Abstract
The brain shrinks with age, but the timing of this process and the extent of its malleability are unclear. We measured changes in regional brain volumes in younger (age 20–31) and older (age 65–80) adults twice over a six months period, and examined the association between changes in volume, history of hypertension, and cognitive training. Between two MRI scans, 49 participants underwent intensive practice in three cognitive domains for 100 consecutive days, whereas 23 control group members performed no laboratory cognitive tasks. Regional volumes of seven brain structures were measured manually and adjusted for intracranial volume. We observed significant mean shrinkage in the lateral prefrontal cortex, the hippocampus, the caudate nucleus, and the cerebellum, but no reliable mean change of the prefrontal white matter, orbital-frontal cortex, and the primary visual cortex. Individual differences in change were reliable in all regions. History of hypertension was associated with greater cerebellar shrinkage. The cerebellum was the only region in which significantly reduced shrinkage was apparent in the experimental group after completion of cognitive training. Thus, in healthy adults, differential brain shrinkage can be observed in a narrow time window, vascular risk may aggravate it, and intensive cognitive activity may have a limited effect on it.
Keywords: aging, cerebellum, cognitive training, plasticity, vascular risk, longitudinal, MRI
1. Introduction
Aging is accompanied by profound changes in brain structure and function, but the pace of change varies significantly across brain regions and is characterized by substantial individual differences (for reviews see Kemper 1994; Hedden and Gabrieli 2004; Raz and Rodrigue 2006). Reliable volume reduction in multiple cortical, subcortical, and cerebellar regions as well as thinning of the cerebral cortex have been observed in vivo in many samples, with various analytical tools, and on different imaging platforms (Pfefferbaum et al. 1998; Resnick et al. 2003; Scahill et al. 2003; Raz et al. 2003a b; 2004; 2005; 2010; Fjell et al. 2009). In healthy individuals, regional changes in brain volume have been detected within time spans from five (Pfefferbaum et al. 1998; Rusinek et al. 2003; Raz et al. 2005; Driscoll et al, 2009) to less than two (Fjell et al. 2009; Raz et al. 2010) years to six months (Murphy et al., 2010), and in the hippocampus, reliable shrinkage was noted within a span of four months (Lövdén et al., 2012). However, the localization and extent of regional change vary across studies and even across different samples studied in the same laboratory (e.g., Raz et al. 2005 vs. Raz et al. 2010). Moreover, the samples in which the changes were observed cover different parts of the adult age span and vary in the mean age by more than two decades (e.g., from 52 years in Raz et al., 2005 to 76 years in Murphy et al., 2010). Notably, changes in brain structure are not limited to older adulthood. Shrinkage of at least some brain regions (e.g., HC) has been observed in younger adults (Delisi et al., 1997; Scahill et al., 2003; Raz et al., 2005; Lövdén et al., 2012), although not at the same rate as noted after the sixth decade of life (Raz et al., 2005).
Age-related shrinkage of the brain is not benign, and persons who evidence significant reduction in local brain volumes display reduced cognitive performance (Rodrigue and Raz, 2004; Raz et al. 2008) and run increased risk of developing dementia (Murphy et al. 2010). Because brain shrinkage and its effects on cognition vary substantially among individuals, it is important to determine the factors that affect the rate of decline. An example of such moderator of brain decline is arterial hypertension, a common vascular risk factor that increases the rate and widens the spread of shrinkage across the brain (Raz et al. 2005; 2007; 2008).
Once age-related shrinkage of the brain is observed, it is only natural to ask what can be done about it. To date, several interventions aimed at slowing age-related brain change yielded mixed results. Whereas treating accelerators of brain aging, such as hypertension, does not slow the rate of decline (Jennings et al., 2012), other interventions such as aerobic exercise (Erickson et al., 2011) inspire cautious optimism regarding the malleability of the aging brain. One increasingly popular approach to combatting age-related brain shrinkage is perceptual-motor and cognitive training. Intensive practice on perceptual-motor and cognitive tasks of a wide range of complexity may result in significant enlargement of circumscribed brain regions and changes in white matter diffusion properties in young adults (Draganski et al. 2004; Takeuchi et al., 2010; Schmidt-Wilcke et al., 2010; Mackey et al., 2012). Most important, older brains show practice-related improvements in grey matter volume (Boyke et al. 2008; Lövdén et al. 2012) and regional white matter integrity (Lövdén et al. 2010b) as well, albeit not always and not to the same extent as the younger brains do (e.g., Wenger et al. 2012).
These findings suggest that intensive and systematic cognitive training may slow down the advancement of brain aging. However, even in younger adults the evidence of experience-induced change is far from overwhelming and significant methodological problems persist to dampen the enthusiasm about the findings. According to a recent review, 80% of extant studies of experience-dependent changes in brain structure relied on voxel-based morphometry (VBM) and a minority use other semi-automated approaches such as FreeSurfer (Thomas & Baker, 2012). Systematic comparisons between VBM and manual morphology (Allen et al. 2008; Kennedy et al. 2009) provide ample basis for caution in interpreting differences between groups and conditions revealed by VBM. On the other hand, hypothesis-driven intervention studies with manual measurements are rare and thus far have been limited to investigation of one or two regions of interest (ROIs, e.g., Lövdén et al. 2012). In general, the hypothesis-free approach taken by the vast majority of the extant studies is problematic, especially when only small clusters of voxels that showed longitudinal change are compared between the training group and the controls (Thomas & Baker, 2012). Notably, among the extant studies of structural plasticity, only two (Lövdén et al., 2012; Wenger et al., 2012), conducted on the same sample, compared practice-related changes in younger and older adults, and most have not evaluated the critically important group × time interaction (Thomas & Baker, 2012). In addition, with a couple of notable exceptions, intervention studies of structural change relied on small samples, with a median size of 38 and a range of 11–120 participants (Thomas & Baker, 2012, Table 1). In summary, Thomas and Baker (2012) concluded that in studies of experience-based change in brain structure, the effects are small, highly localized, poorly replicated, transient, and restricted to younger adults.
Table 1.
Longitudinal Change in Regional Cerebral Volumes: A Summary of the Univariate Latent Change Models.
| ROI | Baseline Mean | Mean Change Baseline - Follow-Up | Baseline, Variance | Variance of Change Baseline - Follow-Up | Annual %Change |
|---|---|---|---|---|---|
| Hippocampus | 3551 | −50* | 170709* | 15048* | −1.42 |
| Lateral Prefrontal Cortex | 10569 | −104* | 3813815* | 77429* | −2.51 |
| Orbital Frontal Cortex | 4924 | −33 | 1031243* | 44333* | −0.83 |
| Primary Visual Cortex | 3026 | −7 | 200748* | 5195* | −0.48 |
| Caudate | 4160 | −39* | 412269* | 7988* | −1.21 |
| Cerebellum | 59703 | −494* | 40457233* | 650285* | −1.86 |
| Prefrontal White | 19656 | 80 | 10247462* | 433687* | 1.28 |
Notes. All volumes are in mm3, adjusted for intracranial size.
p<0.05.
