Abstract
Despite obvious differences such as the ability to fly, the fruit fly Drosophila melanogaster is similar to humans at many different levels of complexity. Studies of development, cell growth and division, metabolism, and even cognition, have borne out these similarities. For example, Drosophila bearing mutations in the fly gene homologue of the known human disease Fragile X, are affected in fundamentally similar ways as affected humans. The ramification of this degree of similarity is that Drosophila, as a model organism, is a rich resource for learning about human cells, development and even human cognition and behavior. Drosophila has a short generation time of ten days, is cheap to propagate and maintain and has a vast array of genetic tools available to it; making Drosophila an extremely attractive organism for the study of human disease. Here, we summarize research from our lab and others using Drosophila to understand the human neurological disease, called Fragile X. We focus on the Drosophila model of fragile X, its characterization, and use as a tool to identify potential drugs for the treatment of Fragile X. Several clinical trials are in progress now that were motivated by this research.
Keywords: Disease Modeling, Fragile X, Drosophila, Pharmacological Treatments, Autism, Cognitive impairment
Fragile X
Fragile X syndrome (FXS) is the leading single gene cause of intellectual disability (ID) and autism [1]. Male patients with Fragile X typically have an IQ below 100 (with the average being close to 50), as well as memory, executive function and sleep deficits. They also have recognizable physical features such as large ears, an elongated face, a high-arched palate and macro-orchidism in post-pubertal males [2–5]. Co-morbid autism afflicts 25–67% of males with fragile X [1, 6, 7], with the severity of autism generally increasing with the severity of intellectual disability [6]. Far fewer fragile X females are diagnosed with autism. The autistic symptoms (communication, social skills, and repetitive behaviors) in Fragile X patients have historically worsened with age. As in autism, sleep problems are common in Fragile X patients. Examination of brain tissue from fragile X patients shows dendritic spine immaturity.
In most cases, the disease occurs when patients inherit an FMR1 gene with an aberrant 5′ untranslated region (5′UTR) containing increases in the normal number of a CGG repeat sequence. The normal range of repeats is 5 to 30, but when the repeat length increases to above 200 a cellular response is triggered that increases local DNA methylation and histone modification changes, leading to transcriptional repression of the gene locus [5, 8]. The subsequent loss of the fragile X mental retardation protein, FMRP [9, 10] causes the disease symptoms.
Drosophila has a single highly conserved FMR1 gene, called dfmr1, which is 35% identical and 60% similar to the human Fragile X gene, FMR1 [11]. The majority of the gene sequence identity corresponds to known protein-protein interaction domains, and nucleotide-binding domains needed for FXS function. In addition, the developmental expression pattern of dfmr1 is analogous to that of the mouse and human FMR1 proteins [11–13]. Drosophila mutants bearing either point mutations, or that lack all- or most of- the dfmr1 gene-coding region lack detectable FMRP expression, providing the basis of a Drosophila fragile X model [13–16]. Studies using this Drosophila Fragile X model have uncovered behavioral, neuroanatomical and biochemical phenotypic similarities with human FXS patients (Table I) [17–19]. That this model is relevant to human FXS is underscored by the fact that pharmacological treatments that rescue Drosophila FXS phenotypes also rescue related mouse FXS phenotypes and more recently, human Fragile X patient symptoms [20–27] in clinical trials. We will now focus the rest of this review on our approaches and that of others to use the Drosophila model of FXS to identify potential treatments for Fragile X.
Table I.
Dfmr1 mutant phenotypes and effective pharmacological treatments that rescue them.
| Analysis | Phenotype | Drug Rescue | Reference |
|---|---|---|---|
| Neuronal Anatomy | Central Neuron targeting defects (mushroom body cross-over) |
mGluR antagonist Lithium,MPEP,MPPG, MTPG and LY341495 |
[19] |
|
GABA agonist GABA, Creatinine and Nipecotic acid |
[79] | ||
|
Muscarinic ACH antagonist Pilocarpine nitrate, Aminobenztropine |
[79] | ||
|
Antibiotic Minocycline |
[26] | ||
| Peripheral Synaptic Defects (over-elaboration of NMJ structure) |
mGluR antagonist MPEP and genetic reduction of DmGluRA |
[71] | |
|
Antibiotic Minocycline |
[26] | ||
