Abstract
Background
Sense of mastery, a personal resource, is likely to have an inverse association with alcohol dependence. Previous evidence, however, is sparse. In addition, the extent to which an association is due to genetic or environmental factors is unknown.
Methods
Data were from 3,983 male twins and 2,630 female twins who had ever used alcohol, interviewed in the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders. Mastery was measured by a 6-item scale. Lifetime diagnosis of alcohol dependence was based on DSM-IV criteria assessed in a structured diagnostic interview. Univariate analyses modeled the relative contributions of genetic and environmental factors to mastery and alcohol dependence using Mx software. Bivariate Cholesky models were fit to the mastery and alcohol dependence raw data.
Results
In the best-fitting model of mastery, genetic factors accounted for about 33% of the observed variance. Nonshared environmental factors, including random measurement error, accounted for the remaining 67%. Fifty-six percent of the variance in liability to alcohol dependence was genetic, and the other 44% was explained by the nonshared environment. The phenotypic polychoric correlation between mastery and alcohol dependence of −0.18 was primarily (67% in the best-fitting model) explained by genes common to both low mastery and alcohol dependence; the rest was explained by nonshared environmental factors.
Conclusions
The findings indicate that genetic risk for alcohol dependence overlaps with genetic factors that influence sense of mastery. Key challenges for future research are to identify the genes that influence mastery and alcohol dependence, as well as the environmental pathways by which they come to be linked.
Keywords: Alcohol Dependence, Mastery, Genetics, Twins
Alcohol dependence, A major health issue, affects 4 to 5% of the U.S. population at any given time (Li et al., 2007) with a lifetime prevalence of 12.5% (Hasin et al., 2007). Family, twin, and adoption studies have shown a substantial genetic contribution to alcohol-related outcomes (Heath et al., 1997; Kaprio et al., 1991; Kendler et al., 1994; Li et al., 2007; McGue et al., 1992).
Risk factors for developing alcohol use disorders include aspects of temperament, such as neuroticism (Kendler et al., 2011; Littlefield and Sher, 2010). A neglected potential protective factor for alcohol use disorders is mastery. Mastery—also termed self-efficacy, sense of control, and locus of control—is the belief that one has control over one’s outcomes. People with a high sense of mastery believe they can handle whatever comes their way and that they—not other people or fate—will determine how things turn out. It is a personal resource on which people draw to deal with challenges and guide their lives in preferred directions (Bandura, 1999, 2006; Mirowsky and Ross, 1998, 2007).
Some evidence suggests that mastery should reduce the risk of alcohol use disorders, including alcohol dependence. Mastery is related to lower alcohol dependence (Poikolainen, 1997), alcohol consumption (Shamloo and Cox, 2010), and the likelihood of any substance use disorders, including alcohol use disorders (Kiecolt et al., 2009). Similar, domain-specific constructs involving self-efficacy for alcohol use also are associated with reduced severity of alcohol use disorders (Williams et al., 1998; Witkiewitz et al., 2012). In many studies, though, samples are small and limited to treatment-seekers (Surgenor et al., 2006).
In addition, mastery influences outcomes over the life course that may alter the risk of alcohol dependence. Among adolescents, it predicts educational attainment (Murasko, 2007; Ross and Broh, 2000). Mastery also has positive effects on physical health and healthy lifestyles (Bovier et al., 2004; Ross and Broh, 2000; Taylor and Stanton, 2007; Thoits, 1995).
Mastery, like self-esteem, is partially heritable (Kendler et al., 1998; Raevuori et al., 2007; Roy et al., 1995). The lone study of which we are aware estimated the heritability of mastery at 0.33 for male and female twins aged 25 to 74 years from the MIDUS twin and sibling subsample (Kessler et al., 2004). No studies to our knowledge have examined common genetic and environmental sources of mastery and alcohol dependence.
In this study, we address this question, using data on male and female twins from the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (VATSPSUD) (Kendler and Prescott, 2006). First, we assess genetic and environmental contributions to mastery and alcohol dependence. Second, we determine whether mastery is related to a lower risk of lifetime alcohol dependence. Third, we investigate the genetic and environmental sources of the association of mastery and alcohol dependence, and we test whether the strength of their effects differs by gender.
MATERIALS AND METHODS
Sample
The sample consisted of twins who participated in the VATSP-SUD, a population-based longitudinal study of psychopathology in adult twins. The study, begun in 1988 (Kendler and Prescott, 2006), identified Caucasian female–female (FF) twins through the population-based Virginia Twin Registry (now the Mid Atlantic Twin Registry). These twins, born in Virginia 1934 to 1974, were eligible to be interviewed if they had completed a mailed questionnaire in 1987 or 1988, which had a response rate of 64%. Zygosity was determined using co-twins’ self-reports on standard questions, photographs, and data on DNA polymorphisms in 496 twin pairs (Kendler and Prescott, 2006). The present study used data from FF twins who were interviewed face-to-face at wave 1 in 1987 to 1989 and by telephone at wave 4 in 1995 to 1997, with response rates of 92 and 85%, respectively. Data also were drawn from a parallel study, begun in 1993, of male-male (MM) and male-female (MF) twins born 1940 to 1974. They were interviewed by telephone at wave 1 in 1993 to 1996 and at wave 2 in 1994 to 1998, with response rates of 72 and 83%, respectively. The present study used data from wave 2.
As often occurs in twin samples (Lykken et al., 1987), monozygotic (MZ) twins (especially males in the present sample) are overrepresented. Possibly identical twins are more willing to participate, due to their identical status. Opposite-sex dizygotic (DZ) twins also are overrepresented, due possibly to greater cooperation rates. Nevertheless, the sample is broadly representative of native-born white Virginians of these age groups (Kendler and Prescott, 2006). For details on recruitment and nonparticipation, see Kendler and Prescott (2006).
The analytic sample consisted of FF, MM, and MF twins of known zygosity on whom we had data for mastery and lifetime alcohol dependence, excluding lifetime abstainers from alcohol. The sample of 6,613 individuals was composed of 766 monozygotic female (MZF) twins, 541 dizygotic female (DZF) twins, 1,579 monozygotic male (MZM) twins, 1,186 dizygotic male (DZM) twins, and 2,541 opposite-sex (OSDZ) twins.
