Abstract
Background
Deficiencies in vitamins A, D and E have been linked to night blindness, bone health, and post-liver transplant reperfusion injury.
Aims
To determine the prevalence and predictive factors of fat soluble vitamin deficiencies in liver transplantation candidates.
Methods
We reviewed medical records of liver transplantation candidates at our center from 1/2008–9/2011. Etiology of cirrhosis, MELD scores, Child Pugh class, BMI, vitamin A, vitamin E, and vitamin 25-OH-D levels were recorded. Patients were excluded for incomplete laboratory data, short gut syndrome, celiac disease, pancreatic insufficiency, or prior liver transplantation.
Results
Sixty three patients were included. The most common etiologies of liver disease were alcohol (23), hepatitis C (19), and NASH (5). Vitamin A and D deficiency was noted in 69.8% and 80.9%, respectively. Only 3.2% of patients were vitamin E deficient. There were no documented cases of night blindness. Of 55 patients with bone density measurements, 25 had osteopenia and 10 had osteoporosis. Four patients had vertebral fractures. There was one case of post-transplant reperfusion injury in a patient with vitamin E deficiency. In a multivariate analysis, there were no statistically significant predictors for vitamin D deficiency. Child Pugh class (OR=6.84 {1.52–30.86};p = 0.012), elevated total bilirubin (OR=44.23{5.02–389.41}; p < .001), and elevated BMI (OR=1.17{1.00–1.36};p = 0.045) were found to be predictors of vitamin A deficiency.
Conclusions
The majority of liver disease patients evaluated for liver transplantation at our center had vitamin A and D deficiency. Presence or absence of cholestatic liver disease did not predict the deficiency, whereas Child Pugh class, bilirubin level, and elevated BMI predicted vitamin A deficiency.
Keywords: Fat soluble vitamins, cholestasis, cirrhosis, liver failure
Introduction
Malnutrition is commonly encountered in patients with end stage liver disease (ESLD) being reported in up to 80% of all patients with cirrhosis and in up to 25% of patients with Child Pugh class A cirrhosis (1, 2). It has also been associated with higher morbidity and mortality (1, 2). In fact, the original Child-Turcotte classification included nutritional status as a prognostic parameter (3). Cirrhosis is associated with a hypermetabolic state, increased protein catabolism, decreased glycogen storage and glucose oxidation as well as increased lipid oxidation, all of which contribute to poor nutritional status(4), but the precise etiology of malnutrition in cirrhosis is not established.
In patients with cirrhosis and chronic cholestasis, there is inadequate delivery of bile salts into the intestinal lumen. This can lead to insufficient absorption and fat soluble vitamin deficiency (5). For example, vitamin A deficiency has been observed in patients with primary biliary cirrhosis and may be associated with night blindness (6, 7). Several studies have also shown vitamin D deficiency in both cholestatic and non-cholestatic liver disorders (8–10). It is, however, not clear whether such vitamin deficiencies are due to poor nutritional intake, hepatic dysfunction, malabsorption, or a combination of these factors. There are little data on the prevalence of fat soluble vitamin deficiency among patients with ESLD awaiting liver transplantation (11). The aims of this study were to assess the prevalence of fat soluble vitamin deficiency in patients being evaluated for liver transplantation and to elucidate the predictive factors for any such deficiency.
Methods
We retrospectively reviewed charts of patients who presented for outpatient evaluation for liver transplant at the Medical College of Wisconsin Hepatology Clinic from January 2008 through September 2011. The study protocol was reviewed and approved by the Medical College of Wisconsin’s Institutional Review Board. The etiology of cirrhosis, MELD scores, Child Pugh score, body mass index (BMI), vitamin A, vitamin E, and vitamin 25-OH-D were recorded. Patient demographics including age, gender, smoking history, alcohol use, and drug use were reviewed.
Serum vitamin A and E levels were determined using high-pressure liquid chromatography (HPLC) with UV detection (Agilent Technologies, Santa Clara, CA). Serum vitamin D 25-OH was determined using radioimmunoassay (DiaSorin, Stillwater, MN). Vitamin A, D and E deficiency were defined as < 19 μg/dl, < 32 ng/ml and < 3 mg/L respectively and were based on normal ranges used at our institution. Severe vitamin D deficiency was defined as < 10 ng/ml.
