Abstract
Pulmonary arterial hypertension (PAH) is a chronic and progressive disease characterized by pulmonary vasculopathy with elevation of pulmonary artery pressure, often culminating in right ventricular failure. GATA-6, a member of the GATA family of zinc-finger transcription factors, is highly expressed in quiescent vasculature and is frequently lost during vascular injury. We hypothesized that endothelial GATA-6 may play a critical role in the molecular mechanisms underlying endothelial cell (EC) dysfunction in PAH. Here we report that GATA-6 is markedly reduced in pulmonary ECs lining both occluded and nonoccluded vessels in patients with idiopathic and systemic sclerosis-associated PAH. GATA-6 transcripts are also rapidly decreased in rodent PAH models. Endothelial GATA-6 is a direct transcriptional regulator of genes controlling vascular tone [endothelin-1, endothelin-1 receptor type A, and endothelial nitric oxide synthase (eNOS)], pro-inflammatory genes, CX3CL1 (fractalkine), 5-lipoxygenease-activating protein, and markers of vascular remodeling, including PAI-1 and RhoB. Mice with the genetic deletion of GATA-6 in ECs (Gata6-KO) spontaneously develop elevated pulmonary artery pressure and increased vessel muscularization, and these features are further exacerbated in response to hypoxia. Furthermore, innate immune cells including macrophages (CD11b+/F4/80+), granulocytes (Ly6G+/CD45+), and dendritic cells (CD11b+/CD11c+) are significantly increased in normoxic Gata6-KO mice. Together, our findings suggest a critical role of endothelial GATA-6 deficiency in development and disease progression in PAH.
Pulmonary arterial hypertension (PAH) is a severe vascular disorder characterized by an increase in resistance and blood pressure in the pulmonary artery (PA) or lung vasculature.1 The key pathological features of PAH occur in small PAs and include vasoconstriction, thrombosis, and inflammation, leading to intimal proliferation and fibrosis. Elevations in pulmonary vascular resistance and PA stiffness lead to pressure and volume overloading of the right ventricle, and eventually cause right heart failure and death. PAH is one of five groups within the pulmonary hypertension World Health Organization clinical classification system, which contains various forms including patients with idiopathic pulmonary arterial hypertension (IPAH) and familial pulmonary arterial hypertension (FPAH), and is associated with connective tissue diseases, primarily systemic sclerosis (SSc) pulmonary arterial hypertension (SSc-PAH).1 SSc-PAH has similar clinical and histopathological features such as IPAH; however, it is unclear whether the molecular mechanisms responsible for the pathogenesis of the two forms are similar.2
The cellular and molecular processes underpinning pathological vascular remodeling in PAH are complex and involve phenotypic alterations in different cell types within the vascular wall with further contributions from the circulating immune and progenitor cells.3 Although the inciting events remain poorly defined, current theories suggest that PAH is initiated by the disruption of EC homeostasis leading to an imbalance of vasoactive factors and production of prothrombotic and pro-inflammatory mediators. Genetic studies have linked dysregulated bone morphogenetic protein signaling to the pathogenesis of FPAH and IPAH. Heterozygous mutations of the bone morphogenetic protein receptor type II (BMPR2) were found in 50% to 70% of FPAH cases and 11% to 40% sporadic of IPAH cases.4 Furthermore, a subset of patients with hereditary hemorrhagic telangiectasia carrying mutations in activin receptor-like kinase or endoglin genes also develops PAH.5 Consistently, mice with the genetic ablation of Bmpr2 gene are predisposed to develop PAH.6 In contrast to FPAH and IPAH, BMPR2 and activin receptor-like kinase 1 mutations, so far, were not found in patients with SSc-PAH, suggesting that other molecular mechanisms may underlie the disease process in SSc-PAH.7,8 Notably, patients with SSc-PAH have higher disease mortality and are less responsive to currently used therapies compared to patients with IPAH.9 In addition to autoimmunity, structural changes in systemic microcirculation and inflammation, present from an early stage of the disease, appear to be more severe in patients with SSc-PAH, and are likely to contribute to SSc-PAH.9 Recent comprehensive gene analyses that compared lung tissues from patients with IPAH and SSc-PAH have further supported the role of inflammation in both forms of PAH.10 The latter study has also revealed common and unique gene expression patterns in each disease. Despite recent advances in the elucidation of the cellular processes contributing to the development of PAH, the role of ECs in the initiation and progression of PAH remains poorly understood.
GATA-6 is one of six mammalian GATA factors that are highly conserved transcription factors with two tandem zinc fingers that interact with other transcriptional regulators and bind the canonical DNA motif, (G/A)GATA(A/T).11 Human GATA-6 is expressed in a wide array of tissues (heart, lung, liver, kidney, pancreas, spleen, ovary, and small intestine), where it is believed to maintain the differentiated phenotype of the cells within these tissues.12 GATA-6 is expressed in quiescent vascular smooth muscle cells (VSMCs) and may contribute to the maintenance of the contractile phenotype of VSMCs.13,14 Furthermore, GATA-6 is rapidly decreased in proliferating VSMCs in vitro12 and in injured vasculature.15 Rescuing GATA-6 levels in a balloon-mediated injury of carotid arteries results in a higher degree of VSMC differentiation and significant reduction of neointimal formation in the rat.15 Furthermore, GATA-6 functions as a transcriptional repressor of Tenascin C,16 an extracellular matrix protein associated with progression of PAH.17 These studies suggest that loss of GATA-6 may be a key component in the pathogenesis of injury-induced vascular lesions. GATA-6 is also significantly reduced in intramyocardial arteries of spontaneously hypertensive rats.18 Importantly, GATA-6 transcript levels were found to be down-regulated concurrent with development and progression of pulmonary hypertension in rats.19 Administration of simvastatin ameliorated increased pulmonary hypertension and vascular remodeling in this model, which correlated with normalization of GATA-6 expression levels.19 Taken together, these observations indicate that GATA-6 may play a role in the phenotypic changes occurring during vascular remodeling in PAH.
Although GATA-6 vascular function has been investigated, mostly in VSMCs, a recent study has demonstrated that GATA-6 plays a vital role in angiogenesis and EC survival.20 Importantly, compared transcriptomes of dermal microvascular ECs from normal subjects and patients affected by SSc revealed that GATA-6 may be down-regulated more than twofold in lesional skin with vasculopathy.21 Given that GATA-6 is down-regulated in various animal models of vascular injury, the present study was undertaken to investigate the potential contribution of GATA-6 to the development of PAH focusing on the role of GATA-6 in ECs. We demonstrate that GATA-6 is down-regulated in pulmonary vascular lesions of PAH patients. Furthermore, the expression of GATA-6 is rapidly reduced in the monocrotaline (MCT) rat model and in the chronic hypoxia mouse model of PAH. Consistent with these findings, mice with the conditional knockdown of GATA-6 in ECs spontaneously develop elevated pulmonary arterial pressure and demonstrate increased vessel muscularization, as well as increased pulmonary inflammation. Microarray analysis coupled with chromatin immunoprecipitation assays reveal that GATA-6 regulates a set of genes linked to EC dysfunction previously associated with PAH. CX3CL1 (fractalkine) was among the genes that were significantly upregulated in GATA-6 deficient ECs, suggesting that GATA-6 down-regulation might also directly contribute to the increased inflammatory milieu. Together, these studies provide novel insights into the role of EC dysfunction during pathogenesis of PAH.
Materials and Methods
Immunohistochemical Analysis of Skin and Lung Specimens
The study group for the lung analysis included five IPAH lung specimens, nine SSc-PAH lung specimens, and four control specimens. Lung samples were obtained from patients with IPAH or SSc-PAH who underwent lung transplantation at the University of Pittsburgh Medical Center, under a protocol approved by institutional board review. Normal lung tissue specimens were obtained from donors, whose lungs were not used for tissue transplantation. Immunohistochemistry was performed on 4 μm serial sections using a Vectastain ABC kit (Vector Laboratories, Burlingame, CA) according to the manufacturer's instructions. Sections were subjected to a 5-minute antigen retrieval treatment in a stainless steel pressure cooker (Fagor, Basque Country, Spain) with antigen unmasking solution (Vector Laboratories, Burlingame, CA). Antibodies used included GATA-6 (1:100; Santa Cruz Biotechnology, Santa Cruz Biotechnology) or CD31 (1:1500; Santa Cruz Biotechnology). Binding of primary antibody to the tissue was visualized with 3.3′-diaminobenzidine solution (Vector Laboratories, Burlingame, CA). Hematoxylin was used as a counterstain. Either normal rabbit or goat IgG was used as a control. The number of positively stained ECs in arterioles, venules, and capillaries were counted in more than 25 fields for each specimen. More than 120 vessels and 340 ECs were counted per specimen.