There were 310 MZ and 205 DZ FF twin pairs, 634 MZ and 426 DZ MM twin pairs, and 953 OSDZ twin pairs. Thirty-six additional twin pairs were formed from 9 sets of triplets with 2 members in the sample, 7 complete sets of triplets, and 1 complete quadruplet set. Another 1,527 respondents had no co-twin. The mean age in the analytic sample was 37.13 (SD = 8.9); mean education was 13.59 (SD = 2.5) years. In the VATSPSUD sample, alcohol dependence is comorbid with major depression, generalized anxiety disorder, phobia, drug abuse and dependence, adult antisocial behavior, and conduct disorder (Kendler et al., 2003).
This project received approval from human subject committees at Virginia Commonwealth University. Written informed consent was secured before the in-person interviews, and verbal consent before the telephone interviews. IRB approval also was granted from Virginia Tech for the secondary analyses.
Measures
Alcohol Dependence
Lifetime diagnosis of alcohol dependence was based on DSM-IV criteria assessed in a structured diagnostic interview, adapted from the Structured Clinical Interview for DSM Disorders (Spitzer & Williams, 1985) and administered by clinically trained interviewers. The initial question asked about lifetime alcohol use. Of respondents with scores on mastery, 4.17% (N = 288) reported never having consumed a full drink; they were excluded from the analyses. Respondents who reported ever having an alcoholic drink were assessed for alcohol dependence if they (i) had ever consumed ≥ 13 (men) or ≥ 7 (women) drinks in a single day; and/or (ii) answered yes to any of 3 screening questions: “Have you ever had a period in your life when:…you drank too much?; …you drank instead of spending time with hobbies, family, or friends?; and… someone else objected to your drinking?” Respondents who met either criterion were asked additional questions about the time “when you used alcohol the most” or “when this problem was at its worst.” Alcohol dependence was assessed at wave 4 for FF twins and at wave 2 for MM and MF twins.
Mastery
Sense of mastery was assessed by 6 items (Maddi et al., 1979). Two items from the original 8-item scale, “What happens to me in the future mostly depends on me” and “I can do just about anything I really set my mind to,” were dropped because they loaded on another dimension. Item responses ranged from 4 (strongly disagree) to 1 (strongly agree). The items were reverse coded so that higher values indicated higher mastery. The resulting raw sum score ranged from 6 to 24. Table 1 shows the 6-item factor loadings for a single-factor model. Cronbach’s a was 0.77. In the biometric twin modeling, mastery was polychotomized as a 5-category variable (r = 0.92 with the sum score), for use with the Mx raw ordinal data option. Mastery was measured at wave 1 for FF twins and at wave 2 for MM and MF twins.
Table 1.
Factor Loadings for a Single Factor Extracted froman Exploratory Factor Analysis of 6 Mastery Items Using Maximum Likelihood Estimation (N = 6,613)
| Item | Factor loadings |
|---|---|
| There is really no way I can solve some of the problems I have |
0.53 |
| Sometimes I feel that I’m being pushed around in life | 0.62 |
| I have little control over things that happen to me | 0.58 |
| I often feel helpless in dealing with the problems of life | 0.66 |
| There is little I can do to changemany of the important things inmy life |
0.63 |
| Things never work out the way I want them to | 0.63 |
| Eigenvalue | 2.84 |
% of variance 36.93.
Other Measures
Supplementary analyses involved age, years of education, and scales of self-esteem (Rosenberg, 1965), optimism (Scheier and Carver, 1985), and neuroticism (Eysenck et al., 1985).
Statistical Analyses
Descriptive analyses were conducted using Stata Version 10.1 (Stata Statistical Software [computer program], 2007). Generalized estimating equation (GEE) models examined how gender, zygosity (MZ vs. DZ), and having a same-sex versus opposite-sex twin were related to mastery, alcohol dependence, age, and education. In addition, we investigated whether the relationship between mastery and alcohol dependence held when controlling for the related constructs of self-esteem, optimism, and neuroticism. Significance tests were adjusted for twin-pair clustering. Only mastery and alcohol dependence were included in subsequent analyses. Age was unrelated to mastery. Education had a small correlation with mastery (r = 0.22, p < 0.001), but in a logistic regression of alcohol dependence on mastery, the coefficient for mastery (OR = 0.92, p < 0.001) was unchanged when education was added as a predictor (OR = 0.93, p < 0.001). Mastery was correlated with the related constructs of self-esteem (r = 0.67, p < 0.001), optimism (r = 0.67, p < 0.001), and neuroticism (r = −0.47, p < 0.001). When each of those constructs was added as a predictor of alcohol dependence, in each case the odds ratio for mastery increased to 0.95, but remained significant (p < 0.001).
The phenotypic association between mastery and alcohol dependence was estimated as a polychoric correlation. We assessed resemblance in twin pairs by estimating polychoric cross-twin correlations for the 2 variables, and cross-twin, cross-trait correlations (i.e., mastery in twin 1 with alcohol dependence in twin 2 and vice versa).
The analyses assume that although mastery is treated as an ordinal variable, the underlying latent response giving rise to this observed variable is normally distributed in the population. Liability to alcohol dependence also is assumed to be normally distributed, where people who exceed some threshold exhibit the disorder. To test whether bivariate normality is a viable assumption for estimating polychoric correlations, we performed likelihood ratio chi-squared tests (G2) of correlations involving mastery using the polychoric procedure in Stata (Stata Statistical Software [computer program], 2007).
Twin models can partition the phenotypic variance of an observed characteristic into 4 sources of variance: (i) additive genetic factors (A) from genes whose allelic effects combine additively, (ii) dominance genetic factors (D) from nonadditive interactions between alleles at the same locus, (iii) the shared (or common) environment (C) that increases similarity between twins, and (iv) the nonshared environment (E), which includes nonshared experiences and measurement error. Heritability is the proportion of total observed variance due to genetic differences between individuals. C and D cannot simultaneously be estimated in samples of twins reared together. Because MZ pairs share their genotypes, both additive and nonadditive genetic effects are correlated at 1.0. As DZ pairs share half their genes, on average, additive and nonadditive effects are correlated at 0.5 and 0.25, respectively. Shared environmental effects are correlated at 1.0 for MZ and DZ twin pairs, and unshared environmental effects are uncorrelated.