Patients were excluded if they had short gut syndrome, celiac disease, pancreatic insufficiency, previous liver transplantation or incomplete laboratory analysis.
Statistical analysis
Univariate comparisons of patient characteristics between vitamin deficient and non-deficient subjects were performed using Student’s t-test for continuous variables (age, BMI, and MELD score), chi-square test for binary variables (sex, race, ALT/AST/bilirubin/albumin/alkaline phosphate status, and contribution of alcohol/non-alcoholic steatohepatitis (NASH)/Hepatitis C/primary sclerosing cholangitis (PSC) to the disease etiology), and Wilcoxon’s rank sum test for ordinal variables (Child Pugh score). Exact chi-square test was used when the expected number of subjects in a category was 5 or fewer. Similar methods were used for the comparison of excluded and included subjects.
Logistic regression was used for the multivariate analysis of predictors of vitamin deficiency. Due to the large number of potential predictors, model building was performed by forward stepwise selection with 10% significance level for entry and 5% significance level for staying in the model. The predictive power of the final model for vitamin A deficiency was evaluated using ROC analysis.
All analyses were performed using SAS 9.2 (SAS Institute, Cary, NC).
Results
Of 211 patients referred for liver transplantation, 63 met inclusion criteria. Incomplete laboratory data (139 patients) was the most common reason for exclusion and this was due to varying adherence of different providers in our practice to screening and not based on clinical suspicion of vitamin deficiency. Subjects’ characteristics are summarized in Table 1. There were no statistically significant differences in age, race, gender, BMI, etiology of liver disease, Child Pugh class, MELD score, alcohol use or smoking history between the included and excluded subjects. The most common etiologies of chronic liver disease were alcohol abuse (23) and hepatitis C (19) (Table 2). The remaining patients had hepatitis C and alcohol (5), NASH (5), PSC (4), primary biliary cirrhosis (PBC) (3), autoimmune hepatitis (AIH) (2), hepatic carcinoid tumor (1), and AIH and PSC (1). None of the included patients had acute liver disease or failure. The majority of patients were deficient in vitamin A and vitamin D (69.8% and 80.9%, respectively), however, only 2/63 (3.2%) patients were vitamin E deficient, both of whom had PSC. Over half of the patients were deficient in both vitamin A and D (36/63, 57.1%). Severe vitamin D deficiency (< 10ng/ml) was found in only 4 patients (6.3%). When 20ng/ml was used as the lower limit of normal for defining vitamin D deficiency, 47/63 patients (75%) were deficient. The one patient who was underweight but with normal fat soluble vitamin levels had hepatitis C cirrhosis.
Table 1.
Vitamin deficiency per patient characteristics
| Vitamin A (n=63)[n (%)] | Vitamin D (n=63)[n (%)] | Vitamin E (n=63)[n (%)] | |||||
|---|---|---|---|---|---|---|---|
| Deficient (<19 μg/dL) | Normal (19–83 μg/dL) | Deficient (<32 ng/mL) | Normal (32–100 ng/mL) | Deficient (<3 mg/L) | Normal (3–15 mg/L) | ||
