Abstract
In a successful pregnancy, the semiallogeneic fetus is not rejected by the maternal immune system, which implies tolerance mechanisms protecting fetal tissues from maternal immune attack. Here we report that the ICOS-B7h costimulatory pathway plays a critical role in maintaining the equilibrium at the fetomaternal interface. Blockade of this pathway increased fetal resorption and decreased fetal survival in an allogeneic pregnancy model (CBA female × B6 male). Locally in the placenta, levels of regulatory markers such as IDO and TGF-β1 were reduced after anti-B7h monoclonal antibody treatment, whereas levels of effector cytokines (eg, IFN-γ) were significantly increased. In secondary lymphoid organs, enhanced IFN-γ and granzyme B production (predominantly by CD8+ T cells) was observed in the anti-B7h–treated group. The deleterious effect of B7h blockade in pregnancy was maintained only in CD4 knockout mice, not in CD8 knockout mice, which suggests a role for CD8+ T cells in immune regulation by the ICOS-B7h pathway. In accord, regulatory CD8+ T cells (in particular, CD8+CD103+ cells) were significantly decreased after anti-B7h monoclonal antibody treatment, and adoptive transfer of this subset abrogated the deleterious effect of B7h blockade in fetomaternal tolerance. Taken together, these data support the hypothesis that B7h blockade abrogates tolerance at the fetomaternal interface by enhancing CD8+ effector response and reducing local immunomodulation mediated by CD8+ regulatory T cells.
More than 50 years ago, Medawar and colleagues1 proposed that immunological tolerance should be present during pregnancy, to protect the fetus against an aggressive maternal alloimmune response directed at the paternal antigens expressed by the fetus. Since that initial hypothesis, several mechanisms have been proposed to function actively in the protection of the fetus from the maternal immune system.2 Among them, the presence of regulatory T cells3–7 and the expression of immune regulatory molecules in the fetal–maternal interface have been identified as crucial factors for fetomaternal tolerance.8–12
T cells play a major role in coordinating immune response. Although T-cell activation depends on the initial antigen-specific signal provided to T-cell receptors via the antigen-loaded major histocompatibility complex, additional signals provided by costimulatory molecules fine-tune this response, determining its strength, nature, and duration. Some costimulatory pathways activate effector T cells, but others inhibit T-cell activation and/or promote regulatory T cells. One of these inhibitory molecules is PD-L1, the expression of which is prevalent at the uteroplacental interface.11 Moreover, placental PD-L1 has been shown to protect murine allogeneic conceptus from maternal T-cell–mediated attack.11,13 Treatment of pregnant CBA mice with a blocking anti–PD-L1 monoclonal antibody (mAb) resulted in loss of allogeneic but not syngeneic conceptus. Similarly, PD-L1–deficient mice had a substantial increase in the rate of spontaneous fetal resorption and a decrease in fetal survival.11 PD-L1 protective effect on fetomaternal tolerance was dependent on CD4+ regulatory T cells.14
Inducible costimulatory molecule (ICOS) and its ligand (B7h; alias B7-H2, ICOS-L) are also considered important costimulatory molecules that influence T-cell activation and differentiation.15 In particular, ICOS–B7h interactions have been shown to promote regulation in a diabetes autoimmune model and to protect against atherosclerosis in a LDL receptor-deficient mice.16,17 Whether ICOS-B7h is important for fetomaternal tolerance remains to be determined. In the present study, we investigated the role of the ICOS-B7h pathway in modulating the effector/regulatory balance at the fetomaternal interface and its role in preventing immune attack to the fetus.
Materials and Methods
Mice
CBA/CaJ (CBA) and C57BL/6 (B6) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice deficient in the tryptophan-depleting enzyme Indoleamine 2,3-dioxygenase 1 (IDO-1; here, just IDO) have been described previously [Dr. Andrew Mellor (Medical College of Georgia, Augusta, GA)].18 Foxp3–green fluorescent protein (GFP) reporter mice [a kind gift from Dr. Yurij Rudensky (University of Washington)] have been described previously.19 Animal care and experimental procedures followed institutional guidelines.
Timed Matings and Resorption Rates
Virgin CBA/CaJ females (8 to 10 weeks of age) were mated with C57BL/6 (allogeneic) or CBA/CaJ (syngeneic) males (6 to 12 weeks of age). Females were inspected daily for vaginal plugs and the day a plug was observed was designated as day 0.5 of pregnancy. Plugged females were either monitored until parturition, when the number of pups born was recorded, or they were sacrificed at 10.5, 13.5, and 16.5 days after copulation (days post coitum, dpc) for examination of the number of implanting embryos and resorbing sites. The rate of resorption was calculated by counting the number of resorbing versus healthy embryos at 13.5 dpc.
Treatment Protocol
Pregnant females were injected intraperitoneally at 6.5, 8.5, 10.5, and 12.5 dpc with anti-mouse B7h mAb (clone HK5.3) or polyclonal rat IgG (control) at a dosage of 500 μg (at 6.5 and 8.5 dpc) and 250 μg (at 10.5 and 12.5 dpc). For certain experiments, anti–CTLA-4 mAb (AC10-4F10) was used with a similar dosing protocol.
Histology
At predetermined intervals, spleen and placentae were removed for histological analysis and immunostaining. Placentae were embedded in Tissue-Tek OCT optimal cutting temperature compound (Sakura Finetek, Torrance, CA) and were snap-frozen in liquid nitrogen. Immunohistochemistry was performed on frozen tissue sections (5 mm thick) with antibodies to B7h (clone HK5.3, rat IgG2a). Sections were fixed in cold acetone, washed in PBS, quenched in 0.3% hydrogen peroxide in PBS, washed again in PBS, blocked with normal serum, and incubated with primary antibody overnight at 4°C. After another PBS wash, slides were incubated with biotinylated antibody for 40 minutes at room temperature, followed by another incubation with avidin-conjugated peroxidase (Vectastain ABC vector Elite kit; Vector Laboratories, Burlingame, CA) before being developed with 3,3′-diaminobenzidine. The tissue sections were finally counterstained with hematoxylin; positive staining was indicated by a red-brown coloration, as described previously.11 Isotype-matched antibodies (rat IgG2a or rat IgG2b) were used as negative controls. Immunostaining was also performed with cytokeratin 7 antibody (polyclonal, rabbit IgG), to assess the colocalization of B7h to trophoblast cells.
ELISPOT Assay
Splenocytes from CBA/CaJ (×C57BL/6) pregnant mice treated with anti-B7h mAb or control IgG were obtained as single-cell suspensions and used as responder cells. Irradiated (30 Gy) splenocytes from C57BL/6 and CBA/CaJ males were used as stimulators. The enzyme-linked immunosorbent spot (ELISPOT) assay was adapted to measure cells secreting IFN-γ and IL-4, as described previously.11
Flow Cytometric Analysis
Placentae and spleen were removed from pregnant mice at defined numbers of days after copulation. Spleens were processed and single-cell suspensions were prepared. Placentae were washed briefly in cold PBS and diced before digestion in medium containing collagenase IV (Roche Applied Science, Indianapolis, IN) and DNase I (Sigma-Aldrich, St. Louis, MO) for 1 hour. After single-cell suspensions were prepared, leukocytes were separated using Percoll equilibrium density gradients between the high and low densities (1.095 and 1.030, respectively). The isolated cells were then washed twice and counted. To study the phenotype of splenocytes and cells infiltrating the placenta, staining was performed with anti–CD4-PerCP, anti–CD8-APC, anti–CD25-fluorescein isothiocyanate, anti–Foxp3-phycoerythrin (PE), anti–CD103-fluorescein isothiocyanate, anti–CD44-APC, and anti–CD62L-PE mAbs in the case of spleens/lymph nodes, and with anti–CD45-APC (pan-leukocyte marker), anti–IFN-γ-PE, and anti–Foxp3-PE mAbs for cells infiltrating the placenta. Intracellular IFN-γ and Foxp3 staining were performed using a Cytofix/Cytoperm intracellular staining kit (BD Biosciences, San Jose, CA). Briefly, cells were blocked with Fc block (to prevent nonspecific binding), fixed, and permeabilized for 16 hours in the dark at 4°C and then were stained for IFN-γ for 60 minutes. All antibodies and reagents for flow cytometry analysis were purchased from BD Biosciences or eBioscience (San Jose, CA). Cells were analyzed on a FACSCalibur fluorescence-activated cell-sorting system using CellQuest software version 5.2 (both from BD Biosciences).
