortion in pine voles (Microtus pinetorum) induced by strange males and pheromones of strange males. Biol Reprod. 1981;25:295–297. doi: 10.1095/biolreprod25.2.295. [DOI] [PubMed] [Google Scholar]
- Schank JC. Can pseudo entrainment explain the synchrony of estrous cycles among golden hamsters (Mesocricetus auratus)? Horm Behav. 2000;38:94–101. doi: 10.1006/hbeh.2000.1603. [DOI] [PubMed] [Google Scholar]
- Schank JC. Do Norway rats (Rattus norvegicus) synchronize their estrous cycles? Physiol Behav. 2001a;72:129–139. doi: 10.1016/s0031-9384(00)00395-4. [DOI] [PubMed] [Google Scholar]
- Schank JC. Menstrual-cycle synchrony: problems and new directions for research. J Comp Psychol. 2001b;115:3–15. doi: 10.1037/0735-7036.115.1.3. [DOI] [PubMed] [Google Scholar]
- Schank JC. Do human menstrual-cycle pheromones exist? Hum Nature-Int Bios. 2006;17:448–470. doi: 10.1007/s12110-006-1006-y. [DOI] [PubMed] [Google Scholar]
- Schank JC, McClintock MK. A coupled-oscillator model of ovarian-cycle synchrony among female rats. J Theor Biol. 1992;157:317–362. doi: 10.1016/s0022-5193(05)80614-9. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serguera C, Triaca V, Kelly-Barrett J, Banchaabouchi MA, Minichiello L. Increased dopamine after mating impairs olfaction and prevents odor interference with pregnancy. Nat Neurosci. 2008;11:949–956. doi: 10.1038/nn.2154. [DOI] [PubMed] [Google Scholar]
- Setchell JM, Kendal J, Tyniec P. Do non-human primates synchronise their menstrual cycles? A test in mandrills. Psychoneuroendocrinology. 2011;36:51–59. doi: 10.1016/j.psyneuen.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Singer AG, Agosta WC, O’Connell RJ, Pfaffmann C, Bowen DV, Field FH. Dimethyl disulfide: an attractant pheromone in hamster vaginal secretion. Science. 1976;191:948–950. doi: 10.1126/science.1251205. [DOI] [PubMed] [Google Scholar]
- Singer AG, Beauchamp GK, Yamazaki K. Volatile signals of the major histocompatibility complex in male mouse urine. Proc Natl Acad Sci U S A. 1997;94:2210–2214. doi: 10.1073/pnas.94.6.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AG, Clancy AN, Macrides F, Agosta WC. Chemical studies of hamster vaginal discharge: male behavioral responses to a high molecular weight fraction require physical contact. Physiol Behav. 1984;33:645–651. doi: 10.1016/0031-9384(84)90385-8. [DOI] [PubMed] [Google Scholar]
- Singer AG, Clancy AN, Macrides F, Agosta WC, Bronson FH. Chemical properties of a female mouse pheromone that stimulates gonadotropin secretion in males. Biol Reprod. 1988;38:193–199. doi: 10.1095/biolreprod38.1.193. [DOI] [PubMed] [Google Scholar]
- Singer AG, Macrides F. Aphrodisin: pheromone or transducer? Chemical Senses. 1990;15:199–203. [Google Scholar]
- Sipos ML, Kerchner M, Nyby JG. An ephemeral sex pheromone in the urine of female house mice (Mus domesticus) Behav Neural Biol. 1992;58:138–143. doi: 10.1016/0163-1047(92)90375-e. [DOI] [PubMed] [Google Scholar]
- Sipos ML, Wysocki CJ, Nyby JG, Wysocki L, Nemura TA. An ephemeral pheromone of female house mice: perception via the main and accessory olfactory systems. Physiol Behav. 1995;58:529–534. doi: 10.1016/0031-9384(95)00089-2. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Smale L. Influence of male gonadal hormones and familiarity on pregnancy interruption in prairie voles. Biol Reprod. 1988;39:28–31. doi: 10.1095/biolreprod39.1.28. [DOI] [PubMed] [Google Scholar]
- Smith TD, Bhatnagar KP, Shimp KL, Kinzinger JH, Bonar CJ, Burrows AM, Mooney MP, Siegel MI. Histological definition of the vomeronasal organ in humans and chimpanzees, with a comparison to other primates. Anat Rec. 2002;267:166–176. doi: 10.1002/ar.10095. [DOI] [PubMed] [Google Scholar]
- Solomon NG, Vandenbergh JG, Wekesa KS, Barghusen L. Chemical cues are necessary but insufficient for reproductive activation of female pine vole (Microtus pinetorum) Biol Reprod. 1996;54:1038–1045. doi: 10.1095/biolreprod54.5.1038. [DOI] [PubMed] [Google Scholar]