| Central Synaptic Defects (Over-elaboration of sLNv neurons) |
Antibiotic Minocycline |
[26] | |
| Neurotransmitter- containing vesicle Defects (elevated presynaptic vesicle pool) |
mGluR antagonist MPEP and genetic reduction of DmGluRA |
[71] | |
| Behavior | Repetitive Behaviors (Excessive grooming behavior) |
Neurotransmitter transport (into vesicles) antagonist Reserpine |
[35] |
| Behavior | Social Behaviors (naïve courtship) |
mGluR antagonist Lithium, MPEP,MPPG, MTPG, LY341495 |
[19] |
|
GABA agonist GABA, Creatinine, Nipecotic acid |
[79] | ||
|
Muscarinic ACH antagonist Pilocarpine nitrate and Aminobenztropine |
[79] | ||
| Cognition | 2 minute memory (Drosophila “Immediate recall memory”) |
mGluR antagonist rescue Lithium, MPEP, MPPG, MTPG. LY341495 |
[19] |
| 60 minute memory (Drosophila “short-term memory”) |
mGluR antagonist Lithium, MPEP, MPPG, MTPG, LY341495 |
[19] | |
| One day memory (Drosophila “long-term memory”-protein synthesis-dependent) |
mGluR antagonist MPEP |
[43] | |
| Age-dependent learning decline |
mGluR antagonist Lithium, MPEP,MPPG, MTPG, LY341495 |
[19] |
Social Defects in the Fragile X model
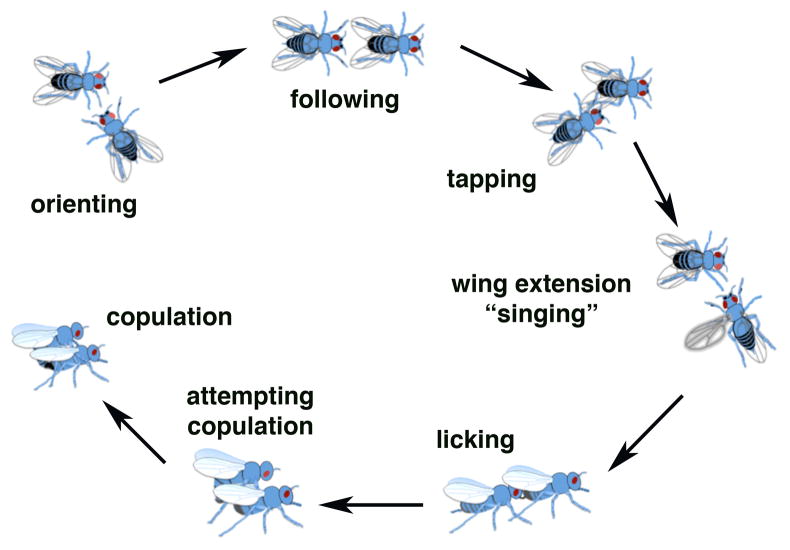
The Drosophila male is born with an innate ability to perform a stereotypic courtship ritual to entice a receptive female to mate. In courtship, male flies perform a characteristic sequence of behaviors (Figure 1) [28–30]. These behaviors are repeated with some variation until successful copulation occurs. This social behavior can be quantified by measuring the percentage of time a male engages in courtship activity during a 10-minute test interval. This percentage is referred to as the courtship index (CI) and gives a measure of overall courtship activity. The quality of courtship behavior can be measured by determining the percentage of time spent performing each of the steps in the courtship ritual (Figure 1).
Figure 1. Drosophila courtship behavior.
Adult males will perform an innate stereotypic behavior to entice receptive females to mate. This courtship ritual involves seven basic steps that are generally performed in the order presented in the figure. The courtship starts by the male orienting toward the female and following her. The male then taps the female to pick up pheromonal cues and then initiates “singing” by extending and vibrating a wing. The male then licks the female abdomen and if the female displays receptive behavior he will attempt and if successful initiate copulation.
The courtship index of naïve dfmr1 mutant males paired with virgin females, was significantly reduced, compared to naive control males (Figure 2) [15]. More specifically, fragile X flies failed to sustain courtship, resulting in a lower percent of fragile X flies that progressed to later steps of courtship (genital licking and copulation). These results suggested a social deficit in the Drosophila model for fragile X [15].
Figure 2. Naïve courtship and memory phenotypes displayed by the Drosophila fragile X model.
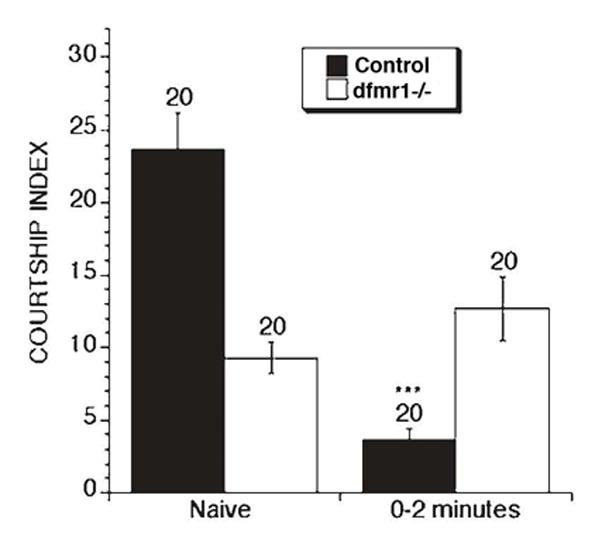
Measuring the total amount of time a male courts in a 10 min test interval can quantitate the level of naïve courtship activity of a particular strain of fly. The total courtship time is divided by 10 mins to derive a courtship index (C.I.). Dfmr1 mutant males (white bar) display reduced naïve courtship relative to controls (black bar). This deficit in courtship activity is not due to sensory or locomotor defects [15]. Learning can be tested in the courtship paradigm by placing a male in a courtship chamber with an unreceptive female. A normal male will learn not to court the female within a one-hour training session. Dfmr1 mutants display a normal learning profile with respect to controls (not shown, see [17]). Once trained, males remember the negative experience of the training and fail to court even receptive females for up to three hours after training. The right two bars in this figure show immediate recall memory that is tested within 2 mins of training, by placing a freshly trained male with a receptive female. Control males displays significant reduction in courtship (indicated by asterisks, p<0.001), whereas no difference is observed between the level of naïve courtship and the level of courtship at 2 mins post-training displayed by the dfmr1 mutants.
Another behavior that can be monitored in Drosophila, is grooming behavior. This behavior is repetitive in nature, but brief; normally lasting for a few seconds. In contrast to wild type flies, dfmr1 mutant Drosophila engage in excessively long time periods of grooming behavior, suggesting that these males are more prone to repetitive behaviors, as is the case in autistic patients [31].