All twin modeling was conducted with the Mx statistical package. Models were fit to the raw ordinal data using maximum likelihood estimation (Neale et al., 2006). This approach works well when sample sizes vary across zygosity groups. We first performed univariate analyses of mastery and alcohol dependence. Because the cross-twin, cross-trait correlations were similar for MZ and DZ pairs, we fit a series of models to estimate the degree to which A, C, and E (path coefficients a, c, and e, respectively) contributed to the phenotypic variability of each variable. Because the MZ crosstwin within-trait correlations were more than twice those for DZ twins, we also fit ADE models. Akaike’s information criterion (AIC; χ2 − 2 df) (Akaike, 1987) was used to evaluate fit. The lower its value is, the better is the balance between parsimony and explanatory power (Williams and Holahan, 1994). The AICs in the ACE and ADE models differed only slightly. We report ACE models, given our limited power to discriminate between additive and dominance genetic effects.
First, in univariate analyses of mastery and alcohol dependence, a general sex-limitation model estimated qualitative sex-specific effects. This model allows for the possibility that different genes contribute to a phenotype in males and females, by freely estimating the genetic correlation ra between male and female OSDZ twins. It also allows the strength of the A, C, and E effects to differ by sex. Second, a common sex-limitation model examined quantitative sex differences. This model assumes the same genetic influences in males and females by constraining the genetic correlation between male and female OSDZ twins to 0.5, but allows the strength of effects (A, C, and E) to differ by sex. Third, a no sex-limitation model constrained those parameters to be equal. Next, models dropping the C component and both the A and C components, respectively, were fit by fixing their respective values to zero. In successive models, if model-data fit did not significantly deteriorate, the more restricted parsimonious model was retained.
The bivariate Cholesky ACE model, shown in Fig. 1 for a single twin, uses information from cross-twin, cross-trait correlations to estimate the extent to which phenotypic covariation is due to shared genetic and/or environmental influences. As the univariate analyses showed no evidence of qualitative sex-specific genetic effects, the bivariate analysis began with a common sex-limitation model, followed by a no sex-limitation model. In subsequent models, individual parameters were tested for significance by setting them to zero, and the model with the lowest AIC was deemed the best-fitting model. In all the twin models, male and female thresholds were allowed to differ.
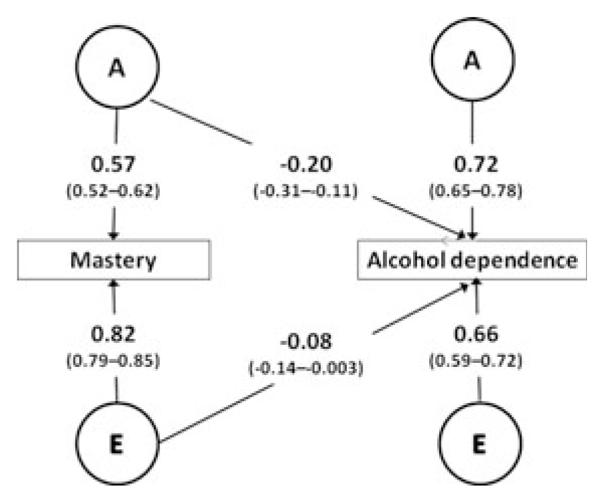
Fig. 1.
Path diagram of bivariate Cholesky model of mastery and alcohol dependence. A, additive genetic effect; C, environmental effect shared by co-twins; E, environmental effect not shared by co-twins.
RESULTS
Descriptive Statistics
Table 2 shows descriptive statistics for mastery and alcohol dependence, by sex and zygosity. It also shows GEE analyses of the effects of sex, zygosity, and having an opposite-sex twin. Mastery was lower among women than men (z = −7.66, p < 0.001), did not differ by zygosity (z = −1.36, p = 0.173), and was higher in opposite-sex than same-sex twins (z = 2.28, p = 0.023). Further analysis of the last result showed that women from OSDZ pairs had higher mastery than women in same-sex pairs (z = 3.42, p = 0.001), but that co-twin’s gender was unrelated to mastery among men (z = −0.32, p = 0.749).
Table 2.
Mean and Frequency Comparisons for Mastery and Alcohol Dependence Across Gender, Zygosity, and Gender by Zygosity Subgroups
| Variable | Women |
Men |
Difference by | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MZ | DZ | OSDZ | MZ | DZ | OSDZ | ||||
|
|
|
||||||||
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | Gender (Female) |
Zygosity (DZ twin) |
Oppositesex twin |
|
| Mastery | 17.07 (3.04) | 17.21 (3.35) | 17.55 (3.15) | 18.12 (3.00) | 17.82 (2.95) | 17.95 (3.00) | −0.62*** | −0.14 | 0.23* |
| Alcohol dependence %dependent |
8.4% | 8.0% | 12.6% | 23.0% | 27.2% | 26.4% | −1.08*** | 0.18* | 0.08 |
| Age | 36.82 (7.68) | 38.34 (8.08) | 36.88 (8.93) | 36.29 (9.13) | 38.31 (9.18) | 37.03 (8.96) | −0.01 | 1.67*** | −1.08*** |
| Education | 13.66 (2.00) | 13.42 (2.06) | 13.68 (2.33) | 13.66 (2.53) | 13.26 (2.81) | 13.62 (2.57) | 0.14* | −0.35*** | 0.29** |
| Self-esteem | 31.44 (4.48) | 31.59 (4.64) | 31.53 (4.82) | 32.92 (4.43) | 32.53 (4.33) | 32.32 (4.53) | −0.94*** | −0.21 | −0.18 |
| Optimism | 17.60 (2.79) | 17.58 (2.97) | 17.43 (2.85) | 18.01 (2.61) | 17.79 (2.63) | 17.80 (2.72) | −0.30*** | −0.12 | −0.10 |
| Neuroticism | 4.03 (3.18) | 4.05 (3.29) | 4.16 (3.25) | 2.80 (2.93) | 3.03 (3.07) | 3.25 (3.19) | 0.97*** | 0.16 | 0.20 |
MZ, monozygotic twins; DZ, same-sex dizygotic twins; OSDZ, opposite-sex dizygotic twins. Coefficients from generalized estimating equation (GEE) analyses show effects of gender (female), zygosity (DZ as compared toMZ twin), and gender of twin (opposite-sex compared with same-sex) on mastery and of % lifetime alcohol dependence. The analyses corrected for the nested structure of the data.
p < 0.05
p < 0.01
p < 0.001.
