Abstract
Across taxa, cooperative breeding has been associated with high reproductive skew. Cooperatively breeding golden lion tamarins (Leontopithecus rosalia) were long thought to have a monogynous mating system in which reproduction was limited to a single dominant female. Subordinates with few reproductive opportunities delayed dispersal and remained in the natal group to provide alloparental care to siblings, thus allowing dominant reproductive females to meet the energetic needs associated with high rates of reproduction and successful infant rearing. The goal of this study was to re-assess monogyny in wild golden lion tamarin groups based upon pregnancy diagnoses that used non-invasive enzyme immunoassay for progesterone and cortisol, combined with weekly data on individual weight gain, bi-annual physical examinations noting pregnancy and lactation status and daily behavioral observations. We established quantitative and qualitative criteria to detect and determine the timing of pregnancies that did not result in the birth of infants. Pregnancy polygyny occurred in 83% of golden lion tamarin groups studied. The loss of 64% of subordinate pregnancies compared to only 15% by dominant females limited reproductive success mainly to dominant females, thus maintaining high reproductive skew in female golden lion tamarins. Pregnancy loss by subordinate adults did not appear to result from dominant interference in subordinate hormonal mechanisms, but more likely resulted from subordinate abandonment of newborn infants to mitigate dominant aggression.
Keywords: cooperative breeding, reproductive skew, polygyny, reproductive suppression, pregnancy loss, dominant control, subordination, progesterone, cortisol, Leontopithecus rosalia
INTRODUCTION
In cooperatively breeding species, reproduction is commonly limited to one or a few dominant individuals of each sex (Clutton-Brock, 2009). The young born to breeding females are reared by all group members, including non-reproductive helpers. Several mechanisms have been suggested to explain high reproductive skew in female cooperative breeders, including lower concentrations of reproductive hormones in subordinates (Creel et al., 1992; Faulkes and Bennett, 2001; Mays et al., 1991; Schoech et al., 1991), suppression of subordinate ovulation (Abbott, 1984; Faulkes and Bennett, 2001; French, 1997; Solomon et al., 2001), dominant interference in subordinate mating (Abbott, 1984), behavioral suppression of subordinate reproduction (Clutton-Brock et al., 2008; De Vleeschouwer et al., 2001; Inglett et al., 1989; Kleiman, 1979; Kutsukake and Clutton-Brock, 2006), stress-induced infertility and pregnancy loss (Gilchrist, 2006a; Pottinger, 1999; Wasser and Barash, 1983; Young et al., 2006), infanticide (Clutton-Brock et al., 1998a; Gilchrist, 2006a; Hoogland, 1985; Saltzman et al., 2009) and ecological constraints on subordinate reproduction (Clutton-Brock et al., 2001a; Creel and Creel, 1991; Emlen, 1982; Hatchwell and Komdeur, 2000; Kleiman, 1977b). If multiple females produce litters at the same time the amount of help received by the offspring of the dominant female may be reduced, resulting in lower infant growth and/or survival (Clutton-Brock et al., 2001b; Digby, 1995a; Hodge, 2009) and reduced dominant fecundity (Clutton-Brock et al., 1998b; Fite et al., 2005; Russell et al., 2003). Therefore, dominant females able to limit subordinate reproduction should have a selective advantage. Indeed, monopolization of reproduction by dominant group members by means of suppression of subordinate reproduction has been documented in several taxa of cooperative breeders including mammals (Solomon and French, 1997), birds (Mays et al., 1991; Reyer et al., 1986; Schoech et al., 1991), fishes (Fitzpatrick et al., 2008) and invertebrates (Hamilton, 2004).
As long-term field research continues, the presence of multiple breeding females within cooperatively breeding groups originally described as monogynous or singular breeding has been observed in an increasing number of species including blue tits (Parus caeruleus (Kempenaers, 1994)), wolves (Canis lupus (Mech, 2000)), naked mole-rats (Heterocephalus glaber (Faulkes and Abbott, 1997)), dwarf mongooses (Helogale parvula (Creel and Waser, 1991)), common marmosets (Callithrix jacchus (Arruda et al., 2005; Digby, 1995b; Digby and Ferrari, 1994; Hubrecht, 1984)), pygmy marmosets (Cebuella pygmaea (Soini, 1982)), cotton-top tamarins (Saguinus oedipus (Savage et al., 1996)), saddle-back tamarins (Saguinus fuscicollis (Goldizen et al., 1996)) and moustached tamarins (Saguinus mystax (Garber et al., 1993; Garber et al., 1984; Ramirez, 1984; Smith et al., 2001)). Though multiple breeding females may be present, high reproductive skew remains, with subordinates having lower reproductive success than dominant females in banded mongooses (Mungos mungo (Gilchrist, 2006a, 2006b)), dwarf mongooses (Creel and Waser, 1997), meerkats (Suricata suricatta (Clutton-Brock et al., 2008; Young et al., 2006)), marmosets (Arruda et al., 2005; Digby, 1995a; Saltzman et al., 2009; Saltzman et al., 2008; Sousa et al., 2005) and tamarins (Garber, 1997; Goldizen et al., 1996). In captive and wild groups of cotton-top tamarins only one female per group gives birth regardless of the number of other pregnant females in the group (Price and McGrew, 1991; Savage et al., 1996). Non-invasive hormonal assays to determine the reproductive status of all group members can be used to reassess mating patterns in cooperatively breeding species as well as illuminate the mechanisms responsible for maintaining high reproductive skew in spite of the presence of multiple breeding females.
Marmosets and tamarins, neotropical primates in the family Callitrichidae (genera Callithrix, Cebuella, Saguinus and Leontopithecus), were long thought to have a monogynous mating system in which reproduction was limited to a single dominant female (Goldizen, 1987; Sussman and Garber, 1987). Golden lion tamarins (GLTs, Leontopithecus rosalia) are cooperative breeders that display a high degree of reproductive skew, with reproduction limited to one or a few dominant individuals of each sex even in groups containing as many as 13 individuals (Dietz and Baker, 1993). Dietz and Baker (1993) described the mating system in GLTs as monogyny with about a 10% incidence of polygyny. However, when examining only those groups containing more than one potentially reproductive female, 44.3% showed pregnancy polygyny (i.e. more than one female was confirmed to be pregnant within the same breeding season) and 26.2% showed rearing polygyny (more than one female reared offspring to weaning) (Baker et al., 2002; Dietz and Baker, 1993). Lion tamarin offspring typically delay dispersal and reproduction, and remain in their natal group to help with the care of infant siblings (Dietz and Baker, 1993). Cooperative care has been suggested to be instrumental in the ability of callitrichids to meet the energetic needs associated with successfully rearing the litters of twins they are capable of producing once or twice a year (Baker et al., 1993; Dietz and Baker, 1993; Kleiman, 1977a; Sussman and Garber, 1987). Therefore, lion tamarins represent a good candidate species for evaluating the occurrence of, and mechanisms underlying, singular vs. plural breeding in a cooperatively breeding mating system.
The goal of this study was to re-assess the degree of polygyny in wild GLT groups based upon pregnancy diagnoses that used non-invasive hormonal enzyme immunoassay for progesterone and cortisol combined with weekly data on individual weight gain, pregnancy and lactation status from bi-annual capture records and behavioral observations. We collected the following data on females residing within seven groups of GLTs over three reproductive years: group demography, dominance status, reproductive status and reproductive success defined as pregnancies that resulted in the birth of live offspring. We tested two competing predictions derived from the hypothesis that reproduction is limited to a single dominant female in the majority of GLT groups. To the extent that dominant females fully ‘control’ reproduction in subordinates, we predicted that subordinate adult female GLTs would not become pregnant while residing within their natal group. In the case where dominant female control was incomplete, we predicted that pregnancies by dominant adult female GLTs would result in the birth of live offspring; whereas pregnancies by subordinate adult female GLTs would not. We also examined two alternative methods by which dominant females may control subordinate reproduction to maintain high reproductive skew. If dominant females suppress subordinate reproduction via hormonal mechanisms, we predicted that subordinates would not get pregnant, or at the least, that hormonal patterns during subordinate pregnancy would be abnormal, resulting in higher rates of subordinate pregnancy loss. Alternatively, if dominant females suppress subordinate reproduction via behavioral mechanisms, we predicted that subordinates might become pregnant, but that dominant aggression and harassment of pregnant subordinates would result in higher rates of pregnancy loss. We also predicted that the offspring of subordinate females might not survive because they would not receive infant care from group members attempting to avoid dominant aggression.
METHODS
Study site and species
Data were collected within the 6300 ha Poço das Antas Biological Reserve (PDA), Rio de Janeiro State, Brazil (22° 30′ – 33′ S, 42° 15′ – 19′ W) (Miller and Dietz, 2006). PDA holds the largest remaining population of GLTs in the wild, with an estimated 350 GLTs in the secondary forests protected by the reserve (Ruiz-Miranda et al., 2008; Rylands et al., 2002).