Learning and memory in Drosophila
Several learning and memory paradigms have been developed in Drosophila. The two most popular are a classical avoidance conditioning paradigm (an associative memory paradigm also known as the odor-shock paradigm) and a conditioned courtship paradigm (an associative memory paradigm also referred to as the courtship conditioning paradigm).
In the odor-shock paradigm, Drosophila memory is measured by the rate at which flies learn to distinguish between an odor associated with an adverse event (electric shock to the foot [32–38]), and one associated with a neutral event (no shock). After a single training session, 0–2 minutes after training is referred to as immediate or immediate-recall memory and is measured behaviorally by the percentage of flies that move to the chamber lacking a shock. This form of memory had been previously referred to as learning, with the terminology changing in the mids 1990s, although to this day is still sometimes referred to as learning. Short-term memory is measured at 60 minutes after training and medium term memory 2–7 hours after training. There are two components of long-term memory, anesthesia resistant memory (ARM) and long-term memory (LTM). ARM lasts for up to 48 hours after training and is not dependent on de novo protein synthesis and is typically tested 1 day after massed training. LTM is de novo protein synthesis dependent, can last at least 8 days but is typically tested 1–4 days after spaced training (for review see Skoulakis EM, Grammenoudi S., 2006). Memory is tested after the delay interval by giving the flies a choice between the two odors, in a T-maze. The flies that have learned to pair the correct odor with the shock, choose the part of the T-maze with the other odor.
In the conditioned courtship paradigm, a male fly is paired for an hour with a previously-mated female (unreceptive female), and tested to see if the male remembers the female cues and suppresses his courtship behavior when subsequently paired with a virgin (receptive) female [28–30, 38–42]. This memory requires the male to pair complex female avoidance behaviors with associated sensory signals [43–49]. In this assay, learning-during-training (LDT) can be measured by comparing the CI during the first ten minutes, with the CI in the last ten-minutes of the pairing. This is sometimes simply referred to as learning, but is more related to working memory since it happens while the environmental stimulus is still present. Generally a 40% reduction or more in courtship activity is observed during the learning-during-training assay.
Memory is assessed in the next step of the paradigm. Males with typical memory will exhibit depressed levels of courtship behavior for 2–3 hours after the learning experience/training [40]. This is evaluated by comparing the behavior of the trained versus an untrained male, when each is paired with a receptive (virgin) female. A trained male with normal memory should show relatively depressed levels of courtship. A modified version of the conditioned courtship paradigm can be utilized to establish and measure long-term memory lasting out to 9 days after training [50].
dfmr1 mutants had learning and memory deficits by both the odor-shock and conditioned courtship memory paradigms, and in a fashion consistent with the cognitive deficits of patients with Fragile X syndrome. Specifically, dfmr1 mutants had impairments in learning (immediate memory) with less choosing the shock-free chamber compared to control flies, and forgot what they learned within one day [39]. In the courtship-based assay dfmr1 mutants showed both immediate-recall memory and short-term memory deficits. First, learning-during-training was initially normal at 5 days of age, but no longer detectable at 20 days of age, perhaps due to cognitive decline with age in the fragile X model [51]. Second, although learning-during-training was normal at 5 days of age, dfmr1 mutants had immediate-recall memory deficits at 0–2 minutes after training (Figure 2), short-term memory deficits at 60 minutes after training, and long-term memory deficits after one day of training. In Drosophila, each of these time points corresponds to a different, genetically separable, form of memory (immediate-recall, short-term memory, and long-term memory, respectively) [17, 52]. Finally, in addition to learning-during-training decaying with age, repetitive-type behaviors increased with age in dfmr1 mutants: The percentage of time dfmr1 mutants groomed themselves is elevated over controls, and increases with age [31].
Neuroanatomical defects in the Fragile X model
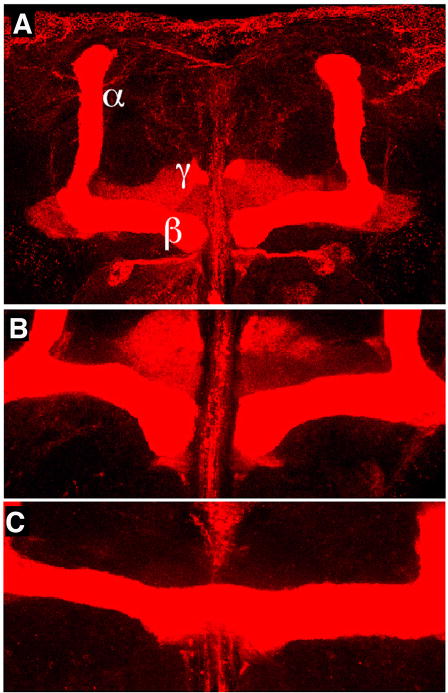
Despite significant symptoms, the brains of patients with Fragile X look quite healthy; however closer examination shows some localized size variation, in addition to reliable differences at the level of neurons [53]. The overall brain size and structure of the dfmr1 mutant brain also appears normal, however more detailed analysis has identified consistent defects in select sets of neurons in the central and peripheral nervous systems. For example, examination of the neuromuscular junction in dfmr1 mutant larvae, reveals an over elaboration of “bou-tons”; the sites of synapse formation [13] (Table I). Additional gross neuranatomical defects are also observed in the fruitfly: in dfmr1 mutants the mushroom bodies required for short- and long-term memory formation exhibit neuron based structures indicative of inappropriate midline crossing of neurons, compared to control flies [17, 54, 55] (Figure 3).