The prevalence of lifetime alcohol dependence was 25.3% for men and 10.4% for women (not shown). The estimate for men was somewhat higher than in one study (Kessler et al., 1994), but similar to that of 23.7% for male lifetime drinkers in another study (Grant, 1997). The GEE analysis in Table 2 shows that the likelihood of alcohol dependence was lower among women than men (z = −14.54, p < 0.001); slightly higher among DZ than MZ twins (z = 2.12, p = 0.012); and no different in opposite-sex than same-sex twins (z = 0.94, p = 0.348). Follow-up analyses showed that the effect of zygosity held for both for men (z = 2.40, p = 0.017) and women (z = 2.19, p = 0.028). The difference was small, and although statistically significant, probably not meaningful.
The phenotypic association between mastery and alcohol dependence was negative, as expected. The polychoric correlation point estimates were modest but significant, at −0.19 for men and −0.15 for women (p < 0.001). Table 3 shows cross-twin correlations for mastery and alcohol dependence, by zygosity and by sex for OSDZ twins. Cross-twin correlations were stronger for alcohol dependence than for mastery. Cross-twin correlations for mastery were more than twice as large for MZ twins as for DZ twins. This pattern also held for alcohol dependence among female twins, suggesting the possibility of nonadditive genetic effects. As noted previously, however, ADE models did not fit better than ACE models. The cross-twin, cross-trait correlations for MZ pairs were not larger than those for DZ pairs, suggesting little common genetic influence on mastery and alcohol dependence.
Table 3.
Cross-Twin and Cross-Twin, Cross-Trait Correlations by Zygosity and Sex, by Zygosity Subgroups
| MZ twins |
Same-sex DZ twins |
OSDZ twins |
|||
|---|---|---|---|---|---|
| Women | Men | Women | Men | Overall | |
| Cross-twin correlations | |||||
| Mastery | 0.35*** | 0.34*** | 0.12* | 0.13*** | 0.15*** |
| Alcohol dependence |
0.66*** | 0.53*** | 0.10 | 0.33*** | 0.26*** |
| Cross-twin, cross-trait correlations |
|||||
| Mastery T1— Alcohol dependence T2 |
0.04 | −0.10 | 0.12 | −0.14* | −0.06 |
| Mastery T2— Alcohol dependence T1 |
0.01 | −0.15* | 0.03 | −0.09 | −0.13** |
MZ, monozygotic twins; DZ, same-sex dizygotic twins; OSDZ, oppositesex dizygotic twins. Coefficients for mastery are polychoric correlations. Coefficients for alcohol dependence are tetrachoric correlations, which assume an underlying latent continuous distribution.
p < 0.05
p < 0.01
p < 0.001.
We tested the 20 correlations involving mastery (phenotypic, cross-twin for mastery, and cross-twin, cross-trait for the 5 twin groups) for evidence of deviations from bivariate normality. Of those, 2 were significant at p < 0.05. That the proportion is slightly higher than expected by chance may reflect the sensitivity of the likelihood ratio chi-squared test to sample size.
Univariate Twin Models of Mastery and Alcohol Dependence
Table 4 shows fit statistics for a series of univariate ACE twin models of mastery and alcohol dependence. For both variables, model 1 was a general sex-limitation model. It estimated latent additive genetic (A), shared environmental (C), and nonshared environmental (E) sources of variability independently for men and women, along with the genetic correlation between OSDZ twins. Model 2, a common sex-limitation model, also estimated A, C, and E independently, but fixed the genetic correlation to 0.5 for OSDZ twins. Model 3, a no sex-limitation model, constrained A, C, and E to be equal across sex. Model 4 was a no sex-limitation model that dropped the C parameter, and model 5 dropped both the A and C parameters.
Table 4.
Goodness-of-Fit Results from TwinModels for Univariate Models of Mastery and Alcohol Dependence
| Model | Compared with model |
df | Δ χ 2 | p | AIC | |
|---|---|---|---|---|---|---|
| Mastery | ||||||
| 1 | General sex- limitation ACE |
— | 6642 | — | — | 7850.86 |
| 2 | Common sex- limitation ACE |
1 | 6643 | 0.17 | 0.68 | 7849.03 |
| 3 | No sex- limitation ACE |
2 | 6646 | 0.16 | 0.98 | 7843.19 |
| 4 | No sex- limitation AE |
3 | 6647 | 0.00 | 1.00 | 7841.19 |
| 5 | No sex- limitation E |
3 | 6648 | 122.25 | <0.001 | 7961.44 |
| Alcohol dependence | ||||||
| 1 | General sex- limitation ACE |
— | 6648 | — | — | −7103.44 |
| 2 | Common sex-limitation ACE |
1 | 6649 | 0.004 | 0.95 | −7105.44 |
| 3 | No sex- limitation ACE |
2 | 6652 | 1.05 | 0.79 | −7110.38 |
| 4 | No sex- limitation AE |
3 | 6653 | 0.00 | 1.00 | −7112.38 |
| 5 | No sex- limitation E |
3 | 6650 | 116.10 | <0.001 | −6998.28 |
Mastery was a 5-category ordinal variable. Model descriptors correspond to additive genetic (A), common environmental (C), and unique environmental (E) influences. Results for the best-fitting models are shown in bold.