Individual identification and weighing
The animals under study at PDA are native and non-manipulated except for bi-annual live captures necessary for replacing radio collars to facilitate group location. During these routine captures, usually in May or early June and again in December or January, individuals are given identifiable markings (hair dye and tattoos), weighed and evaluated for growth and body condition including notes regarding female nipple length (reflecting parity), lactation and pregnancy (Dietz and Baker, 1993; Dietz et al., 1994). During the current study weights were also obtained weekly from August through December of each year using baited scales in the field (Bales, 2000; Bales et al., 2002; Siani, 2009).
Group demography
We collected data on 7 groups of wild GLTs at PDA, each containing 2 to 13 individuals and 1 to 3 adult females. All individuals were habituated to the presence of human observers. Data were collected over three reproductive years: 2004–2005, 2005–2006 and 2006–2007. A reproductive year was defined as the 1st of March through the 28th of February in order to encompass the mating period, pregnancy and the first annual peak in infant births (October through November (Dietz et al., 1994)) as well as post-partum ovulation, mating and pregnancy that lead to the second annual peak in infant births (February (Dietz et al., 1994)). Six of these groups were observed from March 2004 through February 2007. Another group was added to the study in June of 2005, and was observed until the end of the study in February of 2007. Group sizes fluctuated, with losses typically filled by immigrants. Group composition was recorded daily including all births, deaths, emigrations and immigrations.
The ages of individuals born within study groups are known from long-term demographic data or estimated to year based upon weight, the eruption of permanent teeth and degree of tooth wear and discoloration noted at semi-annual captures (Bales et al., 2001; Dietz et al., 1994; French et al., 2003). Adults were defined as individuals older than 18 months of age (Dietz and Baker, 1993), corresponding to the average age of sexual maturation (Dietz et al., 1994; French et al., 2002; French et al., 1989; French and Stribley, 1987). Subadults were defined as individuals from 8 to 18 months of age.
Each group contained one adult female that was behaviorally dominant to other females in the group. A status of ‘dominant female’ was assigned to the predominant aggressor based upon archwalks, mounts and chases and winner of fights, and ‘subordinate female’ to the adult-aged females within each group that were targets of these agonistic displays (Bales et al., 2005; Dietz and Baker, 1993).
Fecal sample collection
We collected 1176 fecal samples from individually identified female GLTs in seven free-ranging social groups from March 2004 through February 2007. Feces were collected from a total of 21 GLT females including 14 adults, 5 females that became adults and 2 females that became subadults during the study period (Table 1). We collected samples year-round during reproductive and non-reproductive months of the year. We collected fecal samples from each female once or twice per week as they left their sleeping locations or during subsequent observations. We attempted to restrict fecal collection to the morning hours to reduce diurnal variation in concentrations of fecal progesterone metabolites (French et al., 2003; Sousa and Ziegler, 1998). As a result, 28% of samples were collected before 0900h and 83% by 1200h. No more than 10 hours passed between fecal sample collection and storage in a freezer. Samples remained frozen until analysis at the Endocrine Bioservices Laboratory, University of Nebraska at Omaha (UNO).
Table 1.
Reproductive summary for 21 GLT females from seven free-ranging GLT groups sampled from March of 2004 through February of 2007.
| Group | Group exhibits polygyny | Female ID | Dominance status | Age category (years) | Age (years) at first pregnancy if during study | Successful pregnancies | Infants born | Unsuccessful pregnancies | Fecal samples |
|---|---|---|---|---|---|---|---|---|---|
| 3M5 | N | 782 | Dominant | Adult (4.5–6.7) | 0 | 0 | 1 | 32 | |
| AL | Y | 539 | Dominant then subordinate | Adult (10.6–13.3) | 2 | 2 | 2 | 97 | |
| AL | Y | 846 | Subordinate then dominant | Adult (2.8–5.3) | 2.9 | 3 | 6 | 0 | 78 |
| AL | Y | 1267 | Subordinate | Infant to subadult (0.2–1.3) | Did not become pregnant | 0 | 0 | 0 | 21 |
| BO2 | Y | 720 | Dominant | Adult (9.4–12.3) | 6 | 11 | 0 | 125 | |
| BO2 | Y | 848 | Subordinate | Adult (2.4–5.3) | 2.5 | 3 | 6 | 2 | 136 |
| BO2/POR | Y/N | 880 | Subordinate/dominant | Subadult to adult (1.4–4.1) | 2.8 | 3 | 6 | 0 | 88 |
| BO2/POR. | Y/N | 899 | Subordinate | Subadult to (1.1–2.4) | Did not become pregnant | 0 | 0 | 0 | 39 |
| BO2/POR | Y/N | 1227 | Subordinate | Subadult to adult (0.7–2.9) | Did not become pregnant | 0 | 0 | 0 | 68 |
| BO2/POR | Y/N | 1241 | Subordinate | Subadult to adult (0.8–2.0) | Did not become pregnant | 0 | 0 | 0 | 52 |
| GF | Y | 766 | Dominant | Adult (5.6–6.9) | 2 | 4 | 0 | 35 | |
| GF | Y | 889 | Subordinate | Adult (1.6–3.0) | 2.5 | 0 | 0 | 1 | 18 |
| GF | N | 1266 | Dominant | Adult (2.9–4.0) | 3.4 | 1 | 1 | 0 | 32 |
| PA | Y | 869 | Dominant | Adult (4.7–7.3) | 4 | 8 | 0 | 98 | |
| PA | Y | 884 | Subordinate | Adult (1.7–3.3) | 2.7 | 0 | 0 | 1 | 60 |
| PA | Y | 1271 | Subordinate | Juvenile to subadult (0.4–1.4) | Did not become pregnant | 0 | 0 | 0 | 32 |
| PP3 | N | 750 | Dominant | Adult (8.7–10.5) | 1 | 2 | 3 | 77 | |
| PP3 | N | 1238 | Subordinate | Juvenile to adult (0.3–1.9) | Did not become pregnant | 0 | 0 | 0 | 56 |
| PP3 | Y | 1264 | Dominant | Adult (4.0–4.3) | 3.9 | 1 | 2 | 0 | 12 |
| PP3 | Y | 1265 | Subordinate | Adult (3.3–4.2) | 3.9 | 0 | 0 | 1 | 10 |
| PP3 | N | T0PP3 | Dominant | Adult (Unknown) | Did not become pregnant | 0 | 0 | 0 | 10 |
| x̄ = 3.1±0.2 | Σ = 26 | Σ= 48 | Σ = 11 | Σ = 1176 |
Pregnanediol-3-glucuronide (PdG) and cortisol extraction from feces
PdG is a metabolite of progesterone excreted in feces and has been validated as a reliable indicator of circulating progesterone concentrations in lion tamarins (Bales, 2000; French et al., 2003). Cortisol is excreted directly into the feces. PdG and cortisol were extracted simultaneously from fecal samples. Fecal samples were allowed to thaw at room temperature, and large seeds, leafy material and undigested insect parts were removed. Fecal samples were dried in a drying oven at 37° C prior to weighing for hormonal extraction. Hormonal extraction was performed by briefly vortexing and then shaking 0.125 g of dried fecal matter in 2.5 ml of solubilizing extraction buffer (40% methanol (MeOH): 60% phosphate buffered saline (PBS)) for 12–16 hours on a shaker rack. Samples were then briefly vortexed to remove residue along the tube walls and centrifuged for 15 min at 2000 rpm at 6° C. The supernatant was decanted into a clean test tube and refrozen for storage until further dilution for assay.
PdG immunoassay
PdG concentrations were determined through standardized enzyme immunoassay techniques (EIA) and calculations resulting from a four-parameter sigmoidal curve-fitting function as previously described (Bales, 2000; French et al., 2003; French et al., 1996). Internal quality control pools consisted of female Geoffroy’s marmoset (Callithrix geoffroyi) urine since a more recent and extensive assay history existed for comparison. Quality control pools were run at high (1:80 dilution in 1:5 extraction buffer:PBS) and low concentrations (1:640 dilution in 1:5 extraction buffer:PBS). The intra- and inter-assay coefficients of variation for high and low concentrations of the urine quality control pool were 9.1% and 19.9% (high), and 7.4% and 26.7% (low), respectively. This EIA assay for fecal PdG was previously tested for accuracy and validated against circulating hormone levels (Bales, 2000; French et al., 2003).
Hormone concentrations that were within 10% above and below the standard curve (11,000 pg/well through 70.2 pg/well) were accepted and used at face value in calculations to arrive at ng PdG/g feces. Concentrations above (n=28) or below (n=167) the 10% cut off were assigned values of 11,000 and 70.2 pg/well, respectively. The large number of samples with very low PdG concentrations was expected due to the large number of samples from young females. This bias could not be avoided since dilution of fecal extracts by less than 1:5 or bringing more fecal extract to the EIA well would have caused interference in the assay due to the increase in methanol concentrations. As such, the risk of false positive pregnancy diagnoses based on PdG concentrations was minimal since overestimation was limited to very low PdG values, with very high PdG values conservatively underestimated.