Figure 3. Mushroom body phenotype of dfmr1 mutants.
A) Whole mount immunostaining of a Drosophila brain with anti-fasicillin II reveals the mushroom body (MB) of the fly brain, which contains three bilaterally symmetric lobes, α, β, and γ. The MB is the major learning and memory center of the fly brain and is thought to be analogous to the vertebrate hippocampus. B) An image of the β–lobes of a control MB shows that the β–lobes grow toward, but do not cross the mid-line of the brain. C) An image of the β–lobes of a dfmr1 mutant MB shows a severe cross-over phenotype that is observed in some of the mutant brains.
Drug treatments in the Drosophila Fragile X model
The Drosophila reproductive cycle is 10–14 days long; which makes orally-delivered drug testing quick and simple. We have successfully used our Drosophila model for Fragile X to identify drug candidates for the treatment of this disorder. These drugs are currently in different phases of clinical trials.
The appropriate balance of mGluR signaling pathways relative to GABA signaling pathways is required for maximum learning and memory in Drosophila, and in other mammals [17, 51] [56–65]. Our earlier studies indicated that a shift in this balance in favor of mGluR activity was causing the learning and memory defect in our model for Fragile X (see [17] for details). Furthermore results from cells from human patients indicated impaired cAMP signaling [57, 61] and mouse studies indicated that there was enhanced mGluR signaling in the hippocampus of the FXS model brain[66], motivating us to test the effect of decreasing mGluR signaling via pharmacological treatment[17].
The mammalian genomes contain eight different metabotropic glutamate receptors (mGluRs), which are subdivided into three groups (I, II and III), based on downstream signaling events. In contrast, the Drosophila genome contains a single mGluR called DmGluRA, which in neurons is connected to the Drosophila homologues of mammalian Group I and Group II mGluR receptor signaling pathways [17, 67–69]. We added several mGluR antagonists, and lithium, (which acts downstream of mGluR, but in the same pathways) to the fly food to reduce mGluR signaling and increase cAMP signaling [17]. The drug was added to the food at different time periods, including during the larval growth period (development), adulthood, or during both time periods. Flies were tested in adulthood for fragile X-related symptoms. Interestingly all drug treatments, but not vehicle containing food, rescued the naïve courtship (social interaction) phenotype, immediate-recall and short-term memory (Figure 4). In contrast, drug needed to be added during development to rescue the mushroom body phenotype of neurons [17]. These results demonstrated that inhibiting the mGluR pathway rescued relevant FXS phenotypes.
Figure 4. Rescue of naïve courtship and memory by treatment with the mGluR antagonist MPEP.
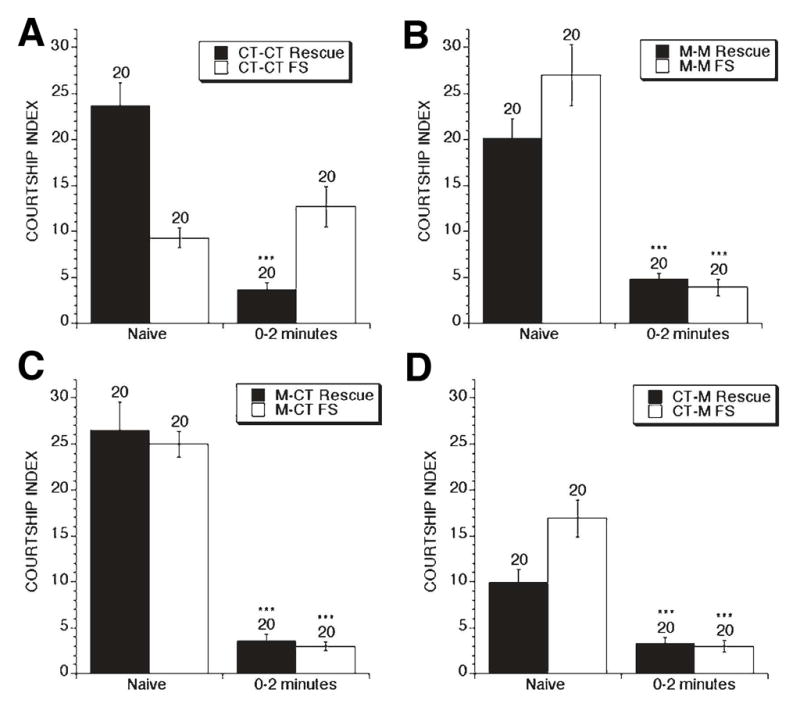
Control (Rescue) and dfmr1 mutants (FS) were: A) raised in control food during development and adults (CT-CT); B) raised in food containing the mGluR antagonist MPEP (drug name) during development and as adults before testing (M-M); C) raised in MPEP containing food during development and then put on control food as adults before testing (M-CT); D) raised on control food during development and then placed on MPEP containing food during adulthood before testing. A) Treatment with only control food reveals the same dfmr1 phenotypes displayed in Figure 2, i.e. reduced naïve courtship and no detectable immediate recall memory. A–C) Treatment of the dfmr1 mutants with MPEP containing food during development and adulthood, development alone or adulthood alone, leads to significant rescue of the naïve courtship and memory deficits, indicated with asterisks, p<0.001.