In this study, we addressed four questions, while trying to take into account threats to validity outlined by previous studies and summarized in Thomas and Baker (2012). First, we inquired whether differential age-related shrinkage in healthy young and old adults happens fast enough to be noticed within a six-month time window. In pursuit of that objective, we selected regions that have been shown to change in relatively short periods (the prefrontal cortex, the hippocampus, the cerebellum, and the caudate nucleus) and a control region that evidenced no significant shrinkage in previous longitudinal studies – the primary visual cortex. Second, we examined if individuals reliably differ in their rates of brain shrinkage, even when observed over relatively short intervals. The third question was whether vascular risk moderates age-related declines in brain volume. Based on the reviewed evidence, we expected vascular risk to exacerbate shrinkage of the gray matter in the prefrontal cortex and the hippocampus. The fourth objective was to evaluate whether persons who undergo intensive cognitive intervention show lesser regional brain shrinkage than their counterparts who do not participate in the program. We addressed these questions by examining short-term changes in the regional brain volumes of healthy adults who participated in intensive training of multiple cognitive skills. We hypothesized that healthy adults, especially the older among them, would show significant shrinkage within six months at least in the brain regions with established vulnerability, such as the hippocampus, the prefrontal cortex, and the cerebellum. We expected to observe significant individual differences in the rate of change, and we hypothesized that persons with hypertension would show greater shrinkage than their normotensive peers would. Finally, we expected that the brains of people who underwent intensive cognitive practice would evidence lesser shrinkage than those of the control group participants. We expected to observe the benefits of practice in the regions with known associations to higher cognitive activities that are tapped by the cognitive tasks employed in this study: episodic memory, working memory and perceptual speed. Thus we selected the hippocampus, in which extensive training related to high memory load is believed to induce functional, metabolic and structural changes (Groussard et al., 2010; Maguire et al., 2000; Mårtensson et al., 2012; Roche et al., 2009), the prefrontal cortex that showed changes after memory, attention and working memory training (Engvig et al., 2010; Hoekzema et al., 2011; Mårtensson et al., 2012), and the cerebellum which evidenced structural growth after working memory training (Hoekzema et al., 2011).
2. Method
2.1 Participants
The participants were paid volunteers, residents of Berlin, Germany, who responded to advertisement in newspapers, word-of-mouth recommendations, and fliers that invited them to join a study on the effects of intensive cognitive training on brain and behavior (the COGITO study; see Schmiedek et al. 2010, for details). The main study involved 101 younger (aged 20–31 years) and 103 older adults (aged 65–80 years). These individuals took part in an intensive 100-days program of cognitive training, during which they practiced several tasks covering three domains of cognition: working memory (three tasks), episodic memory (three tasks), and perceptual speed (six tasks; see Schmiedek et al. 2010 for details). Out of this sample, we recruited for the MRI study individuals with no history of psychiatric, neurological, metabolic, cardiovascular or endocrine disease. Some participants reported diagnosis of essential hypertension and were taking anti-hypertensive medications. MR imaging was performed before and after the cognitive training intervention, with the delays between scans ranging from 135 to 232 days. There were no differences in delay between the scans by age group, sex, hypertension diagnosis or training assignment: all F<1. All measures were tested for association with the delay, and none were found. Therefore, delay was not included in the statistical models. A no-contact control group met the same exclusion and inclusion criteria.
The sample of this study consisted of 35 younger (age 25.27±3.18) and 37 older (age 70.88±3.82) adults. The age groups did not differ in sex composition (61% vs. 53% men among the younger and the older participants, respectively; χ2=.452, p=.501) or inter-scan delay (t=.145, p=.885). Twenty-five younger and 24 older participants formed the training group, whereas 10 younger and 13 older adults constituted a no-contact control group. The control group that was originally selected in the COGITO study (see Schmiedek et al., 2010) was made unavailable to this study because of MRI equipment problems. We therefore recruited a new control group from the same populations using the same advertising methods and the same exclusion and inclusion criteria.
The experimental and control groups did not differ in age, t(67)=.808, p=.420, delay between the MRI scans, t=1.07, p=.290, or sex composition, χ2=.973, p=.323; 53% men and 65% men in the experimental and control group, respectively. There were two notable differences between the groups. First, eight of the training group participants but none of the controls had hypertension, χ2=4.450, p=.044. Second, whereas, as expected, younger participants performed better than their older counterparts: (66.13±1.42 vs. 46.63±1.34, F(1,65) = 99.76, p <.00001), the control group participants performed better than the experimental group: 60.71±1.60 vs. 52.04±1.12; F(1,65)=19.69, p=.00004. As indicated by Age × Training Group interaction (F (1,65) =4.36, p=.040), this difference was mainly due to substantially better performance of younger controls compared to the younger experimental group participants: 72.20±2.40 vs. 59.76±1.52; Tukey HSD test p=.00018. The difference between the older controls and older experimental group participants was not-significant: 48.92±2.11 vs. 44.33±1.67, Tukey p=.32, ns. Older participants attained higher scores on the word-knowledge test than the younger participants did: F(1,65)=43.13, p<.00001. No difference between the control and experimental groups were observed on the vocabulary test (F<1). Thus, controls happened to be somewhat better on at least two important indicators of physical and cognitive health than the experimental group participants were.
2.2 Cognitive Training Protocol
Participants practiced daily for one hour on individual computerized testing stations, up to six participants per lab room, for an average of 100.89 (92–109) sessions distributed over the period between pre and posttest. The cognitive training battery consisted of three working memory, three episodic memory, and six perceptual speed tasks. Individual sessions occurred on up to six days a week. At the end of each session, participants received feedback on their performance on all tasks, including average accuracies and reaction times. For a detailed description of the tasks, see Schmiedek et al. 2010.
2.2.1 Pretest – Posttest Assessment
Participants underwent 10 days of behavioral pre-testing in group sessions that lasted 2–2.5 hours. Measurements consisted of self-report questionnaires, cognitive tasks included in the daily phase, and transfer tasks (for a detailed description, see Schmiedek et al. 2010). Ten posttest group sessions (1.5–2 hours each) consisted of re-administration of the pre-test cognitive tasks and additional self-report measures. In this study, we measured cognitive performance at baseline and follow-up in three major cognitive domains: working memory (three index tasks), episodic memory (three index tasks), and perceptual speed (six index tasks). All tasks were administered during the daily training phase of the study.
2.3 MRI Protocol
2.3.1 Acquisition
A pre-test brain-imaging session was conducted after the behavioral pretest and immediately before the daily assessment phase; the post-test images were acquired shortly after the completion of the behavioral posttest. The images were acquired at both occasions on the same GE Signa LX 1.5 Tesla system (General Electric, Milwaukee, WI) with actively shielded magnetic field gradients (maximum amplitude 40 mTm−1). The MR protocol included a T1-weighted sagittal 3D scan (contrast-optimized spoiled gradient-echo sequence, 124 slices, slice thickness = 1.5 mm, FOV 250 × 250 mm2; 256 × 256 matrix; TE = 8 ms; TR = 24 ms; flip angle = 30°).
2.3.2 Image Processing and Manual Volumetry
Image processing and regional volumetry are described in detail elsewhere (Raz et al. 2004; Raz et al. 2005; Raz et al. 2010). The baseline and follow-up images were coded, mixed, and assigned randomly to two tracers. The tracers were blind to the time of acquisition and to the demographic characteristics of the participants. Reliability of all manual ROI measures assessed by a conservative index, intraclass correlation for random raters (ICC [2], Sprout and Fleiss, 1979), exceeded .93. The volumes of the intracranial vault (ICV) and seven regions of interest (ROI) – lateral prefrontal cortex (LPFC), orbital frontal cortex (OFC), the adjacent prefrontal white matter (PFw), primary visual (calcarine) cortex (VC), the hippocampus (HC), the caudate nucleus (Cd), and the cerebellar hemispheres (Cb) – were computed from measured areas (see Raz et al. 2003a; 2003b; and 2004 for details).