Microbead Cytokine Assay
At 8 weeks of age, CBA/CaJ females were mated with C57BL/6 males and were injected with four doses of anti-B7h monoclonal antibody according to the protocol described above. Lymphocytes were isolated from spleen and lymph nodes of pregnant females at 11.5 and 13.5 dpc and were cultured in RPMI 1640 medium with 10% fetal bovine serum in 96 well plates at a density of 0.5 × 106 cells/well. The stimulator cells were isolated from C57BL/6 males, irradiated with 30 Gy, and added to the culture at a density of 0.5 × 106 cells/well. The mixed lymphocyte culture was incubated for 72 hours at 37°C in 5% CO2. Using a Luminex (Austin, TX) microbead assay, we measured the level of TGF-β1 and IL-17 in cell culture supernatant at 48 and 72 hours according to the manufacturer's instructions. A Luminex 100 IS instrument with STarStation acquisition software version 2 (Applied Cytometry Systems, Sheffield, UK) was used to process the data. All samples were run in single wells, except that the standard curve points were run in duplicate according to the manufacturer's recommendations.
Isolation of Effector CD8+ T Cell Population
Female C57/BL6 Foxp3.GFP reporter mice (in which the sequence encoding GFP is knocked in into the gene encoding Foxp3) were mated with CBA/CAJ males and were inspected daily for plugs. Females with visible plugs were considered at day 0.5 of pregnancy and received four intraperitoneal injections of anti-B7h (or control IgG) mAb according to the protocol described above. At 11.5 and 13.5 dpc, spleen and lymph nodes (uterine, renal, pancreatic, inguinal) were harvested and were mechanically disrupted to prepare single-cell suspensions. Cells from treated and control mice were pooled, and CD8+ T cells were purified using a CD8+ T-cell isolation kit (Miltenyi Biotec, Bergisch-Gladbach, Germany; Auburn, CA) according to the manufacturer's instructions. Isolated CD8+ T cells were then fractioned into Foxp3.GFP+ cells and GFP− cells with a MoFlo cell sorter (Beckman Coulter, Brea, CA).
In Vitro Suppression Assay and Adoptive Transfer of CD8+ Regulatory T Cells
To assess the regulatory function of CD8+CD103+ T cells in vitro, a mixed lymphocyte reaction assay was set up in which T cells isolated from spleen/lymph nodes of pregnant CBA/CaJ females were cultured with irradiated B6 splenocytes and CD8+CD103+ T cells at various ratios (1:0, 1:4, 1:8) for 72 hours. Cells were pulsed with [3H] thymidine (1 μCi per well) for the final 16 hours of incubation, and incorporation of [3H] thymidine was measured with a MicroBeta liquid scintillation counter (PerkinElmer, Waltham, MA). The regulatory function of CD8+CD103+ T cells was assessed by the suppressive effect on the proliferation of effector T cells. Whole T cells were isolated by negative selection with a mouse Pan T-cell isolation kit II (catalog no. 130-095-130; Miltenyi Biotec), and CD8+CD103+ cells were flow-sorted after staining with fluorochrome antibodies for CD8 and CD103, achieving greater than 99% purity. Using a similar isolation technique as described above, CD8+CD103+ cells were adoptively transferred to pregnant CBA/CaJ females (350,000 cells) intraperitoneally 3 days after plugging, and fetal resorption was assessed at 13.5 dpc in the IgG-treated and the anti-B7h-treated groups.
RNA Extraction and qPCR
CD8+GFP+ and CD8+GFP− cells were then suspended in TRIzol reagent (Life Technologies–Invitrogen, Carlsbad, CA), and total RNA isolation was performed according to the manufacturer's protocol. RNA was treated with DNase (Life Technologies–Invitrogen), and 5 μg of RNA was then reverse-transcribed to synthesize 60 μL of cDNA. Each quantitative real-time PCR (qPCR) reaction consisted of 20 μL containing 250 ng of cDNA, 10 μL of SYBR Green master mix (Life Technologies–Applied Biosystems, Foster City, CA), and 250 nmol of sense and antisense primer. Primers were designed using Primer Express software version 2.0 (Life Technologies–Invitrogen). The reaction conditions were 50°C for 2 minutes, 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute; fluorescence was measured during the annealing/extension phase. The following primer sequences were used: B7h forward 5′-TCTTGGAAGAGGTGGTCAGG-3′, reverse 5′-TGGAGCTATCAGAGGTGCTG-3′; IDO forward 5′-CCTTCTGGGAATAAAACACGA-3′, reverse 5′-GGCTGGAGGCATGTACTCTC-3′; IL-10 forward 5′-ATCGATTTCTCCCCTGTGAA-3′, reverse 5′-TGGCCTTGTAGACACCTTGG-3′; PD-L1 forward 5′-AAGCGAATCACGCTGAAAGT-3′, reverse 5′-ATGCTCAGAAGTGGCTGGAT-3′; IL-17 forward 5′-TTCAGGGTCGAGAAGATGCT-3′, reverse 5′-AAACGTGGGGGTTTCTTAGG-3′; IL-4 forward 5′-TCATCGGCATTTTGAACGAG-3′, reverse 5′-CGTTTGGCACATCCATCTCC-3′; IL-5 forward 5′-AAAGAGAAGTGTGGCGAGGAGA-3′, reverse 5′-CACCAAGGAACTCTTGCAGGTAA-3′; IFN-γ forward 5′-AACGCTACACACTGCATCTTGG-3′, reverse 5′-GCCGTGGCAGTAACAGCC-3′; granzyme B forward 5′-CCCAGGCGCAATGTCAAT-3′, reverse 5′-CCAGGATAAGAAACTCGA-3′; GAPDH forward 5′-GGCAAATTCAACGGCACAGT-3′, reverse 5′-AGATGGTGATGGGCTTCCC-3′; and TGF-β1 forward 5′-TGAGTGGCTGTCTTTTGACG-3′, reverse 5′-GGTTCAGTCATGGATGGTG-3′. Expression was measured as copies of any given gene divided by copies of the housekeeping gene GAPDH.
Statistical Analysis
The nonparametric U-test was used for comparison of means. P < 0.05 was considered statistically significant.
Results
Expression of B7h and ICOS at the Fetomaternal Interface
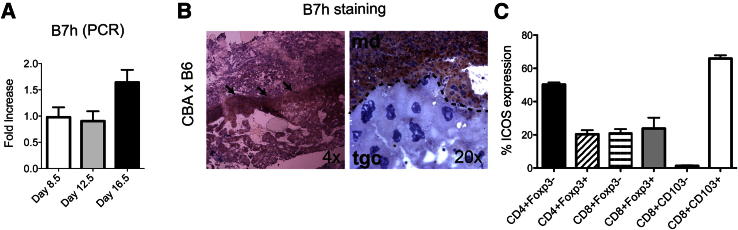
First, we studied expression of B7h (ICOS-L) in the placenta. Placentae were removed at 8.5, 12.5, and 16.5 dpc from pregnant CBA/CaJ females mated with C57BL/6 (B6) males. Expression of B7h was determined by qPCR (Figure 1A). Immunohistochemical staining on cryosection of placenta at 12.5 dpc localized expression of B7h in the maternal decidua of placenta (Figure 1B) and not the decidua basalis; these two areas are structurally distinct under the microscope. This expression pattern differs from that observed for PD-L1, in which expression was localized in the decidua basalis.11 B7h is not expressed on trophoblast cells, as evident from costaining with cytokeratin 7 antibody (data not shown). Furthermore, B7h was not expressed in placental sections obtained from syngeneic matings (CBA×CBA) (Supplemental Figure S1A). Specificity of this expression is demonstrated by lack of expression of B7h in a B7h-deficient on B6 background mice (Supplemental Figure S1B).
Figure 1.