- Spehr M, Kelliher KR, Li XH, Boehm T, Leinders-Zufall T, Zufall F. Essential role of the main olfactory system in social recognition of major histocompatibility complex peptide ligands. J Neurosci. 2006;26:1961–1970. doi: 10.1523/JNEUROSCI.4939-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel E, Hutchison JB. Olfactory recognition in the male hamster: effect of non-aromatizable androgens, 17 beta-hydroxy-17 alpha-methyl-estra-4,9,11-triene-3-one (R 1881) and 5 alpha-dihydrotestosterone, in combination with oestrogen. J Endocrinol. 1986;110:525–531. doi: 10.1677/joe.0.1100525. [DOI] [PubMed] [Google Scholar]
- Stern JJ. Responses of male rats to sex odors. Physiol Behav. 1970;5:519–524. doi: 10.1016/0031-9384(70)90260-x. [DOI] [PubMed] [Google Scholar]
- Stern K, McClintock MK. Regulation of ovulation by human pheromones. Nature. 1998;392:177–179. doi: 10.1038/32408. [DOI] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Stowers L, Marton TF. What is a pheromone? Mammalian pheromones reconsidered. Neuron. 2005;46:699–702. doi: 10.1016/j.neuron.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Strassmann BI. Menstrual synchrony pheromones: cause for doubt. Hum Reprod. 1999;14:579–580. doi: 10.1093/humrep/14.3.579. [DOI] [PubMed] [Google Scholar]
- Swann J, Rahaman F, Bijak T, Fiber J. The main olfactory system mediates pheromone-induced fos expression in the extended amygdala and preoptic area of the male Syrian hamster. Neuroscience. 2001;105:695–706. doi: 10.1016/s0306-4522(01)00227-5. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Lisk RD, Burnett AL., 2nd Dual estradiol action in diencephalon and the regulation of sociosexual behavior in female golden hamsters. Brain Res. 1985;359:194–207. doi: 10.1016/0006-8993(85)91429-5. [DOI] [PubMed] [Google Scholar]
- Thiessen D, Rice M. Mammalian scent gland marking and social behavior. Psychol Bull. 1976;83:505–539. [PubMed] [Google Scholar]
- Thom MD, Stockley P, Jury F, Ollier WE, Beynon RJ, Hurst JL. The direct assessment of genetic heterozygosity through scent in the mouse. Curr Biol. 2008;18:619–623. doi: 10.1016/j.cub.2008.03.056. [DOI] [PubMed] [Google Scholar]
- Thorpe JB, deCatanzaro D. Oestradiol treatment restores the capacity of castrated males to induce both the Vandenbergh and the Bruce effects in mice (Mus musculus) Reproduction. 2012;143:123–132. doi: 10.1530/REP-11-0251. [DOI] [PubMed] [Google Scholar]
- Tinbergen N. The study of instinct. Clarendon Press/Oxford University Press; 1951. [Google Scholar]
- Trinh K, Storm DR. Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium. Nat Neurosci. 2003;6:519–525. doi: 10.1038/nn1039. [DOI] [PubMed] [Google Scholar]
- Trotier D, Eloit C, Wassef M, Talmain G, Bensimon JL, Doving KB, Ferrand J. The vomeronasal cavity in adult humans. Chem Senses. 2000;25:369–380. doi: 10.1093/chemse/25.4.369. [DOI] [PubMed] [Google Scholar]
- Utsumi M, Ohno K, Kawasaki Y, Tamura M, Kubo T, Tohyama M. Expression of major urinary protein genes in the nasal glands associated with general olfaction. J Neurobiol. 1999;39:227–236. doi: 10.1002/(sici)1097-4695(199905)39:2<227::aid-neu7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Van Der Lee S, Boot LM. Spontaneous pseudopregnancy in mice. Acta Physiol Pharmacol Neerl. 1955;4:442–444. [PubMed] [Google Scholar]
- Van Der Lee S, Boot LM. Spontaneous pseudopregnancy in mice. II. Acta Physiol Pharmacol Neerl. 1956;5:213–215. [PubMed] [Google Scholar]
- Vandenbergh JG. Male odor accelerates female sexual maturation in mice. Endocrinology. 1969;84:658–660. doi: 10.1210/endo-84-3-658. [DOI] [PubMed] [Google Scholar]
- Vandenbergh JG, Whitsett JM, Lombardi JR. Partial isolation of a pheromone accelerating puberty in female mice. J Reprod Fertil. 1975;43:515–523. doi: 10.1530/jrf.0.0430515. [DOI] [PubMed] [Google Scholar]
- Vaughn AA, delBarco-Trillo J, Ferkin MH. Sperm investment in male meadow voles is affected by the condition of the nearby male conspecifics. Behav. Ecol. 2008;19:1159–1164. doi: 10.1093/beheco/arn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker DW, Ludwig M. Vasopressin, oxytocin, and social odor recognition. Horm Behav. 2012;61:259–265. doi: 10.1016/j.yhbeh.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Weller L, Weller A. Human menstrual synchrony: a critical assessment. Neurosci Biobehav Rev. 1993;17:427–439. doi: 10.1016/s0149-7634(05)80118-6. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Rissman EF. Oestrogen receptor alpha is essential for female-directed chemo-investigatory behaviour but is not required for the pheromone-induced luteinizing hormone surge in male mice. J Neuroendocrinol. 2000;12:103–110. doi: 10.1046/j.1365-2826.2000.00418.x. [DOI] [PubMed] [Google Scholar]