Cortisol immunoassay
Fecal cortisol concentrations were determined through standardized EIA techniques and calculations resulting from a four-parameter sigmoidal curve-fitting function as previously described (Bales, 2000; Bales et al., 2005). Internal quality control pools consisted of female Geoffroy’s marmoset urine. Quality control pools were run at high (1:2,560 dilution in 1:10 extraction buffer:PBS) and low concentrations (1:20,480 dilution in 1:10 extraction buffer:PBS). The intra- and inter-assay coefficients of variation for high and low concentrations of the urine quality control pool were 3.9% and 11.4% (high), and 3.9% and 20.7% (low), respectively. This EIA assay for fecal cortisol was previously tested for accuracy and validated against circulating hormone levels (Bales, 2000; Bales et al., 2005).
Samples that were more than 10% above the highest standard were re-assayed after further dilution. A small number of samples produced results that were below the lowest standard value by 10% or more (15 cases distributed over 10 females with no female having more than 3 samples with low values). These samples were assigned the value corresponding to 10% below the lowest standard (7.02 pg/well) that was used to calculate ng cortisol/g feces.
Detection of ovulation, pregnancy, parturition and abortion
We plotted PdG and cortisol concentrations over time to help visualize reproductive patterns for individual GLT females. Variation in progesterone levels corresponds to ovulation, the formation of the corpus luteum and placental development (Nelson, 2005; Ojeda, 1996) and has been used to trace ovarian cycles and pregnancy in GLTs (Bales, 2000; French et al., 2003; French et al., 2002; French and Stribley, 1985, 1987). We examined progesterone profiles of subordinate adult GLT females from 18 months of age until they became pregnant for the first time. Subordinate adult females were considered ovulatory if non-pregnant progesterone profiles showed cyclical elevation in PdG concentrations averaging 20 days in periodicity (French et al., 2002).
Pregnancy was diagnosed primarily by the prolonged elevation of PdG concentrations (Bales, 2000; French et al., 2003; French et al., 2002). Cortisol, a glucocorticoid responsible for mobilizing fat and protein reserves for use by the body in times of food deprivation or stress (Nelson, 2005), also shows a significant increase in concentration during the 3rd trimester of GLT pregnancy (Bales, 2000; Bales et al., 2005), so can be used as a late pregnancy diagnostic. In addition, pregnant GLT females demonstrate steady and considerable weight gain beginning in their 2nd trimester of pregnancy and continuing throughout gestation (Bales et al., 2001; Hankerson, 2008). Parturition was diagnosed by a sudden drop in PdG concentrations to baseline levels (Bales, 2000; French et al., 2003; French et al., 2002) accompanied by a rapid and large amount of weight loss (mean=112.7 g; range 73 to 142 g; n=12 full term pregnancies for which immediate pre- and post-partum weights were available (Siani, 2009)). We defined successful pregnancies as those that were carried to full term and that resulted in observations of new infants carried and nursed during the first week after birth by the female identified as the mother. Birth dates were recorded within one or two days of parturition for most infants and within one week for all observed infants born during this study. To establish a calendar for each successful pregnancy we divided the 125-day gestation period (French et al., 2002) into trimesters. Trimesters were assigned by counting back from known parturition dates: 84–125 days (3rd trimester), 42–83 days (2nd) and 0–41 days (1st) (French et al., 2002; French and Stribley, 1985). Because hormone profiles are highly individualized, for each reproductive female we calculated average PdG concentrations during each trimester of pregnancy and during non-pregnant periods to provide “typical” values for successful pregnancies. These trimester averages were used as a guide in diagnosing suspected pregnancies for which infants were not observed and to determine the probable trimester in which pregnancies were aborted.
Pregnancy diagnoses and assignment of trimesters for pregnancies that did not result in observed infants were made by examining multiple sources of information on a case-by-case basis. We used average trimester PdG values for successful pregnancies as a reference to assign trimesters to unsuccessful pregnancies. Females that produced offspring showed mean PdG concentrations of 1500 ng PdG/g feces when they were not pregnant. Their mean PdG concentrations rose to 3500 ng PdG/g feces during the 1st trimester of pregnancy. Mean PdG concentrations above 5000 ng PdG/g feces were considered indicative of a pregnancy that had progressed into the 2nd trimester. Concentrations averaging 10,000 ng PdG/g feces or higher were considered indicative of 3rd trimester pregnancy. Consistently high levels of PdG that suddenly dropped to baseline levels indicated pregnancy loss or parturition depending on when concentrations returned to baseline values (Bales, 2000; French et al., 2003; French et al., 2002).
Results from cortisol assays were also used as a crosscheck when diagnosing pregnancy. A large and consistent rise in cortisol reaching a mean of 15,000 ng cortisol/g feces was used to diagnose pregnancies that had proceeded into the middle to late 3rd trimester. Short rises in PdG concentrations that were not accompanied by weight gain often coincided with temporary increases in cortisol concentrations and potentially stressful events such as bi-annual captures, immigration of new individuals into social groups and increased group encounter frequencies. Extremes in nutrition and stress can affect any assay using a metabolite (PdG) rather than measuring the hormone of interest (progesterone) directly (Griffin, 1996). Thus, cortisol was also used as a crosscheck to prevent false positive early pregnancy diagnoses based upon elevated PdG concentrations resulting from stress.
We used patterns of weight gain or loss in GLT females to establish guidelines for diagnosing pregnancy from the 2nd trimester onward. Since no significant weight gain occurs during the 1st trimester of GLT pregnancy (Bales et al., 2001; Hankerson, 2008; Siani, 2009), we did not attempt to use weight to diagnose pregnancies that did not progress past the 1st trimester. Females gaining 5–10% of their non-pregnant average weight in a consistently rising pattern were considered to be in their 2nd trimester of pregnancy. Steady weight gain in excess of 10% of a female’s non-pregnant body weight was used as an indicator of a 3rd trimester pregnancy. Females that gained approximately 20% of their body mass were considered to have reached full term, since neonatal weight for Leontopithecus is on average 20% of maternal body weight (Tardif et al., 1993). Average weight loss for 12 births during the current study for which immediate pre- and post-partum weights were available was 18.6% of the females’ post-partum weight (Siani, 2009). Rapid weight loss was considered indicative of parturition or the loss of a 2nd or 3rd trimester pregnancy. Using weight gain or loss based upon percentages of normative body weight allowed us to account for individual differences in non-pregnant body mass (Dietz et al., 1994; Siani, 2009) while remaining consistent with the average patterns of weight gain throughout pregnancy for our study population.
We used bi-annual capture records from 2004 through 2007 to provide data points during early pregnancy in late May or June when field weights were not available and pregnancy diagnoses were not often possible given hormonal concentrations that remain low during this early stage. These physical examinations included each female’s weight (considered together with the weights obtained without capture as described above), nipple length (parous females having nipple lengths >3 mm in length), whether a female was lactating or not (indicative of a recent parturition whether infants were observed or not) and uterus palpation for pregnancy and trimester diagnosis at the time of capture (Dietz and Baker, 1993; Dietz et al., 1994).
Ad libitum field observations regarding female appearance (e.g. distended abdomen) and behavioral changes (e.g. remaining at the rear of encounters with neighboring groups and reduced daily travel lengths shortly before parturition) provided additional confirmation, though were not considered diagnostic.
PdG and cortisol averages by trimester
For pregnancies without the observation of live infants and thus unknown dates of parturition we used the guidelines above, counting either forward or backward from an established trimester to obtain surrounding trimester cut off dates as well as probable conception and projected parturition dates. We then calculated average PdG and cortisol concentrations for each trimester for all pregnancies, successful and unsuccessful, as well as for non-pregnant adult females.
STATISTICAL ANALYSES
Hormonal changes associated with pregnancy
We performed a linear mixed model analysis of variance (ANOVA) using the GLIMMIX procedure in SAS 9.2 (SAS Institute, Cary, North Carolina) to determine whether PdG and cortisol concentrations changed significantly as females became pregnant and as pregnancy proceeded from one trimester to another. To avoid hormonal variation due to differences in reproductive maturity (French et al., 2003; French et al., 2002; French et al., 1989; French and Stribley, 1987), only data for adult females were included in analyses. The data set tested consisted of 367 monthly hormonal averages from 18 adult females from seven GLT groups (n=18 females contributing 179 samples while non-pregnant, n=14 females contributing 65 samples during their 1st trimester of pregnancy, n=14 females contributing 65 samples during their 2nd trimester of pregnancy and n=14 females contributing 58 samples during their 3rd trimester of pregnancy). Samples from both successful and unsuccessful pregnancies were included. Using GLIMMIX we were able to account for correlations within the data by assigning both random effects, such as the home range occupied by each female, and repeated effects, such as individual female identity and the identity of each pregnancy. As such, our analysis addressed the problem of pseudoreplication that occurs when multiple observations are drawn from a single female or from multiple females occupying a single home range (Bales et al., 2002; Bales et al., 2001; Diggle et al., 1999). The data were log transformed to meet the assumptions of normality prior to analysis. Pairwise comparisons were performed on least squared means using a Tukey-Kramer adjustment for multiple comparisons.