For mastery, model 2 fit no worse than model 1, indicating an absence of qualitative differences in genetic or environmental effects. Model 3, which constrained the A, C, and E parameters to be equal across sex, fit no worse than model 2. Model 4, an AE model, provided the best fit. In this model, the parameter estimate for the A component was 0.58 [95% CI 0.53 to 0.62], and the estimate for the E component was 0.82 [95% CI 0.78 to 0.85]. That is, genetic factors accounted for about 33% of the variance in mastery. Nonshared environmental factors accounted for the remaining 67%, which by definition also includes random measurement error. Model 5, which dropped the A and C components, fit worse than model 3.
In the univariate analysis of alcohol dependence shown in Table 4, model fit did not worsen from model 1, a general sex-limitation model, to model 3, a no sex-limitation model. Model 4, an AE model, provided the best fit. The parameter estimate for the A component was 0.75 [95% CI 0.75 to 0.80], and the estimate for the E component was 0.66 [95% CI 0.59 to 0.73]. Genetic factors accounted for 56% of the variance in liability to alcohol dependence; nonshared environmental factors and measurement error accounted for the remaining 44%. Model 5, an E model that dropped the A and C components, fit worse than model 3.
Bivariate Twin Models of Mastery and Alcohol Dependence
Table 5 shows goodness-of-fit statistics for a series of bivariate Cholesky decomposition models that examined the covariation between mastery and alcohol dependence. A common sex-limitation bivariate model was the baseline model (model 1, Table 5). Model 2, a no sex-limitation model, constrained the paths from A, C, and E to be equal across sex. Goodness of fit did not decrease. Subsequent models were estimated and compared with model 2 to determine whether a given path or set of paths significantly contributed to the covariation between mastery and alcohol dependence. The best-fitting model was model 3, an AE model that dropped all 3 paths from shared environmental influences (c11, c21, c22). Model 4 was an AE model that dropped the a21 path, the effect of the additive genetic influences shared with mastery. This model fit more poorly, indicating that genetic influences on mastery and alcohol dependence overlap to some extent. Model 5 was an AE model that dropped the e21 path. The model fit did not significantly deteriorate. Nevertheless, based on AIC, the best-fitting model for mastery and alcohol dependence remained the AE model, which dropped all the pathways from shared environmental influences (Table 5, model 3). Finally, model 6, an E model which dropped the 3 paths from genetic influences (a11, a21, and a22), fit significantly worse than model 2.
Table 5.
Goodness-of-Fit Results from Bivariate Models ofMastery and Alcohol Dependence
| Model | Compared with model |
df | Δ χ 2 | p | AIC | |
|---|---|---|---|---|---|---|
| 1 | Common sex- limitation ACE |
— | 13286 | — | — | 645.36 |
| 2 | No sex-limitation ACE |
1 | 13295 | 5.80 | 0.76 | 633.11 |
| 3 | No sex-limitation AE |
2 | 13298 | 0.11 | 0.99 | 627.23 |
| 4 | No sex-limitation AE, re |
2 | 13299 | 17.36 | 0.002 | 642.47 |
| 5 | No sex-limitation AE, ra |
2 | 13299 | 4.32 | 0.36 | 629.43 |
| 6 | No sex-limitation E | 2 | 13301 | 237.92 | <0.001 | 859.03 |
Models: A, additive genetic effect; C, environmental effect shared by co-twins; E, environmental effect not shared by co-twins. Results for the best-fitting model are shown in bold.
The parameter estimates are given in Table 6. Most of the parameter estimates are similar across models. The common sex-limitation models have rather wide confidence intervals, and many of them include zero. In the best-fitting (AE) model, none of the parameter confidence intervals include zero.
Table 6.
Parameter Estimates for the Bivariate ACE Models ofMastery and Alcohol Dependence
| Model | Variable | a 2 | c 2 | e 2 |
|---|---|---|---|---|
| ACE, CSL, ra, rc, re: Males | Mastery | 0.32 (0.10 to 0.39) | 0.00 (0.00 to 0.19) | 0.68 (0.61 to 0.76) |
| Alcohol dependence | 0.43 (0.03 to 0.65) | 0.12 (0.00 to 0.37) | 0.45 (0.35 to 0.58) | |
| Shared components | −0.13 (−0.24 to 0.08) | −0.01 (−0.19 to 0.07) | −0.05 (−0.12 to 0.02) | |
| ACE, CSL, ra, rc, re: Females | Mastery | 0.30 (0.04 to 0.45) | 0.04 (0.00 to 0.25) | 0.66 (0.63 to 0.77) |
| Alcohol dependence | 0.58 (0.08 to 0.81) | 0.06 (0.00 to 0.46) | 0.36 (0.19 to 0.58) | |
| Shared components | −0.07 (−0.23 to 0.17) | 0.04 (−0.14 to 0.16) | −0.12 (−0.25 to 0.00) | |
| ACE, ra, rc, re | Mastery | 0.32 (0.20 to 0.38) | 0.00 (0.00 to 0.09) | 0.67 (0.62 to 0.73) |
| Alcohol dependence | 0.55 (0.30 to 0.65) | 0.02 (0.00 to 0.20) | 0.43 (0.35 to 0.53) | |
| Shared components | −0.11 (−0.18 to −0.02) | 0.00 (−0.10 to 0.03) | −0.06 (−0.12 to −0.003) | |
| AE, ra, re | Mastery | 0.33 (0.27 to 0.38) | 0.00 | 0.67 (0.62 to 0.73) |
| Alcohol dependence | 0.56 (0.48 to 0.65) | 0.00 | 0.44 (0.35 to 0.52) | |
| Shared components | −0.12 (−0.18 to −0.06) | 0.00 | −0.06 (−0.11 to −0.002) | |
| AE, re | Mastery | 0.31 (0.26 to 0.37) | 0.00 | 0.69 (0.63 to 0.74) |
| Alcohol dependence | 0.53 (0.44 to 0.62) | 0.00 | 0.46 (0.38 to 0.56) | |
| Shared components | 0.00 | 0.00 | −0.15 (−0.19 to −0.12) | |
| AE, ra | Mastery | 0.33 (0.28 to 0.39) | 0.00 | 0.67 (0.62 to 0.72) |
| Alcohol dependence | 0.58 (0.48 to 0.66) | 0.00 | 0.42 (0.34 to 0.52) | |
| Shared components | −0.17 (−0.20 to −0.13) | 0.00 | 0.00 |
Models: CSL, common sex-limitation; A, additive genetic effect; C, environmental effect shared by co-twins; E, environmental effect not shared by cotwins; a2, proportion additive genetic variance; c2, proportion environmental variance shared by co-twins; e2, proportion environmental variance not shared by co-twins; ra, mastery/alcohol dependence genetic correlation; rc, mastery/alcohol dependence shared environmental correlation; re, mastery/alcohol dependence nonshared environmental correlation/error. Results for the best-fittingmodel are shown in bold.