| Variables | Total [n (%)](n=63) | n=44 (69.8%) | n=19 (30.2%) | n=51 (80.9%) | n=12 (19.1%) | n=2 (3.2%) | n=61 (96.8%) |
| Age (years) [n (%)] | |||||||
| ≥18–29 | 3 (4.8) | 2 (3.2) | 1 (1.6) | 3 (4.8) | 0 | 1 (1.6) | 2 (3.2) |
| ≥30–44 | 3 (4.8) | 2 (3.2) | 1 (1.6) | 2 (3.2) | 1 (1.6) | 0 | 3 (4.8) |
| ≥45–59 | 48 (76.2) | 34 (54.1) | 14 (22.2) | 39 (61.9) | 9 (14.3) | 1 (1.6) | 47 (74.6) |
| ≥60 | 9 (14.3) | 6 (9.5) | 3 (4.8) | 7 (11.1) | 2 (3.2) | 0 | 9 (14.3) |
| Total (mean ± STD) | 52.5 ± 8.3 | ||||||
| Gender [n (%)] | |||||||
| Male | 43 (68.2) | 30 (47.6) | 13 (20.6) | 33 (52.4) | 10 (15.8) | 1 (1.6) | 42 (66.7) |
| Female | 20 (31.7) | 14 (22.2) | 6 (9.5) | 18 (28.6) | 2 (3.2) | 1 (1.6) | 19 (30.2) |
| Race [n (%)] | |||||||
| Caucasian | 49 (77.7) | 35 (35.6) | 14 (22.2) | 38 (60.3) | 11 (17.5) | 2 (3.2) | 47 (74.6) |
| African American | 7 (11.1) | 5 (7.9) | 2 (3.2) | 7 (11.1) | 0 | 0 | 7 (11.1) |
| Hispanic | 4 (6.3) | 3 (4.8) | 1 (1.6) | 3 (4.8) | 1 (1.6) | 0 | 4 (6.3) |
| Native American | 2 (3.2) | 1 (1.6) | 1 (1.6) | 2 (3.2) | 0 | 0 | 2 (3.2) |
| Middle Eastern | 1 (1.6) | 0 | 1 (1.6) | 1 (1.6) | 0 | 0 | 1 (1.6) |
| BMI (kg/m2) [n (%)] | |||||||
| <18.5 (underweight) | 1 (1.6) | 0 | 1 (1.6) | 0 | 1 (1.6) | 0 | 1 (1.6) |
| 18.5–24.99 (normal) | 14 (22.2) | 10 (15.8) | 4 (6.3) | 11 (17.5) | 3 (4.8) | 0 | 14 (22.2) |
| 25–29.99 (overweight) | 22 (34.9) | 14 (22.2) | 8 (12.7) | 20 (31.7) | 2 (3.2) | 2 (3.2) | 20 (31.7) |
| 30–39.99 (obese) | 21 (33.3) | 16 (25.4) | 5 (7.9) | 15 (23.8) | 6 (9.5) | 0 | 21 (33.3) |
| ≥40 (morbidly obese) | 5 (7.9) | 4 (6.3) | 1 (1.6) | 5 (7.9) | 0 | 0 | 5 (7.9) |
| Total (mean ± STD) | 29.3 ± 6.2 | ||||||
| Alcohol Use [n (%)] | |||||||
| Active | 5 (7.9) | 4 (6.3) | 1 (1.6) | 4 (6.3) | 1 (1.6) | 0 | 5 (7.9) |
| Former | 32 (50.8) | 24 (38.1) | 8 (12.7) | 26 (41.3) | 6 (9.5) | 0 | 32 (50.8) |
| Never | 26 (41.3) | 16 (25.4) | 10 (15.8) | 21 (33.3) | 5 (7.9) | 2 (3.2) | 24 (38.1) |
| Smoking Use [n (%)] | |||||||
| Active | 6 (9.5) | 6 (9.5) | 0 | 6 (9.5) | 0 | 0 | 6 (9.5) |
| Former | 29 (46) | 18 (28.6) | 11 (17.5) | 24 (38.1) | 5 (7.9) | 0 | 29 (46) |
| Never | 28 (44.4) | 20 (31.7) | 8 (12.7) | 21 (33.3) | 7 (11.1) | 2 (3.2) | 26 (41.3) |
Table 2.
Vitamin deficiency per liver disease etiology and severity
| Vitamin A (n=63)[n (%)] | Vitamin D (n=63)[n (%)] | Vitamin E (n=63)[n (%)] | |||||
|---|---|---|---|---|---|---|---|
| Deficient (<19 μg/dL) | Normal (19–83 μg/dL) | Deficient (<32 ng/mL) | Normal (32–100 ng/mL) | Deficient (<3 mg/L) | Normal (3–15 mg/L) | ||
| Variables | Total (n=63) | n=44 (69.8%) | n=19 (30.2%) | n=51 (80.9%) | n=12 (19.1%) | n=2 (3.2%) | n=61 (96.8%) |
| Alcohol | 23 (36.5) | 19 (30.2) | 4 (6.3) | 17 (27) | 6 (9.5) | 0 | 23 (36.5) |
| HCV | 19 (30.2) | 11 (17.5) | 8 (12.7) | 16 (25.4) | 3 (4.8) | 0 | 19 (30.2) |
| Alcohol and HCV | 5 (7.9) | 3 (4.8) | 2 (3.2) | 4 (6.3) | 1 (1.6) | 0 | 5 (7.9) |
| NASH | 5 (7.9) | 3 (4.8) | 1 (1.6) | 3 (4.8) | 1 (1.6) | 0 | 5 (7.9) |
| PSC | 4 (6.3) | 2 (3.2) | 2 (3.2) | 4 (6.3) | 0 | 2 (3.2) | 2 (3.2) |
| PBC | 2 (3.2) | 2 (3.2) | 0 | 2 (3.2) | 0 | 0 | 2 (3.2) |
| AIH | 2 (3.2) | 1 (1.6) | 1 (1.6) | 1 (1.6) | 1 (1.6) | 0 | 2 (3.2) |
| Hepatic Carcinoid | 1 (1.6) | 0 | 1 (1.6) | 1 (1.6) | 0 | 0 | 1 (1.6) |