B7h/ICOS expression at the fetomaternal interface of CBA/CaJ mice. Virgin female CBA/CaJ mice were mated with C57BL/6 males. A: Placentae were removed at 8.5, 12.5, and 16.5 dpc, and B7h expression was analyzed by qPCR. B: Placentae at 12.5 dpc were also immunostained for B7h (arrows). Representative photomicrographs show staining in the whole decidua (md, maternal decidua); trophoblastic giant cells (tgc) of placenta are also shown. Original magnification (×4 or ×20) is indicated for each image. Results are representative of two independent sets of experiments. C: ICOS expression in leukocytes isolated from placenta at 12.5 dpc. Data are expressed as means ± SEM. n = 3 per experiment.
To evaluate the binding partner of B7h, the percentage of ICOS expression was characterized by flow cytometry in leukocytes isolated from placenta. A high expression of ICOS was observed in CD8+CD103+ T cells (65.9% ±1.9) and CD4+Foxp3− cells (50.2 ± 1.2%), compared with CD4+Foxp3+ (20.4 ± 2.3%), CD8+Foxp3+ (23.7 ± 6.5%), CD8+Foxp3− (21 ± 2.4%), and CD8+CD103− cells (1.2 ± 0.3%) (Figure 1C).
Effect of B7h Blockade on the Rate of Spontaneous Resorption and Litter Size
To study the role of B7h in fetomaternal tolerance, we used a model of allogeneic pregnancy, mating CBA/CaJ females with C57BL/6 males. The reported rate of spontaneous resorption in this model is approximately 20%.10,20 We assessed the rate of resorption and litter size after blocking B7h with a monoclonal antibody. The resorption rate increased significantly, from 26% in the control IgG group to 46% in the anti-B7h mAb–treated group (P = 0.0014) (Figure 2A). We confirmed our results by assessing the number of pups born from this same allogeneic mating. B7h-treated mice had a mean litter size of 5.5 pups, compared with 7.2 in the control IgG group (P = 0.0419) (Figure 2B). The effect of anti-B7h antibody treatment was alloantigen specific, because there was no increase in resorption in CBA/CaJ×CBA/CaJ syngeneic mating after treatment with this antibody (data not shown).
Figure 2.
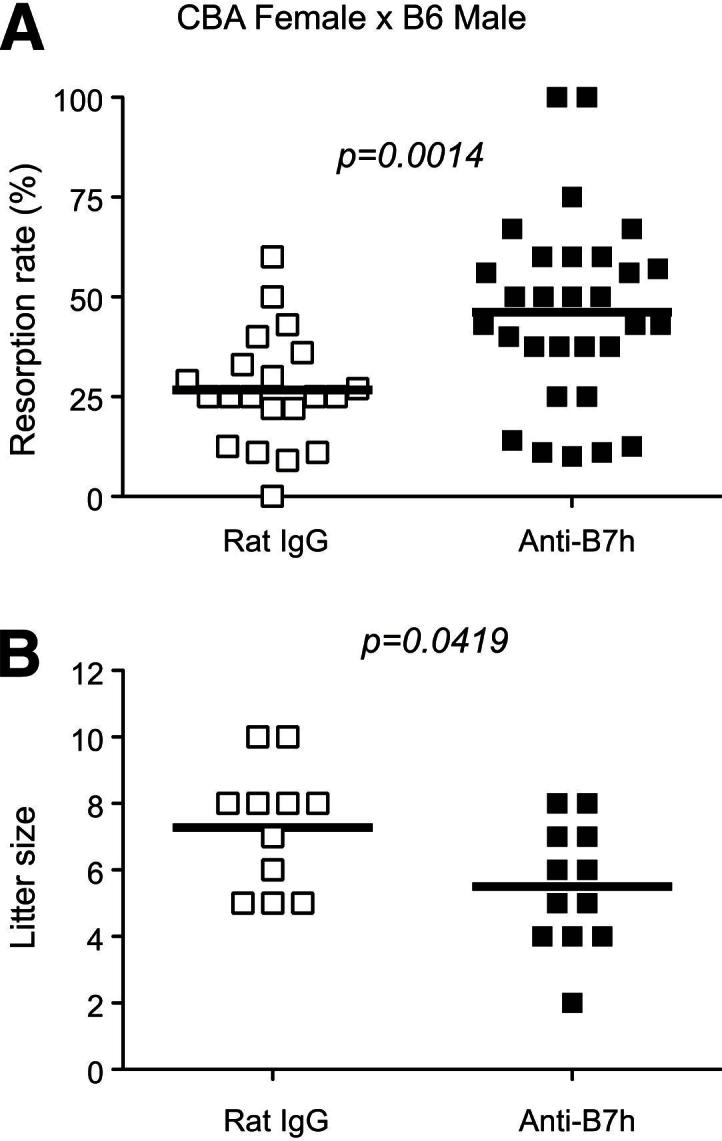
B7h blockade significantly increases fetal resorption and reduces litter size in the CBA/CaJ×C57BL/6 allogeneic pregnancy model. A: Pregnant CBA/CaJ (×C57BL/6) females were injected intraperitoneally with anti-B7h mAb or control IgG, and the fetal resorption rate was determined at 13.5 dpc in pregnant CBA/CaJ mice of both groups. There was a significant increase in fetal resorption rate in the anti-B7h–treated mice, compared with control. B: Some mice treated with anti-B7h or rat control IgG were allowed to go to term and litter size was recorded. There was a significant reduction in litter size in the anti-B7h–treated mice, compared with control. n = 21 (A) or 11 (B), controls; n = 30 (A) or 12 (B), treatment group. Horizontal bars indicate means.
B7h Blockade Enhances Effector Function But Decreases Regulation in the Placental Interface
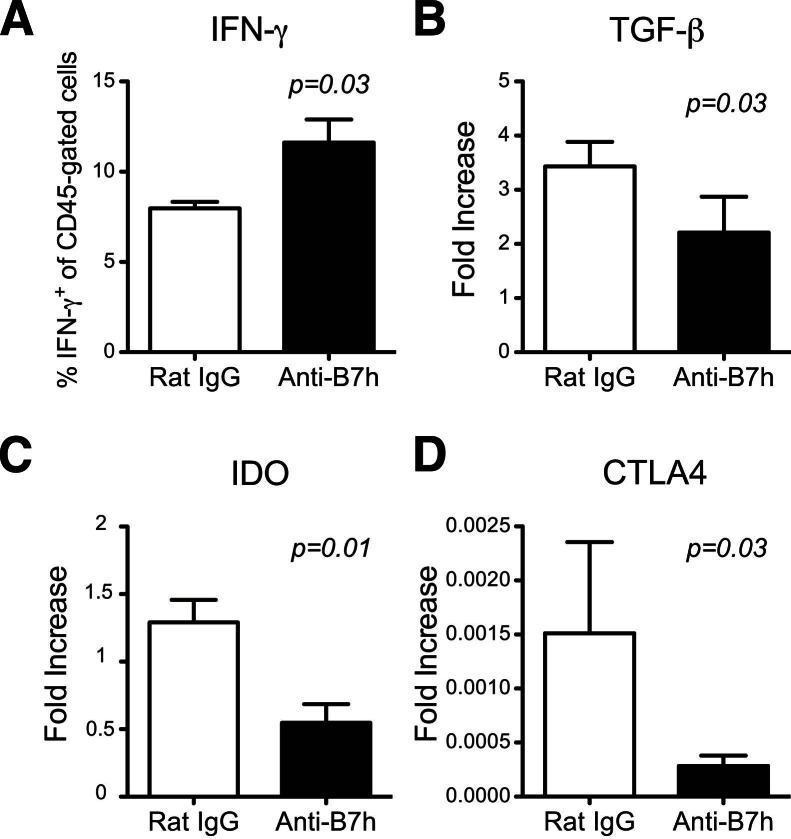
The placenta is the central organ involved in immune regulation during pregnancy, because it contains both maternal and fetal cells in close proximity.2 To characterize the role of B7h in fetomaternal tolerance, we evaluated effector and regulatory markers in the placenta. First, we isolated leukocytes (CD45+) from the placenta and observed a significant increase in the percentage of IFN-γ–producing leukocytes after allostimulation in the anti-B7h–treated mice, compared with controls (11 ± 1.28 versus 7 ± 0.36; P = 0.03) (Figure 3A). Next, we quantified regulatory markers in the placenta by qPCR. B7h blockade significantly decreased mRNA of the regulatory molecules TGF-β1, IDO, and CTLA-4 (Figure 3, B–D). IL-10 showed a trend toward lower expression, but this did not reach statistical significance (1.45 ± 0.45 versus 2.27 ± 0.45, respectively; P = 0.27). Because the coinhibitory molecule PD-L1 is crucial for fetal tolerance,11 PD-L1 expression was also measured, but was found to be similar in both groups (data not shown). In sum, B7h blockade significantly affected immunoregulation at the fetomaternal interface.