Effect of dominance status and group size on reproductive success
We used logistic regression analyses to test for a statistical relationship between the dominance status of the pregnant female and the likelihood that the pregnancy would result in live infants. We also included group size in the model to test whether pregnant females occupying larger groups had a greater likelihood of giving birth to live young. We performed backward selection eliminating non-significant variables (those with the largest p-value>0.05) one at a time. All 37 pregnancies detected during the course of the study were included in this analysis including 26 successful and 11 unsuccessful pregnancies to 14 dominant and subordinate females. We controlled for multiple pregnancies by including FEMID as a covariate in the model. The final model was accepted when every variable remaining in the model was significant, the model as a whole was significant and all assumptions for the model were met.
RESULTS
Pregnancy polygyny in free-ranging GLTs
All adult females over 3.9 years of age became pregnant (Table 1). A total of 37 pregnancies to 14 adult GLT females were detected. Ten females gave birth to 48 infants as the result of 26 successful pregnancies. Eleven pregnancies to seven females were unsuccessful. Of the 21 GLT females sampled, only five females did not become pregnant after reaching adulthood. Four of the five were younger than three years of age at the conclusion of the study but did show cyclical elevations in PdG concentrations indicative of ovulation. The fifth female was of unknown age and remained within a habituated study group for just three months during which time she did not become pregnant.
Six of the seven GLT groups studied contained more than one adult (post-pubertal) female, thus were potentially polygynous. Five of these six groups (83%) exhibited pregnancy polygyny. Only four pregnancies by two subordinate adult females in two groups resulted in the birth of live young.
Effect of dominance status and group size on reproductive success
Both dominance status of the pregnant female (Wald Chi-Square=7.69, df=1, p=0.0055) and the number of individuals in her group (Wald Chi-Square=4.52, df=1, p=0.0335) were significant predictors of a successful pregnancy according to a significant logistic regression model that also controlled for FEMID (Likelihood Ratio Chi-Square=15.37, df=3, p=0.0015). A pregnant female holding dominant status in her group during her pregnancy was 40.4 (95% confidence limits=3.0–552.1) times as likely to give birth to live young as a pregnant female holding a subordinate ranking. Dominant females became pregnant on 26 occasions, 22 (85%) pregnancies were successful and 4 (15%) unsuccessful. Two pregnancies to dominant females were lost in the second trimester and two were lost in the third trimester. Subordinate females became pregnant on 11 occasions, 4 (36%) pregnancies were successful and 7 (64%) unsuccessful. Infants were never observed for five subordinate pregnancies though they were carried to full-term. In addition, one subordinate female lost a pregnancy during the second trimester and another subordinate female lost a pregnancy during the third trimester.
Pregnant females residing in larger groups were more likely to have a successful pregnancy, regardless of whether they held dominant or subordinate status. For every additional group member, pregnant females increased their chances of success by 1.7 times (95% confidence limits=1.0–2.9). Given information on the dominance status of the pregnant female and the number of individuals residing within her group, we correctly classified a pregnancy as successful 88.5% of the time.
Hormonal changes associated with pregnancy
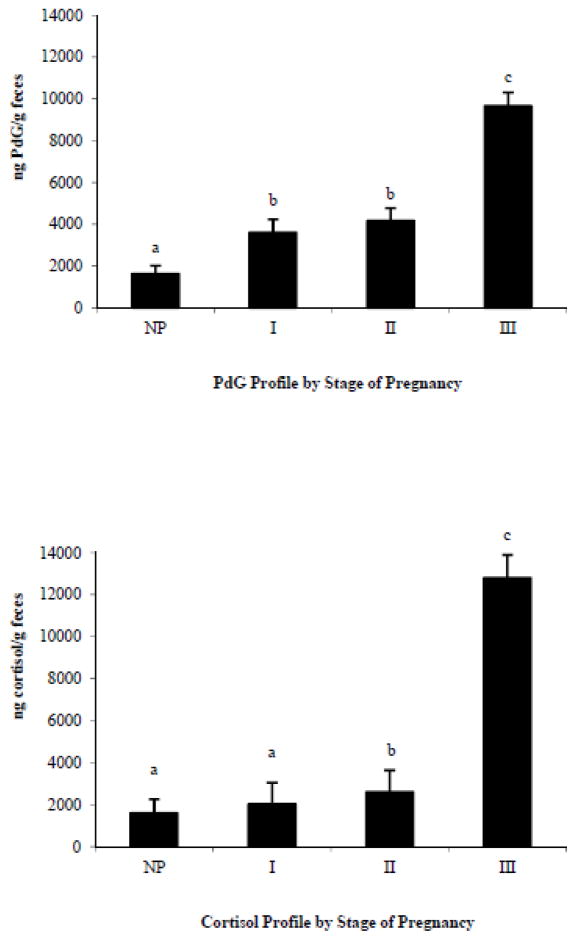
Both PdG (F(3,342)=50.34, p<0.0001) and cortisol (F(3,342)=41.95, p<0.0001) concentrations were significantly affected by stage of pregnancy (Figure 1). Mean PdG concentrations increased significantly following conception (non-pregnant vs. 1st trimester; t(342)=5.86, adjusted p<0.0001). PdG levels did not increase significantly from the 1st to the 2nd trimester (t(342)=0.44, adjusted p=0.9711). A significant increase in PdG occurred from the 2nd trimester to the 3rd trimester of pregnancy (t(342)=4.85, adjusted p=0.0001). Cortisol did not increase significantly following conception (non-pregnant vs. 1st trimester; t(342)=0.04, adjusted p=1.000). However, cortisol increased significantly as females moved from the 1st to the 2nd trimester of pregnancy (t(342)=2.67, adjusted p=0.0395). The largest increase in cortisol was from the 2nd to the 3rd trimester of pregnancy (t(342)=6.58, adjusted p<0.0001).
Figure 1.
Mean (± standard error of the mean) fecal pregnanediol-3-glucuronide (PdG) and cortisol concentrations (ng hormone/g feces) for adult females while non-pregnant (NP, n=179 samples, from 18 females) and during each trimester of pregnancy (1st trimester, I, n=65, 14; 2nd trimester, II, n=65, 14; 3rd trimester, III, n=58, 14). Statistically significant differences in means are indicated by different letters.
DISCUSSION
High rates of pregnancy polygyny under stable group compositions
In the current study, all females over 3.9 years of age became pregnant regardless of whether they still resided in their natal group. Pregnancy was more common in this GLT population than previously reported (Baker and Dietz, 1993; Dietz et al. 1994; Baker et al. 2002; French et al., 2003). Subordinate females in the seven groups studied were pregnant at the same time as dominant females 11 times during three reproductive years. The 83% rate of pregnancy polygyny observed in this study represents an almost doubling of the 44.3% rate reported by Baker et al. (2002). The large increase in pregnancy polygyny reported here is likely due to the use of improved methods of pregnancy detection. The combination of non-invasive fecal hormone enzyme immunoassay for progesterone and cortisol, baited scales for obtaining weekly data on individual weight gain without capture, evaluation of reproductive status during biannual live captures and the observation of behavioral changes occurring during pregnancy allowed the detection of mid- to late-term pregnancies that went unobserved in previous studies. Consistency between the trimester-based hormone profiles in this and previous studies of successful pregnancies in GLTs (Bales, 2000; Bales et al., 2005; French et al., 2003) and other callitrichids (Smith and French, 1997; Ziegler et al., 1995; Ziegler and Sousa, 2002; Ziegler et al., 2004) provides support for our pregnancy diagnoses.
Polygyny was common in large GLT groups. With each additional group member, the likelihood of a successful pregnancy increased 1.7 times, regardless of the dominance status of the female. Multiple breeding females lead to larger groups, but larger groups also provide the support necessary to care for multiple infants that arrive during the same reproductive season (in birds (Brown, 1987), coyotes (Canis latrans (Bekoff and Wells, 1982)), lions (Panthera leo (Bygott et al., 1979)), African wild dogs (Lycaon pictus (Malcolm and Marten, 1982)), blackbacked jackals (Canis mesomelas (Moehlman, 1979)), dwarf mongooses (Rood, 1990) and badgers (Meles meles (Kruuk, 1989))). Both the current study and earlier reports from the same research site (Baker et al., 2002) included data collected during periods when predation levels and population turnover were low. Study groups were large, stable and dominant females tended to be older, a set of circumstances that have been shown to promote polygyny in GLTs (Baker et al., 2002; Dietz, 2004; Dietz and Baker, 1993; French et al., 2003). With most or all breeding positions occupied by tenured females, eldest subordinate GLT daughters residing within large, stable groups attempted reproduction within their natal groups, which led to high rates of pregnancy polygyny.
Our report of pregnancy polygyny occurring in 83% of GLT groups may still be an underestimate. Although progesterone concentrations rise significantly during the 1st trimester of pregnancy (Figure 1), weight gain during this time is not significant (Bales et al., 2001; Hankerson, 2008; Siani, 2009), uterine palpation fails to detect fetuses (Henry and Dietz, unpublished data) and maternal behavior does not change (Henry, personal observation). The lack of corroborating sources of evidence to diagnose early pregnancy may have resulted in non-detection of pregnancies lost during the 1st trimester.