| AIH and PSC | 1 (1.6) | 1 (1.6) | 0 | 1 (1.6) | 0 | 0 | 1 (1.6) |
| NASH and PBC | 1 (1.6) | 1 (1.6) | 0 | 1 (1.6) | 0 | 0 | 1 (1.6) |
| MELD [n (%)] | |||||||
| < 15 | 27 (42.9) | 17 (27) | 10 (15.8) | 22 (34.9) | 5 (7.9) | 0 | 27 (42.9) |
| 15–19 | 21 (33.3) | 16 (25.4) | 5 (7.9) | 16 (25.4) | 5 (7.9) | 2 (3.2) | 19 (30.2) |
| 20–29 | 12 (19.0) | 9 (14.2) | 3 (4.8) | 11 (17.5) | 1 (1.6) | 0 | 12 (19.0) |
| 30–39 | 1 (1.6) | 1 (1.6) | 0 | 0 | 1 (1.6) | 0 | 1 (1.6) |
| 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total (mean ± STD) | 15.3 ± 4 | ||||||
| Child Pugh Class | |||||||
| A | 14 (22.2) | 5 (7.9) | 9 (14.3) | 12 (19.0) | 2 (3.2) | 0 | 14 (22.2) |
| B | 38 (60.3) | 28 (44.4) | 10 (15.8) | 26 (41.3) | 2 (3.2) | 1 (1.6) | 37 (58.7) |
| C | 11 (17.5) | 11 (17.5) | 0 | 3 (4.8) | 8 (12.7) | 1 (1.6) | 10 (15.8) |
There were no documented cases of night blindness in our cohort. Vertebral fractures were documented in 4 patients, all of whom were vitamin D deficient. Bone density as measured by DEXA scan was available on 55 patients. Osteopenia was noted in 25 patients and osteoporosis was seen in 10 patients, whereas normal bone density was noted in 20 patients. In this group with bone density data, vitamin D deficiency was noted in 18/25 (72%) patients with osteopenia, 10/10 (100%) patients with osteoporosis and 16/20 (80%) patients with normal bone density. Vertebral fractures were documented in 4 patients all of whom had vitamin D deficiency and osteopenia on DEXA scan. One of our patients who had vitamin E deficiency prior to transplant had reperfusion injury post-operatively.
Most patients were not on any vitamin supplementation (40/63, 63.5%) prior to assessment in the hepatology clinic. Seventeen patients (26.9%) were taking a multivitamin supplement. However, 13/17 (76.5%) of these patients were vitamin D deficient, 12/17 (70.6%) were vitamin A deficient, and 3/17 (17.6%) had no vitamin deficiencies. The content of fat soluble vitamins in the multivitamin pill and patients’ compliance with taking it could not be determined from the chart review. Only 6 patients were taking vitamin D (5 of whom were on > 1000 IU/day dosing) and 2 patients were taking vitamin E supplementation. Compliance with taking these supplements could not be verified. In the limited number of patients in this cohort who had follow up data, oral supplementation with vitamin A and D resulted in correction of vitamin A and D deficiency in 3 of 5 (60%) and 12 of 16 (75%)of patients, respectively.
All patients with Child Pugh class C cirrhosis (11) were deficient in vitamin A. In a multivariate analysis, there were no statistically significant predictors for vitamin D deficiency. Child Pugh class (OR=6.84 {CI 1.52–30.86};p = 0.012), elevated total bilirubin (OR=44.23 {CI 5.02–389.41}; p < .001), and elevated BMI (OR=1.17 {CI 1.00–1.36}; p = 0.045) were found to be predictors of vitamin A deficiency(Table 3). With the full model including Child Pugh class, bilirubin and BMI, the AUROC for this model was 0.91(CI 0.84–0.98) and was higher than a model including Child Pugh class and bilirubin or bilirubin alone [0.87 (CI 0.77–0.96) and 0.78 (CI 0.66–0.89) respectively] (Figure 1).