As expected, men had significantly greater ICV than women did, F(1,62)=41.069, p<.00001, but we observed no sex × ICV interactions for any of the baseline regional volumes (F’s ranging from .12 to 1.67, all ns) or at follow-up (all F’s < 1). The ICV at follow-up was significantly larger than at baseline: a small but consistent difference of .47%. Moreover, the ICV volume increase was smaller for the control group (.26%) than for the experimental group (.85%), t(70)=2.836, p=.006. The correlation over time between the ICV measures was r=.990, p<.001. The sources of the observed ICV differences are unclear. It may arise from changes in head positioning, gradients, RF, and participant’s hydration level, to name a few possibilities. The assumption is that all these factors affect everything that is imaged in the scanner inside a given cranium. Incidentally, other studies reported longitudinal ICV change. In one study we found a 0.3% increase in ICV between baseline and the first retest but not between the first and the second retest after the same delay (Lövdén et al, 2012). In another study, we observed a similar small difference in the opposite direction, i.e. a reduction of .3% (Raz et al., 2005). In the present study, there was no interaction between ICV and training group membership or age at any measurement occasions for any of the ROI volumes: all F< 1. We could therefore adjust for individual and sex differences in body size, as well as variations in MRI scanning across two occasions, by adjusted all regional volumes for the ICV through a regression-ANCOVA approach described in previous publications (Jack et al. 1989; Raz et al. 2004).
2.4 Statistical Analyses
To improve reliability of the cognitive measures, we computed unit-weighted composites of performance on the training tasks in each cognitive domain. Each measure was first z- transformed using the pretest means and standard deviations of the total sample, so that age and time differences were preserved. Next, the measures of working memory (three tasks), episodic memory (three tasks), and perceptual speed (six tasks) were averaged to form the three cognitive domain composites. Because the cognitive data were available only for the intervention group, we analyzed performance for that group with a series of univariate 2 (time; pretest vs. posttest) × 2 (age group; young vs. old) mixed analyses of variance (ANOVAs) for each cognitive composite.
To evaluate the change in regional brain volumes between two occasions, we used the latent difference score (LDS) model. This structural equation modeling technique is well suited for evaluating individual differences in change with two-occasion data (Rogosa, and Willett, 1985; McArdle and Nesselroade, 1994; McArdle, 2009), and has been used in previous investigations of brain aging (McArdle et al. 2004; Raz, et al. 2005, 2008, 2010). We used LDS models to generate latent factors for each ROI at pretest and posttest using left and right volumes as indicator variables. Change between measurement occasions is included in the model by regressing the posttest factor on the pretest factor with a fixed regression weight of one and an additional latent change factor on which the posttest factor has a fixed loading of one. Intercept and residual variance of the posttest factor are fixed at zero. Factor loadings, intercepts, and residual variances of the indicator variables are constrained as equal across pretest and posttest (for details, see Raz et al. 2005). Thus, the mean and the variance of the latent change factor represent average change and individual differences in change, respectively, at the level of latent factors. Both parameters can be tested for significance with likelihood ratio tests, using comparisons to models in which these parameters are fixed to zero. A major advantage of the LDS approach is that it enables assessment of mean change and individual differences in change separately from measurement error, such as unsystematic sources of inaccuracy in volume tracing. In addition, demonstrating metric invariance of the measurement models across time, as was the case in this study, guarantees that the regions of interest are represented in the same way at both measurement occasions, and thus further enhances the validity of the results.
We tested separate LDS models for each ROI in a two-step process. First, for each region, we fitted a model that contained volume of that region at baseline and follow up, without predictors of level and change in the model. All these models fit the data reasonably well, all χ2s < 5.82, dfs = 3, ps > .121, CFIs > .995, and RMSEAs < .113. The 95% confidence intervals of the RMSEA included .05 in all cases. At the second step, we evaluated the effects of age at baseline, sex, training group, and hypertension by conducting multiple regressions of the latent factors of level and change in brain volume on the corresponding independent variable. For tests of statistical significance, in the second step, we used Bonferroni corrections for multiple comparisons.
3. Results
3.1 Changes in Regional Brain Volumes
3.1.1 Latent Difference Scores Models
Table 1 summarizes the univariate LDS models for change in regional cerebral volumes between the baseline assessment and the follow-up. The results of these analyses indicate that the mean volumes of four out of seven examined regions shrunk within less than six months. Significant mean shrinkage occurred in the hippocampus, the lateral prefrontal cortex, the caudate nucleus, and the cerebellum. Notably, all seven measured ROIs evidenced significant variance in change, including those that showed no mean change. To illustrate the mean change in volume, we computed annualized percentage change (APC) index by subtracting the baseline volume from the follow-up value, dividing the result by the baseline volume, multiplying by 100 and dividing by the delay duration in months (i.e., fraction of years). The APC is an imperfect index of change. It was computed on raw scores, and its limitations include measurement errors and the fact that it does not take into account variability. The APCs for all regions also appear in Table 1.
3.1.2 Age, Sex, Training, and Hypertension Effect on Volume Change
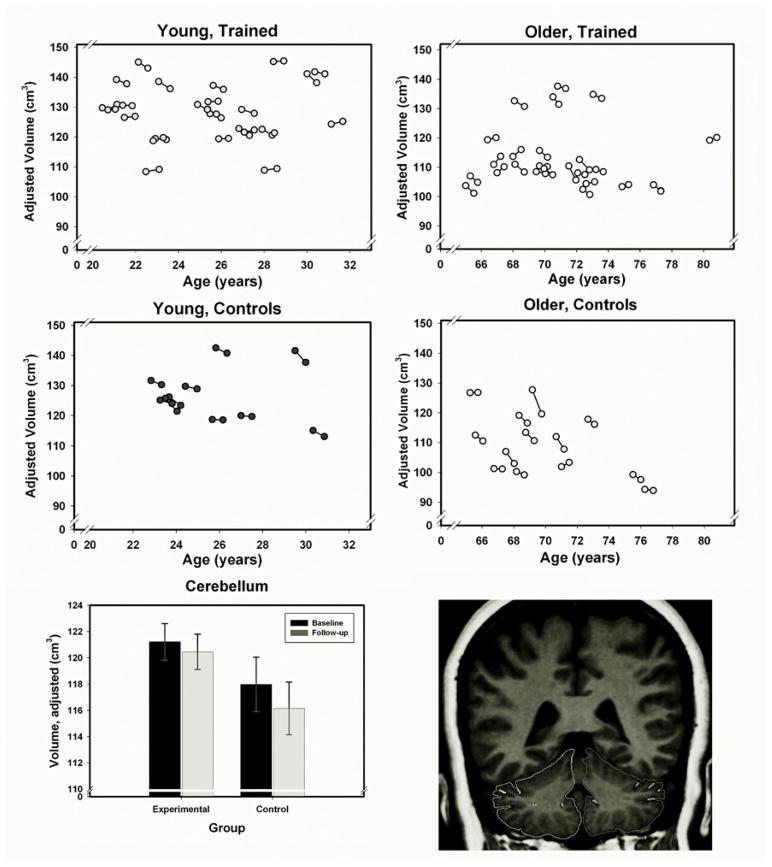
The results of step 2 analyses, at which age at baseline, sex, training status, and hypertension entered the LDS model as predictors of level and change in brain volume appear in Table 2. The effects of age on volume (age-related differences) were significant for all examined structures, with the greatest advantage of the younger participants in the volume of the prefrontal cortex and the smallest in the volume of the primary visual cortex. Men had a significantly greater volume of prefrontal white matter even after adjustment for intracranial volume. However, there were no sex differences in change. The diagnosis of hypertension had significant influence on change only in the cerebellum. The effect of training (Group) was significant only for the cerebellum (see Figure 1 for individual change trajectories, and training-related differences).
Table 2.