In Situ Hybridization
In situ hybridization was performed as described earlier.22 Briefly, a 712-bp human GATA-6 probe was synthesized from a GATA-6 cDNA expression plasmid using the following primers: GATA-6 forward 5′-ATGACTCCAACTTCCACCTCT-3′; GATA-6 reverse 5′-CAGCCTCCAGAGATGTGTAC-3′. The PCR product was subject to digoxygenin-UTP labeling using the DIG (Sp6/T7) RNA labeling kit (Roche Diagnostics, Basel, Switzerland) and purification by RNeasy clean up kit (Qiagen, Hilden, Germany). Sections were incubated overnight with the GATA-6 sense or antisense probe (final concentration 200 ng/mL). Immuno-BCIP/NBT liquid substrate (MP Biomedicals, Santa Ana, CA) was used to detect hybridization. A poly d(T) probe was used as a control for intact RNA.
Cell Culture
Human PA ECs (HPAECs) were purchased from Lonza (Walkersville, MD) and cultured in complete endothelial growth medium-2. The cells were used at passage number 5–8 for all experiments.
Transfection of siRNA Oligos
Confluent cultures of HPAECs were transfected with 50 nmol/L of siRNA directed against GATA-6 (Dharmacon, Waltham, MA) and nonsilencing siRNA (Qiagen) using Genesilencer (Genlantis, San Diego, CA). Cells were incubated for 72 hours and either total RNA was prepared using TRI reagent (MRC, Inc., Cincinnati, OH) according to the manufacturer's protocol or whole cell extracts were prepared.
Quantitative RT-PCR
Quantitative RT-PCR was performed as previously described.23 Sequences for all primers used in these studies are shown in Tables 1 and 2.
Table 1.
Primers Used for Human RT-qPCR
| Gene | Primers | |
|---|---|---|
| PLA2G4C | 5′-CCACTCACAACTTCCTGTACAAAC-3′ | Forward |
| 5′-ATGGCTAAACCAGCATCCA-3′ | Reverse | |
| FLAP | 5′-GTCTGCGGGGCTACTTTG-3′ | Forward |
| 5′-TGCCTCACAAACAAGTACATCA-3′ | Reverse | |
| RhoB | 5′-TAAGGGTGGTGATGGGTGAG-3′ | Forward |
| 5′-GGGTTGGAAAGATGGTCAAG-3′ | Reverse | |
| MMP-10 | 5′-TGGACAGAAGATGCATCAGG-3′ | Forward |
| 5′-CTTCAGTGTTGGCTGAGTGAA-3′ | Reverse | |
| MMP-1 | 5′-TCTGGGGTGTGGTGTCTCA-3′ | Forward |
| 5′-GCCTCCCATCATTCTTCAGGTT-3′ | Reverse | |
| PAI-1 | 5′-CCCAGCTCATCAGCCACT-3′ | Forward |
| 5′-GAGGTCGACTTCAGTCTCCAG-3′ | Reverse | |
| CX3CL1 | 5′-CCACCTTCTGCCATCTGAC-3′ | Forward |
| 5′-ATGTTGCATTTCGTCACACC-3′ | Reverse | |
| eNOS | 5′-AGGAACCTGTGTGACCCTCA-3′ | Forward |
| 5′-TATCCAGGTCCATGCAGACA-3′ | Reverse | |
| ACE | 5′-AACATGAGCAGGATCTACTCCAC-3′ | Forward |
| 5′-AGCCAGGATGTTGGTGAGA-3′ | Reverse | |
| EDNRA | 5′-CTCAACCTCTGCGCTCTTAGTG-3′ | Forward |
| 5′-CCAAAGGAATCCCAATTCCC-3′ | Reverse | |
| ET-1 | 5′-GCTCGTCCCTGATGGATAAA-3′ | Forward |
| 5′-CCATACGGAACAACGTGCT-3′ | Reverse | |
| GATA-6 | 5′-TTGTGGACTCTACATGAAACTCCA-3′ | Forward |
| 5′-TTATGTTCTTAGGTTTTCGTTTCCTG-3′ | Reverse |
Table 2.
Primers Used for Mouse RT-qPCR
| Gene | Primers | |
|---|---|---|
| PLA2G4C | 5′-GAGGACCTTCTGGCTGATTG-3′ | Forward |
| 5′-CCAGCATGATGAGGAGTGAA-3′ | Reverse | |
| Flap | 5′-CTGCTTCTCATCCCCTGATT-3′ | Forward |
| 5′-TTGCGTTATGATGCGTCTCT-3′ | Reverse | |
| RhoB | 5′-CAGACTGCCTGACATCTGCT-3′ | Forward |
| 5′-GTGCCCACGCTAATTCTCAG-3′ | Reverse | |
| MMP-10 | 5′-AGGAAGTGACCCCACTCAC-3′ | Forward |
| 5′-GGGTAAAAGTCTCCGTGTTCTC-3′ | Reverse | |
| Pai-1 | 5′-AGGATCGAGGTAAACGAGAGC-3′ | Forward |
| 5′-GCGGGCTGAGATGACAAA-3′ | Reverse | |
| CX3CL1 | 5′-CGCGTTCTTCCATTTGTGTA-3′ | Forward |
| 5′-CATGATTTCGCATTTCGTCA-3′ | Reverse | |
| eNOS | 5′-CCAGTGCCCTGCTTCATC-3′ | Forward |
| 5′-GCAGGGCAAGTTAGGATCAG-3′ | Reverse | |
| Ace | 5′-TTGATGGAAGCATCACCAAG-3′ | Forward |
| 5′-GGGCACAGACCCTGATACTT-3′ | Reverse | |
| EDNRA | 5′-TGATCGTTTCATCTTCTTTCAATG-3′ | Forward |
| 5′-CCTCATCAGACGGTCTTCCT-3′ | Reverse | |
| Et-1 | 5′-CTGCTGTTCGTGACTTTCCA-3′ | Forward |
| 5′-TCTGCACTCCATTCTCAGCTC-3′ | Reverse | |
| Gata-6 (mouse) | 5′-GGTGCTCCACAGCTTACAGG-3′ | Forward |
| 5′-GCCGTCTCGTCTCCACAG-3′ | Reverse | |
| Gata-6 (rat) | 5′-ACGCATGCGGTCTCTACAGT-3′ | Forward |
| 5′-AGTCCAAGCCGTCGTGAT-3′ | Reverse | |
| Hif1a | 5′-GCACTAGACAAAGTTCACCTGAGA-3′ | Forward |
| 5′-CGCTATCCACATCAAAGCAA-3′ | Reverse | |
| Hif1b | 5′-TGCCTCATCTGGTACTGCTG-3′ | Forward |
| 5′-TGTCCTGTGGTCTGTCCAGT-3′ | Reverse | |
| Hif1a | 5′-GGTTAAGGAACCCAGGTGCT-3′ | Forward |
| 5′-GGGATTTCTCCTTCCTCAGC-3′ | Reverse |
Immunoblotting for Cell Extracts
Nitrocellulose membranes were blocked with nonfat dry milk in T-TBS and then probed overnight with anti–GATA-6 (sc-9055; 1:500; Santa Cruz Biotechnology), anti-RhoB (sc-180; 1:250; Santa Cruz Biotechnology), anti–endothelial Nitric Oxide Synthase (eNOS; sc-654; 1:500; Santa Cruz Biotechnology), anti–MMP-1 (MAB3307; 1:1000; Millipore, Billerica MA), anti-MMP10 (MS-8220-PO; 1:500; NeoMarkers, Fremont, CA), or PAI-1 (395R; 1:250; American Diagnostics, Stamford, CT). Blots were incubated for at least 1 hour in the appropriate horseradish peroxidase coupled-secondary antibodies (1:3000) and developed using the Chemiluminescent detection kit (Pierce, Waltham, MA). Band intensities were determined by densitometric analysis.