These studies were the first to indicate that drug treatment after the bulk of brain development could rescue a developmental brain disorder. These studies demonstrated that social and cognitive impairments were not set in stone by immutable developmental circuitry, but that adulthood signaling was important in social behavior and memory and that modulating adulthood signaling could ameliorate social impairments. [70–76] Interestingly the administration of the specific group II antagonist LY341495 or lithium in full adult mice at eight weeks of age has recently been shown to reverse phenotypes in adult FXS mice [77, 78] as has treatment with the group I mGluR antagonist CTEP started soon after weening but before adulthood [78].
In another study, a relatively high-throughput screen has also been employed to identify drugs that rescue relevant Drosophila FXS mutant phenotypes. By taking advantage of the observation that elevated levels of glutamate in fly food is toxic to dfmr1 mutants, Chang et al., 2008, performed a drug screen to identify compounds that rescued the lethality of dfmr1 mutants. They screened the Spectrum collection of 2,000 FDA approved drugs collection and identified a few that not only rescued the lethality but also rescued the naïve courtship and mushroom body cross-over defects. Three of the identified compounds have the commonality in that they act to promote γ-aminobutyric acid (GABA) receptor activity [79]. Interestingly the identification of these compounds matches findings that there are deficiencies of GABA(A) receptor signaling in the Drosophila and mouse fragile X models [80].
Another compound that has efficacy in the fly fragile X model is the drug minocycline. This derivative of tetracycline was tested in the fly model as it was shown to rescue defects in the neuronal morphology displayed by the mouse FXS model [25]. In the fly study the effects of minocycline treatment were examined in three different classes of neurons: motor neurons, circadian neurons, and mushroom body neurons. In all three neurons the synaptic connectivity defects were rescued by minocycline treatment and the results were validated by genetic manipulations [24, 81]. Interestingly in the mouse, minocycline treatment has been shown to inhibit MMP9 activity, which is also inhibited by cAMP signaling [82, 83]. The overall data from both the fly and mouse models indicate that minocycline treatment should be examined as an approach to treat FXS symptoms.
Pharmacological treatments for the excessive grooming phenotype of the dfmr1 mutants have also been identified. Unlike other phenotypes, such as naïve courtship, memory and the mushroom body defects, this phenotype was exacerbated by treatment with mGluR antagonists, but interestingly was rescued by treatment with reserpine. Basic research into the grooming behavior of Drosophila has demonstrated that the addition of monoamines dopamine, octopamine and serotonin to decapitated flies increases grooming behavior. Interestingly monoamine synthesis has been found to be elevated in dfmr1 mutants [84] and over-expression of Drosophila vesicular monoamine transporter (VMAT) transporter that loads monoamines into synaptic vesicles also increases grooming behavior. Examination of dfmr1 mutants revealed elevated levels of VMAT mRNA and protein [31]. Reserpine is a known antagonist of VMAT and treatment with this drug was found to suppress the excessive grooming behavior [31]. As excessive grooming is a phenotype displayed by the mouse FXS model, it clearly important to explore this therapeutic route in the mouse model. Also as reserpine has broad effects on monoamine transport more selective inhibitors of the specific monoamines should be explored in the fly and mouse models as a route to suppress excessive grooming behavior which might relate to the repetitive behaviors displayed by fragile X patients.
As discussed in this review, the development of a Drosophila fragile X model and its initial characterization has led to the realization that it displays several seemingly relevant phenotypes. The relevance of these phenotypes is highlighted by the fact that they have been useful in combination with basic research and with findings from studies using the mouse fragile X model to suggest routes to pursue for pharmacological testing in Fragile X patients. However the final validation from any model comes from the ability of the model to guide the identification of treatments that have efficacy in human patients. The Drosophila model has reached this benchmark. Clinical trials, utilizing lithium, mGluR antagonists and minocycline have all indicated promising results, suggesting further focus on such compounds in full placebo controlled trials is warranted [20, 21, 85](Table I). The Drosophila model has provided initial data in the cases of lithium and mGluR antagonists and GABA agonists to pursue. It has also demonstrated great utility in how it can be used from hypothesis testing based on basic research findings to unbiased drug screening. Thus its utilization in the study of other human diseases affecting cognition and behavior should be considered.
