To the Editor,
Howell and colleagues [1] have recently provided important insights into the relationship among viral kinetic patterns, including the triphasic pattern (Fig.1 in [1]), ethnicity (African Americans, AA, and Caucasian Americans, CA) and IL28B genotypes (rs12979860; CC, TC and TT genotypes) during pegylated interferon-alpha-2a (IFN)+ribavirin(RBV) treatment. Their study strongly suggests differences in viral kinetic patterns under IFN+RBV between AA and CA are associated with IL28B genotypes and elegantly showed that the difference disappears in AA or CA subjects with the same favorable allele (CC). Interestingly, Howell et al. described subjects in whom a static or increasing viral phase (termed here a shoulder) was observed during days 2 to 7 after treatment initiation (Fig. 1 in [1]). While the nature of the shoulder phase is still not known, Howell et al. [1] related this phase to “a delay in the pharmacologic activity of ribavirin” or “lower IFN effectiveness”. We sought here to clarify the current theory behind the nature of the shoulder phase and provide further insights into the subject.
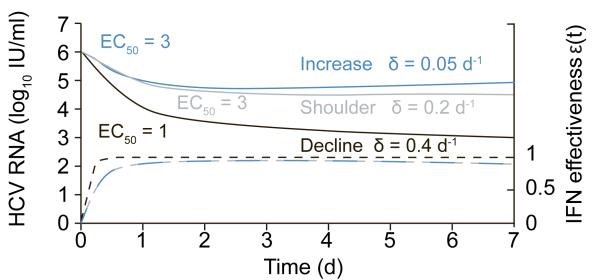
Figure 1.
Predicting the viral kinetic patterns observed in Howell et al. during the first week of IFN+RBV treatment using the standard model of HCV infection [6] but allowing the drug effectiveness, ( dashed lines), to vary with PEG-IFN concentration,(C(t) = FD / Vdka(ke−Ka)(e−kat − e−ket)), where the IFN dose, D, is assumed to reach the injection site immediately, is absorbed into the blood with rate constant ka, is eliminated from the blood with rate constant ke, and is distributed through a volume Vd where only a fraction, F, of the drug is bioavailable. The constant EC50 is the PEG-IFN concentration at which the drug’s effectiveness in blocking viral production is half its maximum and n is a parameter called the Hill coefficient, which determines how steeply the effectiveness rises with increasing drug concentration [4]. Predicted HCV RNA kinetics (solid lines) and its related IFN effectiveness (dashed lines) are represented with the same color. Here we show that this model can generate a shoulder (blue line). Further, while EC50 affects IFN effectiveness, the loss rate of infected cells, δ, of the standard model [6] dictates if viral load will increase, plateau or decrease until day 7. Since only EC50 and δ (values shown in Fig) were found significantly different between subject with CC and TC/TT genotypes [7], other model parameters were fixed to the median values found in [4] as follows: FD/Vd =15 ng/ml; ka=1 d−1; ke=0.1 d−1 ; n=2. The other parameter of the standard model [6], .i.e., the viral clearance rate, c, was fixed to 6 d−1.
A transient viral decline was observed in some HCV-monoinfected subjects during the first week of RBV monotherapy [2]. In a recent study in which subjects were treated with RBV alone for 4 weeks, there was no association between HCV RNA kinetics measured weekly and IL28B genotypes (submitted). In HIV/HCV coinfected subjects treated with pegylated-interferon-alpha-2b and RBV, median RBV area under the curve levels were lower in sustained viral responders (SVRs) compared with non-SVRs at days 3 and 7 and was associated with a continued viral decline during days 3 to 7 (in SVRs) compared to a shoulder in non-SVRs (Fig. 2C in [3]). Unfortunately, data on IL28B genotypes is not yet available to be linked to our previous studies [2, 3], but if indeed subjects with IL28B CC genotypes in Howell et al. are associated with early lower RBV levels and/or transient viral decline during the first week then it could, partly, explain their findings.
A more evident cause of the viral shoulder phase is the known pharmacokinetics of pegylated interferon-alpha which peaks and then declines during the first week of treatment [4, 5]. To explain the shoulder phase with the standard model [6] the pegylated interferon-alpha concentration in serum, C(t), was coupled with its effectiveness, ε(t), in blocking viral production/release [4, 5] (see Fig.1 for equations and parameters definition). Using this theory the observed viral kinetic patterns from Howell et al. can be predicted (Fig. 1). Interestingly, we recently showed, in HIV/HCV (genotype 1/3) co-infected subjects who were treated with IFN+RBV, that the significantly higher effectiveness in subjects with CC genotype was associated with significantly lower EC50 (median 1.3) compared to TC/TT subjects (median 3.3) [7]. In addition, the second phase of viral decline was faster and the infected cells loss rate, δ, was larger in CC than in TC/CC subjects (δ=0.25 vs 0.13 d−1) [7]. Accordingly, the higher IFN effectiveness and the continued viral decline between days 2 and 7 in subjects with a CC genotype from Howell et al. may be partly related to lower EC50 and higher δ, compared to TC/TT patients (Fig. 1; black vs. red/blue lines). Model simulations suggest that the shoulder phase in CT/TT genotype patients is not necessarily due to low IFN effectiveness but may also be due to lower δ. Clearly, further studies that combine IFN and RBV pharmacokinetics/pharmacodynamics with frequent viral kinetics during the first week of treatment are necessary to pinpoint the causes of the shoulder phase observed in TC/TT subjects.