ELISA
Cell culture supernatants were subjected to enzyme-linked immunosorbent assay (ELISA) using a human Quantikine CX3CL1 ELISA kit (R&D Systems, Minneapolis, MN) following the manufacturer's instructions.
Chromatin Immunoprecipitation
Chromatin immunoprecipitation was performed as previously described.24 Relative fold enrichment of DNA was determined by PCR using specific primers for the indicated gene promoters (Table 3), followed by agarose gel electrophoresis. Primers that flank a region of genomic DNA between the glyceraldehydes-3-phosphate dehydrogenase gene and the CNAP1 gene were used a negative control.
Table 3.
Primers Used for ChIP
| Promoter | Primers | |
|---|---|---|
| Negative control | 5′-ATGGTTGCCACTGGGGATC-3′ | Forward |
| 5′-TGCCAAAGCCTAGGGGAAGA-3′ | Reverse | |
| PLA2G4C | 5′-ATGGCTCCACCAGGTAGGTA-3′ | Forward |
| 5′-AGCTCATGAGGCTCTCCACA-3′ | Reverse | |
| FLAP | 5′-CAGTGGTCCATGTTCCCTTT-3′ | Forward |
| 5′-AGGTGGCTGCCTGGTATTCT-3′ | Reverse | |
| RhoB | 5′-ACAGGCATGAGGTACTGTGC-3′ | Forward |
| 5′-CGGTCTCTGTCAGTTGTTG-3′ | Reverse | |
| MMP-10 | 5′-TACCAAGCTTGTCAGCTCTG-3′ | Forward |
| 5′-GGTGATACAGCCTACATCAG-3′ | Reverse | |
| MMP-1 | 5′-AATGCTGCCTAGCACCAAGG-3′ | Forward |
| 5′-AGAGCCTTACCTGAGAAGAC-3′ | Reverse | |
| PAI-1 | 5′-AGGCAGGAGAACCACTTGAA-3′ | Forward |
| 5′-ACTGGCTAGCAGTGGGTGAG-3′ | Reverse | |
| CX3CL1 | 5′-CTTCGGTGAGAGCCTGTTG-3′ | Forward |
| 5′-CCCTGTTAGCCCAAAGAACA-3′ | Reverse | |
| eNOS | 5′-TGGTGCCACATCACAGAAGG-3′ | Forward |
| 5′-TCTAAAGCCTCAGCTCCACG-3′ | Reverse | |
| EDNRA | 5′-CGCTGGAACCTTCCATAGTC-3′ | Forward |
| 5′-AAGCTTAGAGGCCGTTGAGG-3′ | Reverse | |
| ET-1 | 5′-ATCTCCCCCTGGTGTTCTTC-3′ | Forward |
| 5′-TTAGGCACATGCCCAGTCTT-3′ | Reverse | |
| VE-Cadherin | 5′-GGTTCTTCTGGGCTCTGATC-3′ | Forward |
| 5′-GGATATTGGGTGGAGTCAAG-3′ | Reverse |
ChIP, chromatin immunoprecipitation.
Mice
Gata6flox/flox mice were purchased from Jackson Laboratory (Bar Harbor, ME). The mouse line, which contains LoxP elements in introns flanking exon 2 of the Gata-6 gene, was bred with mice expressing Cre recombinase under the control of the endothelial-specific VE-cadherin promoter (Jackson Laboratory). For hypoxic conditions, mice were placed in a hypoxic chamber (10% oxygen) (BioSpherix, Lacona, NY).
Mouse EC Isolation
Two different methods were used to isolate mouse ECs. In the first method, lung and hearts were excised aseptically and transferred to ice-cold Dulbecco's modified Eagle's medium. Under the hood, the tissues were minced finely, followed by digestion in 15 mL of 1 mg/mL warm collagenase (Roche Diagnostics) at 37°C for 45 minutes with gentle agitation. The digested tissue was aspirated and transferred to a 20 mL syringe with a 14 g cannula attached and the clumps were triturated. The single cell suspension was then passed through a 70 μm cell strainer and subjected to centrifugation. The cell pellet was resuspended in 0.1% bovine serum albumin/PBS and anti-platelet EC adhesion molecule-1 antibody conjugated Dynabeads (Invitrogen, Carlsbad, CA) were added for 12 minutes with rotation. Beads were prepared according to the manufacturer's instructions. After washing, cells were plated on gelatin-coated 60 mm dishes. When cells were 70% to 80% confluent, they were sorted a second time with anti–ICAM-2 antibody-conjugated Dynabeads. Cultured ECs were characterized by a cobblestone morphology, uptake of fluorescent acetylated LDL (Biomedical Technologies Inc, Stoughton, MA), and specific staining for CD31. Cells were harvested at passages 2 to 3 for analyses.
In the second method, which was used for isolation of fresh cells for mRNA analyses, mouse lung tissue was finely minced and digested with 0.1 collagenase A (Roche Diagnostics), 2.4 units/mL dispase (Roche Diagnostics), and 6 units/mL DNase I (Qiagen) at 37°C for 1 hour. Debris was removed by sequential filtration through 70 and 40 μm filters (BD Biosciences, San Jose, CA). Cells were stained with fluorochrome-conjugated mouse-specific antibodies CD31-FITC/CD45-APC from BD Biosciences and then CD31+/CD45− cells were sorted by MoFlo High Speed Cell Sorter (BD Biosciences).
Hemodynamic Analysis
Systolic blood pressure, diastolic blood pressure, mean pressure, heart pulse rate, and blood volume were measured noninvasively by determining the tail blood volume with a volume pressure recording sensor and an occlusion tail-cuff (CODA System; Kent Scientific, Torrington, CT).
Echocardiography
Transthoracic echocardiography was performed using a Vevo 770 High-Resolution Imaging System with 30-MHz RMV-707b scanning head (VisualSonics, Toronto, Canada). Pulmonary acceleration time (PAT) and PAT as a fraction of ejection time were measured from the pulse-wave Doppler recordings of the PA blood flow as previously described at baseline and after 1 month and 2 months of hypoxia.25
Measurement of RV Pressure
After the open chest method,26 right ventricular (RV) pressures were measured using a high-fidelity pressure sensor catheter inserted directly into the right ventricle. Briefly, mice were anesthetized using isoflurane and mechanically ventilated through a 22-gauge cannula [130 breaths per minute, Harvard Apparatus (Holliston, MA) rodent ventilator]. Body temperatures were maintained on a heating pad. The thoracic cage was pulled upward by the xiphoid cartilage and the diaphragm was carefully removed to expose the heart. Cauterization of the tissue was used to minimize blood loss. The heart was superfused with a few drops of warm saline. The tip of a 25-guage needle was immersed in heparin solution (10,000 USP units/mL) and gently inserted into the right ventricle using the right coronary artery as a guide. The needle was retracted and the tip of a pressure catheter transducer (Micko-Tip, SPR-839; Millar Instruments, Houston, TX) was inserted through the small aperture. Pressure waveforms were recorded for at least 2 minutes for each mouse in real-time using the PowerLab Chart 5 version 5.3 data acquisition system and analysis software (ADInstruments, Colorado Springs, CO). RV pressures were calculated by averaging at least 20 cardiac cycles for each mouse.