Table 3.
Multivariate analysis
Predictors of vitamin A deficiency Stepwise logistic regression results
| Vitamin | Variable | OR (95% CI) | P-value |
|---|---|---|---|
| Vitamin A | Intercept | ||
| Child Pugh class | 6.84 (1.52 – 30.86) | 0.012 | |
| Total Bilirubin | 44.23 (5.02 – 389.41) | <0.001 | |
| BMI | 1.17 (1.00 – 1.36) | 0.045 |
Figure 1.
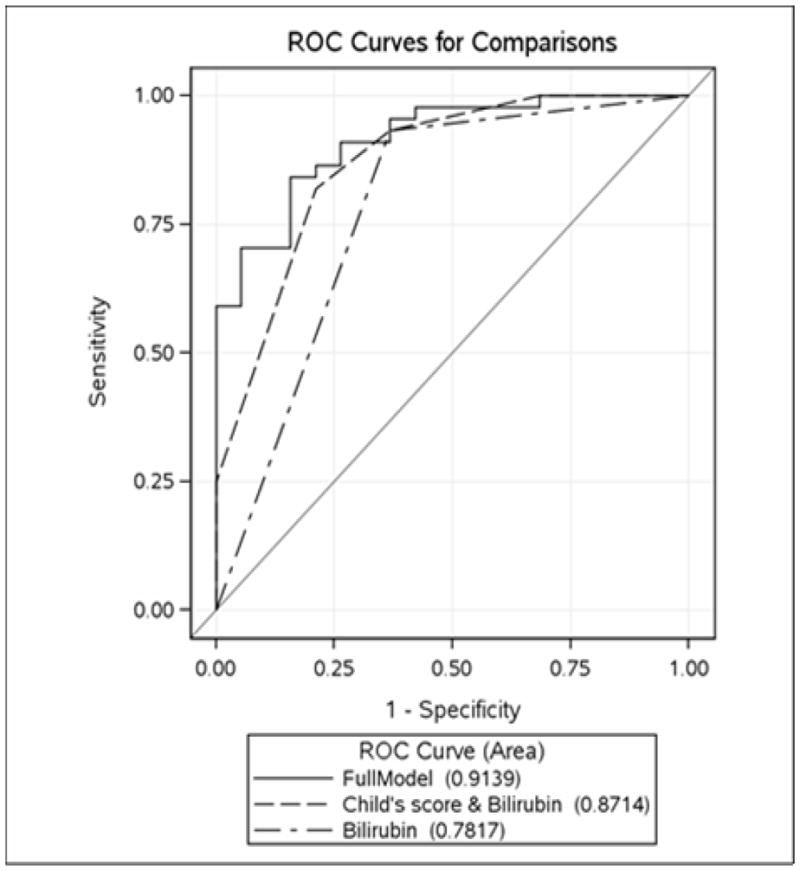
ROC graph for models predicting vitamin A deficiency
Discussion
The majority of ESLD patients evaluated for liver transplantation at our center were deficient in vitamin A and vitamin D. Child Pugh class, serum bilirubin and elevated BMI were predictors of vitamin A deficiency. There were no predictors for vitamin D deficiency. Interestingly, the etiology of liver disease was not predictive of fat soluble vitamin deficiency.
Malnutrition has been established as a negative prognostic indicator in patients with end stage liver disease (12–18). Previous studies have evaluated fat soluble vitamin deficiency in patients with chronic cholestatic liver disorders, in which impaired bile flow is the proposed underlying mechanism that leads to malabsorption of fat soluble vitamins. There are few studies that have evaluated this in patients with non-cholestatic liver disease, and most have focused on vitamin D deficiency.