Footnotes
Conflict of interest statement: The authors declare that they do not have a conflict of interest regarding the content of this review.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jacquemont S, et al. Fragile-X syndrome and fragile X-associated tremor/ataxia syndrome: two faces of FMR1. Lancet Neurol. 2007;6(1):45–55. doi: 10.1016/S1474-4422(06)70676-7. [DOI] [PubMed] [Google Scholar]
- 2.Hagerman R, et al. Fragile X syndrome and targeted treatment trials. Results Probl Cell Differ. 2012;54:297–335. doi: 10.1007/978-3-642-21649-7_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McBride SM, Bell AJ, Jongens TA. Behavior in a Drosophila model of fragile X. Results Probl Cell Differ. 2012;54:83–117. doi: 10.1007/978-3-642-21649-7_6. [DOI] [PubMed] [Google Scholar]
- 4.Tessier CR, Broadie K. Molecular and genetic analysis of the Drosophila model of fragile X syndrome. Results Probl Cell Differ. 2012;54:119–56. doi: 10.1007/978-3-642-21649-7_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Donnell WT, Warren ST. A decade of molecular studies of fragile X syndrome. Annu Rev Neurosci. 2002;25:315–38. doi: 10.1146/annurev.neuro.25.112701.142909. [DOI] [PubMed] [Google Scholar]
- 6.Hatton DD, et al. Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. Am J Med Genet A. 2006;140A(17):1804–13. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- 7.Zafeiriou DI, Ververi A, Vargiami E. Childhood autism and associated comorbidities. Brain & development. 2007;29(5):257–72. doi: 10.1016/j.braindev.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Sutcliffe JS, et al. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum Mol Genet. 1992;1(6):397–400. doi: 10.1093/hmg/1.6.397. [DOI] [PubMed] [Google Scholar]
- 9.Verkerk AJ, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65(5):905–14. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 10.Ashley CT, Jr, et al. FMR1 protein: conserved RNP family domains and selective RNA binding. Science. 1993;262(5133):563–6. doi: 10.1126/science.7692601. [DOI] [PubMed] [Google Scholar]
- 11.Wan L, et al. Characterization of dFMR1, a Drosophila melanogaster homolog of the fragile X mental retardation protein. Mol Cell Biol. 2000;20(22):8536–47. doi: 10.1128/mcb.20.22.8536-8547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darnell JC, et al. Discrimination of common and unique RNA-binding activities among Fragile X mental retardation protein paralogs. Hum Mol Genet. 2009;18(17):3164–77. doi: 10.1093/hmg/ddp255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang YQ, et al. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell. 2001;107(5):591–603. doi: 10.1016/s0092-8674(01)00589-x. [DOI] [PubMed] [Google Scholar]
- 14.Morales J, et al. Drosophila fragile X protein, DFXR, regulates neuronal morphology and function in the brain. Neuron. 2002;34(6):961–72. doi: 10.1016/s0896-6273(02)00731-6. [DOI] [PubMed] [Google Scholar]
- 15.Dockendorff TC, et al. Drosophila lacking dfmr1 activity show defects in circadian output and fail to maintain courtship interest. Neuron. 2002;34(6):973–84. doi: 10.1016/s0896-6273(02)00724-9. [DOI] [PubMed] [Google Scholar]
- 16.Inoue S, et al. A role for the Drosophila fragile X-related gene in circadian output. Current biology : CB. 2002;12(15):1331–5. doi: 10.1016/s0960-9822(02)01036-9. [DOI] [PubMed] [Google Scholar]
- 17.McBride SM, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron. 2005;45(5):753–64. doi: 10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 18.Dolen G, Bear MF. Courting a cure for fragile X. Neuron. 2005;45(5):642–4. doi: 10.1016/j.neuron.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Bhogal B, Jongens TA. Fragile X syndrome and model organisms: identifying potential routes of therapeutic intervention. Dis Model Mech. 2010;3(11–12):693–700. doi: 10.1242/dmm.002006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berry-Kravis E, et al. Open-label treatment trial of lithium to target the underlying defect in fragile X syndrome. J Dev Behav Pediatr. 2008;29(4):293–302. doi: 10.1097/DBP.0b013e31817dc447. [DOI] [PubMed] [Google Scholar]
- 21.Jacquemont S, et al. Epigenetic modification of the FMR1 gene in fragile X syndrome is associated with differential response to the mGluR5 antagonist AFQ056. Sci Transl Med. 2011;3(64):64ra1. doi: 10.1126/scitranslmed.3001708. [DOI] [PubMed] [Google Scholar]
- 22.Yan QJ, et al. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49(7):1053–66. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Dolen G, et al. Correction of fragile X syndrome in mice. Neuron. 2007;56(6):955–62. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siller SS, Broadie K. Neural circuit architecture defects in a Drosophila model of Fragile X syndrome are alleviated by minocycline treatment and genetic removal of matrix metalloproteinase. Dis Model Mech. 2011;4(5):673–85. doi: 10.1242/dmm.008045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bilousova TV, et al. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J Med Genet. 2009;46(2):94–102. doi: 10.1136/jmg.2008.061796. [DOI] [PubMed] [Google Scholar]
- 26.Yuskaitis CJ, et al. Lithium ameliorates altered glycogen synthase kinase-3 and behavior in a mouse model of fragile X syndrome. Biochem Pharmacol. 2010;79(4):632–46. doi: 10.1016/j.bcp.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Min WW, et al. Elevated glycogen synthase kinase-3 activity in Fragile X mice: key metabolic regulator with evidence for treatment potential. Neuropharmacology. 2009;56(2):463–72. doi: 10.1016/j.neuropharm.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dickson BJ. Wired for sex: the neurobiology of Drosophila mating decisions. Science. 2008;322(5903):904–9. doi: 10.1126/science.1159276. [DOI] [PubMed] [Google Scholar]
- 29.Siwicki KK, Ladewski L. Associative learning and memory in Drosophila: beyond olfactory conditioning. Behav Processes. 2003;64(2):225–238. doi: 10.1016/s0376-6357(03)00137-2. [DOI] [PubMed] [Google Scholar]