Mouse Histology and Pulmonary Vascular Morphometry
Mice were euthanized by CO2 gas and the right lung (with the heart) was inflated and fixed overnight in 4% paraformaldehyde. After fixation, the lung was dehydrated through a series of alcohol gradients and embedded in paraffin. Five μm sections were cut, deparaffinized in Histo-Clear (National Diagnostics, Atlanta, GA), and stained with an anti–α-smooth muscle actin antibody (dilution 1:800, clone 1A4; Sigma, St. Louis, MO), an anti–human von Willebrand factor antibody (dilution 1:800; Dako, Hamburg, Germany), and an anti–mouse Mac3 antibody (dilution 1:50; BD Pharminogen, San Jose, CA) using a Vectastain ABC kit (Vector Laboratories). Peripheral PAs ranging in 20 to 70 μm in size were counted in at least four fields at ×20 magnification with a Zeiss (Thornwood, NY) Axiovert-35 inverted microscope. Counted vessels were categorized into nonmuscularized, partially muscularized (1% to 74% of medial layer has positive α-smooth muscle actin [SMA] staining), or fully muscularized (75% to 100% of medial layer has positive α-SMA staining). Percentages of vessels in each category were calculated by dividing the total number of vessels in each category by the total number of vessels counted in the field. Percent wall thickness (the thickness between the inner and outer boundary of the α-SMA-staining medial layer) was measured in fully muscularized PAs at two sites along the blood vessel using ImageJ software version 1.45s (NIH, Bethesda, MD). External diameters were measured concurrently for the same vessel and the percentage medial wall thickness was calculated as (wall thickness1 + wall thickness2) × 100 per external diameter.
Analysis of Cardiac Valves in Wt and Gata6 Conditional Knockout Mice
Hearts isolated from adult wild-type (Wt) and Gata6 conditional knockout mice were fixed in a 4% paraformaldehyde. Heart tissues were sectioned at 10 μm and stained with H&E, similar to previously described methods.27 Valve histology was analyzed by comparison of serial sections of Wt (2) and Gata6-KO (3) mice under normoxic and hypoxic conditions.
Ventricular Weight Measurements
The ventricles were excised and weighed. The weight ratio of the right ventricle to the left ventricle plus septum was calculated as indices of RV hypertrophy.
MCT-Induced Rat Model of PAH
Male Sprague-Dawley rats (200 to 225 g body weight) were dosed with a single 60 mg/kg body mass subcutaneous injection of MCT. Control rats received an equal volume of saline. Rats were housed in a 12/12-light/dark cycle and given standard rat chow and water ad libitum. They were euthanized at 1, 3, 5, 10, and 20 days postinjection.
RNA Isolation from Mouse and Rat Lungs and Real Time-PCR
RNA was isolated and purified using the Qiagen RNeasy kit (Qiagen) following the manufacturer's instructions. RT-PCR was performed as previously described using the primers listed in Table 2.
Immunoprecipitation and Immunoblotting for Rat Lungs
Lungs were homogenized in radioimmunoprecipitation assay buffer using a douncer. Then 1000 μg of extract was pre-cleared with protein G-Sepharose beads (Amersham Biosciences, Pittsburgh, PA), and incubated with 1 μg of monoclonal mouse anti-GATA6 antibody (R&D Systems) overnight at 4°C with gentle rotation. Protein G-Sepharose beads were added and the precipitated proteins were subjected to SDS-PAGE. Immunoblotting for Gata-6 was performed as previously described for cell extracts. As an input control, 10 μL of the pre-cleared extract was subjected to SDS-PAGE and membranes were incubated with a mouse anti–β-actin antibody (1:5000, Sigma).
Rat Lung Histology and Immunohistochemistry
Sections (5 μm thick) were stained with Masson's trichrome stain and H&E stain using standard techniques. Immunohistochemical analysis was performed using the standard avidin-biotin-peroxidase method as previously described. Sections were incubated in primary antibody against α-SMA (Thermo Scientific, Neomarkers, Kalamazoo, MI).
Flow Cytometry Analysis
Flow cytometry was performed on an LSRII cytometer (BD Biosciences) and data were analyzed using FlowJo software version 6.3.2 (Tree Star Inc., Ashland, OR). Mouse lung tissue was finely minced and digested with 0.1 collagenase A (Roche Diagnostics), 2.4 units/mL dispase (Roche Diagnostics), and 6 units/mL DNase I (Qiagen) at 37°C for 1 hour. Debris was removed by sequential filtration through 70 and 40 μm filters (BD Biosciences). Cells were stained with fluorochrome-conjugated mouse-specific antibodies (T cells CD3-PE/CD45-APC, B cells B220-PE/CD45-APC, macrophages CD11b-FITC/F4/80-PE, granulocytes Ly6G-FITC/CD45-APC, and dendritic cells CD11b-FITC/CD11c-PerCP) from BD Biosciences.
Statistical Analysis
All data were analyzed by Student's paired t-test. Mouse data were also subjected to one-way analysis of variance with Bonferroni post hoc). The level for statistical significance was set at P < 0.05.
Results
GATA-6 Levels are Markedly Decreased in the Pulmonary Vasculature of SSc-PAH and IPAH Patients
We first examined expression of GATA-6 protein in lung specimens from nine patients with SSc-PAH, five patients with IPAH, and four healthy controls. Representative stainings are shown in Figure 1, A and B. In healthy controls, GATA-6 protein was detected in the nuclei of alveolar type II cells, VSMCs, and ECs; however, peripheral, slight, or no nuclear staining could be detected in ECs of SSc-PAH tissue and slight or no nuclear staining for GATA-6 could be seen in ECs of IPAH tissue. GATA-6 positive EC nuclei were counted in arterioles and venules for each lung specimen (Figure 1, A and B, respectively). The analysis revealed a significant reduction of GATA-6 in both vessel types, including occluded and nonoccluded, in both SSc-PAH and IPAH patients. Reduced expression of GATA-6 was also observed in the capillaries of PAH patients. In addition, GATA-6 protein levels were drastically decreased in VSMCs, but there were no apparent changes in the expression of GATA-6 in alveolar type II cells. To further analyze if GATA-6 is decreased at the mRNA level, we performed in situ hybridization studies (Figure 1C). The results demonstrated that GATA-6 is abundantly expressed in ECs lining arterioles and venules in normal controls, but is strikingly decreased in ECs lining both vessel types in IPAH patients. These observations suggest that GATA-6 deficiency may contribute to vessel remodeling during PAH.
Figure 1.
GATA-6 levels are decreased in the pulmonary vasculature of SSc-PAH and IPAH patients. A and B: Paraffin-embedded tissue sections from four healthy controls, five IPAH, and nine SSc-PAH patients were analyzed for GATA-6 using the standard avidin-biotin-peroxidase methodology. Arrows indicate positively or negatively stained ECs in occluded and nonoccluded arterioles (A), and venules (B) of healthy individuals, IPAH patients, and SSc-PAH patients. C: Percentage of positively stained ECs in both occluded and nonoccluded arterioles and venules [in normal (bullets), IPAH (open diamonds) and SSC-PAH (triangles) lung tissues] were plotted on a scatter plot. ∗P < 0.01. In situ hybridization of paraffin-embedded tissue from healthy individuals and IPAH patients were used to analyze GATA-6 mRNA levels Arrows point to GATA-6 positive or negative ECs in the indicated vessels of healthy controls (top panel) or IPAH patients (bottom panel). Original magnification, ×400. Scale bars: 50 μm (A–C).
GATA-6 Regulates Expression of Genes Involved in Vascular Remodeling
In an effort to begin to understand how loss of GATA-6 in ECs may contribute to vascular remodeling, we sought to identify genes regulated by GATA-6. To this end, we used a commercially available EC PCR array (SABiosciences, Valencia, CA) and performed the analyses in HPAECs after suppression of GATA-6 with siRNA oligos. This screen identified genes involved in vessel tone and permissibility (eNOS, ACE, EDNRA, ET-1), EC activation (CX3CL1, PAI-1, RhoB, 5-lipoxygenease-activating protein [FLAP]), matrix remodeling (MMP1, MMP10), and EC injury (PLA2G4C) (for complete list of genes, see Table 4). mRNA levels of putative target genes were examined by RT-qPCR. As shown in Figure 2A, levels of mRNA for PLA2G4C, FLAP, RhoB, MMP1, MMP10, PAI-1, CX3CL1, ACE, EDNRA, and ET-1 were elevated, whereas levels of eNOS were decreased after suppression of GATA-6. Western blot analysis and ELISA also confirmed differential expression of eNOS, MMP-1, MMP-10, PAI-1, RhoB, and CX3CL1 at the protein level (Figure 2, B and C, respectively). These data strongly suggest that GATA-6 may play a role in PAH by regulating genes that promote vascular remodeling and dysfunction. To further investigate if GATA-6 is a direct transcriptional regulator of these genes, we performed chromatin immunoprecipitation analysis. Putative GATA sites were identified using Tfsitescan. As shown in Figure 2D and Table 5, GATA-6 occupied one or more sites on the promoters of FLAP, RhoB, MMP10, MMP1, PAI-1, CX3CL1, eNOS, EDNRA, and ET-1, indicating that GATA-6 is a direct regulator of these genes. We were not able to detect GATA-6 binding on the PLA2G4C promoter. Finally, as an additional negative control, we were not able to immunoprecipitate the promoter of VE-Cadherin, a gene that is not regulated by GATA-6 according to our microarray data.