The results for the best-fitting bivariate model are shown in Fig. 2. The models predicted a total correlation between mastery and alcohol dependence of −0.18. The genetic correlation (ra) between mastery and alcohol dependence was estimated at −0.27. This negative correlation indicates that genetic factors that increase mastery tend to decrease the risk of alcohol dependence. The bivariate heritability equaled: . Thus, about two-thirds of the phenotypic correlation was attributable to shared genetic effects. The other one-third was due to nonshared environmental effects, which include random measurement error. The nonshared environmental correlation (re) between mastery and alcohol dependence equaled −0.12. Therefore, the bivariate e2 equaled: . Hence, genetic influences and nonshared environmental influences explain the association between mastery and alcohol dependence.
Fig. 2.
Best-fitting bivariate Cholesky model of mastery and alcohol dependence (model 3, Table 5) with parameter estimates and 95% confidence intervals. All coefficients are significant at p < 0.05. A, additive genetic effect; E, effect of nonshared environment. Thresholds were estimated but not shown.
DISCUSSION
This study examined the etiology of the association between mastery and alcohol dependence using a large sample of male and female twins. Mastery, the sense that one can control one’s outcomes, was hypothesized to be negatively related to the risk of alcohol dependence. We investigated the extent to which genetic and environmental factors explained variability in mastery and alcohol dependence, as well as the association between the two.
In the univariate analyses, genetic factors accounted for 56% of the variance in liability to alcohol dependence, similar to previous estimates from the data (Kendler and Prescott, 2006; Kendler et al., 1994, 2010; Prescott and Kendler, 1999). Nonshared environmental factors and measurement error accounted for the remaining 44%. Similarly, both genetic and nonshared environmental factors influenced mastery, whereas the shared environment did not. The same genetic factors influenced mastery for men and women, and to the same extent. Genetic factors explained ~33% of the variance in mastery, just as in a previous study with a different sample (Kessler et al., 2004). Nonshared environmental factors and measurement error explained the remaining 67%. This component of variance likely includes the portion of respondents’ socioeconomic status independent of familial influences, as well as circumstances and choices over the life course that can enhance or erode mastery (Bandura, 1999; Mirowsky and Ross, 2007).
Not surprisingly, men had higher average mastery scores than women did. In addition, gender interacted with the gender of one’s co-twin. Men’s average mastery scores did not vary with the gender of their co-twin, but women with a male co-twin scored higher on mastery than women with a female co-twin. To the extent that women’s relative status in opposite-sex twin pairs parallels their somewhat lower status in society, women may “try harder” and boost their mastery in so doing. Alternatively, perhaps identification with a twin who has higher mastery fosters greater mastery.
Mastery had a modest but significant inverse relationship with alcohol dependence. Genetic factors common to both alcohol dependence and mastery explained about two-thirds of the association between the two. Shared environmental factors had no discernible influence on individual differences for either variable or the association between them. Nonshared environmental factors explained the other one-third of the association. The unshared environmental component may include stressful life events or chronic strains that undermine mastery and increase the risk of alcohol dependence. Alternatively, the environmental effect may be primarily causal. Mastery enables people to cope better with stressors, and it may help people avoid stressful situations and exploit opportunities that lead them away from alcohol-related problems.
The bivariate twin models revealed patterns not evident from the cross-twin, cross-trait correlations, which had suggested little common genetic influence on mastery and alcohol dependence. Such correlations can hint at the results of twin models, but do not always directly correspond with them. Model fitting takes into account more than just discrete correlations, as it involves simultaneous joint modeling of all correlations. Especially when the phenotypic association is modest, a small amount of cross-twin, cross-trait correlation can explain a high percentage of the total covariance, as is the case here.
The findings have some possible clinical implications for intervention and treatment. Interventions designed to raise mastery may help people who are susceptible to alcohol use disorders to control or reduce their alcohol consumption. Especially pertinent are treatments involving constructs related to mastery. For example, drink refusal training increased self-efficacy in abstaining from alcohol and reduced drinking frequency after treatment for alcohol dependence (Witkiewitz et al., 2012). Similarly, training in alcohol-related coping skills, another likely correlate of mastery, reduced drinking in high-risk situations (Litt et al., 2009; Witkiewitz et al., 2012).
The results presented here should be interpreted in light of 3 potential limitations. First, the sample consists of Caucasian twins born in Virginia. Although patterns of alcohol dependence in this sample are broadly consistent with those of adults in the United States (Kendler and Prescott, 2006), the prevalence for men was somewhat higher than in other studies (Kessler et al., 1994). In addition, the findings may differ in other racial/ethnic groups. Second, lifetime alcohol dependence was measured retrospectively, so inaccuracies in recall are possible. Short-term test-retest reliability on alcohol dependence was estimated at κ = 0.72 (95% CI 0.61 to 0.82) for 382 randomly selected twin respondents who were re-interviewed after an average of 30 days (Kendler and Prescott, 2006). Third, although mastery was unrelated to age in this sample, it is sometimes found to be lower in later life, due to widowhood, retirement, and declining health (Schieman, 2001). It probably fluctuates over time, as people experience or resolve stressful life events, hardships, and other changes in life circumstances. Measures of mastery over time would more clearly indicate its relation to alcohol dependence.