Longitudinal Change in Regional Cerebral Volumes: Independent effect of age, sex, group, and hypertension on level and change as estimated by multiple regressions of the level and change factors on these variables.
| HC | DLPFC | OF | VC | CD | CB | FW | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | C | L | C | L | C | L | C | L | C | L | C | L | C | |
| Age | −.64* | −.33 | −.76* | −.02 | −.80* | .31 | −.52* | .11 | −.77* | .22 | −.63* | .02 | −.67* | −.05 |
| Sex | −.22 | .00 | −.16 | −.10 | −.04 | −.03 | −.08 | .06 | −.19* | .15 | −.21* | .26 | −.32* | −.06 |
| Group | −.24 | .06 | −.08 | .07 | −.07 | −.26 | .01 | −.19 | .04 | −.06 | −.11 | −.40* | −.09 | .02 |
| Hypertension | .09 | −.10 | −.10 | −.09 | −.08 | −.36 | .00 | −.43 | −.03 | −.11 | −.04 | −.34* | −.09 | .01 |
significant after Bonferroni adjustment (critical p=.007). Coefficients are standardized regression coefficients, and can be interpreted as correlations. Sex is coded as 1= man and 2 = women. Group 1=experimental and 2=control; Hypertension 0=normotensive and 1=hypertensive; L – level, C – change.
Abbreviations: HC – hippocampus, DLPFC – dorsolateral prefrontal cortex, OF – orbitofrontal cortex, VC – primary visual (pericalcarine) cortex, CD – caudate nucleus, CB – cerebellum, FW – prefrontal white matter.
Figure 1.
Cerebellar volume in experimental (trained) and control groups at baseline and follow-up. Top four panels – spaghetti plots of cerebellar volume adjusted for the intracranial volume as a function of baseline age. Lower panel – mean cerebellar volumes (with ± SE, standard error bars) at baseline and follow-up for participants who underwent cognitive training and controls. Volumes of cerebellar hemispheres include the cortex, the white matter, and the deep nuclei were traced as shown.
In the intervention group, cognitive performance (see Table 3) improved over time in each measured domain: working memory, F(1,47) = 254.23, p < 0.001, episodic memory, F(1,47) = 99.37, p < 0.001, and perceptual speed, F(1,47) = 141.98, p < 0.001. Younger adults had higher scores on all cognitive composites: working memory, F(1,47) = 35.87, p < 0.001, episodic memory, F(1,47) = 47.76, p < 0.001, and perceptual speed, F(1,47) = 84.20, p < 0.001. The age group × time interaction reached significance for perceptual speed, F(1,47) = 41.17, p < 0.001, and episodic memory, F(1,47) = 24.01, p < 0.001, indicating larger increases over time for younger relative to older adults. No other effects were significant.
Table 3.
Cognitive performangce before and after practice.
| Cognitive Domain | Young | Old | ||||||
|---|---|---|---|---|---|---|---|---|
| Pretest | Posttest | Pretest | Posttest | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Working memory | 0.50 | 0.69 | 1.78 | 0.81 | −0.52 | 0.46 | 0.85 | 0.56 |
| Episodic memory | 0.48 | 0.88 | 1.75 | 1.31 | −0.50 | 0.46 | −0.06 | 0.82 |
| Perceptual speed | 0.54 | 0.65 | 2.11 | 0.53 | −0.56 | 0.75 | −0.10 | 0.82 |
Note: The measures are averages of z-standardized (using the Mean and SD of the total sample at pretest) individual measures of working memory (three tasks), episodic memory (three tasks), and perceptual speed (six tasks), respectively.
To assess the specificity of training effect on changes in the cerebellar volume, we examined the correlations between individual differences in volume change and changes in performance on specific tasks among the experimental group participants. We modeled the pre- and post-test latent factors in each cognitive domain (working memory, episodic memory, and perceptual speed) with the LDS model, in a similar way as we did with the brain volume measures (see Schmiedek et al. 2010, for details). The relations between the cerebellum volume and changes therein to level and changes in performance were tested in separate bivariate LDS models for each cognitive domain. In none of the models did level of cognitive performance or change therein correlate with cerebellum volume (all rs < .38, ps > .117).
4. Discussion
4.1 Neuroanatomical Changes
4.1.1 Regional Brain Shrinkage – How Fast?
The results of this study show that significant shrinkage of selected brain structures occurs in healthy adults within less than six months. We observed mean volume changes in four out of seven examined brain regions. The pace of change in these regions was equivalent to 1.4 to 2.5% per annum, with the lateral prefrontal cortex exhibiting the fastest rate of shrinkage. These shrinkage rates are greater than the ones reported in a five-year longitudinal study (Raz et al., 2005) but smaller than those found in another sample (Raz et al., 2010) evaluated with the tracing method identical to the one employed here. The sources of this heterogeneity in shrinkage await systematic study. Some portion of this heterogeneity may reflect differences among the populations from which the different samples were drawn: Memphis, Tennessee (Raz et al., 2005) vs. Detroit, Michigan (Raz et al., 2010) vs. Berlin, Germany (this sample). In addition, variations in the duration of inter-measurement intervals may contribute to differences in shrinkage rates, even when expressed as APC. A quantitative evaluation (meta-analysis) of contributions to this heterogeneity would most useful but it will have to wait for accumulation of a sufficient number of longitudinal studies..
4.1.2 Individual differences in brain shrinkage
The finding of individual differences in volume changes in this sample contributes to understanding the shape of brain aging trajectories. General heterochronicity of life-span trajectories of brain development and aging is well established (Lenroot and Giedd, 2006; Raz and Kennedy, 2009). However, variability of age-related neuroanatomical change is substantial (Raz et al., 2005; 2010). Even a cursory examination of individual trajectories in this and other longitudinal studies reveals that for some individuals, the slope of change between two measurement occasions is reversed relative to the mean trajectory, and to most other individual trajectories. The nature of such reversals is unclear, as the neurobiological mechanisms of brain shrinkage observed on MRI are unclear. Gross volume reduction may reflect changes in neural number and size (Bobinski et al., 2000), loss of intralaminar myelin (Courchesne et al., 2000), rarefaction of arterioles and reduction in density of capillary networks (Riddle et al., 2003), and fluctuation in hydration (Duning et al., 2005). It is also conceivable that changes in dendritic arborization and local capillary density contribute to variation in brain volumes. Regional brain volumes may be determined by multiple factors that fluctuate and counteract, thus creating a state of dynamic equilibrium that changes in a quasi-periodic fashion but shifts downwards over time.
The MRI technique used in this study is too coarse to present a true picture of such quasi-periodic process as it is incapable of tracking changes in individual cortical laminae, not to mention single neurons and dendritic branches, and it is incapable of assessing changes in density of small blood vessels in a given ROI. The Nyquist frequency of such a process is unknown but it is highly unlikely to be faithfully captured even in a relatively short (six–month) window used in this study. However, some sources of individual differences in age-related change can be discerned even with current methods. The methods that are best suited to discover neurobiologically meaningful change remain to be established. Current MRI-derived measures of volume and cortical thickness are still very coarse. Moreover, their neurobiological validity is not well-established. For instance, unlike manual MRI measures (Bobinski et al., 2000), VBM-derived estimates of local gray matter density do not correlate with actual neuron counts, neuronal nuclear antigen expression or presence of glial fibrillary acidic protein (Eriksson et al., 2009). Carefully performed manual volumetry, with all its limitations, is probably a preferred method of measurement, provided that it is conducted by experienced well-trained operators who attain high reliability. Significant limitations of semi-automated methods have been outlined in several studies (e.g., Kennedy et al., 2009, see Thomas & Baker, 2012 for a review). For example, FreeSurfer produces volume estimates that overestimate the actual volume of structures such as the hippocampus by about 25% (Cherbuin et al., 2009) and correlate only .77 – .81 with manual measures (Cherbuin et al., 2009; Shen et al., 2010), probably because of over-inclusive definition of the hippocampus in the FreeSurfer labeling. Clearly, future developments in MRI technology and software are needed to improve the resolution and validity of the methods that are relied upon in research on structural changes in healthy human brains.