Figure 3.
Characterization of placental markers after anti-B7h treatment. A: Percentage of IFN-γ–producing leukocytes (CD45+) isolated from placenta of anti-B7h–treated versus control IgG-treated mice at 13.5 dpc. B–D: qPCR analysis of TGF-β1, IDO, and CTLA-4 in the placenta from both groups at similar time points . Data are expressed as means ± SEM. n = 8 or 9 per group.
Both CTLA-4 and IDO Regulatory Components Play a Role in the Deleterious Effect of B7h Blockade
Because expression of both IDO and CTLA-4 was down-regulated in the placentae of the anti-B7h mAb–treated group, we decided to further examine whether these regulatory components are functionally important for the enhanced fetal loss observed on B7h blockade. Allogeneically mated pregnant IDO-deficient females treated with anti-B7h mAb did not exhibit a significant change in the rate of fetal resorption, compared with IgG-treated controls (n = 7; P = 0.25) (Supplemental Figure S2A). Furthermore, using a blocking antibody against CTLA-4 (anti–CTLA-4 mAb) in wild-type allogeneic pregnancy model (CBA×B6), we observed that neutralization of CTLA-4 also abrogated the enhanced fetal loss observed with anti-B7h treatment (n = 4 to 7; P = 0.42) (Supplemental Figure S2B). These data indicate that the increased rate of fetal resorption after B7h blockade is in part mediated by regulatory signals (eg, IDO and CTLA-4).
Effect of anti-B7h mAb in Secondary Lymphoid Organs
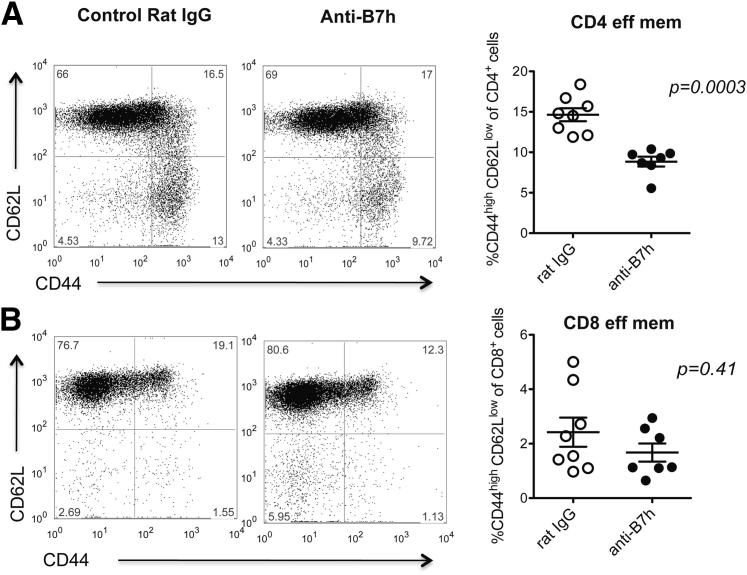
To assess the effect of B7h blockade on the periphery, we started by comparing the frequency of CD4+ and CD8+ effector memory T cells in the spleen and lymph nodes of treated and control pregnant CBA/CaJ (×C57BL/6) females at 11.5 and 13.5 dpc. We observed a transient decrease in the percentage of CD4+ effector memory T cells in the anti-B7h–treated group at 11.5 dpc (P = 0.0003) (Figure 4A), whereas the percentage of CD8+ effector memory T cells was the same between groups (P = 0.41) (Figure 4B). At 13.5 dpc, CD4+ and CD8+ T-cell subsets were similar in number in both groups (data not shown). Next, we performed mixed leukocyte cultures in vitro to further characterize the alloimmune response. For these studies, splenocytes from pregnant allogeneically mated CBA females treated with anti-B7h mAb or control IgG were isolated at 11.5 dpc and were cultured in the presence of irradiated splenocytes from C57BL/6 males. An ELISPOT assay was performed for determining the frequency of IFN-γ–producing cells.
Figure 4.
Analysis of effector memory T cells on spleens after anti-B7h treatment. A and B: Splenocytes from CBA/CaJ (×C57BL/6) pregnant mice treated with anti-B7h mAb or control IgG were processed at 11.5 dpc and stained for CD4 (A) and CD8 (B) in combination with effector memory markers (CD44, CD62L). Effector memory T cells were defined by high expression of CD44 and low expression of CD62L. Quantification data are expressed as means ± SEM. n = 7 or 8 per group.
For monitoring other cytokines, cell culture supernatants were collected and analyzed using a Luminex microbead assay. We observed an increase in IFN-γ production by splenocytes from the anti-B7h–treated group (17 ± 4 spots versus 5 ± 1 spots for controls; n = 5; P = 0.03), although the levels were low overall. IL-4 and IL-17 production did not differ significantly between groups (data not shown). In addition, there was a trend toward lower TGF-β1 levels in the anti-B7h–treated group, compared with controls, after 48 to 72 hours of incubation. Using intracellular cytokine staining after allostimulation, we determined that the major producers of IFN-γ in the periphery were CD8+ T cells and that the percentage of CD8+ IFN−γ+ T cells was significantly higher in the spleen and lymph nodes of anti-B7h–treated mice, compared with controls (15.4 ± 4 versus 0.01 ± 0.006 for controls; n = 5; P = 0.01). These data suggest that B7h blockade leads to enhanced alloimmunity in pregnancy, with a predominant increase in IFN-γ–producing CD8+ cells.
CD8+ T Cells Play a Crucial Role in Fetomaternal Tolerance on B7h Blockade
To further investigate the roles of CD4+ and CD8+ T cells in B7h-mediated fetomaternal tolerance, we used CD4+ and CD8+ T-cell–deficient mice. Injection of anti-B7h mAb into CD4+ T-cell–deficient mice resulted in a statistically significant decrease in litter size (P = 0.001) (Figure 5A), whereas injection of anti-B7h mAb into mice deficient for CD8+ T cells abrogated this deleterious effect on litter size (P = 0.47) (Figure 5B). These findings in CD8+ T-cell–deficient mice suggest a critical role for CD8+ cells in mediating the effects of B7h blockade on tolerance at the fetomaternal interface.
Figure 5.