Mechanisms responsible for maintaining high reproductive skew in free-ranging GLT groups
Reproduction in subordinate adult female GLTs appears to be limited by pregnancy loss. Pregnancy polygyny followed by high rates of subordinate pregnancy loss occurs in several cooperatively breeding species including banded mongooses (Gilchrist, 2006a, 2006b), meerkats (Clutton-Brock et al., 2008; Young et al., 2006), marmosets (Arruda et al., 2005; Digby, 1995a; Saltzman et al., 2009; Saltzman et al., 2008; Sousa et al., 2005), tamarins and lion tamarins (Dietz and Baker, 1993; Garber, 1997; Goldizen et al., 1996). Banded mongooses have low preparturition reproductive skew, but high rates of subordinate pregnancy loss resulting from stress-induced abortions and infanticide tip the scales back in favor of dominant reproductive success (Gilchrist, 2006a).
Dominant female GLTs were 40 times more likely to give birth to live infants than were subordinate females. This is not surprising given the association between cooperative breeding and high reproductive skew seen across taxa (Clutton-Brock, 2009). When simultaneous breeding by multiple females dilutes the investment of helpers and/or reduces the survival or growth of offspring born to the dominant (Clutton-Brock et al., 2001b; Hodge, 2009), selection should favor dominant females able to control subordinate reproduction through hormonal and/or behavioral mechanisms.
Dominant control via hormonal mechanisms
Other studies suggest that dominant females exert control over subordinate reproduction through the reduction of reproductive hormone concentrations (Florida scrub jays (Aphelocoma coerulescens coerulescens (Schoech et al., 1991)), Harris’ hawks (Parabuteu unicinctus (Mays et al., 1991)), dwarf mongooses (Creel et al., 1992) and African mole-rats (Cryptomys spp. and Heterocephalus spp. (Faulkes and Bennett, 2001))). Singular breeding in many callitrichid species has been shown to be maintained by hormonal suppression of subordinate female ovulation and the inhibition of subordinate sexual behavior (in marmosets (Abbott, 1984, 1993; Abbott and Hearn, 1978; Abbott et al., 1981; Albuquerque et al., 2001; Digby, 1999; Evans and Hodges, 1984; French, 1997; Hubrecht, 1989; Rothe, 1975; Saltzman et al., 1997; Sousa et al., 2005) and tamarins (Abbott, 1993; Epple and Katz, 1984; French, 1997; French et al., 1984)).
The genus Leontopithecus differs from other callitrichids in that dominant females do not appear to control subordinate reproduction through the suppression of ovulation in either captive (French, 1987; French et al., 2002; French et al., 1989; French and Stribley, 1987; Inglett, 1993; Monfort et al., 1996) or free-ranging populations (Henry, 2011). Subordinate GLT females older than 18 months of age ovulate, as demonstrated by cyclical elevations of progesterone concentrations, while residing within their natal group (Henry, 2011) and all females regardless of dominance status became pregnant by the time they were 3.9 years of age. In addition, consistency between the trimester-based hormone profiles in this study (Figure 1) and previous studies of successful GLT pregnancies (Bales, 2000; Bales et al., 2005; French et al., 2003) do not lend support to the hypothesis that pregnancies by subordinate females fail as a result of dominant interference in subordinate reproduction via hormonal mechanisms.
Dominant control via behavioral mechanisms
Five of seven unsuccessful pregnancies by subordinate females were carried to full-term but infants were never observed. Aggression, eviction, stress-induced abortion and infanticide have been shown to skew post-conceptive reproductive success in favor of dominant females in a wide range of cooperatively breeding species (Arabian babblers (Turdoides squamiceps (Zahavi, 1990)), meerkats (Clutton-Brock et al., 2001a; Clutton-Brock et al., 1998a; Clutton-Brock et al., 2008; Kutsukake and Clutton-Brock, 2006; Young et al., 2006), dingos (Canis familiaris dingo (Corbett, 1988)), African wild dogs (Creel et al., 1997), dwarf mongooses (Creel and Waser, 1991, 1997; Rasa, 1994), banded mongooses (Cant et al., 2010; Gilchrist, 2006a), naked mole-rats (Faulkes and Abbott, 1997), prairie dogs (Cynomys ludovicianus (Hoogland, 1985)) and in Mongolian gerbils (Meriones unguiculatus (Saltzman et al., 2006))). In the current study, five of seven unsuccessful subordinate GLT pregnancies were carried to full term. With no hormonal evidence to indicate abnormal fetal or placental development, it seems unlikely that pregnancies lost at full term were stillborn. In common marmoset groups, where the potential hormonal and behavioral mechanisms responsible for reproductive skew have been examined in detail in captive and wild groups, infanticide appears to be responsible for limiting reproductive success in subordinate females (Saltzman, 2003; Saltzman et al., 2009). In meerkats, simultaneous litters by dominant and subordinate females dilute helping investment and reduce food intake and growth of pups, variables closely associated with survival (Clutton-Brock et al., 2001b; Hodge, 2009). Infanticide in meerkats has been interpreted as a means to reduce competition for resources among litters (Young and Clutton-Brock, 2006). In contrast, infanticide has never been observed in the long term study of GLTs at PDA (Dietz, personal communication). However, overt aggression by dominant females toward subordinate females has been suggested as a potential mechanism limiting reproduction by subordinate lion tamarin females (De Vleeschouwer et al., 2001; French et al., 2002; Inglett et al., 1989; Kleiman, 1979). Attempts to evict subordinates upon giving birth within the natal group could lead to the abandonment of litters in order to re-enter the group as has been shown to occur in banded mongooses (Gilchrist, 2006a). In the face of dominant aggression toward themselves, their newborn infants, or toward any group member offering assistance to subordinate mothers, subordinate females may simply abandon their newborn offspring shortly after birth in favor of being allowed to remain peaceably within the group. The contradiction presented here between high rates of pregnancy polygyny and low reproductive success by subordinate females encourages a re-examination of the classical explanations for singular breeding in callitrichids.
Highlights.
All female golden lion tamarins became pregnant by 3.9 yrs of age.
Two females became pregnant in 83% of groups with multiple adult females.
Pregnancies by dominant females were 40 times more likely to succeed.
5 of 7 subordinate pregnancies reached full-term but infants were never observed.
Subordinate reproduction was limited via behavioral, not hormonal mechanisms.
Acknowledgments
Funding for this project was provided by the American Society of Mammalogists, the Copenhagen Zoo Lion Tamarins of Brazil Fund, the University of Maryland Center for Biodiversity, a Darwin Fellowship from the University of Maryland BEES Program and a Wylie Dissertation Fellowship from the University of Maryland Graduate School to M. Henry and by the National Science Foundation (SBR-9727687; BCS-0216096) to J. Dietz. The Endocrine Bioservices Laboratory at UNO was supported in part by funds from the National Institutes of Health (HD 042882) to J. French. We thank the Brazilian Science Council (CNPq), Brazilian Institute for the Environment and Renewable Natural Resources (IBAMA) and Associação Mico-Leão-Dourado (AMLD) for logistic support and permission to conduct this study. This research complies with the guidelines of the University of Maryland Animal Care and Use Committee and all applicable Brazilian laws. Importation of fecal samples into the U.S. was conducted under the auspices of CITES, USDA and CDC. We thank AMLD research assistants Otávio Narciso, Synval de Melo, Jadir Ramos and Andréia Martins for help with data collection in the field. We thank Jeff Fite, Kate Townley and Tom Shirazi for assistance with endocrine analyses. We also thank Karen Bales for setting the precedent for endocrine work in this population of tamarins.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
MaLinda D. Henry, Email: malinda.henry@unmc.edu.
Sarah J. Hankerson, Email: sjhank@stthomas.edu.
Jennifer M. Siani, Email: jmsiani@gmail.com.
Jeffrey A. French, Email: jfrench@unomaha.edu.
James M. Dietz, Email: jmdietz@umd.edu.