- Westberry JM, Meredith M. Pre-exposure to female chemosignals or intracerebral GnRH restores mating behavior in naive male hamsters with vomeronasal organ lesions. Chem Senses. 2003;28:191–196. doi: 10.1093/chemse/28.3.191. [DOI] [PubMed] [Google Scholar]
- Whissell-Buechy D, Amoore JE. Odour-blindness to musk: simple recessive inheritance. Nature. 1973;242:271–273. doi: 10.1038/242271a0. [DOI] [PubMed] [Google Scholar]
- White NR, Colona LC, Barfield RJ. Sensory cues that elicit ultrasonic vocalizations in female rats (Rattus norvegicus) Behav Neural Biol. 1991;55:154–165. doi: 10.1016/0163-1047(91)80136-3. [DOI] [PubMed] [Google Scholar]
- Whitten W. Pheromones and regulation of ovulation. Nature. 1999;401:232–233. doi: 10.1038/45720. [DOI] [PubMed] [Google Scholar]
- Whitten WK, Bronson FH, Greenstein JA. Estrus-inducing pheromone of male mice: transport by movement of air. Science. 1968;161:584–585. doi: 10.1126/science.161.3841.584. [DOI] [PubMed] [Google Scholar]
- Williams GW, McGinnis MY, Lumia AR. The effects of olfactory bulbectomy and chronic psychosocial stress on serum glucocorticoids and sexual behavior in female rats. Physiol Behav. 1992;52:755–760. doi: 10.1016/0031-9384(92)90410-4. [DOI] [PubMed] [Google Scholar]
- Wilson HC. Female axillary secretions influence women’s menstrual cycles: a critique. Horm Behav. 1987;21:536–550. doi: 10.1016/0018-506x(87)90012-2. [DOI] [PubMed] [Google Scholar]
- Wilson HC. A critical review of menstrual synchrony research. Psychoneuroendocrinology. 1992;17:565–591. doi: 10.1016/0306-4530(92)90016-z. [DOI] [PubMed] [Google Scholar]
- Winman A. Do perfume additives termed human pheromones warrant being termed pheromones? Physiol Behav. 2004;82:697–701. doi: 10.1016/j.physbeh.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wood RI. Estradiol, but not dihydrotestosterone, in the medial amygdala facilitates male hamster sex behavior. Physiol Behav. 1996;59:833–841. doi: 10.1016/0031-9384(95)02204-x. [DOI] [PubMed] [Google Scholar]
- Wood RI. Thinking about networks in the control of male hamster sexual behavior. Horm Behav. 1997;32:40–45. doi: 10.1006/hbeh.1997.1403. [DOI] [PubMed] [Google Scholar]
- Wood RI. Integration of chemosensory and hormonal input in the male Syrian hamster brain. Ann N Y Acad Sci. 1998;855:362–372. doi: 10.1111/j.1749-6632.1998.tb10594.x. [DOI] [PubMed] [Google Scholar]
- Wood RI, Brabec RK, Swann JM, Newman SW. Androgen and estrogen concentrating neurons in chemosensory pathways of the male Syrian hamster brain. Brain Res. 1992;596:89–98. doi: 10.1016/0006-8993(92)91536-n. [DOI] [PubMed] [Google Scholar]
- Wood RI, Newman SW. The medial amygdaloid nucleus and medial preoptic area mediate steroidal control of sexual behavior in the male Syrian hamster. Horm Behav. 1995;29:338–353. doi: 10.1006/hbeh.1995.1024. [DOI] [PubMed] [Google Scholar]
- Wood RI, Swann JM. The bed nucleus of the stria terminalis in the Syrian hamster: subnuclei and connections of the posterior division. Neuroscience. 2005;135:155–179. doi: 10.1016/j.neuroscience.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Woodley SK, Baum MJ. Effects of sex hormones and gender on attraction thresholds for volatile anal scent gland odors in ferrets. Horm Behav. 2003;44:110–118. doi: 10.1016/s0018-506x(03)00126-0. [DOI] [PubMed] [Google Scholar]
- Woodley SK, Cloe AL, Waters P, Baum MJ. Effects of vomeronasal organ removal on olfactory sex discrimination and odor preferences of female ferrets. Chem Senses. 2004;29:659–669. doi: 10.1093/chemse/bjh069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt TD. Pheromones and animal behaviour: communication by smell and taste. Cambridge University Press; Cambridge, U.K.; New York: 2003. [Google Scholar]