4.1.3 The role of vascular risk factors in brain aging variability
The results of this study are in agreement with the extant reports of major vascular risk factors such as hypertension exacerbating age-related regional brain shrinkage. When using a conservative adjustment for multiple comparisons, hypertension reliably affected shrinkage rate at least in one region, the cerebellum. The finding of negative impact of hypertension on cerebellar volume is in agreement with early reports (Strassburger et al., 1997), although not with more recent longitudinal studies (e.g., Raz et al., 2005). With a less conservative decision rule, we note the same effect in the pericalcarine cortex, a region that exhibits relative stability in most of the extant investigations of the aging brain but may be sensitive to age-related increase in vascular risk (Raz and Kennedy, 2009 for a review). We did not replicate previously observed exacerbation of hippocampal (Korf et al., 2004; Raz et al., 2005), and prefrontal (Raz et al., 2005) shrinkage by hypertension. Given the low statistical power of this study, especially with regards to effects of hypertension, lack of replication should be interpreted with great caution. It is plausible, however, that specific targets of hypertension effects on parenchymal integrity vary by the participants’ age and duration of follow-up. Longitudinal studies with greater density of measures and a larger number of hypertensive participants are needed to examine the influence of vascular risk on individual differences in brain aging.
4.2. Neuroanatomy and Cognitive Performance
4.2.1 Attenuation of Brain Shrinkage by Cognitive Practice
The effort to attenuate brain decline with intensive cognitive intervention showed a promise of success at least for one brain structure out of seven examined – the cerebellum. We were unable to demonstrate association of cognitive practice and change in the other brain regions, some of which (e.g., the hippocampus) play a pivotal role in support of the cognitive operations that our participants practiced for 100 days, whereas others (e.g., primary visual cortex) were selected as control locations. Although cognitive performance clearly improved after practice, especially in the younger participants, the reduced cerebellar shrinkage did not significantly relate to the initial cognitive attainment or to the success in training program. The observed benefits of cognitive training may therefore represent a general response to increase in environment complexity. In animal models, environmental manipulations of complexity produce extensive neuro-, glia-, angio- and synaptogenesis as well as dendritic and axonal proliferation (Black et al, 1987; 1990; Kleim et al., 2007; Mora et al. 2007), and different types of environmental intervention may cause different types of structural change (Black et al., 1990; Kleim et al., 2007). At the current state of knowledge, it is impossible to determine which of these physiological and anatomical changes occurred and which ones account for the observed effect on the MRI-based measures. Note also that the lack of specific association between performance changes and brain volume should, given limited power, be carefully interpreted. Other recent findings have shown associations between brain changes and performance gains, at least in younger adults (e.g., Mårtensson et al., 2012). It is unlikely, however that training intensity played a role in our relatively isolated and unspecific findings: our participants underwent longer practice (100 days of about 90 minutes of practice) than participants in other studies that reported significant change (e.g., Engvig et al., 2010 - 50–60 days, Hoekzema et al., 2011 – 14 days).
4.2.2 The Role of Cerebellum and Cognitive Performance
The effect of cognitive training on the cerebellum is a novel finding and, to the best of our knowledge, there are no comparable investigations involving this region. Our results are in accord with findings of significant cellular plasticity in cerebellar neurons (Abrahamsson et al. 2012), and suggest that cerebellar plasticity deserves greater attention in studies of cognitive training (cf. Diamond, 2000). Although the observed reduction in cerebellar shrinkage is unlikely to reflect a specific effect of training on one or many of the tasks, the link between cerebellar volume and these tasks is plausible. The cerebellum is a sophisticated multipurpose processing device with long-reaching connections to the motor, premotor and prefrontal association cortices (Strick et al., 2009). It is involved in a wide range of motor, affective, and cognitive functions, all of which could have improved in the course of 100 days of training. For instance, even relatively simple working memory tasks activate lateral cerebellum (Cabeza and Nyberg, 2000), and age differences in performance on speeded working memory tasks are associated with structural differences in the cerebellum (Eckert et al., 2010). The neural substrates of cognitive skill map on cerebellar and cortical regions that are distinct from the motor circuits, for example, the lobules VII–VIII of the cerebellum (especially Crus II region) and the LPFC (Salmi et al., 2010). Unfortunately, our method lacked resolution to assess the changes in those circumscribed regions of the cerebellum. Moreover, the gross volume measures may not have been sufficiently sensitive to rapid experience-induced changes. Other indicators such as white matter diffusion properties appear to respond to learning in a much more rapid manner (Lövdén et al., 2010b; Landi et al., 2011; Engvig et al., 2012; Sagi et al. 2012) and may undergo subtle regional changes that were missed by the method employed in this study.
4.3 Implications for models of cognitive and brain plasticity
The results of this study support the argument that the relationship between brain and cognition is a two-way street. The brain is important for cognitive performance but the former can also supply the capacity to respond to raising demands on the latter (Lövdén et al., 2010a). It is interesting that the only region that showed significant change after cognitive practice was also one of the most vulnerable to aging, the cerebellum (e.g., Andersen et al., 2003; see Raz and Kennedy, 2009 for a review). In a way, the “dark side of plasticity” principle (Raz, 2000; Arendt, 2003), according to which brain structures that display the highest degree of plasticity are those that are destined to show the greatest impact of aging, was turned on its head, with one of the most age-sensitive structures evidencing the greatest ability to change.
It is unclear what physiological phenomena the reported changes in volume and density of the brain tissue represent. Given significant loss of arteriolar and capillary density, alteration of their microstructure, and reduction in the pace of angiogenesis with aging (Riddle et al., 2003), it is plausible that microarchitecture of the cerebrovascular system is the primary respondent to volume gains brought by cognitive intervention. Indeed, according to some findings, this is a distinct possibility (Pereira et al., 2007), and because synaptogenesis and angiogenesis are far from independent, the contributions of vascular and neural mechanisms (Farkas and Luiten, 2001) to experience-related plasticity may be difficult to disentangle.
To date, the research efforts aimed at understanding the neural substrates of cognitive plasticity centered exclusively on the cerebral cortex and the hippocampus (e.g., May, 2011), with the cerebellum largely ignored or assigned a role of a motor learning organ (see Ito, 2002, for a historical review). The results reported here call the attention of researchers back to the cerebellum, which for quite a while has been known as not just an organ of motor learning but a full participant in many complex cognitive processes (Botez et al, 1989). Moreover, comparison to non-human primates reveals a significant evolutionary development in the human cerebellum such as increase in the relative volume of Crus I and Crus II, the cerebellar compartments that project to the tertiary association areas of the prefrontal cortex (Balsters et al., 2010). In the extant literature, one can find references to changes in cerebellum after cognitive training of children with attention deficit disorder (Hoekzema et al., 2011). Our findings show that cerebellar changes accompany cognitive practice in healthy adults as well.
4.4 Limitations
Consideration of several limitations of this study should help in interpreting its results. First, the lack of intermittent measures of regional brain volumes precluded a more nuanced analysis of the volume change at various stages of training. The cerebellum may play different roles and attain a variable degree of prominence at different stages of skill acquisition. For example, activity in some cerebellar regions connected to the tertiary association areas of the cortex (e.g. area 46) increases as training of a cognitive skill progresses to automaticity (Balsters and Ramnani, 2011) and spatial navigation learning shows different degree of dependence on hippocampus and cerebellum at different stages of information transfer (Rochefort et al., 2011). Thus, it is possible that sensitivity of cerebellar volume to training reflects the high degree of automaticity of performance after a lengthy and intensive process of acquisition. Denser measurements would have facilitated uncovering the links between volume change and cognitive improvement. A study of young adults provides an example of such investigation of the time course of practice-related neuroanatomical change (Driemeyer et al, 2008). Having only two measurement occasions and a relatively narrow age range within age groups precluded the estimation or assessment of nonlinear change.