Table 4.
The Human Endothelial Cell Biology RT2Profiler PCR Array Gene List
| Description | Up-regulation and down-regulation (compared to control group) |
|
|---|---|---|
| Symbol | Group 1 |
|
| Fold regulation | ||
| Angiotensin I converting enzyme (peptidyl-dipeptidase A) 1 | ACE | 2.3457 |
| ADAM metallopeptidase domain 17 | ADAM17 | 1.2746 |
| Angiotensinogen (serpin peptidase inhibitor, clade A, member 8) | AGT | −1.014 |
| Angiotensin II receptor, type 1 | AGTR1 | −1.014 |
| Arachidonate 5-lipoxygenase | ALOX5 | 1.954 |
| Angiopoietin 1 | ANGPT1 | −1.014 |
| Annexin A5 | ANXA5 | 1.0425 |
| BCL2-associated X protein | BAX | −1.1647 |
| B-cell CLL/lymphoma 2 | BCL2 | −1.3566 |
| BCL2-related protein A1 | BCL2A1 | 1.454 |
| BCL2-like 1 | BCL2L1 | 1.5801 |
| Chemokine (C-X-C motif) receptor 5 | CXCR5 | −1.014 |
| Caspase 1, apoptosis-related cysteine peptidase (interleukin 1, beta, convertase) | CASP1 | −1.0281 |
| Caspase 3, apoptosis-related cysteine peptidase | CASP3 | 1.007 |
| Caspase 6, apoptosis-related cysteine peptidase | CASP6 | 1.1728 |
| Chemokine (C-C motif) ligand 2 | CCL2 | 1.3379 |
| Chemokine (C-C motif) ligand 5 | CCL5 | −1.0644 |
| Cadherin 5, type 2 (vascular endothelium) | CDH5 | 1.1173 |
| CASP8 and FADD-like apoptosis regulator | CFLAR | 1.3104 |
| Collagen, type XVIII, alpha 1 | COL18A1 | 1.2834 |
| Carboxypeptidase B2 (plasma) | CPB2 | 2.941 |
| CASP2 and RIPK1 domain containing adaptor with death domain | CRADD | 2.0705 |
| Colony stimulating factor 2 (granulocyte-macrophage) | CSF2 | −1.014 |
| Chemokine (C-X3-C motif) ligand 1 | CX3CL1 | 2.7895 |
| Thymidine phosphorylase | TYMP | 1.3472 |
| Endothelin 1 | EDN1 | 1.4044 |
| Endothelin 2 | EDN2 | −1.014 |
| Endothelin receptor type A | EDNRA | 2.395 |
| Fas (TNF receptor superfamily, member 6) | FAS | −1.1019 |
| Fas ligand (TNF superfamily, member 6) | FASLG | −1.014 |
| Fibroblast growth factor 1 (acidic) | FGF1 | −1.014 |
| Fms-related tyrosine kinase 1 (vascular endothelial growth factor/vascular permeability factor receptor) | FLT1 | 1.1408 |
| Fibronectin 1 | FN1 | 1.5263 |
| Intercellular adhesion molecule 1 | ICAM1 | 1.434 |
| Interferon, beta 1, fibroblast | IFNB1 | 1.7654 |
| Interleukin 11 | IL11 | 1.7901 |
| Interleukin 1, beta | IL1B | 1.5134 |
| Interleukin 3 (colony-stimulating factor, multiple) | IL3 | −1.014 |
| Interleukin 6 (interferon, beta 2) | IL6 | −1.1408 |
| Interleukin 7 | IL7 | −1.1567 |
| Integrin, alpha 5 (fibronectin receptor, alpha polypeptide) | ITGA5 | 1.2834 |
| Integrin, alpha V (vitronectin receptor, alpha polypeptide, antigen CD51) | ITGAV | 1.0425 |
| Integrin, beta 1 (fibronectin receptor, beta polypeptide, antigen CD29 includes MDF2, MSK12) | ITGB1 | 1 |
| Integrin, beta 3 (platelet glycoprotein IIIa, antigen CD61) | ITGB3 | −1.2924 |
| Kinase insert domain receptor (a type III receptor tyrosine kinase) | KDR | 1.2658 |
| V-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog | KIT | −1.0867 |
| Kallikrein-related peptidase 3 | KLK3 | −1.014 |
| Matrix metallopeptidase 1 (interstitial collagenase) | MMP1 | 2.0705 |
| Matrix metallopeptidase 2 (gelatinase A, 72kDa gelatinase, 72kDa type IV collagenase) | MMP2 | 1.2658 |
| Matrix metallopeptidase 9 (gelatinase B, 92kDa gelatinase, 92kDa type IV collagenase) | MMP9 | 3.7412 |
| Nitric oxide synthase 2, inducible | NOS2 | 1.2658 |
| Nitric oxide synthase 3 (endothelial cell) | NOS3 | −2.3134 |
| Natriuretic peptide B | NPPB | −1.014 |
| Natriuretic peptide receptor A/guanylate cyclase A (atrionatriuretic peptide receptor A) | NPR1 | 1.1329 |
| Occludin | OCLN | −1.014 |
| Platelet-derived growth factor receptor, alpha polypeptide | PDGFRA | 1.9211 |
| Platelet/endothelial cell adhesion molecule | PECAM1 | 1.1567 |
| Platelet factor 4 | PF4 | −1.0425 |
| Placental growth factor | PGF | 1.0867 |
| Phospholipase A2, group IVC (cytosolic, calcium-independent) | PLA2G4C | 1.8703 |
| Plasminogen activator, tissue | PLAT | 1.4845 |
| Plasminogen activator, urokinase | PLAU | 1.5476 |
| Plasminogen | PLG | −1.014 |
| Prostaglandin I2 (prostacyclin) synthase | PTGIS | −1.5443 |
| Ras homolog gene family, member B | RHOB | 1.7443 |
| Receptor (TNFRSF)-interacting serine-threonine kinase 1 | RIPK1 | 1.0497 |
| Selectin E | SELE | −1.1251 |
| Selectin L | SELL | −1.9754 |
| Selectin P ligand | SELPLG | −1.014 |
| Serpin peptidase inhibitor, clade E (nexin, plasminogen activator inhibitor type 1), member 1 | SERPINE1 | 2.2658 |
| Superoxide dismutase 1, soluble | SOD1 | 1.0353 |
| Sphingosine kinase 1 | SPHK1 | 1.2483 |
| TEK tyrosine kinase, endothelial | TEK | 1.1567 |
| Tissue factor pathway inhibitor (lipoprotein-associated coagulation inhibitor) | TFPI | −1.0943 |
| Thrombomodulin | THBD | −1.1251 |
| Thrombospondin 1 | THBS1 | 1.3013 |
| TIMP metallopeptidase inhibitor 1 | TIMP1 | −1.1096 |
| Tumor necrosis factor | TNF | −1.014 |
| Tumor necrosis factor, α-induced protein 3 | TNFAIP3 | 1.4044 |
| Tumor necrosis factor receptor superfamily, member 10c, decoy without an intracellular domain | TNFRSF10C | −1.0792 |
| Tumor necrosis factor (ligand) superfamily, member 10 | TNFSF1 | −1.2397 |
| Vascular cell adhesion molecule 1 | VCAM1 | 1.2397 |
| Vascular endothelial growth factor A | VEGFA | 1.7171 |
| Von Willebrand factor | VWF | 1.1567 |
| Beta-2-microglobulin | B2 mol/L | −1.2142 |
| Hypoxanthine phosphoribosyltransferase 1 | HPRT1 | 1.1173 |
| Ribosomal protein L13a | RPL13A | −1.0281 |
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | 1.1173 |
| Actin, beta | ACTB | 1.5052 |
| Human genomic DNA contamination | HGDC | −1.014 |
| Reverse transcription control | RTC | 1.2658 |
| Reverse transcription control | RTC | 1.257 |
| Reverse transcription control | RTC | 1.3379 |
| Positive PCR control | PPC | 1.0497 |
| Positive PCR control | PPC | −1.4743 |
| Positive PCR control | PPC | −1.2658 |
ADAM, A-disintegrin and metalloproteinase; BCL2, B cell lymphoma 2; CASP8, cysteine-aspartic protease 8; CLL, chronic lymphoid leukemia; FADD, fas-associated protein with death domain; TEK, tyrosine kinase endothelial; TIMP, tissue inhibitor of metalloproteinases; TNF, tumor necrosis factor.