References
- Abbott DH. Behavioral and physiological suppression of fertility in subordinate marmoset monkeys. Am J Primatol. 1984;6:169–186. doi: 10.1002/ajp.1350060305. [DOI] [PubMed] [Google Scholar]
- Abbott DH. Social conflict and reproductive suppression in marmoset and tamarin monkeys. In: Mason WA, Mendoza SP, editors. Primate social conflict. State University of New York Press; Albany, NY: 1993. pp. 331–372. [Google Scholar]
- Abbott DH, Hearn JP. Physical, hormonal, and behavioural aspects of sexual development in the marmoset monkey, Callithrix jacchus. J Reprod Fertil. 1978;53:155–166. doi: 10.1530/jrf.0.0530155. [DOI] [PubMed] [Google Scholar]
- Abbott DH, McNeilly AS, Lunn SF, Hulme MJ, Burden FJ. Inhibition of ovarian function in subordinate female marmoset monkeys (Callithrix jacchus jacchus) J Reprod Fertil. 1981;63:335–345. doi: 10.1530/jrf.0.0630335. [DOI] [PubMed] [Google Scholar]
- Albuquerque ACSR, Sousa MBC, Santos HM, Ziegler TE. Behavioral and hormonal analysis of social relationships between oldest females in a wild monogamous group of common marmosets (Callithrix jacchus) Int J Primatol. 2001;22:631–645. [Google Scholar]
- Arruda MF, Araujo A, Sousa MBC, Albuquerque FS, Albuquerque ACSR, Yamamoto ME. Two breeding females within free-living groups may not always indicate polygyny: alternative subordinate female strategies in common marmosets (Callithrix jacchus) Folia Primatol (Basel) 2005;76:10–20. doi: 10.1159/000082451. [DOI] [PubMed] [Google Scholar]
- Baker AJ, Bales KL, Dietz JM. Mating system and group dynamics in lion tamarins. In: Kleiman DG, Rylands AB, editors. Lion tamarins: biology and conservation. Smithsonian Institution Press; Washington, D.C: 2002. pp. 188–212. [Google Scholar]
- Baker AJ, Dietz JM, Kleiman DG. Behavioural evidence for monopolization of paternity in multi-male groups of golden lion tamarins. Anim Behav. 1993;46:1091–1103. [Google Scholar]
- Bales KL. Mammalian monogamy: dominance, hormones, and maternal care in wild golden lion tamarins, Biology. University of Maryland; College Park, MD: 2000. [Google Scholar]
- Bales KL, French JA, Dietz JM. Explaining variation in maternal care in a cooperatively breeding mammal. Anim Behav. 2002;63:453–461. [Google Scholar]
- Bales KL, French JA, Hostetler CM, Dietz JM. Social and reproductive factors affecting cortisol levels in wild female golden lion tamarins (Leontopithecus rosalia) Am J Primatol. 2005;67:25–35. doi: 10.1002/ajp.20167. [DOI] [PubMed] [Google Scholar]
- Bales KL, O’Herron M, Baker AJ, Dietz JM. Sources of variability in numbers of live births in wild golden lion tamarins (Leontopithecus rosalia) Am J Primatol. 2001;54:211–221. doi: 10.1002/ajp.1031. [DOI] [PubMed] [Google Scholar]
- Bekoff M, Wells MC. The behavioral ecology of coyotes: social organization, rearing patterns, space use, and resource defense. Z Tierpsychol. 1982;60:281–305. [Google Scholar]
- Brown JL. Helping and communal breeding in birds. Princeton University Press; Princeton: 1987. [Google Scholar]
- Bygott JD, Bertram BCR, Hanby JP. Male lions in large coalitions gain reproductive advantages. Nature. 1979;282:838–840. [Google Scholar]
- Cant MA, Hodge SJ, Bell MBV, Gilchrist JS, Nichols HJ. Reproductive control via eviction (but not the threat of eviction) in banded mongooses. Proc R Soc B. 2010;277:2219–2226. doi: 10.1098/rspb.2009.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock TH. Stucture and function in mammalian societies. Phil Trans R Soc B. 2009;364:3229–3242. doi: 10.1098/rstb.2009.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock TH, Brotherton PNM, Russell AF, O’Riain MJ, Gaynor D, Kansky R, Griffin A, Manser M, Sharpe L, McIlrath GM, Small T, Moss A, Monfort SL. Cooperation, control, and concession in meerkat groups. Science. 2001a;291:478–481. doi: 10.1126/science.291.5503.478. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH, Brotherton PNM, Smith R, McIlrath GM, Kansky R, Gaynor D, O’Riain MJ, Skinner JD. Infanticide and expulsion of females in a cooperative mammal. Proc R Soc B. 1998a;265:2291–2295. doi: 10.1098/rspb.1998.0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock TH, Gaynor D, Kansky R, MacColl ADC, McIlrath GM, Chadwick P, Brotherton PNM, O’Riain JM, Manser M, Skinner JD. Costs of cooperative behaviour in suricates (Suricata suricata) Proc R Soc B. 1998b;265:185–190. doi: 10.1098/rspb.1998.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock TH, Hodge SJ, Flower TP. Group size and the suppression of subordinate reproduction in Kalahari meerkats. Anim Behav. 2008;76:689–700. [Google Scholar]
- Clutton-Brock TH, Russell AF, Sharpe L, Brotherton PNM, McIlrath GM, White S, Cameron EZ. Effects of helpers on juvenile development and survival in meerkats. Science. 2001b;293:2446–2449. doi: 10.1126/science.1061274. [DOI] [PubMed] [Google Scholar]
- Corbett LK. Social dynamics of a captive dingo pack: population regulation by dominant female infanticide. Ethology. 1988;78:177–198. [Google Scholar]
- Creel SR, Creel NM. Energetics, reproductive suppression and obligate communal breeding in carnivores. Behav Ecol Sociobiol. 1991;28:263–270. [Google Scholar]
- Creel SR, Creel NM, Mills GL, Monfort SL. Rank and reproduction in cooperatively breeding African wild dogs: behavioral and endocrine correlates. Behav Ecol. 1997;8:298–306. [Google Scholar]
- Creel SR, Creel NM, Wildt DE, Monfort SL. Behavioral and endocrine mechanisms of reproductive suppression in Serenge dwarf mongooses. Anim Behav. 1992;43:231–245. [Google Scholar]
- Creel SR, Waser PM. Failures of reproductive suppression in dwarf mongooses (Helogale parvula): accident or adaption. Behav Ecol. 1991;2:7–15. [Google Scholar]
- Creel SR, Waser PM. Variation in reproductive suppression among dwarf mongooses: interplay between mechanisms and evolution. In: Solomon NG, French JA, editors. Cooperative breeding in mammals. Cambridge University Press; Cambridge: 1997. pp. 150–198. [Google Scholar]
- De Vleeschouwer K, Van Elsacker L, Leus K. Multiple breeding females in captive groups of golden-headed lion tamarins (Leontopithecus chrysomelas): causes and consequences. Folia Primatol (Basel) 2001;72:1–10. doi: 10.1159/000049913. [DOI] [PubMed] [Google Scholar]
- Dietz JM. Kinship structure and reproductive skew in cooperative breeding primates. In: Chapais B, Berman C, editors. Kinship and behavior in primates. Oxford University Press; New York, NY: 2004. pp. 233–241. [Google Scholar]
- Dietz JM, Baker AJ. Polygyny and female reproductive success in golden lion tamarins, Leontopithecus rosalia. Anim Behav. 1993;46:1067–1078. [Google Scholar]
- Dietz JM, Baker AJ, Miglioretti D. Seasonal variation in reproduction, juvenile growth, and adult body mass in golden lion tamarins (Leontopithecus rosalia) Am J Primatol. 1994;34:115–132. doi: 10.1002/ajp.1350340204. [DOI] [PubMed] [Google Scholar]
- Digby LJ. Infant care, infanticide, and female reproductive strategies in polygynous groups of common marmosets (Callithrix jacchus) Behav Ecol Sociobiol. 1995a;37:51–61. [Google Scholar]
- Digby LJ. Social organization in wild population of Callithrix jacchus II Intragroup social behavior. Primates. 1995b;36:361–375. [Google Scholar]
- Digby LJ. Sexual behavior and extragroup copulations in a wild population of common marmosets (Callithrix jacchus) Folia Primatol (Basel) 1999;70:136–145. doi: 10.1159/000021686. [DOI] [PubMed] [Google Scholar]
- Digby LJ, Ferrari SF. Multiple breeding females in free-ranging groups of Callithrix jacchus. Int J Primatol. 1994;15:389–397. [Google Scholar]
- Diggle PJ, Liang K, Zeger SL. Analysis of longitudinal data. Clarendon Press; Oxford: 1999. [Google Scholar]
- Emlen ST. The evolution of helping. I An ecological constraints model. Am Nat. 1982;119:29–39. [Google Scholar]
- Epple G, Katz Y. Social influences on estrogen excretion and ovarian cyclicity in saddleback tamarins (Saguinus fuscicollis) Am J Primatol. 1984;6:215–227. doi: 10.1002/ajp.1350060309. [DOI] [PubMed] [Google Scholar]
- Evans S, Hodges JK. Reproductive status of adult daughters in family groups of common marmosets (Callithrix jacchus jacchus) Folia Primatol (Basel) 1984;42:127–133. doi: 10.1159/000156155. [DOI] [PubMed] [Google Scholar]
- Faulkes CG, Abbott DH. The physiology of a reprodutive dictatorship: regulation of male and female reproduction by a single breeding female in colonies of naked mole-rats. In: Solomon NG, French JA, editors. Cooperative breeding in mammals. Cambridge University Press; Cambridge: 1997. pp. 302–334. [Google Scholar]