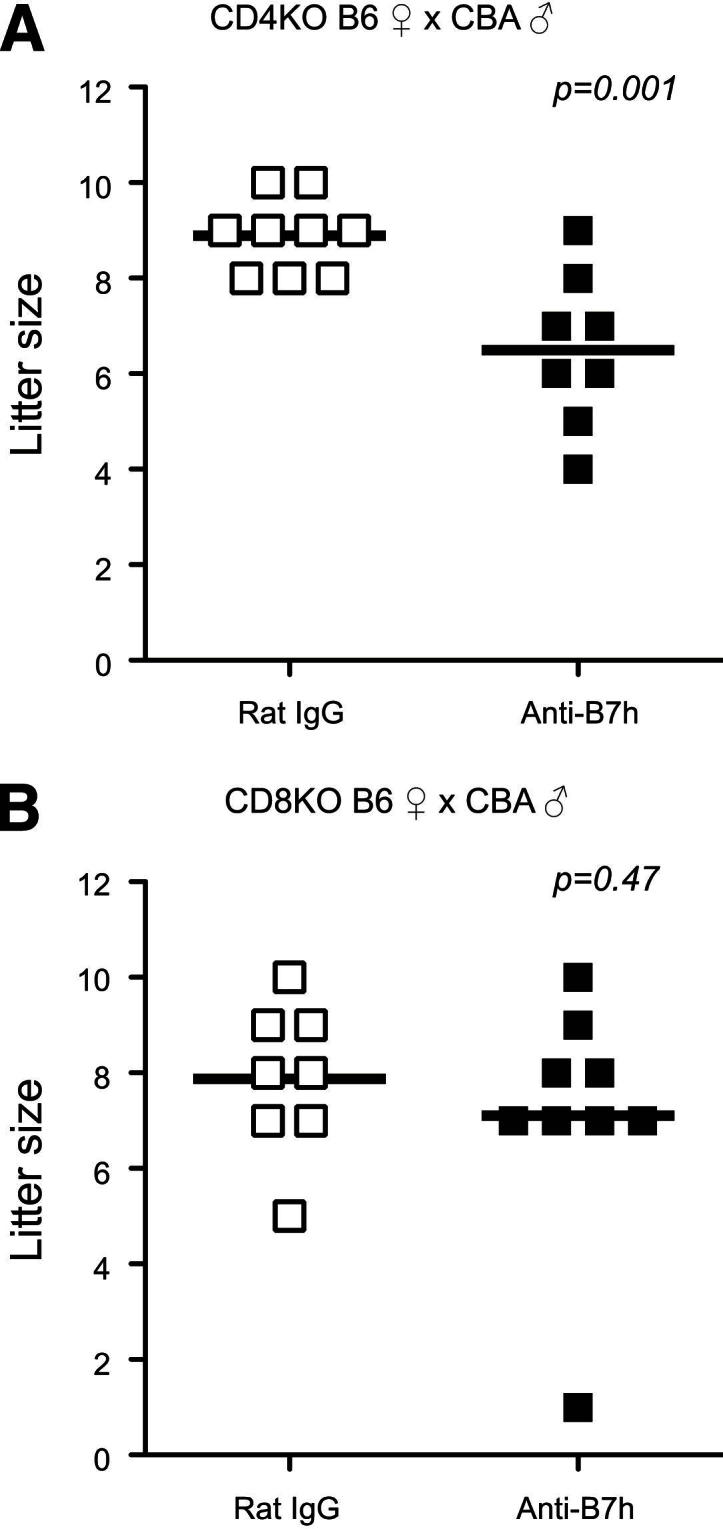
Reduced litter size in CD4+ T-cell–deficient but not CD8+ T-cell–deficient mice after anti-B7h treatment. CD4+ (A) or CD8+ (B) T-cell–deficient females on C57BL/6 background were mated with CBA/CaJ males and treated with anti-B7h mAb or control IgG. Litter size was determined at term. There was a significant reduction in litter size in CD4-deficient mice but not in CD8-deficient mice. n = 8 or 9 per group. KO, knockout.
Regulatory CD8+ T Cells Are Significantly Affected by B7h Blockade
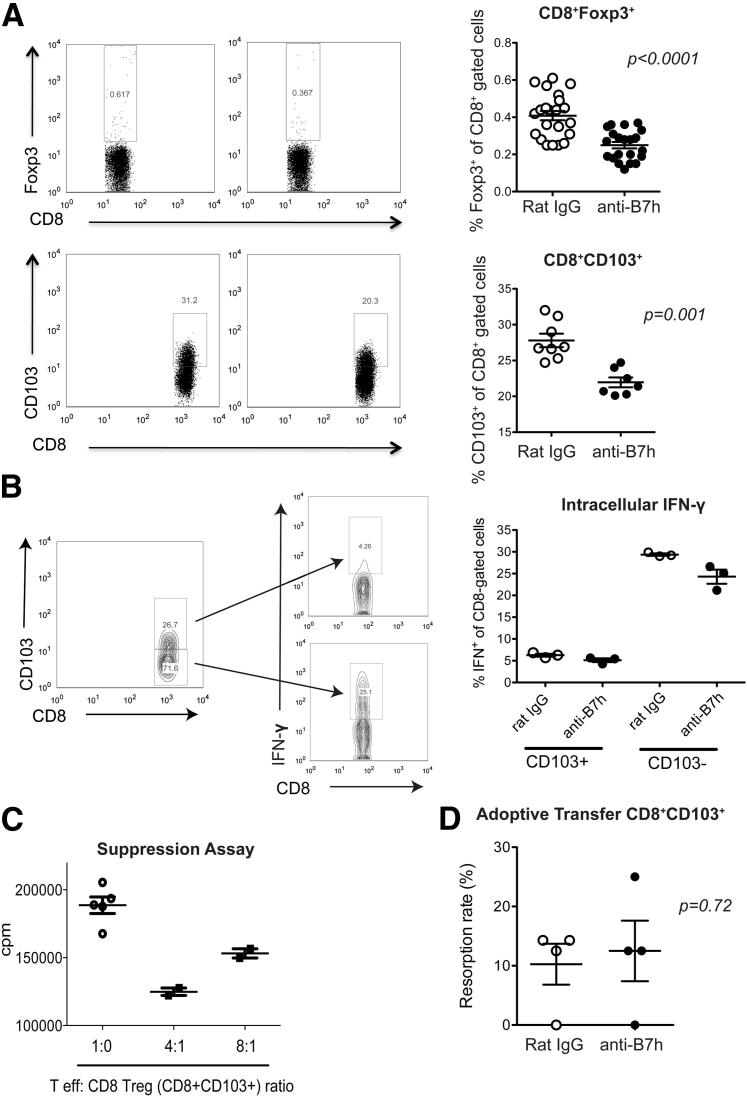
To evaluate the role of regulatory T cells in our model, we started by measuring the frequency of CD4+ regulatory T cells in spleens and lymph nodes by flow cytometry. No differences in the percentage of CD4+CD25+Foxp3+ cells was observed between pregnant CBA/CaJ female mice treated with anti-B7h mAb or control IgG (Supplemental Figure S3). There was, however, a significant decrease in two subpopulations of CD8+ regulatory T cells characterized by expression of Foxp3 or CD103 (P < 0.001) (Figure 6A).
Figure 6.
Evaluation of CD8+ regulatory T cells on B7h blockade. Splenocytes from CBA/CaJ (×C57BL/6) pregnant mice treated with anti-B7h mAb or control IgG were processed at 11.5 dpc. A: Representative dot plots of CD8+Foxp3+ and CD8+CD103+ subpopulations are shown (left panel), with the percentages of these subsets combining multiple experiments (right panel). B: Determination of IFN-γ production by intracellular cytokine staining in CD8+CD103− and CD8+CD103+ cells. C: In vitro suppression assay of CD8+ regulatory T cells (flow-sorted CD8+CD103+ cells) in combination with effector T cells at different ratios, as described under Materials and Methods. D: Adoptive transfer of CD8+ regulatory T cells (flow-sorted CD8+CD103+ cells) into pregnant CBA/CaJ females at 3 days after plugging. Fetal resorption was assessed at 13.5 dpc in IgG and anti-B7h–treated groups. n = 4 per group.
To further characterize these regulatory T-cell subsets, we used a Foxp3-GFP reporter mouse; this model permits the isolation of CD8+GFP.Foxp3+ and CD8+GFP.Foxp3− cells from the spleen and lymph nodes of pregnant females. The yield of CD8+Foxp3+ cells after flow sorting was very low, even after pooling samples from more than one mouse. We therefore used qPCR to compare the cytokine profile between groups. IFN-γ expression on CD8+Foxp3+ cells was similar between the anti-B7h–treated and IgG control groups, whereas expression of IL-4, IL-5, IL-10, and IL-17 was undetectable (data not shown). Analysis of the CD8+Foxp3− subset by qPCR showed a 5.3-fold increase in granzyme B expression and a 2.6-fold increase in IFN-γ expression in CD8+Foxp3− cells isolated from anti-B7h–treated group, compared with controls. These findings are in agreement with our observations (noted above) in the placenta and in whole splenocytes. In addition, we determined IFN-γ production by the other subset of regulatory CD8+ cells (ie, CD8+CD103+ cells). These cells demonstrated a lower production of IFN-γ by intracellular cytokine staining after allostimulation in vitro, compared with CD8+CD103− cells (Figure 6B). Furthermore, flow-sorted CD8+CD103+ T cells were able to suppress T-cell proliferation in vitro (Figure 6C), supporting the evidence of a regulatory role for this subset.21 Last of all, adoptive transfer of CD8+CD103+ cells to pregnant CBA/CaJ females abrogated the increased fetal resorption elicited by B7h blockade (Figure 6D). Taken together, these findings suggest that ICOS–B7h interaction is important for the generation of regulatory CD8+ cells and that blockade of B7h significantly affects immune regulation at the placental interface.