Second, for unknown reasons, the experimental group exhibited worse health and lower cognitive ability than the controls did. Although hypertension was related to greater shrinkage, the experimental group that included hypertensive participants showed overall lesser decline in the Cb volume than the control group composed of self-reported normotensives. Thus, it seems that presence of hypertension in the experimental group worked against the hypothesis of group differences. It is possible however, that presence of undetected hypertension among the control participants resulted in observed greater Cb shrinkage. Given that all participants came from the same pool in a community with reliable medical care, this is an unlikely scenario. Nevertheless, it is unclear if the ameliorative effect of cognitive training on cerebellar shrinkage emerged in spite the health and performance differences or it occurred because the experimental group had more room for improvement. Close matching of experimental and control group on this important parameter should be implemented in future intervention studies that include older adults.
Third, although we drew our control participants from the same population as we recruited our experimental group, we were unable due to technical problems with the scanning equipment to use the data for the originally selected control group. Thus, this study is a non-randomized controlled observational study and as such it cannot support strong claims of treatment effectiveness. As we discussed above, the differences between controls and participants of the experimental group are such that they are more likely to counter treatment success than to promote it.
Fourth, the logic of hypothesis testing allows claiming the significant impact of cognitive practice on the cerebellum; it precludes, however, a clear declaration of the lack thereof in the other regions examined in this study. This is especially true in light of the relatively low statistical power of the present study. This drawback is one of the problematic aspects of this area of research enumerated in the introduction. Despite the high cost and logistical difficulties inherent in longitudinal investigations, this limitation must be addressed in the future studies.
Fifth, the time scope of this study was relatively limited. It is unclear how long the observed gains, cognitive and neuroanatomical, will last. In studies that first reported significant structural changes in the brains of people who acquired a novel perceptual-motor skill, the neuroanatomical gains were lost with discontinuation of practice (see Draganski and May, 2008 for review). At least one additional follow-up assessment is needed to establish the durability of changes reported here.
Sixth, brain volume and changes therein may be affected by multiple genetic factors such as the ApoEε4 allele (Moffat et al. 2000), or by their interaction with vascular risk (de Frias et al, 2007; Bender & Raz, 2012). Unfortunately, at the time of writing, we had no information about the genotypes of our participants, and thus were unable to examine the possibility of genetic risks contributing to the observed declines.
Finally, in this study, we used only one indicator of structural change, i.e. the regional brain volume. Future studies should address the possibility that training can affect other aspects of brain anatomy, including the local microstructure (e.g., as used in Lövdén et al., 2012) and vascular architecture.
4.5 Conclusion
In healthy adults, age-related shrinkage of selected brain regions is detectable within a period shorter than six months. Self-reported history of treated hypertension was associated with greater shrinkage. In at least one region, the cerebellum, intensive cognitive training was associated with slower shrinkage, although this apparent amelioration of brain change does not relate specifically to improvement in the targeted cognitive skills.
Highlights.
Healthy young and older adults had MRI scans 6 month apart.
The experimental group underwent 100 days of cognitive practice.
Prefrontal, hippocampus, caudate, cerebellum shrunk; calcarine, orbital did not.
In older participants, hypertension exacerbated cerebellar shrinkage.
In experimental group, cerebellar shrinkage was lesser than in controls.
Acknowledgments
This project was supported by the Max Planck Society grant from the Innovation Fund, the German Federal Ministry for Education and Research (BMBF), the Deutsche Forschungsgemeinschaft, the Sofja Kovalevskaja Award administered by the Alexander von Humboldt Foundation (to ML), and by the grant R37 AG-011230 from the National Institute on Aging, USA (to NR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Naftali Raz, Department of Psychology & Institute of Gerontology, Wayne State University, Detroit MI, USA.
Florian Schmiedek, German Center for International Educational Research (DIPF), Frankfurt am Main, Germany.
Karen M. Rodrigue, Center for Vital Longevity, University of Texas at Dallas, Dallas, TX, USA
Kristen M. Kennedy, Center for Vital Longevity, University of Texas at Dallas, Dallas, TX, USA
Ulman Lindenberger, Center for Lifespan Psychology, Max Planck Institute for Human Development, Berlin, Germany.
Martin Lövdén, Aging Research Center, Karolinska Institutet and Stockholm University, Stockholm, Sweden.
References
- Abrahamsson T, Cathala L, Matsui K, Shigemoto R, Digregorio DA. Thin dendrites of cerebellar interneurons confer sublinear synaptic integration and a gradient of short-term plasticity. Neuron. 2012;73:1159–72. doi: 10.1016/j.neuron.2012.01.027. Epub 2012 Mar 21. [DOI] [PubMed] [Google Scholar]
- Andersen BB, Gundersen HJ, Pakkenberg B. Aging of the human cerebellum: a stereological study. J Comp Neurol. 2003;466:356–365. doi: 10.1002/cne.10884. [DOI] [PubMed] [Google Scholar]
- Arendt T. Synaptic plasticity and cell cycle activation in neurons are alternative effector pathways: the ‘Dr. Jekyll and Mr. Hyde concept’ of Alzheimer’s disease or the yin and yang of neuroplasticity. Prog Neurobiol. 2003;71:83–248. doi: 10.1016/j.pneurobio.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Balsters JH, Cussans E, Diedrichsen J, Phillips KA, Preuss TM, Rilling JK, Ramnani N. Evolution of the cerebellar cortex: the selective expansion of prefrontal-projecting cerebellar lobules. Neuro Image. 2010;49:2045–2052. doi: 10.1016/j.neuroimage.2009.10.045. Epub 2009 Oct 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsters JH, Ramnani N. Cerebellar plasticity and the automation of first-order rules. J Neurosci. 2011;31:2305–2312. doi: 10.1523/JNEUROSCI.4358-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender AR, Raz N. Age-related differences in memory and executive functions in healthy APOE ε4 carriers: The contribution of individual differences in prefrontal volumes and systolic blood pressure. Neuropsychologia. 2012;50:704–714. doi: 10.1016/j.neuropsychologia.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JE, Sirevaag AM, Greenough WT. Complex experience promotes capillary formation in young rat visual cortex. Neurosci Lett. 1987;83:351–355. doi: 10.1016/0304-3940(87)90113-3. [DOI] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci U S A. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobinski M, de Leon MJ, Wegiel J, Desanti S, Convit A, Saint Louis LA, Rusinek H, Wisniewski HM. The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer’s disease. Neuroscience. 2000;95:721–725. doi: 10.1016/s0306-4522(99)00476-5. [DOI] [PubMed] [Google Scholar]
- Botez MI, Botez T, Elie R, Attig E. Role of the cerebellum in complex human behavior. Ital J Neurol Sci. 1989;10:291–300. doi: 10.1007/BF02333774. [DOI] [PubMed] [Google Scholar]