- Faulkes CG, Bennett NC. Family values: group dynamics and social control of reproduction in African mole-rats. Trends Ecol Evolut. 2001;16:184–190. doi: 10.1016/s0169-5347(01)02116-4. [DOI] [PubMed] [Google Scholar]
- Fite JE, Patera KJ, French JA, Rukstalis M, Hopkins EC, Ross CN. Opportunistic mothers: female marmosets (Callithrix kuhlii) reduce their investment in offspring when they have to, and when they can. J Hum Evol. 2005;49:122–142. doi: 10.1016/j.jhevol.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick JL, Desjardins JK, Milligan N, Stiver KA, Montgomerie R, Balshine S. Female-mediated causes and consequences of status change in a social fish. Proc R Soc B. 2008;275:929–936. doi: 10.1098/rspb.2007.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA. Reproductive suppression in marmosets and tamarins-absence of social effects in the lion tamarins (Leontopithecus rosalia) Am J Primatol. 1987;12:342. [Google Scholar]
- French JA. Proximate regulation of singular breeding in callitrichid primates. In: Solomon NG, French JA, editors. Cooperative breeding in mammals. Cambridge Unversity Press; Cambridge: 1997. pp. 34–75. [Google Scholar]
- French JA, Abbott DH, Snowdon CT. The effect of social environment on estrogen excretion, scent marking, and socio-sexual behavior in tamarins (Saguinus oedipus) Am J Primatol. 1984;6:155–167. doi: 10.1002/ajp.1350060304. [DOI] [PubMed] [Google Scholar]
- French JA, Bales KL, Baker AJ, Dietz JM. Endocrine monitoring of wild dominant and subordinate female Leontopithecus rosalia. Int J Primatol. 2003;24:1281–1300. [Google Scholar]
- French JA, Brewer KJ, Schaffner CM, Schalley J, Hightower-Merritt D, Smith TE, Bell SM. Urinary steroid and gonadotropin excretion across the reproductive cycle in female Wied’s black tufted-ear marmosets (Callithrix kuhli) Am J Primatol. 1996;40:231–245. doi: 10.1002/(SICI)1098-2345(1996)40:3<231::AID-AJP2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- French JA, De Vleeschouwer K, Bales KL, Heistermann M. Lion tamarin reproductive biology. In: Kleiman DG, Rylands AB, editors. Lion Tamarins: biology and conservation. Smithsonian Institution Press; Washington, DC: 2002. pp. 133–156. [Google Scholar]
- French JA, Inglett BJ, Dethlefs TM. The reproductive status of non-breeding group members in captive golden lion tamarin social groups. Am J Primatol. 1989;18:73–86. doi: 10.1002/ajp.1350180202. [DOI] [PubMed] [Google Scholar]
- French JA, Stribley JA. Patterns of urinary oestrogen excretion in female golden lion tamarins (Leontopithecus rosalia) J. Reprod. Fertil. 1985;75:537–546. doi: 10.1530/jrf.0.0750537. [DOI] [PubMed] [Google Scholar]
- French JA, Stribley JA. Synchronization of ovarian cycles within and between social groups in the golden lion tamarin (Leontopithecus rosalia) Am J Primatol. 1987;12:469–478. doi: 10.1002/ajp.1350120403. [DOI] [PubMed] [Google Scholar]
- Garber PA. One for all and breeding for one: cooperation and competition as a tamarin reproductive strategy. Evol Anthropol. 1997;5:187–199. [Google Scholar]
- Garber PA, Encarnación FML, Pruetz JD. Demographic and reproductive patterns in moustached tamarin monkeys (Saguinus mystax): implications for reconstructing platyrrhine mating systems. Am J Primatol. 1993;29:235–254. doi: 10.1002/ajp.1350290402. [DOI] [PubMed] [Google Scholar]
- Garber PA, Moya L, Malaga C. A preliminary field study of the moustached tamarin monkey (Saguinus mystax) in northern Peru: questions concerned witht he evolution of a communal breeding system. Folia Primatol (Basel) 1984;42:17–32. [Google Scholar]
- Gilchrist JS. Female eviction, abortion, and infanticide in banded mongooses (Mungos mungo): implications for social control of reproduction and synchronized parturition. Behav Ecol. 2006a;17:664–669. [Google Scholar]
- Gilchrist JS. Reproductive success in a low skew, communal breeding mammal: the banded mongoose, Mungos mungo. Behav Ecol Sociobiol. 2006b;60:854–863. [Google Scholar]
- Goldizen AW. Tamarins and marmosets: communal care of offspring. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham R, Struhsaker TT, editors. Primate Societies. Chicago University Press; Chicago, IL: 1987. pp. 34–43. [Google Scholar]
- Goldizen AW, Mendelson J, van Vlaardingen M, Terborgh J. Saddle-back tamarin (Saguinus fuscicollis) reproductive strategies: evidence from a thirteen-year study of a marked population. Am J Primatol. 1996;38:57–83. doi: 10.1002/(SICI)1098-2345(1996)38:1<57::AID-AJP6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Griffin JE. Assesment of endocrine function. In: Griffin JE, Ojeda SR, editors. Textbook of endocrine physiology. Oxford University Press; Oxford: 1996. pp. 86–100. [Google Scholar]
- Hamilton IM. A commitment model of reproductive inhibition in cooperatively breeding groups. Behav Ecol. 2004;15:585–591. [Google Scholar]
- Hankerson SJ. Behavior, Ecology, Evolution, and Systematics. University of Maryland; College Park, MD: 2008. Resource and space use in the wild golden lion tamarin, Leontopithecus rosalia. [Google Scholar]
- Hatchwell BJ, Komdeur J. Ecological constraints, life history traits and the evolution of cooperative breeding. Anim Behav. 2000;59:1079–1086. doi: 10.1006/anbe.2000.1394. [DOI] [PubMed] [Google Scholar]
- Henry MD. Behavior, Ecology, Evolution, and Systematics. University of Maryland; College Park, MD: 2011. Proximate mechanisms and ultimate causes of female reproductive skew in cooperatively breeding golden lion tamarins, Leontopithecus rosalia. [Google Scholar]
- Hodge SJ. Understanding variation in reproductive skew: directions for future empirical research. In: Hager R, Jones CB, editors. Reproductive skew in vertebrates: proximate and ultimate causes. Cambridge University Press; Cambridge: 2009. pp. 439–466. [Google Scholar]
- Hoogland JL. Infanticide in prairie dogs: lactating females kill offspring of close kin. Science. 1985;230:1037–1040. doi: 10.1126/science.230.4729.1037. [DOI] [PubMed] [Google Scholar]
- Hubrecht RC. Field observations on group size and composition of the common marmoset (Callithrix jacchus jacchus), at Tapacura, Brazil. Primates. 1984;25:13–21. [Google Scholar]
- Hubrecht RC. The fertility of daughters in common marmoset (Callithrix jacchus jacchus) family groups. Primates. 1989;30:423–432. [Google Scholar]
- Inglett BJ. Psychology. University of Nebraska; Omaha, NE: 1993. The role of social bonds and the female reproductive cycle on the regulation of social and sexual interactions in the golden lion tamarin (Leontopithecus rosalia rosalia) [Google Scholar]
- Inglett BJ, French JA, Simmons LG, Vires KW. Dynamics of intra-family aggression and social reintegration in lion tamarins (Leontopithecus rosalia) Zoo Biol. 1989;8:67–78. [Google Scholar]
- Kempenaers B. Polygyny in the blue tit: unbalanced sex ratio and female aggression restrict mate choice. Anim Behav. 1994;47:943–957. [Google Scholar]
- Kleiman DG. Characteristics of reproduction and sociosexual interactions in pairs of lion tamarins (Leontopithecus rosalia) during the reproductive cycle. In: Kleiman DG, editor. The Biology and Conservation of the Callitrichidae. Smithsonian Institution Press; Washington, D.C: 1977a. pp. 181–190. [Google Scholar]
- Kleiman DG. Monogamy in mammals. Q Rev Biol. 1977b;52:39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- Kleiman DG. Parent-offspring conflict and sibling competition in a monogamous primate. Am Nat. 1979;114:753–760. [Google Scholar]
- Kruuk H. The social badger. Oxford University Press; Oxford: 1989. [Google Scholar]
- Kutsukake N, Clutton-Brock TH. Aggression and submission reflect reproductive conflict between females in cooperatively breeding meerkats Suricata suricatta. Behav Ecol Sociobiol. 2006;59:541–548. [Google Scholar]
- Malcolm JR, Marten K. Natural selection and the communal rearing of pups in African wild dogs (Lycaon pictus) Behav Ecol Sociobiol. 1982;10:1–13. [Google Scholar]
- Mays NA, Vleck CM, Dawson J. Plasma luteinizing hormone, steroid hormones, behavioral role, and nest stage in cooperatively breeding Harris’ hawk (Parabuteo unicinctus) Auk. 1991;108:619–637. [Google Scholar]
- Mech LD. Leadership in wolf, Canis lupus, packs. Can Field-Nat. 2000;114:259–263. [Google Scholar]
- Miller KE, Dietz JM. Effects of individual and group characteristics on feeding behaviors in wild Leontopithecus rosalia. Int J Primatol. 2006;27:911–939. [Google Scholar]