- 30.Griffith LC, Ejima A. Courtship learning in Drosophila melanogaster: diverse plasticity of a reproductive behavior. Learn Mem. 2009;16(12):743–50. doi: 10.1101/lm.956309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tauber JM, Vanlandingham PA, Zhang B. Elevated levels of the vesicular monoamine transporter and a novel repetitive behavior in the Drosophila model of fragile X syndrome. PLoS One. 2011;6(11):e27100. doi: 10.1371/journal.pone.0027100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quinn WG, Harris WA, Benzer S. Conditioned behavior in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 1974;71(3):708–12. doi: 10.1073/pnas.71.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dudai Y, et al. dunce, a mutant of Drosophila deficient in learning. Proceedings of the National Academy of Sciences of the United States of America. 1976;73(5):1684–8. doi: 10.1073/pnas.73.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jellies JA. Associative olfactory conditioning in Drosophila melanogaster and memory retention through metamorphosis. Illinois State University; Normal, IL: 1981. p. 83. [Google Scholar]
- 35.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. Journal of comparative physiology A, Sensory, neural, and behavioral physiology. 1985;157(2):263–77. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 36.Davis RL. Mushroom bodies and Drosophila learning. Neuron. 1993;11(1):1–14. doi: 10.1016/0896-6273(93)90266-t. [DOI] [PubMed] [Google Scholar]
- 37.Tully T, et al. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79(1):35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 38.Skoulakis EM, Grammenoudi S. Dunces and da Vincis: the genetics of learning and memory in Drosophila. Cell Mol Life Sci. 2006;63(9):975–88. doi: 10.1007/s00018-006-6023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolduc FV, et al. Excess protein synthesis in Drosophila fragile X mutants impairs long-term memory. Nat Neurosci. 2008;11(10):1143–5. doi: 10.1038/nn.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siegel RW, Hall JC. Conditioned responses in courtship behavior of normal and mutant Drosophila. Proc Natl Acad Sci U S A. 1979;76(7):3430–3434. doi: 10.1073/pnas.76.7.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hall JC. The mating of a fly. Science. 1994;264(5166):1702–14. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- 42.Hall JC. Control of male reproductive behavior by the central nervous system of Drosophila: dissection of a courtship pathway by genetic mosaics. Genetics. 1979;92(2):437–57. doi: 10.1093/genetics/92.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tompkins L, et al. Conditioned courtship in Drosophila and its mediation by association of chemical cues. Behav Genet. 1983;13(6):565–78. doi: 10.1007/BF01076402. [DOI] [PubMed] [Google Scholar]
- 44.Tompkins L, et al. The role of female movement in the sexual behavior of Drosophila melanogaster. Behavior genetics. 1982;12(3):295–307. doi: 10.1007/BF01067849. [DOI] [PubMed] [Google Scholar]
- 45.Tompkins L. Genetic analysis of sex appeal in Drosophila. Behavior genetics. 1984;14(5):411–40. doi: 10.1007/BF01065443. [DOI] [PubMed] [Google Scholar]
- 46.Ackerman SL, Siegel RW. Chemically reinforced conditioned courtship in Drosophila: responses of wild-type and the dunce, amnesiac and don giovanni mutants. J Neurogenet. 1986;3(2):111–23. doi: 10.3109/01677068609106898. [DOI] [PubMed] [Google Scholar]
- 47.Siwicki KK, et al. The role of cuticular pheromones in courtship conditioning of Drosophila males. Learn Mem. 2005;12(6):636–45. doi: 10.1101/lm.85605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ejima A, et al. Sequential learning of pheromonal cues modulates memory consolidation in trainer-specific associative courtship conditioning. Curr Biol. 2005;15(3):194–206. doi: 10.1016/j.cub.2005.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ejima A, et al. Generalization of courtship learning in Drosophila is mediated by cis-vaccenyl acetate. Curr Biol. 2007;17(7):599–605. doi: 10.1016/j.cub.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McBride SM, et al. Mushroom body ablation impairs short-term memory and long-term memory of courtship conditioning in Drosophila melanogaster. Neuron. 1999;24(4):967–77. doi: 10.1016/s0896-6273(00)81043-0. [DOI] [PubMed] [Google Scholar]
- 51.Choi CH, et al. Age-dependent cognitive impairment in a Drosophila fragile X model and its pharmacological rescue. Biogerontology. 2010;11(3):347–62. doi: 10.1007/s10522-009-9259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banerjee P, et al. Short- and long-term memory are modulated by multiple isoforms of the fragile X mental retardation protein. J Neurosci. 2010;30(19):6782–92. doi: 10.1523/JNEUROSCI.6369-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He CX, Portera-Cailliau C. The trouble with spines in fragile X syndrome: density, maturity and plasticity. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Restifo LL. Mental retardation genes in drosophila: New approaches to understanding and treating developmental brain disorders. Ment Retard Dev Disabil Res Rev. 2005;11(4):286–94. doi: 10.1002/mrdd.20083. [DOI] [PubMed] [Google Scholar]
- 55.Pan L, et al. The Drosophila fragile X gene negatively regulates neuronal elaboration and synaptic differentiation. Curr Biol. 2004;14(20):1863–70. doi: 10.1016/j.cub.2004.09.085. [DOI] [PubMed] [Google Scholar]
- 56.Yin JC, et al. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79(1):49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- 57.Berry-Kravis E, Huttenlocher PR. Cyclic AMP metabolism in fragile X syndrome. Ann Neurol. 1992;31(1):22–6. doi: 10.1002/ana.410310105. [DOI] [PubMed] [Google Scholar]