In conclusion, most research on the etiology of mastery has investigated its environmental determinants (Kessler et al., 2004). The present study adds to evidence that genetic factors also contribute to individual differences in mastery. Mastery has a modest but significant negative association with alcohol dependence. About two-thirds of this association is attributable to common additive genetic factors. The rest is explained by nonshared environmental factors. Key challenges for future research are to further probe and explain the population heritability estimates of this phenotypic relationship. One challenge is to identify genes that influence mastery and alcohol dependence, as well as the biological pathways by which they do so. Leads may emerge from discoveries of genes that are associated with related personality traits (e.g., neuroticism) and alcohol dependence (Judge et al., 2002; Kendler and Prescott, 2006; Kendler et al., 2011).
Another challenge is to explain how mastery, like other personality traits, is linked to alcohol dependence (Littlefield and Sher, 2010). Both externalizing and internalizing pathways are possible. The externalizing pathway to alcohol use disorders, especially common among men (Kendler and Prescott, 2006; Kendler et al., 2011), is marked by strong genetic risk factors and externalizing behaviors that often appear in childhood (Dubow et al., 2008; Englund et al., 2008; Maggs et al., 2008; Pitkanen et al., 2008). Mastery may be negatively associated with externalizing behaviors, as it seems to help people avoid negative life events and chronic difficulties (Thoits, 1995). Alternatively, mastery may overlap with boldness—the “healthiest” dimension of psychopathy—which entails social dominance, resiliency, and venturesomeness. If so, mastery is unlikely to be related to externalizing behavior (Patrick et al., 2009). We would predict a stronger link between mastery and alcohol use disorders through the internalizing pathway. This pathway, which involves depression and anxiety, is equally common among men and women and has a less strong genetic basis (Kendler and Prescott, 2006; Pitkanen et al., 2008). Mastery is well-known to be negatively related to depressive symptoms (Kiecolt et al., 2009; Mirowsky and Ross, 2007; Thoits, 1995). Both phenotypic and genetic models are needed to test these possibilities.
ACKNOWLEDGMENTS
Supported in part by NIH grants MH-80828 and MH/AA/DA-49492. Linda Corey, PhD, provided assistance with the ascertainment of twins from the Virginia Twin Registry, now part of the Mid-Atlantic Twin Registry (MATR). The MATR, now directed by Judy Silberg, PhD, has received support from the NIH, the Carman Trust, and the W.M. Keck, John Templeton, and Robert Wood Johnson Foundations and grant UL1RR031990 from the National Center for Research Resources. KJK received funding from the Virginia Tech Institute for Society, Culture, and Environment and a Dean’s Faculty Research Fellowship from the College of Liberal Arts and Human Sciences.
REFERENCES
- Akaike H. Factor-Analysis and Aic. Psychometrika. 1987;52:317–332. [Google Scholar]
- Bandura A. A sociocognitive analysis of substance abuse: an agentic perspective. Psychol Sci. 1999;10:214–217. [Google Scholar]
- Bandura A. Toward a psychology of human agency. Perspect Psychol Sci. 2006;1:164–180. doi: 10.1111/j.1745-6916.2006.00011.x. [DOI] [PubMed] [Google Scholar]
- Bovier PA, Chamot E, Perneger TV. Perceived stress, internal resources, and social support as determinants of mental health among young adults. Qual Life Res. 2004;13:161–170. doi: 10.1023/B:QURE.0000015288.43768.e4. [DOI] [PubMed] [Google Scholar]
- Dubow EF, Boxer P, Huesmann LR. Childhood and adolescent predictors of early and middle adulthood alcohol use and problem drinking: the Columbia County Longitudinal Study. Addiction. 2008;103(Suppl 1):36–47. doi: 10.1111/j.1360-0443.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Englund MM, Egeland B, Oliva EM, Collins WA. Childhood and adolescent predictors of heavy drinking and alcohol use disorders in early adulthood: a longitudinal developmental analysis. Addiction. 2008;103(Suppl 1):23–35. doi: 10.1111/j.1360-0443.2008.02174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck SBG, Eysenck HJ, Barrett P. A revised version of the psychoticism scale. Personality Individ Differ. 1985;6:21–29. [Google Scholar]
- Grant BF. Prevalence and correlates of alcohol use and DSM-IV alcohol dependence in the United States: results of the National Longitudinal Alcohol Epidemiologic Survey. J Stud Alcohol. 1997;58:464–473. doi: 10.15288/jsa.1997.58.464. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PAF, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol Med. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Judge TA, Erez A, Bono JE, Thoresen CJ. Are measures of self-esteem, neuroticism, locus of control, and generalized self-efficacy indicators of a common core construct? J Pers Soc Psychol. 2002;83:693–710. doi: 10.1037//0022-3514.83.3.693. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Rose RJ, Romanov K, Koskenvuo M. Genetic and environmental determinants of use and abuse of alcohol—the Finnish Twin Cohort Studies. Alcohol Alcohol. 1991;1:131–136. [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Prescott CA. A population-based twin study of self-esteem and gender. Psychol Med. 1998;28:1403–1409. doi: 10.1017/s0033291798007508. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for alcohol use disorders in men. Twin Res Hum Genet. 2011;14:1–15. doi: 10.1375/twin.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Dick D, Prescott CA. The relationship between genetic influences on alcohol dependence and on patterns of alcohol consumption. Alcohol Clin Exp Res. 2010;34:1058–1065. doi: 10.1111/j.1530-0277.2010.01181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Heath AC, Kessler RC, Eaves LJ. A twin-family study of alcoholism in women. Am J Psychiatry. 1994;151:707–715. doi: 10.1176/ajp.151.5.707. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Genes, Environment, and Psychopathology: Understanding the Causes of Psychiatric and Substance Use Disorders. Guilford Press; New York: 2006. [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Gilman SE, Thornton LM, Kendler KS. Health, well-being, and social responsibility in the MIDUS twin and sibling subsamples, in How Healthy Are We? In: Brim OG, Ryff CD, Kessler RC, editors. A National Study of Well-being at Midlife. University of Chicago Press; Chicago, IL: 2004. pp. 124–152. [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kiecolt KJ, Hughes M, Keith VM. Can a high sense of control and John Henryism be bad for mental health? Sociol Quart. 2009;50:693–714. [Google Scholar]