- Moehlman PD. Jackal helpers and pup survival. Nature. 1979;277:382–383. [Google Scholar]
- Monfort SL, Bush M, Wildt DE. Evaluation of natural and induced ovarian synchrony in golden lion tamarins (Leontopithecus rosalia) Biol Reprod. 1996;55:875–882. doi: 10.1095/biolreprod55.4.875. [DOI] [PubMed] [Google Scholar]
- Nelson RJ. An introduction to behavioral endocrinology. 3. Sinauer Associates, Inc; Sunderland, MA: 2005. [Google Scholar]
- Ojeda SR. Female reproductive function. In: Griffin JE, Ojeda SR, editors. Textbook of endocrine physiology. 3. Oxford University Press; Oxford: 1996. pp. 164–200. [Google Scholar]
- Pottinger TG. The impact of stress on animal reproductive activities. In: Baum PHM, editor. Stress physiology in animals. CRC Press; Boca Raton, FL: 1999. pp. 130–177. [Google Scholar]
- Price EC, McGrew WC. Departures from monogamy in colonies of captive cotton-top tamarins. Folia Primatol (Basel) 1991;57:16–27. [Google Scholar]
- Ramirez M. Population recovery in the moustached tamarin (Saguinus mystax): management strategies and mechanisms. Am J Primatol. 1984;7:245–259. doi: 10.1002/ajp.1350070304. [DOI] [PubMed] [Google Scholar]
- Rasa OAE. Altruistic infant care or infanticide: the dwarf mongooses’ dilemma. In: Parmigiani S, Saal FSV, editors. Infanticide and parental care. Harwood Academic Publishers; London, UK: 1994. pp. 301–320. [Google Scholar]
- Reyer HU, Dittami JP, Hall MR. Avian helpers at the nest: are they psychologically castrated? Ethology. 1986;71:216–228. [Google Scholar]
- Rood JP. Group size, survival, reproduction, and routes to breeding in dwarf mongooses. Anim Behav. 1990;39:566–572. [Google Scholar]
- Rothe H. Some aspects of sexuality and reproduction in groups of captive marmosets (Callithrix jacchus) Z Tierpsychol. 1975;37:255–273. doi: 10.1111/j.1439-0310.1975.tb00880.x. [DOI] [PubMed] [Google Scholar]
- Ruiz-Miranda CR, Daudt Grativol A, Procópio-de-Oliveira P. Introdução: A espécie e sua situação na paisagem fragmentada. In: Procópio-de-Oliveira P, Daudt Grativol A, Ruiz-Miranda CR, editors. Conservação do mico-leão-dourado: enfrentando os desafios de uma paisagem fragmentada. Universidade Estadual do Norte Fluminense Darcy Ribeiro; Campos dos Goytacazes: 2008. pp. 6–13. [Google Scholar]
- Russell AF, Brotherton PNM, McIlrath GM, Sharpe LL, Clutton-Brock TH. Breeding success in cooperative meerkats: effects of helper number and maternal state. Behav Ecol. 2003;14:486–492. [Google Scholar]
- Rylands AB, Kierulff MCM, De Souza Pinto LP. Distribution and status of lion tamarins. In: Kleiman DG, Rylands AB, editors. Lion tamarins: biology and conservation. Smithsonian Institution Press; Washington, D.C: 2002. pp. 42–58. [Google Scholar]
- Saltzman W. Reproductive competition among female common marmosets (Callithrix jacchus): proximate and ultimate causes. In: Jones CB, editor. Sexual selection and reproductive competition in primates: new perspectives and directions. American Society of Primatologists; Norman, OK: 2003. pp. 197–229. [Google Scholar]
- Saltzman W, Ahmed S, Fahimi A, Wittwer DJ, Wegner FH. Social suppression of female reproductive maturation and infanticidal behavior in cooperatively breeding Mongolian gerbils. Horm Behav. 2006;49:527–537. doi: 10.1016/j.yhbeh.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Digby LJ, Abbott DH. Reproductive skew in female common marmosets: what can proximate mechanisms tell us about ultimate causes? Proc R Soc B. 2009;276:389–399. doi: 10.1098/rspb.2008.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman W, Liedl KJ, Salper OJ, Pick RR, Abbott DH. Post-conception reproductive competition in cooperatively breeding common marmosets. Horm Behav. 2008;53:274–286. doi: 10.1016/j.yhbeh.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Schultz-Darken N, Abbott DH. Familial influences on ovulatory function in common marmosets (Callithrix jacchus) Am J Primatol. 1997;41:159–177. doi: 10.1002/(SICI)1098-2345(1997)41:3<159::AID-AJP1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Savage A, Giraldo LH, Soto LH, Snowdon CT. Demography, group composition, and dispersal in wild cotton-top tamarin (Saguinus oedipus) groups. Am J Primatol. 1996;38:85–100. doi: 10.1002/(SICI)1098-2345(1996)38:1<85::AID-AJP7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Schoech SJ, Mumme RL, Moore MC. Reproductive endocrinology and mechanisms of breeding inhibition in cooperatively breeding Florida scrub jays (Aphelocoma ccoerulescens) Condor. 1991;93:354–364. [Google Scholar]
- Siani JM. Costs and benefits of cooperative infant care in wild golden lion tamarins (Leontopithecus rosalia) Behavior, Ecology, Evolution, and Systematics. University of Maryland; College Park, MD: 2009. [Google Scholar]
- Smith AC, Tirado Herrera ER, Buchanan-Smith HM, Heymann EW. Multiple breeding females and allo-nursing in a wild group of moustached tamarins (Saguinus mystax) Neotrop Primates. 2001;9:67–69. [Google Scholar]
- Smith TE, French JA. Social and reproductive conditions modulate urinary cortisol excretion in black tufted-ear marmosets (Callithrix kuhlii) Am J Primatol. 1997;42:253–267. doi: 10.1002/(SICI)1098-2345(1997)42:4<253::AID-AJP1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Soini P. Ecology and population dynamics of the pygmy marmoset, Cebuella pygmaea. Folia Primatol (Basel) 1982;39:1–21. doi: 10.1159/000156066. [DOI] [PubMed] [Google Scholar]
- Solomon NG, Brant CL, Callahan PA, Steinly BA., Jr Mechanisms of reproductive suppression in female pine voles (Microtus pinetorum) Reproduction. 2001;122:297–304. doi: 10.1530/rep.0.1220297. [DOI] [PubMed] [Google Scholar]
- Solomon NG, French JA. Cooperative breeding in mammals. Cambridge University Press; Cambridge: 1997. [Google Scholar]
- Sousa MB, Ziegler TE. Diurnal variation on the excretion patterns of fecal steroid in common marmoset (Callithrix jacchus) females. Am J Primatol. 1998;46:105–118. doi: 10.1002/(SICI)1098-2345(1998)46:2<105::AID-AJP1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Sousa MBC, Albuquerque ACSR, Albuquerque FS, Araujo A, Yamamoto ME, Arruda MF. Behavioral strategies and hormonal profiles of dominant and subordinate common marmoset (Callithrix jacchus) females in wild monogamous groups. Am J Primatol. 2005;67:37–50. doi: 10.1002/ajp.20168. [DOI] [PubMed] [Google Scholar]
- Sussman RW, Garber PA. A new interpretation of the social organization and mating system of the Callitrichidae. Int J Primatol. 1987;8:3–92. [Google Scholar]
- Tardif SD, Harrison ML, Simek ML. Communal infant care in marmosets and tamarins: relation to energetics, ecology and social organization. In: Rylands AB, editor. Marmosets and tamarins: systematics, behaviour, and ecology. Oxford University Press; Oxford: 1993. pp. 220–234. [Google Scholar]
- Wasser SK, Barash DP. Reproductive suppression among female mammals: implications for biomedicine and sexual selection theory. Q Rev Biol. 1983;58:513–538. doi: 10.1086/413545. [DOI] [PubMed] [Google Scholar]
- Young AJ, Carlson AA, Monfort SL, Russell AF, Bennett NC, Clutton-Brock TH. Stress and the suppression of subordinate reproduction in cooperatively breeding meerkats. Proc Natl Acad Sci U S A. 2006;103:12005–12010. doi: 10.1073/pnas.0510038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AJ, Clutton-Brock TH. Infanticide by subordinates influences reproductive sharing in cooperatively breeding meerkats. Biol Lett. 2006;2:385–387. doi: 10.1098/rsbl.2006.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahavi A. Arabian babblers: the quest for social status in a cooperative breeder. In: Stacey PB, Koenig WD, editors. Cooperative breeding in birds. Cambridge University Press; Cambridge: 1990. pp. 103–130. [Google Scholar]
- Ziegler TE, Scheffler G, Snowdon CT. The relationship of cortisol levels to social environment and reproductive functioning in female cotton-top tamarins, Saguinus oedipus. Horm Behav. 1995;29:407–424. doi: 10.1006/hbeh.1995.1028. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Sousa MB. Parent-daughter relationships and social controls on fertility in female common marmosets, Callithrix jacchus. Horm Behav. 2002;42:356–367. doi: 10.1006/hbeh.2002.1828. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Washabaugh KF, Snowdon CT. Responsiveness of expectant male cotton-top tamarins, Saguinus oedipus, to mate’s pregnancy. Horm Behav. 2004;45:84–92. doi: 10.1016/j.yhbeh.2003.09.003. [DOI] [PubMed] [Google Scholar]