- Boyke J, Driemeyer J, Gaser C, Büchel C, May A. Training-induced brain structure changes in the elderly. J Neurosci. 2008;28:7031–7035. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cherbuin N, Anstey KJ, Réglade-Meslin C, Sachdev PS. In vivo hippocampal measurement and memory: a comparison of manual tracing and automated segmentation in a large community-based sample. PLoS One. 2009;4(4):e5265. doi: 10.1371/journal.pone.0005265. Epub 2009 Apr 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology; 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- de Frias CM, Bunce D, Wahlin A, Adolfsson R, Sleegers K, Cruts M, Van Broeckhoven C, Nilsson LG. Cholesterol and triglycerides moderate the effect of apolipoprotein E on memory functioning in older adults. J Gerontol B Psychol Sci Soc Sci. 2007;62:P112–P118. doi: 10.1093/geronb/62.2.p112. [DOI] [PubMed] [Google Scholar]
- Delisi LE, Sakuma M, Tew W, Kuschner M, Hoff AL, Grimson R. Schizophrenia as a chronic active brain process: a study of progressive brain structural change subsequent to the onset of schizophrenia. J Psychiatr Res. 1997;74:129–140. doi: 10.1016/s0925-4927(97)00012-7. [DOI] [PubMed] [Google Scholar]
- Draganski B, May A. Training-induced structural changes in the adult human brain. Behav Brain Res. 2008;192:137–142. doi: 10.1016/j.bbr.2008.02.015. Epub 2008 Feb 17. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Bush V, Schuierer G, Boghdan U, May A. Neuroplasticity: Changes in the gray matter introduced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Büchel C, May A. Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci. 2006;26:6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driemeyer J, Boyke J, Gaser C, Büchel C, May A. Changes in gray matter induced by learning--revisited. PLoS One. 2008;3:e2669. doi: 10.1371/journal.pone.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Davatzikos C, An Y, Wu X, Shen D, Kraut M, Resnick SM. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology. 2009;72:1906–1913. doi: 10.1212/WNL.0b013e3181a82634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duning T, Kloska S, Steinsträter O, Kugel H, Heindel W, Knecht S. Dehydration confounds the assessment of brain atrophy. Neurology. 2005;64:548–550. doi: 10.1212/01.WNL.0000150542.16969.CC. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Keren NI, Roberts DR, Calhoun VD, Harris KC. Age-related changes in processing speed: unique contributions of cerebellar and prefrontal cortex. Front Hum Neurosci. 2010;4:10. doi: 10.3389/neuro.09.010.2010.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth Ø, Larsen VA, Walhovd KB. Effects of memory training on cortical thickness in the elderly. Neuro Image. 2010;52:1667–1676. doi: 10.1016/j.neuroimage.2010.05.041. [DOI] [PubMed] [Google Scholar]
- Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth Ø, Larsen VA, Walhovd KB. Memory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging study. Hum Brain Mapp. 2012;33:2390–2406. doi: 10.1002/hbm.21370. Epub 2011 Aug 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–22. doi: 10.1073/pnas.1015950108. Epub 2011 Jan 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson SH, Free SL, Thom M, Symms MR, Martinian L, Duncan JS, Sisodiya SM. Quantitative grey matter histological measures do not correlate with grey matter probability values from in vivo MRI in the temporal lobe. J Neurosci Methods. 2009;181(1):111–118. doi: 10.1016/j.jneumeth.2009.05.001. Epub 2009 May 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM. One-year brain atrophy evident in healthy aging. J Neurosci. 2009;29:15223–15231. doi: 10.1523/JNEUROSCI.3252-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Westlye LT, Østby Y, Tamnes CK, Jernigan TL, Gamst A, Dale AM. When does brain aging accelerate? Dangers of quadratic fits in cross-sectional studies. Neuro Image. 2010;50:1376–1383. doi: 10.1016/j.neuroimage.2010.01.061. Epub 2010 Jan 25. [DOI] [PubMed] [Google Scholar]
- Groussard M, La Joie R, Rauchs G, Landeau B, Chételat G, Viader F, Desgranges B, Eustache F, Platel H. When music and long-term memory interact: effects of musical expertise on functional and structural plasticity in the hippocampus. PLoS One. 2010;5:e13225. doi: 10.1371/journal.pone.0013225.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekzema E, Carmona S, Ramos-Quiroga JA, Barba E, Bielsa A, Tremols V, Rovira M, Soliva JC, Casas M, Bulbena A, Tobeña A, Vilarroya O. Training-induced neuroanatomical plasticity in ADHD: a tensor-based morphometric study. Hum Brain Mapp. 2011;32:1741–1749. doi: 10.1002/hbm.21143. Epub 2011 Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT. Exercise and the brain: Angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J Cereb Blood Flow Metab. 1992;12:110–119. doi: 10.1038/jcbfm.1992.14. [DOI] [PubMed] [Google Scholar]
- Ito M. Historical review of the significance of the cerebellum and the role of Purkinje cells in motor learning. Ann N Y Acad Sci. 2002;978:273–288. doi: 10.1111/j.1749-6632.2002.tb07574.x. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Twomey CK, Zinsmeister AR, Sharbrough FW, Petersen RC, Cascino GD. Anterior temporal lobes and hippocampal formations: normative volumetric measurements from MR images in young adults. Radiology. 1989;172:549–554. doi: 10.1148/radiology.172.2.2748838. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Mendelson DN, Muldoon MF, Ryan CM, Gianaros PJ, Raz N, Aizenstein H. Regional grey matter shrinks in hypertensive individuals despite successful lowering of blood pressure. J Hum Hypertens. 2012;26:295–305. doi: 10.1038/jhh.2011.31. Epub 2011 Apr 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fenema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Markham JA, Vij K, Freese JL, Ballard DH, Greenough WT. Motor learning induces astrocytic hypertrophy in the cerebellar cortex. Behav Brain Res. 2007;178:244–249. doi: 10.1016/j.bbr.2006.12.022. Epub 2007 Jan 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf ES, White LR, Scheltens P, Launer LJ. Midlife blood pressure and the risk of hippocampal atrophy: the Honolulu Asia Aging Study. Hypertension. 2004;44:29–34. doi: 10.1161/01.HYP.0000132475.32317.bb. Epub 2004 May 24. [DOI] [PubMed] [Google Scholar]
- Landi SM, Baguear F, Della-Maggiore V. One week of motor adaptation induces structural changes in primary motor cortex that predict long-term memory one year later. J Neurosci. 2011;31:11808–11813. doi: 10.1523/JNEUROSCI.2253-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lövdén M, Bäckman L, Lindenberger U, Schaefer S, Schmiedek F. A theoretical framework for the study of adult cognitive plasticity. Psychol Bull. 2010a;136:659–676. doi: 10.1037/a0020080. [DOI] [PubMed] [Google Scholar]
- Lövdén M, Bodammer NC, Kühn S, Kaufmann J, Schütze H, Tempelmann C, Heinze HJ, Düzel E, Schmiedek F, Lindenberger U. Experience-dependent plasticity of white-matter microstructure extends into old age. Neuropsychologia. 2010b;48:3878–3883. doi: 10.1016/j.neuropsychologia.2010.08.026. Epub 2010 Sep 15. [DOI] [PubMed] [Google Scholar]
- Lövdén M, Schaefer S, Noack H, Bodammer NC, Kühn S, Heinze HJ, Düzel E, Bäckman L, Lindenberger U. Spatial navigation training protects the hippocampus against age-related changes during early and late adulthood. Neurobiol Aging. 2012;33:620.e9–620.e22. doi: 10.1016/j.neurobiolaging.2011.02.013. Epub 2011 Apr 16. [DOI] [PubMed] [Google Scholar]
- Mackey AP, Whitaker KJ, Bunge SA. Experience-dependent plasticity in white matter microstructure: reasoning training alters structural connectivity. Front Neuroanat. 2012;6:32. doi: 10.3389/fnana.2012.00032. Epub 2012 Aug 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J, Eriksson J, Bodammer NC, Lindgren M, Johansson M, Nyberg L, Lövdén M. Growth of language-related brain areas after foreign language learning. NeuroImage. 2012;63:240–244. doi: 10.1016/j.neuroimage.2012.06.043. Epub 2012 Jun 29. [DOI] [PubMed] [Google Scholar]