Figure 2.
GATA-6 regulates genes involved in vascular remodeling. HPAECs were transfected with control or small interfering RNA against GATA-6 oligos for 72 hours. Samples were assayed by RT-qPCR to determine mRNA levels (A) or by Western blot analysis (B) and ELISA (C) to determine protein levels for the genes indicated. The experiments were performed at least three times and representative blots are shown. Band intensities were quantified by densitometric analysis. Black bars, control siRNA; grey bars, Gata6 siRNA treated cells. ∗P < 0.05, ∗∗P < 0.01. D: Chromatin immunoprecipitation assays were performed in HPAECs using rabbit anti–GATA-6 polyclonal antibody or rabbit IgG. The experiments were performed at least three times and representative gels are shown.
Table 5.
Summary of Results for ChIP Analysis
| Gene | Predicted putative GATA binding site | Amplified regions of target gene promoter | In vivo binding of GATA-6 |
|---|---|---|---|
| PLA2G4C | −982 to 977, −962 to 957 | −1081 to 878 | + |
| FLAP | −1101 to 1096 | −1134 to 966 | + |
| RhoB | −1877 to 1872, −1731 to 1726, | −1908 to 1689 | + |
| −862 to 857 | −1034 to 801 | − | |
| MMP-10 | −1831 to 1826, −1798 to 1793 | −1922 to 1721 | + |
| −1573 to 1568 | −1650 to 1428 | − | |
| −994 to 989 | −1112 to 885 | − | |
| −226 to 221 | −270 to 114 | + | |
| MMP-1 | −1356 to 1351, −1323 to 1318 | −1435 to 1200 | + |
| −1112 to 1107, −982 to 977, −955 to 950 | −1137 to 907 | − | |
| PAI-1 | −1691 to 1686 | −1739 to 1556 | + |
| −572 to 567 | −677 to 490 | + | |
| −431 to 426 | −510 to 305 | + | |
| CX3CL1 | −881 to 876, −801 to 796 | −958 to 755 | + |
| eNOS | −1114 to 1109 | −1220 to 1000 | − |
| −203 to 198 | −300 to 119 | + | |
| EDNRA | −767 to 762 | −846 to 650 | − |
| −495 to 490 | −568 to 421 | − | |
| −328 to 323 | −420 to 261 | + | |
| ET-1 | −1207 to 1202, −1154 to 1149 | −1298 to 1107 | − |
| −745 to 740, −701 to 696, −681 to 676 | −849 to 652 | − | |
| −136 to 131 | −254 to 68 | + |
ChIP, chromatin immunoprecipitation.
GATA-6 Down-Regulation is an Early Event in the Chronic Hypoxia Mouse Model and the MCT Rat Model of PAH
Because endothelial GATA-6 deficiency affected expression of genes previously implicated in the development of PAH, we next determined the levels of expression of GATA-6 in two rodent models of PAH, the chronic hypoxia mouse model, and the MCT rat model. A significant decrease of Gata-6 expression was already noticeable at 3 days after hypoxia and expression decreased further at 7 and 21 days (Figure 3A). Several of the GATA-6 target genes, including Pla2G4C, RhoB, EDNRA, and Pai-1 were increased, whereas, surprisingly, Cx3cl1 gradually decreased starting at day 3 (Figure 3B). Furthermore, hypoxia inducible factor (Hif) subunits Hif1α, Hif1β, and Hif2α showed the most pronounced upregulation at day 3 (Figure 3B) with significantly elevated levels continuing for the duration of the experiment (up to 2 months) (Supplemental Figure S1).
Figure 3.
Gata-6 expression and target genes are altered in the lungs of hypoxic mice. Lung samples from control mice housed under normoxic conditions (baseline) and mice exposed to hypoxic conditions for 3, 7, and 21 days were assayed by RT-qPCR to determine mRNA levels of Gata-6 (A) and putative target genes (B). Levels are relative to baseline (normoxic) animals. ∗P < 0.05, ∗∗P < 0.01 (n = 3 to 5 per group). C: A mouse line, which contains LoxP elements in introns flanking exon 2 of the Gata-6 gene (Jackson Laboratory), were bred with mice expressing Cre recombinase under the control of the endothelial-specific VE-cadherin promoter. The mRNA levels of GATA-6 were determined in pulmonary ECs isolated from Wt and Gata6-KO (KO) neonate mice by RT-qPCR. ∗P < 0.01. D: Pulmonary ECs isolated from both Wt and KO mice exposed to either normoxia or hypoxia for 1, 3, 5, or 7 days. In each group, cells were pulled together from three animals and assayed by RT-qPCR to determine mRNA levels of the genes indicated. E: HPAECs were transfected with control or siGATA-6 oligos for 72 hours. Samples were assayed by RT-qPCR to determine mRNA levels for the genes indicated. ∗P < 0.05, ∗∗P < 0.01.
Next we investigated if Gata-6 expression is decreased after MCT injury (Supplemental Figure S2, A–E). Gata-6 expression was significantly decreased as early as 3 days and further gradually decreased up to 20 days postinjection. Gata-6 protein levels are also decreased in the lung tissue of rats challenged with MCT after 20 days. These findings indicate that GATA-6 expression levels are down-regulated at both early and late stages of disease in two animal models of PAH.
GATA-6 Deficiency Augments mRNA Expression of Endothelial HIF2α and HIF1β
To study the involvement of altered Gata-6 expression in the vasculature in vivo, we selectively reduced expression of the Gata-6 gene in ECs by crossing an established, commercially available mouse line harboring a conditional loss-of-function allele of Gata-6 with mice expressing Cre recombinase under the control of the endothelial-specific VE-Cadherin promoter to generate Gata-6flox/flox/VEcadCre+/− mice (Gata6-KO). Sex-matched littermates lacking the Cre allele were used as controls. Isolation of pulmonary ECs from Gata6-KO mice and controls demonstrated that Gata-6 expression is reduced by 65% (Figure 3C).
After exposure to chronic hypoxia down-regulation of Gata-6 correlated with upregulation of Hif1α and Hifβ and Hif2α, we next assessed the contribution of endothelial Gata-6 to the upregulation of Hif subunits using Wt and Gata6-KO mice. Pulmonary ECs (CD31+/CD45−) were sorted by FACS at days 1, 3, 5, and 7, and were directly used for mRNA analysis. In Wt mice, a gradual decrease of Gata-6 expression starting at day 1 of hypoxia was observed, whereas a rapid increase of Hif1α occurred at day1, then gradually returned to control levels by day 7 (Figure 3D). A similar pattern of expression was also observed for Hif1β and Hif2α. These data suggest that ECs may contribute to the initial upregulation, but not to the prolonged activation of Hif genes in response to hypoxia. Interestingly, pulmonary ECs isolated from Gata6-KO mice showed increased baseline expression of Hif1α and Hif1β, as well as Hif2α. Similar to Wt mice, a further transient increase of Hif gene expression was observed in Gata6-KO ECs. The Gata-6 mRNA levels in Gata6-KO mice were comparable to the levels in Wt mice. To determine whether GATA-6 could directly contribute to the upregulation of HIF genes, HIF1α, Hif1β, and HIF2α were analyzed in HPAECs after suppression of GATA-6 with siRNA oligos. As shown in Figure 3E, expression of HIF1β and HIF2α was significantly increased, whereas expression of HIF1α remained unchanged after depletion of GATA-6. Together, these data suggest that absence of endothelial GATA-6 may exacerbate effects of hypoxia in pulmonary vasculature.