While individual viral kinetics were not shown by Howell et al., it might be true that some patients experienced a longer (up to day 28) shoulder phase as previously documented [4, 8], and with a higher proportion in subjects with TT/TC genotypes compared to CC as recently observed by de Araujo et al. in HIV/HCV coinfected subjects [7]. This longer shoulder phase is explained by other mechanisms that are not related to IFN pharmacokinetics/pharmacodynamics as described in [4, 8, 9].
In the era of direct-acting antiviral agents (DAAs) against HCV, it is important to note that the aforementioned shoulder phase is not commonly seen (e.g., one case in supplementary material of [10]). This may be attributed to the ability of DAAs not only to reduce the synthesis of new intracellular HCV RNA, vRNA, but also to enhance the degradation of vRNA. Based on this theory, the shoulder phase is not observed as intracellular vRNA continuously declines and thus the overall production of virions declines as well, even if the DAA is combined with IFN and its effectiveness declines during the first week of treatment. However, as pegylated interferon-alpha+RBV is still the backbone of the current standard of care and also is used as a lead-in in some protocols, the existence of the shoulder phase is still relevant and should be further be explored in order to better optimize the standard of care.
Acknowledgments
Financial support
U.S. Department of Energy under contract DE-AC52-06NA25396, NIH grants P20-RR018754, R56/R01-AI078881, OD011095, AI028433, and AI065256 and the University of Illinois Walter Payton Liver Center GUILD.
Footnotes
Conflict of interest
The authors declare that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
References
- [1].Howell CD, et al. Single nucleotide polymorphism upstream of interleukin 28B associated with phase 1 and phase 2 of early viral kinetics in patients infected with HCV genotype 1. J Hepatol. 2012;56(3):557–63. doi: 10.1016/j.jhep.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pawlotsky JM, et al. Antiviral action of ribavirin in chronic hepatitis C. Gastroenterology. 2004;126(3):703–14. doi: 10.1053/j.gastro.2003.12.002. [DOI] [PubMed] [Google Scholar]
- [3].Dahari H, et al. Early ribavirin pharmacokinetics, HCV RNA and alanine aminotransferase kinetics in HIV/HCV co-infected patients during treatment with pegylated interferon and ribavirin. J Hepatol. 2007;47(1):23–30. doi: 10.1016/j.jhep.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dahari H, et al. Pharmacodynamics of PEG-IFN-alpha-2a in HIV/HCV co-infected patients: Implications for treatment outcomes. J Hepatol. 2010;53(3):460–7. doi: 10.1016/j.jhep.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Talal AH, et al. Pharmacodynamics of PEG-IFN alpha differentiate HIV/HCV coinfected sustained virological responders from nonresponders. Hepatology. 2006;43(5):943–53. doi: 10.1002/hep.21136. [DOI] [PubMed] [Google Scholar]
- [6].Neumann AU, et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998;282(5386):103–7. doi: 10.1126/science.282.5386.103. [DOI] [PubMed] [Google Scholar]
- [7].de Araujo ES, et al. Pharmacodynamics of PEG-IFN-[alpha]-2a and HCV response as a function of IL28B polymorphism in HIV/HCV-coinfected patients. J Acquir Immune Defic Syndr. 2011;56(2):95–9. doi: 10.1097/QAI.0b013e3182020596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Herrmann E, Lee JH, Marinos G, Modi M, Zeuzem S. Effect of ribavirin on hepatitis C viral kinetics in patients treated with pegylated interferon. Hepatology. 2003;37(6):1351–8. doi: 10.1053/jhep.2003.50218. [DOI] [PubMed] [Google Scholar]
- [9].Dahari H, Ribeiro RM, Perelson AS. Triphasic decline of hepatitis C virus RNA during antiviral therapy. Hepatology. 2007;46(1):16–21. doi: 10.1002/hep.21657. [DOI] [PubMed] [Google Scholar]
- [10].Rong L, Dahari H, Ribeiro RM, Perelson AS. Rapid emergence of protease inhibitor resistance in hepatitis C virus. Sci Transl Med. 2010;2(30):30ra32. doi: 10.1126/scitranslmed.3000544. [DOI] [PMC free article] [PubMed] [Google Scholar]