Discussion
The ICOS–B7h interaction is a complex costimulatory pathway that actively participates in the process of T-cell activation, differentiation, and function.22,23 In addition to its role in effector T cells, this pathway also seems to be important for immune regulation. ICOS-B7h signaling was shown to be critical for the generation of regulatory T cells, capable of suppressing pulmonary inflammation in a model of airway hyperactivity.24 ICOS–B7h interaction was also shown to be essential for oral mucosal tolerance in a model in which the encephalitogenic myelin oligodendrocyte glycoprotein peptide MOG35–55 is administered orally for protection against experimental autoimmune encephalomyelitis.25 Furthermore, ICOS blockade exacerbated diabetes in BDC2.5 transgenic NOD mice, and this effect was dependent on regulatory T cells.16 Interestingly, ICOS–B7h interaction has been shown to have diverse effects, depending on the disease model studied, the type of immune response elicited, and the timing of ICOS-B7h blockade.15 For example, ICOS blockade prolonged cardiac allograft survival in a fully HLA-mismatched murine transplant model, in particular when the protocol involved delayed blockade.23 In contrast, early ICOS blockade accelerated rejection in a minor mismatched cardiac transplant model.23 Some of these discrepancies seem to be related to the diverse role of ICOS in both effector and regulatory T cells.26 Consistent with report of suppressive effects of the ICOS-B7h pathway, blockade of B7h in our allogeneic pregnancy model enhanced fetal resorption and decreased the litter size (Figure 2), with associated reduction in both CD4+ effector memory T cells and CD8+ regulatory T cells.
In general, ICOS signaling appears to support the expansion and survival of differentiated T cells, and, depending on the dominant phenotype of the immune response, it might either accelerate the disease process or provide protection against it.26–28 It does seem that in non–Th1 dominant models, absence of this pathway is deleterious. An example would be asthma, which is caused by a Th2-driven immune response and blockade of ICOS-B7h exacerbates disease.24 In healthy pregnancy, the balance of Th1/Th2 cytokines is considered to be of critical importance, with dominance of a Th2-type immunity and suppression of Th1,29–32 fitting as a non–Th1 dominant model. Indeed, augmented Th1 response is associated with increased rates of abortion.33,34 In supporting of the above hypothesis, we observed an expansion of IFN-γ–producing leukocytes both in the placenta and peripherally in anti-B7h–treated pregnant mice, suggesting that an enhanced Th1 response is a contributing factor in the increased fetal resorption rate observed as a result of B7h blockade. In another study, however, in vitro cocultures of JEG-3 cells (an extravillous trophoblast cell line with documented B7h expression) with T cells and anti-CD3 mAb led to increased IFN-γ secretion.35 We believe that the differences between our present in vivo findings and these other in vitro observations are related to the experimental setting and reflect some of the challenges in attempts to examine isolated cell interactions in vitro with exogenous stimuli (anti-CD3) that do not truly reflect the placental microenvironment.
ICOS signaling has been reported to also play a critical role in Th17 differentiation, inducing expression of the transcription factor c-MAF and consequent IL-21 production.36,37 In the present study, however, we could not detect any differences in IL-17 production after B7h blockade, neither in the periphery nor locally at the fetomaternal interface. Moreover, IL-17 has been detected only in very low levels at the fetomaternal interface in general.34,38 Whether Th17 cells can play a deleterious role in fetomaternal tolerance still remains to be determined.
Regulatory T cells are critical for the maintenance of tolerance at the fetomaternal interface3,39 and significant reductions in regulatory T cells (CD4+CD25+) at the decidual level and in peripheral blood specimens have been demonstrated in spontaneous abortions, compared with healthy pregnancies.5 Most of the studies in pregnancy have shown a role for CD4+ regulatory T cells. However, Shao et al40 reported that placental trophoblasts are able to generate a subpopulation of regulatory CD8+ cells that express CD103. In another study, CD103 was identified as a marker of alloantigen-induced regulatory CD8+ T cells, and CD8+CD103+ T cells were shown to have immunosuppressive function with low proliferative and cytotoxic capacity.21 Consistent with these findings, in the present study we observed a predominant reduction in regulatory CD8+ T cells as a result of B7h blockade. Two different subsets of regulatory CD8+ T cells were identified by the coexpression of either Foxp3 or CD103 (Figure 6A). The effect on the latter was observed to be numerically more significant. Furthermore, CD8-deficient mice treated with anti-B7h mAb did not experience the same enhanced fetal resorption, indicating that the deleterious effect of ICOS-B7h blockade is dependent on CD8+ cells. The adoptive transfer of CD8+ regulatory T cells abrogated the increased fetal resorption in anti-B7h–treated group, confirming the key role of this subset in fetomaternal tolerance (Figure 6D).
In accord with other reports,35,41 ICOS expression was predominant on placental T cells and B7h was present in placental decidua. Our study expanded those findings by showing the greatest expression of ICOS by the CD8+CD103+ T-cell subset and B7h in the maternal decidua. In humans, B7h expression is present in the placenta throughout gestation.41 Although ICOS has been shown to be important for CD4+Foxp3+ regulatory T-cell homeostasis, with ICOS-deficient mice exhibiting a reduced number of CD4+Foxp3+ regulatory T cells,26 the exact of role of ICOS–B7h interaction in the generation of regulatory CD8+ cells still remains to be determined. Based on the high expression of ICOS on CD8+CD103+ cells and the lower numbers of this subset on B7h blockade, ICOS-B7h signaling might be important for the generation of this CD8+ regulatory T-cell subset. Prior work on CD4+ regulatory T cells showed that two subsets of regulatory CD4+ cells were differently regulated by signaling through ICOS or CD28; whether this is also true for CD8+ regulatory T cells warrants further investigation.42
Regulatory T cells exert their suppressive effect through multiple mechanisms, including secretion of inhibitory cytokines (eg, TGF-β1, IL-10) and cytotoxic enzymes (eg, granzyme B), as well as direct cell–cell interaction via inhibitory receptors (eg, CTLA-4). We have previously shown that the PD-1–PD-L1 pathway plays a critical role in tolerance at the fetomaternal interface and that this effect is dependent on regulatory T cells.11,13,14 In the present study, however, we did not observe a significant difference of PD-L1 expression in the placenta of anti-B7h–treated mice, suggesting that the deleterious effect of B7h blockade is independent of PD-L1 signaling.
IDO has also been shown to play an important role in conferring tolerance at the fetomaternal interface,10 and inhibition of IDO was shown to result in increased fetal rejection associated with massive T-cell infiltration and complement deposition at the uteroplacental interface.20 In the present study, blockade of the ICOS-B7h pathway resulted in down-regulation of IDO, TGF-β1, and CTLA-4 locally at the fetomaternal interface. TGF-β1 is an important regulator of tolerance during pregnancy and acts via multiple mechanisms.43–45 Interaction of CTLA-4 with the costimulatory molecule B7 (CD80/86) expressed on the dendritic cells induces the activity of IDO.46 CTLA-4 expressed on regulatory T cells has been shown to regulate IDO expression on decidual and peripheral blood antigen-presenting cells, and in spontaneous abortion the expression of CTLA-4 on regulatory T cells was reduced.47 Further investigation will be required to determine the exact relationship between ICOS–B7h interaction and IDO, and how B7h blockade affects the generation of CD8+ regulatory T cells. Our studies with IDO-deficient mice and with anti–CTLA-4 mAb in combination with anti-B7h mAb suggest that they are definitely interconnected.