- Li TK, Hewitt BG, Grant BF. The alcohol dependence syndrome, 30 years later: a commentary. The 2006 H. David Archibald lecture. Addiction. 2007;102:1522–1530. doi: 10.1111/j.1360-0443.2007.01911.x. [DOI] [PubMed] [Google Scholar]
- Litt MD, Kadden RM, Kabela-Cormier E. Individualized assessment and treatment program for alcohol dependence: results of an initial study to train coping skills. Addiction. 2009;104:1837–1838. doi: 10.1111/j.1360-0443.2009.02693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield AK, Sher KJ. The multiple, distinct ways that personality contributes to alcohol use disorders. Soc Personal Psychol Compass. 2010;4:767–782. doi: 10.1111/j.1751-9004.2010.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykken DT, McGue M, Tellegen A. Recruitment bias in twin research: the rule of two-thirds reconsidered. Behav Genet. 1987;17:343–362. doi: 10.1007/BF01068136. [DOI] [PubMed] [Google Scholar]
- Maddi SR, Kobasa SC, Hoover M. An alienation test. J Humanist Psychol. 1979;19:73–76. [Google Scholar]
- Maggs JL, Patrick ME. Childhood and adolescent predictors of alcohol use and problems in adolescence and adulthood in the National Child Development Study. Addiction. 2008;103(Suppl 1):7–22. doi: 10.1111/j.1360-0443.2008.02173.x. [DOI] [PubMed] [Google Scholar]
- McGue M, Pickens RW, Svikis DS. Sex and age effects on the inheritance of alcohol problems: a twin study. J Abnorm Psychol. 1992;101:3–17. doi: 10.1037//0021-843x.101.1.3. [DOI] [PubMed] [Google Scholar]
- Mirowsky J, Ross CE. Education, personal control, lifestyle and health—A human capital hypothesis. Res Aging. 1998;20:415–449. [Google Scholar]
- Mirowsky J, Ross CE. Life course trajectories of perceived control and their relationship to education. Am J Sociol. 2007;112:1339–1382. [Google Scholar]
- Murasko JE. A lifecourse study on education and health: the relationship between childhood psychosocial resources and outcomes in adolescence and young adulthood. Soc Sci Res. 2007;36:1348–1370. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 7th ed. Department of Psychiatry, Virginia Commonwealth University; Richmond, VA: 2006. [Google Scholar]
- Patrick CJ, Fowles DC, Krueger RF. Triarchic conceptualization of psychopathy: developmental origins of disinhibition, boldness, and meanness. Dev Psychopathol. 2009;21:913–938. doi: 10.1017/S0954579409000492. [DOI] [PubMed] [Google Scholar]
- Pitkanen T, Kokko K, Lyyra AL, Pulkkinen L. A developmental approach to alcohol drinking behaviour in adulthood: a follow-up study from age 8 to age 42. Addiction. 2008;103(Suppl 1):48–68. doi: 10.1111/j.1360-0443.2008.02176.x. [DOI] [PubMed] [Google Scholar]
- Poikolainen K. Risk factors for alcohol dependence: a questionnaire survey. Alcohol Clin Exp Res. 1997;21:957–961. [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Raevuori A, Dick DM, Keski-Rahkonen A, Pulkkinen L, Rose RJ, Rissanen A, Kaprio J, Viken RJ, Silventoinen K. Genetic and environmental factors affecting self-esteem from age 14 to 17: a longitudinal study of Finnish twins. Psychol Med. 2007;37:1625–1633. doi: 10.1017/S0033291707000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M. Society and the Adolescent Self-Image. Princeton University Press; Princeton, NJ: 1965. [Google Scholar]
- Ross CE, Broh BA. The roles of self-esteem and the sense of personal control in the academic achievement process. Sociol Educ. 2000;73:270–284. [Google Scholar]
- Roy MA, Neale MC, Kendler KS. The genetic epidemiology of self-esteem. Br J Psychiatry. 1995;166:813–820. doi: 10.1192/bjp.166.6.813. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Carver CS. Optimism, coping, and health: assessment and implications of generalized outcome expectancies. Health Psychol. 1985;4:219–247. doi: 10.1037//0278-6133.4.3.219. [DOI] [PubMed] [Google Scholar]
- Schieman S. Age, education, and the sense of control: a test of the cumulative advantage hypothesis. Res Aging. 2001;23:153–178. [Google Scholar]
- Shamloo ZS, Cox WM. The relationship between motivational structure, sense of control, intrinsic motivation and university students’ alcohol consumption. Addict Behav. 2010;35:140–146. doi: 10.1016/j.addbeh.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-III-R (SCID) Biometrics Research Department. New York State Psychiatric Institute; New York: 1985. [Google Scholar]
- Stata Statistical Software [computer program] Release 10. StataCorp LP; College Station, TX: 2007. [Google Scholar]
- Surgenor LJ, Horn J, Hudson SM, Adamson S, Robertson P. Alcohol dependence and psychological sense of control: refining the links. New Zeal J Psychol. 2006;35:146–152. [Google Scholar]
- Taylor SE, Stanton AL. Coping resources, coping processes, and mental health. Ann Rev Clin Psych. 2007;3:377–401. doi: 10.1146/annurev.clinpsy.3.022806.091520. [DOI] [PubMed] [Google Scholar]
- Thoits PA. Stress, coping, and social support processes: where are we? What next? J Health Soc Behav. 1995:53–79. Extra Issue. [PubMed] [Google Scholar]
- Williams LJ, Holahan PJ. Parsimony-based fit indices for multiple-indicator models: do they work? Struct Equ Modeling. 1994;1:161–189. [Google Scholar]
- Williams RJ, Connor JP, Ricciardelli LA. Self-efficacy for refusal mediated by outcome expectancies in the prediction of alcohol-dependence amongst young adults. J Drug Educ. 1998;28:347–359. doi: 10.2190/WY6A-GKDF-3PBQ-NH3K. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Donovan DM, Hartzler B. Drink refusal training as part of a combined behavioral intervention: Effectiveness and mechanisms of change. J Consult Clin Psychol. 2012;80:440–449. doi: 10.1037/a0026996. [DOI] [PMC free article] [PubMed] [Google Scholar]