Fisher et al (9) evaluated 100 consecutive patients with non-cholestatic liver disease for vitamin D deficiency. The most common etiology of liver disease was alcohol (40) and hepatitis C (38). Vitamin D deficiency was found in 68 patients and was more prevalent in patients with Child Pugh class C cirrhosis. Malhem et al (8)sought to compare vitamin D deficiency in patients with non-cholestatic liver disease versus cholestatic liver disease. In this study, 89 patients with alcoholic cirrhosis and 34 patients with primary biliary cirrhosis were retrospectively evaluated for vitamin D deficiency. Vitamin D deficiency was more prevalent in patients with alcoholic cirrhosis than primary biliary cirrhosis (85% vs. 60%). Arteh et al (10)evaluated vitamin D deficiency in 118 consecutive patients with hepatitis C. They found that 109/118 (92%) of patients had vitamin D deficiency, with severe vitamin deficiency (< 7 ng/ml) more common in patients with cirrhosis. The high incidence of vitamin A and D deficiency was also reported in a recent study by Abbott-Johnson et al(11). In this study, 107 patients with end-stage liver disease who were awaiting liver transplantation were prospectively followed. 75% of patients were deficient in vitamin A, 66% were deficient in vitamin D and only 3% were deficient in vitamin E. Similar to our findings, severity of underlying liver disease as reflected by Child Pugh class was found to be a predictor of fat soluble vitamin deficiency.
Concordant with prior studies, the majority of our patients with end-stage liver disease who were evaluated for liver transplant were found to be deficient in both vitamin A (69.8%) and D (80.9%), but there was a low proportion of patients with vitamin E deficiency (3.2%). MELD score and presence or absence of cholestatic liver disease was not found to be predictive of vitamin deficiency in our patients.
Fat soluble vitamin deficiency may have significant clinical consequences. Osteoporosis is a potential complication after liver transplantation. Vitamin D deficiency prior to liver transplantation may increase the risk for osteoporosis and fracture by causing secondary hyperparathyroidism (19). There was high rate of osteoporosis and osteopenia in our patients and 4 subjects (6.3%) had vertebral fractures. A recent study by Kaemmerer et al evaluated the impact of ibandronate, vitamin D3 and calcium supplements prior to and after liver transplantation in 31 patients with osteopenia or osteoporosis prior to transplantation (20). This regimen increased bone mineral density in the lumbar spine up to 12 months after transplantation, and after 24 months, there were only 2 reported cases of fracture within this group. Although there were no documented cases of night blindness in our patients with vitamin A deficiency, no patients underwent formal ophthalmological evaluation. A study by Abbott-Johnson et al evaluated vitamin A deficiency and night blindness in 8 patients with cirrhosis awaiting liver transplantation (7). Interestingly, 6/8 were unaware of impairment with their vision. All patients showed improvement with dark adaptation following intramuscular supplementation with vitamin A. One of our two patients with vitamin E deficiency had reperfusion injury following liver transplantation. Interestingly, Goode et al demonstrated increased risk of reperfusion injury after liver transplantation in patients with pretransplant vitamin E deficiency(21). We found no predictors for vitamin D deficiency which was contrary to previous studies that had identified cirrhosis, female gender and African American race as predictors for vitamin D deficiency(10, 22). Dietary insufficiency, malabsorption, impaired hepatic hydroxylation of vitamin D, and lack of sunlight exposure may contribute to vitamin D deficiency. Whereas no predictors for vitamin A deficiency were identified in prior reports, advanced Child Pugh class, total bilirubin and elevated BMI were associated with it in our cohort. It is unclear why patients in Child Pugh class C had lower frequency of vitamin D deficiency and higher frequency of vitamin A deficiency in comparison to those in Child Pugh classes A and B.
Our study has several limitations. We were unable to find data in there viewed medical records concerning adequacy of oral intake or other parameters to assess nutritional status such as pre-albumin, triceps skinfold thickness, or midarm muscle circumference. Our patient population is located in Wisconsin (latitude of 43°N) and this may impact the prevalence of vitamin D deficiency as the reduced exposure to sunlight may contribute to lower vitamin D levels. Vitamin E deficiency was not evident in our patient population. There are conflicting studies on whether serum measurement of α-tocopherol or if the α-tocopherol to total lipid ratio is a more accurate measure of vitamin E in patients with chronic liver disease (23, 24). Serum lipid panel from our patient population was not analyzed and we did not calculate this ratio to elucidate if more patients had vitamin E deficiency. Our study was not designed to evaluate the mechanism for these deficiencies, but to identify whether these patients have significant deficiencies in fat soluble vitamins. Although elevated BMI was identified as a predictor of vitamin A deficiency, our BMI calculation was not adjusted for fluid retention. Finally, the exact composition of each patient’s multivitamin is unknown, and therefore, it is unclear how much, if any, impact these supplements may have had on serum values of fat soluble vitamins.