GATA-6 Deficiency Leads to Elevated Pulmonary Arterial Pressure
At 12 weeks of age, mice were subjected to echocardiographical imaging before and after exposure to chronic hypoxia. Using pulsed-wave Doppler of PA flow, PAT, and ejection time were measured (Figure 4A). This noninvasive assessment of PAH has been validated in mice as a sensitive method to assess changes in RV systolic pressure.28 Gata6-KO mice had significantly lower PAT at baseline compared to Wt, which lowered significantly more after exposure to hypoxia for 2 months (Supplemental Figure S3). PAT was decreased in Wt after exposure to chronic hypoxia, indicative of PAH. PAT/ejection time was also lower in Gata6-KO than Wt under normoxic conditions and significantly lower in both Wt and Gata6-KO mice after hypoxic insult for 1 and 2 months. To confirm the presence of increased PA pressure among Gata6-KO mice, we also performed right heart catheterization (Figure 4B). RV systolic pressure was significantly elevated in Gata6-KO mice compared to Wt mice at baseline, and significantly increased in both Wt and Gata6-KO mice after exposure to hypoxia for 1 month.
Figure 4.
GATA-6 deficiency induces hemodynamic changes in mice. Pulmonary acceleration time (PAT) and PAT as a fraction of ejection time (PAT/ET) (A) and RV systolic pressure (RVSP) (B) were measured in Wt and Gata6-KO (KO) mice during normoxia (baseline) and after 1 month of chronic hypoxia (see Materials and Methods). ∗P < 0.05 versus baseline; †P < 0.05 versus Wt. n = 3 to 4 per group.
Systolic blood pressure, diastolic blood pressure, mean pressure, heart pulse rate, and blood volume appeared to be no different in the Gata6-KO mice (Table 6). Heart morphology was investigated by serially sectioning hearts from both Wt and Gata6-KO mice housed in both hypoxic and normoxic conditions. There were no observed defects in valve morphology in Gata6-KO mice in comparison to Wt controls. Furthermore, there was no evidence of intramyocardial shunting of blood, as there were no detectable septal defects in Gata6-KO mice in either hypoxic or normoxic conditions. Myocardial histology also appeared normal (Supplemental Figure S4).
Table 6.
Occlusion Tail-Cuff Measurements
| Animal | SBP | DBP | Mean BP | HR | Volume |
|---|---|---|---|---|---|
| Wt 1 | 92 | 67 | 74 | 628 | 27 |
| Wt 2 | 107 | 79 | 88 | 674 | 39 |
| Wt 3 | 108 | 79 | 89 | 454 | 32 |
| Wt 4 | 82 | 59 | 66 | 606 | 30 |
| KO 1 | 76 | 50 | 59 | 488 | 27 |
| KO 2 | 92 | 69 | 76 | 666 | 34 |
| KO 3 | 80 | 49 | 59 | 519 | 29 |
| KO 4 | 130 | 111 | 117 | 702 | 33 |
BP, blood pressure; DBP, diastolic blood pressure; HR, heart rate; KO, Gata6-KO; SBP, systolic blood pressure; Wt, wild-type.
We concluded from these experiments that a reduction of Gata-6 alone is enough to alter the PA pressure in mice and that loss of Gata-6 leads to more severe changes in hemodynamics under hypoxia.
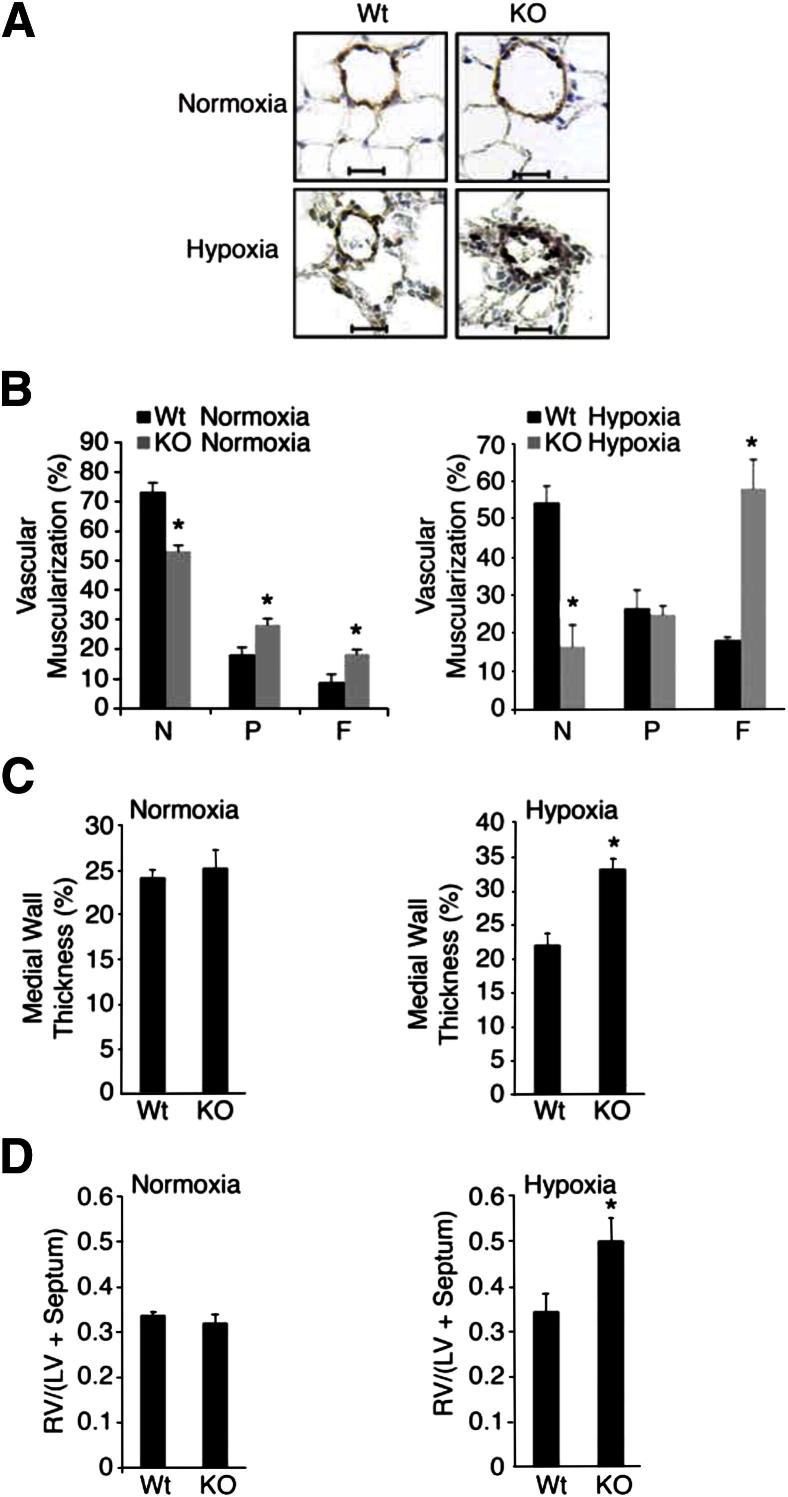
GATA-6 Deficiency Leads to Worsened Vascular Remodeling and RV Hypertrophy in Hypoxic Mice
To investigate the effect of decreased Gata-6 levels on pulmonary vascular remodeling, the degree of muscularization and the medial wall thickness of intra-acinar PAs (20 to 70 μm in diameter) were analyzed in normoxic and hypoxic conditions. Morphometric analysis revealed a significant difference between Gata6-KO mice and control animals under both conditions. Furthermore, severe enhanced PA muscularization seen by enhanced immunoreactivity for α-SMA actin was observed in Gata6-KO mice subjected to hypoxia for 1 month (Figure 5A). The analysis revealed significantly more fully and partially muscularized vessels in the Gata6-KO mice and less nonmuscularized vessels (Figure 5B). Under hypoxia, Gata6-KO mice had an even more dramatic increase in fully muscularized vessels, as well as a marked decrease in nonmuscularized vessels. In addition, the medial wall thickness was significantly increased in Gata6-KO mice under hypoxic conditions (Figure 5C). Finally, we observed that Gata-6-KO hypoxic mice also had significantly larger right ventricles as evidenced by an increase in the ratio of RV to left ventricular (LV) plus septum weight (RV/[LV+S]) as compared to control littermates (Figure 5D).