In summary, the present findings demonstrate that the ICOS-B7h pathway plays a critical role in the regulation of fetomaternal tolerance by enhancing IFN-γ CD8+ effector response and by reducing local immunomodulation mediated by CD8+ regulatory T cells. Further studies should attempt to elucidate the exact function of ICOS-B7h in CD8+ regulatory T-cell generation and the potential interactions with CTLA-4 and IDO pathways in pregnancy.
Footnotes
Supported by NIH grants R21-AI076794-02 and R01-AI084756-02 (I.G.) and the American Heart Association (L.V.R.).
Supplemental Data
Immunostaining of B7h in placenta of syngeneic CBA mice and B7h-deficient mice. Representative immunostaining for B7h on placental sections at 12.5 dpc. A: Staining in CBA/CaJ×CBA/CaJ (syngeneic) placenta. B: Staining on placental sections of B7h-deficient mice mated with CBA mice. Results are representative of two independent sets of experiments. n = 3 per experiment. Original magnification (×4 or ×20) is indicated for each image.
Litter size determination in IDO-deficient mice or in wild-type mice treated with anti–CTLA-4 mAb in an allogeneic pregnancy model. A: IDO-deficient female mice were mated with CBA males and treated with anti-B7h mAb or with control IgG. Litter size was determined at term. B: Anti–CTLA-4 blocking antibody was administered to pregnant female CBA mice, and litter size was determined between different treatment groups.
Evaluation of CD4+ regulatory T cells in the anti-B7h–treated group. Foxp3 expression was determined by intracellular staining in lymphocytes prepared from spleens of female CBA/CaJ (×C57BL/6) mice treated with anti-B7h mAb, compared with control IgG-treated mice, at 13.5 dpc. The percentage of CD4+CD25+ Foxp3+ T cells did not differ between the two groups (P = 0.3829). Representative dot plots are also shown on the left panel.
References
- 1.Billingham R.E., Brent L., Medawar P.B. ‘Actively acquired tolerance’ of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [Republished in J Immunol 2010, 184:5–8] [DOI] [PubMed] [Google Scholar]
- 2.Guleria I., Sayegh M.H. Maternal acceptance of the fetus: true human tolerance. J Immunol. 2007;178:3345–3351. doi: 10.4049/jimmunol.178.6.3345. [DOI] [PubMed] [Google Scholar]
- 3.Aluvihare V.R., Kallikourdis M., Betz A.G. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 4.Zenclussen A.C., Gerlof K., Zenclussen M.L., Sollwedel A., Bertoja A.Z., Ritter T., Kotsch K., Leber J., Volk H.D. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol. 2005;166:811–822. doi: 10.1016/S0002-9440(10)62302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sasaki Y., Sakai M., Miyazaki S., Higuma S., Shiozaki A., Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10:347–353. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- 6.Somerset D.A., Zheng Y., Kilby M.D., Sansom D.M., Drayson M.T. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terness P., Kallikourdis M., Betz A.G., Rabinovich G.A., Saito S., Clark D.A. Tolerance signaling molecules and pregnancy: IDO, galectins, and the renaissance of regulatory T cells. Am J Reprod Immunol. 2007;58:238–254. doi: 10.1111/j.1600-0897.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 8.Ishitani A., Sageshima N., Lee N., Dorofeeva N., Hatake K., Marquardt H., Geraghty D.E. Protein expression and peptide binding suggest unique and interacting functional roles for HLA-E, F, and G in maternal-placental immune recognition. J Immunol. 2003;171:1376–1384. doi: 10.4049/jimmunol.171.3.1376. [DOI] [PubMed] [Google Scholar]
- 9.King A., Burrows T.D., Hiby S.E., Bowen J.M., Joseph S., Verma S., Lim P.B., Gardner L., Le Bouteiller P., Ziegler A., Uchanska-Ziegler B., Loke Y.W. Surface expression of HLA-C antigen by human extravillous trophoblast. Placenta. 2000;21:376–387. doi: 10.1053/plac.1999.0496. [DOI] [PubMed] [Google Scholar]
- 10.Munn D.H., Zhou M., Attwood J.T., Bondarev I., Conway S.J., Marshall B., Brown C., Mellor A.L. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 11.Guleria I., Khosroshahi A., Ansari M.J., Habicht A., Azuma M., Yagita H., Noelle R.J., Coyle A., Mellor A.L., Khoury S.J., Sayegh M.H. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J Exp Med. 2005;202:231–237. doi: 10.1084/jem.20050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin L.P., Fan D.X., Li D.J. Regulation of costimulatory signal in maternal-fetal immune tolerance. Am J Reprod Immunol. 2011;66:76–83. doi: 10.1111/j.1600-0897.2010.00982.x. [DOI] [PubMed] [Google Scholar]
- 13.D'Addio F., Riella L.V., Mfarrej B.G., Chabtini L., Adams L.T., Yeung M., Yagita H., Azuma M., Sayegh M.H., Guleria I. The link between the PDL1 costimulatory pathway and Th17 in fetomaternal tolerance. J Immunol. 2011;187:4530–4541. doi: 10.4049/jimmunol.1002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habicht A., Dada S., Jurewicz M., Fife B.T., Yagita H., Azuma M., Sayegh M.H., Guleria I. A link between PDL1 and T regulatory cells in fetomaternal tolerance. J Immunol. 2007;179:5211–5219. doi: 10.4049/jimmunol.179.8.5211. [DOI] [PubMed] [Google Scholar]
- 15.Li X.C., Rothstein D.M., Sayegh M.H. Costimulatory pathways in transplantation: challenges and new developments. Immunol Rev. 2009;229:271–293. doi: 10.1111/j.1600-065X.2009.00781.x. [DOI] [PubMed] [Google Scholar]
- 16.Herman A.E., Freeman G.J., Mathis D., Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199:1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gotsman I., Grabie N., Gupta R., Dacosta R., MacConmara M., Lederer J., Sukhova G., Witztum J.L., Sharpe A.H., Lichtman A.H. Impaired regulatory T-cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation. 2006;114:2047–2055. doi: 10.1161/CIRCULATIONAHA.106.633263. [DOI] [PubMed] [Google Scholar]
- 18.Baban B., Chandler P., McCool D., Marshall B., Munn D.H., Mellor A.L. Indoleamine 2,3-dioxygenase expression is restricted to fetal trophoblast giant cells during murine gestation and is maternal genome specific. J Reprod Immunol. 2004;61:67–77. doi: 10.1016/j.jri.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Fontenot J.D., Rasmussen J.P., Williams L.M., Dooley J.L., Farr A.G., Rudensky A.Y. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Mellor A.L., Sivakumar J., Chandler P., Smith K., Molina H., Mao D., Munn D.H. Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat Immunol. 2001;2:64–68. doi: 10.1038/83183. [DOI] [PubMed] [Google Scholar]
- 21.Uss E., Rowshani A.T., Hooibrink B., Lardy N.M., van Lier R.A., ten Berge I.J. CD103 is a marker for alloantigen-induced regulatory CD8+ T cells. J Immunol. 2006;177:2775–2783. doi: 10.4049/jimmunol.177.5.2775. [DOI] [PubMed] [Google Scholar]
- 22.Sharpe A.H. Mechanisms of costimulation. Immunol Rev. 2009;229:5–11. doi: 10.1111/j.1600-065X.2009.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harada H., Salama A.D., Sho M., Izawa A., Sandner S.E., Ito T., Akiba H., Yagita H., Sharpe A.H., Freeman G.J., Sayegh M.H. The role of the ICOS-B7h T cell costimulatory pathway in transplantation immunity. J Clin Invest. 2003;112:234–243. doi: 10.1172/JCI17008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akbari O., Freeman G.J., Meyer E.H., Greenfield E.A., Chang T.T., Sharpe A.H., Berry G., DeKruyff R.H., Umetsu D.T. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 25.Miyamoto K., Kingsley C.I., Zhang X., Jabs C., Izikson L., Sobel R.A., Weiner H.L., Kuchroo V.K., Sharpe A.H. The ICOS molecule plays a crucial role in the development of mucosal tolerance. J Immunol. 2005;175:7341–7347. doi: 10.4049/jimmunol.175.11.7341. [DOI] [PubMed] [Google Scholar]