If our findings are validated in other studies, universal screening for vitamin A and D in this population being evaluated for liver transplantation may be reasonable given the high prevalence of these deficiencies. Whether vitamin E deficiency is only limited to patients with PSC also needs to be examined in order to make recommendation on appropriate screening. Whether using a daily multivitamin supplement will reduce these deficiencies has not been prospectively studied, however, it did not seem to impact vitamin A and D deficiency in a subgroup of our patients.
In summary, our study confirms that deficiency of vitamin A and D in patients with ESLD awaiting liver transplantation is very common. Etiology of liver disease did not predict vitamin deficiency, however, severity of liver disease impacted vitamin A deficiency. Identification and correction of these common deficiencies may be an important element in the efforts to optimize this highly morbid patient population prior to liver transplantation.
Acknowledgments
Financial Support
Supported, in part, by grant 1UL1RR031973 from the Clinical and Translational Science Award (CTSI) program of the National Center for Research Resources, National Institutes of Health
Abbreviations
- AIH
autoimmune hepatitis
- ALT
alanine transaminase
- AST
aspartate transaminase
- BMI
body mass index
- ESLD
end-stage liver disease
- HCV
hepatitis C virus
- MELD
model for end stage liver disease
- NASH
non-alcoholic steatohepatitis
- PBC
primary biliary cirrhosis
- PSC
primary sclerosing cholangitis
Footnotes
The authors have no conflicts of interest
References
- 1.Kalaitzakis E, Simren M, Olsson R, Henfridsson P, Hugosson I, Bengtsson M, et al. Gastrointestinal symptoms in patients with liver cirrhosis: Associations with nutritional status and health-related quality of life. Scand J Gastroenterol. 2006 Dec;41(12):1464–72. doi: 10.1080/00365520600825117. [DOI] [PubMed] [Google Scholar]
- 2.Guglielmi FW, Panella C, Buda A, Budillon G, Caregaro L, Clerici C, et al. Nutritional state and energy balance in cirrhotic patients with or without hypermetabolism. multicentre prospective study by the ‘nutritional problems in gastroenterology’ section of the italian society of gastroenterology (SIGE) Dig Liver Dis. 2005 Sep;37(9):681–8. doi: 10.1016/j.dld.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Kondrup J. Nutrition in end stage liver disease. Best Pract Res Clin Gastroenterol. 2006;20(3):547–60. doi: 10.1016/j.bpg.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Tsiaousi ET, Hatzitolios AI, Trygonis SK, Savopoulos CG. Malnutrition in end stage liver disease: Recommendations and nutritional support. J Gastroenterol Hepatol. 2008 Apr;23(4):527–33. doi: 10.1111/j.1440-1746.2008.05369.x. [DOI] [PubMed] [Google Scholar]
- 5.Maillette de Buy Wenniger L, Beuers U. Bile salts and cholestasis. Dig Liver Dis. 2010 Jun;42(6):409–18. doi: 10.1016/j.dld.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Herlong HF, Russell RM, Maddrey WC. Vitamin A and zinc therapy in primary biliary cirrhosis. Hepatology. 1981 Jul-Aug;1(4):348–51. doi: 10.1002/hep.1840010412. [DOI] [PubMed] [Google Scholar]
- 7.Abbott-Johnson WJ, Kerlin P, Abiad G, Clague AE, Cuneo RC. Dark adaptation in vitamin A-deficient adults awaiting liver transplantation: Improvement with intramuscular vitamin A treatment. Br J Ophthalmol. 2011 Apr;95(4):544–8. doi: 10.1136/bjo.2009.179176. [DOI] [PubMed] [Google Scholar]