- Wyatt TD. Fifty years of pheromones. Nature. 2009;457:262–263. doi: 10.1038/457262a. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Katz Y, Bernhard R. Male vomeronasal organ mediates female-induced testosterone surges in mice. Biol Reprod. 1983;28:917–922. doi: 10.1095/biolreprod28.4.917. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Nyby J, Whitney G, Beauchamp GK, Katz Y. The vomeronasal organ: primary role in mouse chemosensory gender recognition. Physiol Behav. 1982;29:315–327. doi: 10.1016/0031-9384(82)90021-x. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Preti G. Facts, fallacies, fears, and frustrations with human pheromones. Anat Rec A Discov Mol Cell Evol Biol. 2004;281:1201–1211. doi: 10.1002/ar.a.20125. [DOI] [PubMed] [Google Scholar]
- Xiao K, Kondo Y, Sakuma Y. Sex-specific effects of gonadal steroids on conspecific odor preference in the rat. Horm Behav. 2004;46:356–361. doi: 10.1016/j.yhbeh.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Xiao K, Kondo Y, Sakuma Y. Differential regulation of female rat olfactory preference and copulatory pacing by the lateral septum and medial preoptic area. Neuroendocrinology. 2005;81:56–62. doi: 10.1159/000084893. [DOI] [PubMed] [Google Scholar]
- Yahr P, Gregory JE. The medial and lateral cell groups of the sexually dimorphic area of the gerbil hypothalamus are essential for male sex behavior and act via separate pathways. Brain Res. 1993;631:287–296. doi: 10.1016/0006-8993(93)91547-6. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Beauchamp GK, Wysocki CJ, Bard J, Thomas L, Boyse EA. Recognition of H-2 types in relation to the blocking of pregnancy in mice. Science. 1983;221:186–188. doi: 10.1126/science.6857281. [DOI] [PubMed] [Google Scholar]
- Yoon H, Enquist LW, Dulac C. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell. 2005;123:669–682. doi: 10.1016/j.cell.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Zacharias R, de Catanzaro D, Muir C. Novel male mice disrupt pregnancy despite removal of vesicular-coagulating and preputial glands. Physiol Behav. 2000;68:285–290. doi: 10.1016/s0031-9384(99)00170-5. [DOI] [PubMed] [Google Scholar]
- Zarrow MX, Estes SA, Denenberg VH, Clark JH. Pheromonal facilitation of ovulation in the immature mouse. J Reprod Fertil. 1970;23:357–360. doi: 10.1530/jrf.0.0230357. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Liu YJ, Zhang JH, Sun L. Dual role of preputial gland secretion and its major components in sex recognition of mice. Physiol Behav. 2008a;95:388–394. doi: 10.1016/j.physbeh.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Rao XP, Sun L, Zhao CH, Qin XW. Putative chemical signals about sex, individuality, and genetic background in the preputial gland and urine of the house mouse (Mus musculus) Chem Senses. 2007;32:293–303. doi: 10.1093/chemse/bjl058. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Soini HA, Bruce KE, Wiesler D, Woodley SK, Baum MJ, Novotny MV. Putative chemosignals of the ferret (Mustela furo) associated with individual and gender recognition. Chem Senses. 2005;30:727–737. doi: 10.1093/chemse/bji065. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Sun L, Zhang JH, Feng ZY. Sex- and gonad-affecting scent compounds and 3 male pheromones in the rat. Chem Senses. 2008b;33:611–621. doi: 10.1093/chemse/bjn028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TE, Schultz-Darken NJ, Scott JJ, Snowdon CT, Ferris CF. Neuroendocrine response to female ovulatory odors depends upon social condition in male common marmosets, Callithrix jacchus. Horm Behav. 2005;47:56–64. doi: 10.1016/j.yhbeh.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Zufall F, Leinders-Zufall T. Mammalian pheromone sensing. Curr Opin Neurobiol. 2007;17:483–489. doi: 10.1016/j.conb.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Zuri I, Halpern M. Modification of odor investigation and discrimination in female opossums (Monodelphis domestica) following the ablation of the accessory olfactory bulbs. Behav Neurosci. 2005;119:612–621. doi: 10.1037/0735-7044.119.2.612. [DOI] [PubMed] [Google Scholar]