- 26.Burmeister Y., Lischke T., Dahler A.C., Mages H.W., Lam K.P., Coyle A.J., Kroczek R.A., Hutloff A. ICOS controls the pool size of effector-memory and regulatory T cells. J Immunol. 2008;180:774–782. doi: 10.4049/jimmunol.180.2.774. [Erratum appeared in J Immunol 2008, 180:3613] [DOI] [PubMed] [Google Scholar]
- 27.Bonhagen K., Liesenfeld O., Stadecker M.J., Hutloff A., Erb K., Coyle A.J., Lipp M., Kroczek R.A., Kamradt T. ICOS+ Th cells produce distinct cytokines in different mucosal immune responses. Eur J Immunol. 2003;33:392–401. doi: 10.1002/immu.200310013. [DOI] [PubMed] [Google Scholar]
- 28.Löhning M., Hutloff A., Kallinich T., Mages H.W., Bonhagen K., Radbruch A., Hamelmann E., Kroczek R.A. Expression of ICOS in vivo defines CD4+ effector T cells with high inflammatory potential and a strong bias for secretion of interleukin 10. J Exp Med. 2003;197:181–193. doi: 10.1084/jem.20020632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaouat G., Menu E., Delage G., Moreau J.F., Khrishnan L., Hui L., Meliani A.A., Martal J., Raghupathy R., Lelaidier C., Bertrand C., Freitas S., Hambartsumian E., Wegmann T.G., Frydman R. Immuno-endocrine interactions in early pregnancy. Hum Reprod. 1995;10(Suppl 2):55–59. doi: 10.1093/humrep/10.suppl_2.55. [DOI] [PubMed] [Google Scholar]
- 30.Chaouat G., Menu E., de Smedt D., Khrihnan L., Hui L., Assal Meliani A., Martal J., Raghupathy R., Wegmann T.G. The emerging role of IL-10 in pregnancy. Am J Reprod Immunol. 1996;35:325–329. doi: 10.1111/j.1600-0897.1996.tb00488.x. [Erratum appeared in Am J Reprod Immunol 1996, 36:127] [DOI] [PubMed] [Google Scholar]
- 31.Piccinni M.P., Beloni L., Livi C., Maggi E., Scarselli G., Romagnani S. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat Med. 1998;4:1020–1024. doi: 10.1038/2006. [DOI] [PubMed] [Google Scholar]
- 32.Wegmann T.G., Lin H., Guilbert L., Mosmann T.R. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 33.Lédée-Bataille N., Dubanchet S., Coulomb-L'hermine A., Durand-Gasselin I., Frydman R., Chaouat G. A new role for natural killer cells, interleukin (IL)-12, and IL-18 in repeated implantation failure after in vitro fertilization. Fertil Steril. 2004;81:59–65. doi: 10.1016/j.fertnstert.2003.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Saito S., Nakashima A., Shima T., Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63:601–610. doi: 10.1111/j.1600-0897.2010.00852.x. [DOI] [PubMed] [Google Scholar]
- 35.Nagamatsu T., Barrier B.F., Schust D.J. The regulation of T-cell cytokine production by ICOS-B7H2 interactions at the human fetomaternal interface. Immunol Cell Biol. 2011;89:417–425. doi: 10.1038/icb.2010.101. [DOI] [PubMed] [Google Scholar]
- 36.Bauquet A.T., Jin H., Paterson A.M., Mitsdoerffer M., Ho I.C., Sharpe A.H., Kuchroo V.K. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paulos C.M., Carpenito C., Plesa G., Suhoski M.M., Varela-Rohena A., Golovina T.N., Carroll R.G., Riley J.L., June C.H. The inducible costimulator (ICOS) is critical for the development of human T(H)17 cells. Sci Transl Med. 2010;2:55ra78. doi: 10.1126/scitranslmed.3000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakashima A., Ito M., Yoneda S., Shiozaki A., Hidaka T., Saito S. Circulating and decidual Th17 cell levels in healthy pregnancy. Am J Reprod Immunol. 2010;63:104–109. doi: 10.1111/j.1600-0897.2009.00771.x. [DOI] [PubMed] [Google Scholar]
- 39.Zenclussen A.C., Gerlof K., Zenclussen M.L., Ritschel S., Zambon Bertoja A., Fest S., Hontsu S., Ueha S., Matsushima K., Leber J., Volk H.D. Regulatory T cells induce a privileged tolerant microenvironment at the fetal-maternal interface. Eur J Immunol. 2006;36:82–94. doi: 10.1002/eji.200535428. [DOI] [PubMed] [Google Scholar]
- 40.Shao L., Jacobs A.R., Johnson V.V., Mayer L. Activation of CD8+ regulatory T cells by human placental trophoblasts. J Immunol. 2005;174:7539–7547. doi: 10.4049/jimmunol.174.12.7539. [DOI] [PubMed] [Google Scholar]
- 41.Petroff M.G., Kharatyan E., Torry D.S., Holets L. The immunomodulatory proteins B7-DC, B7-H2, and B7-H3 are differentially expressed across gestation in the human placenta. Am J Pathol. 2005;167:465–473. doi: 10.1016/S0002-9440(10)62990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ito T., Hanabuchi S., Wang Y.H., Park W.R., Arima K., Bover L., Qin F.X., Gilliet M., Liu Y.J. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008;28:870–880. doi: 10.1016/j.immuni.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blois S.M., Ilarregui J.M., Tometten M., Garcia M., Orsal A.S., Cordo-Russo R., Toscano M.A., Bianco G.A., Kobelt P., Handjiski B., Tirado I., Markert U.R., Klapp B.F., Poirier F., Szekeres-Bartho J., Rabinovich G.A., Arck P.C. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13:1450–1457. doi: 10.1038/nm1680. [Erratum appeared in Nat Med 2009, 15:584] [DOI] [PubMed] [Google Scholar]
- 44.Gorczynski R.M., Hadidi S., Yu G., Clark D.A. The same immunoregulatory molecules contribute to successful pregnancy and transplantation. Am J Reprod Immunol. 2002;48:18–26. doi: 10.1034/j.1600-0897.2002.01094.x. [DOI] [PubMed] [Google Scholar]
- 45.Robertson S.A., Guerin L.R., Bromfield J.J., Branson K.M., Ahlström A.C., Care A.S. Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol Reprod. 2009;80:1036–1045. doi: 10.1095/biolreprod.108.074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munn D.H., Sharma M.D., Mellor A.L. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol. 2004;172:4100–4110. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]
- 47.Miwa N., Hayakawa S., Miyazaki S., Myojo S., Sasaki Y., Sakai M., Takikawa O., Saito S. IDO expression on decidual and peripheral blood dendritic cells and monocytes/macrophages after treatment with CTLA-4 or interferon-gamma increase in normal pregnancy but decrease in spontaneous abortion. Mol Hum Reprod. 2005;11:865–870. doi: 10.1093/molehr/gah246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunostaining of B7h in placenta of syngeneic CBA mice and B7h-deficient mice. Representative immunostaining for B7h on placental sections at 12.5 dpc. A: Staining in CBA/CaJ×CBA/CaJ (syngeneic) placenta. B: Staining on placental sections of B7h-deficient mice mated with CBA mice. Results are representative of two independent sets of experiments. n = 3 per experiment. Original magnification (×4 or ×20) is indicated for each image.
Litter size determination in IDO-deficient mice or in wild-type mice treated with anti–CTLA-4 mAb in an allogeneic pregnancy model. A: IDO-deficient female mice were mated with CBA males and treated with anti-B7h mAb or with control IgG. Litter size was determined at term. B: Anti–CTLA-4 blocking antibody was administered to pregnant female CBA mice, and litter size was determined between different treatment groups.
Evaluation of CD4+ regulatory T cells in the anti-B7h–treated group. Foxp3 expression was determined by intracellular staining in lymphocytes prepared from spleens of female CBA/CaJ (×C57BL/6) mice treated with anti-B7h mAb, compared with control IgG-treated mice, at 13.5 dpc. The percentage of CD4+CD25+ Foxp3+ T cells did not differ between the two groups (P = 0.3829). Representative dot plots are also shown on the left panel.