- 58.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361(6410):315–25. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 59.Lakin-Thomas PL. Effects of inositol starvation on the levels of inositol phosphates and inositol lipids in Neurospora crassa. Biochem J. 1993;292(Pt 3):805–11. doi: 10.1042/bj2920805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berry-Kravis E, Hicar M, Ciurlionis R. Reduced cyclic AMP production in fragile X syndrome: cytogenetic and molecular correlations. Pediatr Res. 1995;38(5):638–43. doi: 10.1203/00006450-199511000-00002. [DOI] [PubMed] [Google Scholar]
- 61.Berry-Kravis E, Ciurlionis R. Overexpression of fragile X gene (FMR-1) transcripts increases cAMP production in neural cells. J Neurosci Res. 1998;51(1):41–8. doi: 10.1002/(SICI)1097-4547(19980101)51:1<41::AID-JNR4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 62.Bullock BP, Habener JF. Phosphorylation of the cAMP response element binding protein CREB by cAMP-dependent protein kinase A and glycogen synthase kinase-3 alters DNA-binding affinity, conformation, and increases net charge. Biochemistry. 1998;37(11):3795–809. doi: 10.1021/bi970982t. [DOI] [PubMed] [Google Scholar]
- 63.Inoue T, et al. Type 1 inositol 1,4,5-trisphosphate receptor is required for induction of long-term depression in cerebellar Purkinje neurons. J Neurosci. 1998;18(14):5366–73. doi: 10.1523/JNEUROSCI.18-14-05366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takei K, et al. Regulation of nerve growth mediated by inositol 1,4,5-trisphosphate receptors in growth cones. Science. 1998;282(5394):1705–8. doi: 10.1126/science.282.5394.1705. [DOI] [PubMed] [Google Scholar]
- 65.Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27(7):370–7. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 66.Huber KM, et al. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99(11):7746–50. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choi CH, et al. Pharmacological reversal of synaptic plasticity deficits in the mouse model of Fragile X syndrome by group II mGluR antagonist or lithium treatment. Brain research. 2011;1380:106–19. doi: 10.1016/j.brainres.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pan L, Broadie KS. Drosophila fragile X mental retardation protein and metabotropic glutamate receptor A convergently regulate the synaptic ratio of ionotropic glutamate receptor subclasses. J Neurosci. 2007;27(45):12378–89. doi: 10.1523/JNEUROSCI.2970-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pan L, et al. Mechanistic relationships between Drosophila fragile X mental retardation protein and metabotropic glutamate receptor A signaling. Mol Cell Neurosci. 2008;37(4):747–60. doi: 10.1016/j.mcn.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moldin SO. Understanding Fragile X syndrome: molecular, cellular and genomic neuroscience at the crossroads. Genes Brain Behav. 2005;4(6):337–40. doi: 10.1111/j.1601-183X.2005.00150.x. [DOI] [PubMed] [Google Scholar]
- 71.Moldin SO. Understanding Fragile X syndrome: molecular, cellular and genomic neuroscience at the crossroads. Genes, brain, and behavior. 2005;4(6):337–40. doi: 10.1111/j.1601-183X.2005.00150.x. [DOI] [PubMed] [Google Scholar]
- 72.Moldin SO, Rubenstein JL, Hyman SE. Can autism speak to neuroscience? The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(26):6893–6. doi: 10.1523/JNEUROSCI.1944-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walsh CA, Morrow EM, Rubenstein JL. Autism and brain development. Cell. 2008;135(3):396–400. doi: 10.1016/j.cell.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Volkmar FR, State M, Klin A. Autism and autism spectrum disorders: diagnostic issues for the coming decade. Journal of child psychology and psychiatry, and allied disciplines. 2009;50(1–2):108–15. doi: 10.1111/j.1469-7610.2008.02010.x. [DOI] [PubMed] [Google Scholar]
- 75.Raymond FL, Tarpey P. The genetics of mental retardation. Hum Mol Genet. 2006;15(Spec2):R110–6. doi: 10.1093/hmg/ddl189. [DOI] [PubMed] [Google Scholar]
- 76.State MW. The genetics of child psychiatric disorders: focus on autism and Tourette syndrome. Neuron. 2010;68(2):254–69. doi: 10.1016/j.neuron.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi CH, et al. Pharmacological reversal of synaptic plasticity deficits in the mouse model of fragile X syndrome by group II mGluR antagonist or lithium treatment. Brain Res. 2011;1380:106–19. doi: 10.1016/j.brainres.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Michalon A, et al. Chronic Pharmacological mGlu5 Inhibition Corrects Fragile X in Adult Mice. Neuron. 2012;74(1):49–56. doi: 10.1016/j.neuron.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang S, et al. Identification of small molecules rescuing fragile X syndrome phenotypes in Drosophila. Nat Chem Biol. 2008;4(4):256–63. doi: 10.1038/nchembio.78. [DOI] [PubMed] [Google Scholar]
- 80.Gantois I, et al. Expression profiling suggests underexpression of the GABA(A) receptor subunit delta in the fragile X knockout mouse model. Neurobiol Dis. 2006;21(2):346–57. doi: 10.1016/j.nbd.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 81.Sanchez AJ, et al. Rolipram impairs NF-kappaB activity and MMP-9 expression in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;168(1–2):13–20. doi: 10.1016/j.jneuroim.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 82.Martin-Chouly CA, et al. Modulation of matrix metalloproteinase production from human lung fibroblasts by type 4 phosphodiesterase inhibitors. Life Sci. 2004;75(7):823–40. doi: 10.1016/j.lfs.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 83.Oger S, et al. Evidence for a role of phosphodiesterase 4 in lipopolysaccharide-stimulated prostaglandin E2 production and matrix metalloproteinase-9 activity in human amniochorionic membranes. J Immunol. 2005;174(12):8082–9. doi: 10.4049/jimmunol.174.12.8082. [DOI] [PubMed] [Google Scholar]
- 84.Liu W, et al. Effect of glutamine on the expression of grp75 in PC12 cells. Shi Yan Sheng Wu Xue Bao. 2005;38(5):423–31. [PubMed] [Google Scholar]
- 85.Paribello C, et al. Open-label add-on treatment trial of minocycline in fragile X syndrome. BMC Neurol. 2010;10:91. doi: 10.1186/1471-2377-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]