- 8.Malham M, Jorgensen SP, Ott P, Agnholt J, Vilstrup H, Borre M, et al. Vitamin D deficiency in cirrhosis relates to liver dysfunction rather than aetiology. World J Gastroenterol. 2011 Feb 21;17(7):922–5. doi: 10.3748/wjg.v17.i7.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher L, Fisher A. Vitamin D and parathyroid hormone in outpatients with noncholestatic chronic liver disease. Clin Gastroenterol Hepatol. 2007 Apr;5(4):513–20. doi: 10.1016/j.cgh.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Arteh J, Narra S, Nair S. Prevalence of vitamin D deficiency in chronic liver disease. Dig Dis Sci. 2010 Sep;55(9):2624–8. doi: 10.1007/s10620-009-1069-9. [DOI] [PubMed] [Google Scholar]
- 11.Abbott-Johnson W, Kerlin P, Clague A, Johnson H, Cuneo R. Relationships between blood levels of fat soluble vitamins and disease etiology and severity in adults awaiting liver transplantation. J Gastroenterol Hepatol. 2011 Sep;26(9):1402–10. doi: 10.1111/j.1440-1746.2011.06746.x. [DOI] [PubMed] [Google Scholar]
- 12.Pikul J, Sharpe MD, Lowndes R, Ghent CN. Degree of preoperative malnutrition is predictive of postoperative morbidity and mortality in liver transplant recipients. Transplantation. 1994 Feb;57(3):469–72. doi: 10.1097/00007890-199402150-00030. [DOI] [PubMed] [Google Scholar]
- 13.Harrison J, McKiernan J, Neuberger JM. A prospective study on the effect of recipient nutritional status on outcome in liver transplantation. Transpl Int. 1997;10(5):369–74. doi: 10.1007/s001470050072. [DOI] [PubMed] [Google Scholar]
- 14.Caregaro L, Alberino F, Amodio P, Merkel C, Bolognesi M, Angeli P, et al. Malnutrition in alcoholic and virus-related cirrhosis. Am J Clin Nutr. 1996 Apr;63(4):602–9. doi: 10.1093/ajcn/63.4.602. [DOI] [PubMed] [Google Scholar]
- 15.Alberino F, Gatta A, Amodio P, Merkel C, Di Pascoli L, Boffo G, et al. Nutrition and survival in patients with liver cirrhosis. Nutrition. 2001 Jun;17(6):445–50. doi: 10.1016/s0899-9007(01)00521-4. [DOI] [PubMed] [Google Scholar]
- 16.Merli M, Riggio O, Dally L. Does malnutrition affect survival in cirrhosis? PINC (policentrica italiana nutrizione cirrosi) Hepatology. 1996 May;23(5):1041–6. doi: 10.1002/hep.510230516. [DOI] [PubMed] [Google Scholar]
- 17.Selberg O, Bottcher J, Tusch G, Pichlmayr R, Henkel E, Muller MJ. Identification of high-and low-risk patients before liver transplantation: A prospective cohort study of nutritional and metabolic parameters in 150 patients. Hepatology. 1997 Mar;25(3):652–7. doi: 10.1002/hep.510250327. [DOI] [PubMed] [Google Scholar]
- 18.Figueiredo F, Dickson ER, Pasha T, Kasparova P, Therneau T, Malinchoc M, et al. Impact of nutritional status on outcomes after liver transplantation. Transplantation. 2000 Nov 15;70(9):1347–52. doi: 10.1097/00007890-200011150-00014. [DOI] [PubMed] [Google Scholar]
- 19.Compston JE. Osteoporosis after liver transplantation. Liver Transpl. 2003 Apr;9(4):321–30. doi: 10.1053/jlts.2003.50044. [DOI] [PubMed] [Google Scholar]
- 20.Kaemmerer D, Benjamin S, Gabriele L, Gunter W, Utz S, Merten H. Treatment of bone loss in patients with chronic liver disease awaiting liver transplantation. Transplant Res. 2012 Jun 1;1(1):7, 1440-1-7. doi: 10.1186/2047-1440-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goode HF, Webster NR, Howdle PD, Leek JP, Lodge JP, Sadek SA, et al. Reperfusion injury, antioxidants and hemodynamics during orthotopic liver transplantation. Hepatology. 1994 Feb;19(2):354–9. [PubMed] [Google Scholar]
- 22.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009 Mar 23;169(6):626–32. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Look MP, Reichel C, von Falkenhausen M, Hahn C, Stockinger K, von Bergmann K, et al. Vitamin E status in patients with liver cirrhosis: Normal or deficient? Metabolism. 1999 Jan;48(1):86–91. doi: 10.1016/s0026-0495(99)90015-x. [DOI] [PubMed] [Google Scholar]
- 24.Nagita A, Ando M. Assessment of hepatic vitamin E status in adult patients with liver disease. Hepatology. 1997 Aug;26(2):392–7. doi: 10.1002/hep.510260220. [DOI] [PubMed] [Google Scholar]