- May A. Experience-dependent structural plasticity in the adult human brain. TICS. 2011;15:475–482. doi: 10.1016/j.tics.2011.08.002. [DOI] [PubMed] [Google Scholar]
- McArdle JJ. Latent variable modeling of differences and changes with longitudinal data. Ann Rev Psychol. 2009;60:577–605. doi: 10.1146/annurev.psych.60.110707.163612. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Nesselroade JR. Using multivariate data to structure developmental change. In: Cohen SH, Reese HW, editors. Life-span developmental psychology: Methodological contributions. Hillsdale, NJ: Lawrence Erlbaum Associates; 1994. pp. 223–267. [Google Scholar]
- McArdle JJ, Hamagami F, Jones K, Jolesz F, Kikinis R, Spiro R, Albert MS. Structural modeling of dynamic changes in memory and brain structure using longitudinal data from the Normative Aging Study. J Gerontol B Psychol Sci Soc Sci. 2004;59:P294–304. doi: 10.1093/geronb/59.6.p294. [DOI] [PubMed] [Google Scholar]
- Mora F, Segovia G, del Arco A. Aging, plasticity and environmental enrichment: structural changes and neurotransmitter dynamics in several areas of the brain. Brain Res Rev. 2007;55:78–88. doi: 10.1016/j.brainresrev.2007.03.011. Epub 2007 Apr 13. [DOI] [PubMed] [Google Scholar]
- Murphy EA, Holland D, Donohue M, McEvoy LK, Hagler DJ, Jr, Dale AM, Brewer JB Alzheimer’s Disease Neuroimaging Initiative. Six-month atrophy in MTL structures is associated with subsequent memory decline in elderly controls. NeuroImage. 2010;53:1310–1317. doi: 10.1016/j.neuroimage.2010.07.016. Epub 2010 Jul 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch Gen Psychiatry. 1998;55:905–912. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. Handbook of Aging and Cognition - II. Mahwah, NJ: Erlbaum; 2000. pp. 1–90. [Google Scholar]
- Raz N, Rodrigue K. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience & Biobehavioral Reviews. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Kennedy KM. A systems approach to age-related change: Neuroanatomic changes, their modifiers, and cognitive correlates. In: Jagust W, D’Esposito M, editors. Imaging the Aging Brain. New York, NY: Oxford University Press; 2009. pp. 43–70. [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Dahle C, Head D, Acker JD. Differential age-related changes in the regional metencephalic volumes in humans: A five-year follow-up. Neurosci Lett. 2003a;349:163–166. doi: 10.1016/s0304-3940(03)00820-6. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Head D, Gunning-Dixon FM, Acker JD. Differential aging of the human striatum: Longitudinal evidence. AJNR. 2003b;24:1849–1856. [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: Vulnerability of the prefrontal regions and executive functions. Beh Neurosci. 2003c;17:1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Williamson A, Rodrigue K, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiol Aging. 2004;25:377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences, and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21:149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Ghisletta P, Rodrigue KM, Kennedy KM, Acker JD. Neuroanatomical correlates of fluid intelligence in healthy adults and persons with vascular risk factors. Cereb Cortex. 2008 Jul 5;18:718–726. doi: 10.1093/cercor/bhm108. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. Trajectories of brain aging in middle-aged and older adults: regional and individual differences. NeuroImage. 2010;51:501–511. doi: 10.1016/j.neuroimage.2010.03.020. Epub 2010 Mar 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle DR, Sonntag WE, Lichtenwalner RJ. Microvascular plasticity in aging. Age Res Rev. 2003;2:149–168. doi: 10.1016/s1568-1637(02)00064-8. [DOI] [PubMed] [Google Scholar]
- Roche RA, Mullally SL, McNulty JP, Hayden J, Brennan P, Doherty CP, Fitzsimons M, McMackin D, Prendergast J, Sukumaran S, Mangaoang MA, Robertson IH, O’Mara SM. Prolonged rote learning produces delayed memory facilitation and metabolic changes in the hippocampus of the ageing human brain. BMC Neurosci. 2009;10:136. doi: 10.1186/1471-2202-10-136.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort C, Arabo A, André M, Poucet B, Save E, Rondi-Reig L. Cerebellum shapes hippocampal spatial code. Science. 2011;334:385–389. doi: 10.1126/science.1207403. [DOI] [PubMed] [Google Scholar]
- Rogosa DR, Willett JB. Understanding correlates of change by modeling individual differences in growth. Psychometrika. 1985;50:203–228. [Google Scholar]
- Rusinek H, De Santi S, Frid D, Tsui WH, Tarshish CY, Convit A, de Leon MJ. Regional brain atrophy rate predicts future cognitive decline: 6-year longitudinal MR imaging study of normal aging. Radiology. 2003;229:691–696. doi: 10.1148/radiol.2293021299. [DOI] [PubMed] [Google Scholar]
- Turner AM, Greenough WT. Differential rearing effects on rat visual cortex synapses. I. Synaptic and neuronal density and synapses per neuron. Brain Res. 1985;329:195–205. doi: 10.1016/0006-8993(85)90525-6. [DOI] [PubMed] [Google Scholar]
- Sagi Y, Tavor I, Hofstetter S, Tzur-Moryosef S, Blumenfeld-Katzir T, Assaf Y. Learning in the fast lane: new insights into neuroplasticity. Neuron. 2012;73:1195–203. doi: 10.1016/j.neuron.2012.01.025. Epub 2012 Mar 21. [DOI] [PubMed] [Google Scholar]
- Salmi J, Pallesen KJ, Neuvonen T, Brattico E, Korvenoja A, Salonen O, Carlson S. Cognitive and motor loops of the human cerebro-cerebellar system. J Cogn Neurosci. 2010;22:2663–2676. doi: 10.1162/jocn.2009.21382. [DOI] [PubMed] [Google Scholar]
- Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol. 2003;60:989–994. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- Schlerf JE, Verstynen TD, Ivry RB, Spencer RM. Evidence of a novel somatopic map in the human neocerebellum during complex actions. J Neurophysiol. 2010;103:3330–3336. doi: 10.1152/jn.01117.2009. Epub 2010 Apr 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Wilcke T, Rosengarth K, Luerding R, Bogdahn U, Greenlee MW. Distinct patterns of functional and structural neuroplasticity associated with learning Morse code. Neuroimage. 2010;51:1234–1241. doi: 10.1016/j.neuroimage.2010.03.042. Epub 2010 Mar 24. [DOI] [PubMed] [Google Scholar]
- Schmiedek F, Lövdén M, Lindenberger U. Hundred days of cognitive training enhance broad cognitive abilities in adulthood: Findings from the COGITO study. Front Aging Neurosci. 2010;2 doi: 10.3389/fnagi.2010.00027.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Saykin AJ, Kim S, Firpi HA, West JD, Risacher SL, McDonald BC, McHugh TL, Wishart HA, Flashman LA. Comparison of manual and automated determination of hippocampal volumes in MCI and early AD. Brain Imaging Behav. 2010;4(1):86–95. doi: 10.1007/s11682-010-9088-x.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassburger TL, Lee HC, Daly EM, Szczepanik J, Krasuski JS, Mentis MJ, Salerno JA, DeCarli C, Schapiro MB, Alexander GE. Interactive effects of age and hypertension on volumes of brain structures. Stroke. 1997;28:1410–1417. doi: 10.1161/01.str.28.7.1410. [DOI] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Sekiguchi A, Taki Y, Yokoyama S, Yomogida Y, Komuro N, Yamanouchi T, Suzuki S, Kawashima R. Training of working memory impacts structural connectivity. J Neurosci. 2010;30:3297–3303. doi: 10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Baker CI. Teaching an adult brain new tricks: A critical review of evidence for training-dependent structural plasticity in humans. Neuroimage. 2012 Mar 30; doi: 10.1016/j.neuroimage.2012.03.069. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Wenger E, Schaefer S, Noack H, Kühn S, Mårtensson J, Heinze H-J, Düzel E, Bäckman L, Lindenberger U, Lövdén M. Cortical thickness changes following spatial navigation training in adulthood and aging. NeuroImage. 2012;59:3389–3397. doi: 10.1016/j.neuroimage.2011.11.015. [DOI] [PubMed] [Google Scholar]