Figure 5.
Loss of GATA-6 leads to worsened vessel muscularization and RV hypertrophy in mice exposed to hypoxia. A: Lungs and hearts from 12- to 16-week-old male Wt and Gata6-KO (KO) mice exposed to either normoxic or hypoxic conditions for 1 month were subjected to morphometric analysis of pulmonary vessels and RV hypertrophy measurements. Lung sections were stained for von Willebrand factor (brown) and α-SMA (purple). Representative microphotographs are shown. Original magnification, ×400. Scale bars: 20 μm. B: Morphometry was performed on the double immunostained lung sections. A total of 60 to 80 intra-acinar vessels ranging in 20 to 70 μm in size were counted and categorized into nonmuscularized (N), partially (P) muscularized, or fully (F) muscularized (see Materials and Methods). ∗P < 0.01. C: Percent wall thickness was measured in round fully muscularized PAs (see Materials and Methods). D: The weight ratios of the right ventricle to the left ventricle plus septum [RV/(LV + Septum)] were calculated as indices of RV hypertrophy. ∗P < 0.05.
GATA-6 Deficiency Induces Pathological Changes in Pulmonary Gene Expression in Vivo
Next we analyzed the levels of GATA-6 target genes found earlier in HPAECs (Figure 2) in the lungs of Gata6-KO and control animals under both normoxic and hypoxic conditions. Under normoxia, the pattern of gene expression in the Gata-6 KO mice largely reproduced that of GATA-6-deficient HPAECs (Figure 6A). In particular, expression of CX3CL1 and EDNRA was significantly increased. Changes in expression of other genes did not reach statistical significance, likely due to variability between the mice and a small number of mice in each group (n = 3 to 4); however, they showed the same trends as HPAECs, with the exception of matrix metalloproteinase 10 and 5-lipoxygenase activating protein, which did not change in the Gata6-KO mice. Several of the GATA-6 target genes, including Pla2G4C, RhoB, EDNRA, and ET-1 were also upregulated in Wt mice exposed to 1 month of hypoxia and were further increased in hypoxic Gata6-KO mice (Figure 6B). A significant increase of RhoB and PAI-1 was observed after 2 months of hypoxia in Wt mice, and an even more pronounced increase of those genes was observed in Gata-6-KO mice (Supplemental Figure S1). Hif1α and Hif1β were modestly elevated in normoxic Gata-6-KO mice, whereas expression of all three factors was significantly increased in Wt and Gata-6-KO mice after 1 and 2 months of hypoxia (Figure 6B and Supplemental Figure S1). Notably, expression of Cx3cl1 gene was significantly reduced in hypoxic Wt mice in comparison to Wt mice from normoxic conditions and was also less prominently increased in Gata6-KO mice from the hypoxic conditions as compared to normoxia. Although, only a limited number of genes have been analyzed, these data suggest that the mechanisms of the vascular injury induced by hypoxia share both common and distinct pathways with those regulated by GATA-6.
Figure 6.
A: Gata-6 regulates genes involved in vessel remodeling in vivo. Lung samples from Gata6-KO (KO) mice and controls were assayed by RT-qPCR to determine mRNA levels of putative target genes. B: Lung samples from KO and control Wt mice subjected to hypoxic conditions for 1 month were assayed by RT-qPCR to determine mRNA levels of Gata-6 target genes relative to control Wt mice housed in normoxic conditions. ∗P < 0.05, ∗∗P < 0.01. n = 3 to 5 per group.
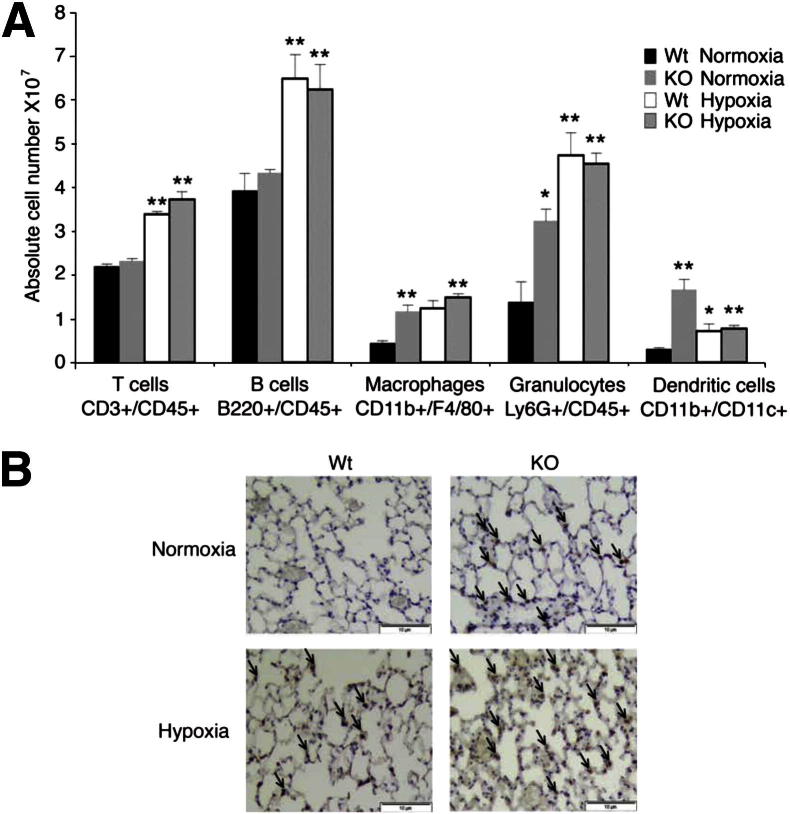
GATA-6 Deficient Mice Show Persistent Inflammation in Vivo
Given the persistently elevated expression of Cx3cl1 in Gata6-KO mice, we sought to determine whether recruitment of inflammatory cells to pulmonary vasculature is affected in these mice. FACS analysis was performed on lung tissues from Wt and Gata-6-KO mice in normoxic conditions and after 1 month of hypoxia challenge. The number of T cells (CD3+/CD45+) and B cells (B220+/CD45+) was similar in Wt and Gata-6-KO mice under normoxia and was similarly increased in both strains under hypoxia (Figure 7A). In contrast, macrophages (CD11b+/F4/80+), granulocytes (Ly6G+/CD45+), and dendritic cells (CD11b+/CD11c+) were significantly increased in Gata-6-KO mice under normoxic conditions, but did not differ significantly in hypoxic mice (Figure 7A). An increased presence of monocytes/macrophages in Gata6-KO mice was further confirmed in lung tissue sections from Wt and Gata6-KO mice (Figure 7B).
Figure 7.
Gata-6 (KO) deficiency leads to increased perivascular inflammation. A: Single cell suspensions isolated from Wt and KO mice exposed to both normoxia and hypoxia for 1 month were stained with fluorochrome-conjugated antibodies for the indicated cell surface proteins and subjected to flow cytometry analysis. ∗P < 0.05, ∗∗P < 0.01. B: Lung sections from Wt and KO mice exposed to normoxic or hypoxic conditions for 2 months were subjected to immunostaining for Mac3 using the standard avidin-biotin-peroxidase methodology. Immunoreactivity was detected using 3, 3′-diaminiobenzidine substrate kit. Hematoxylin was used as a counterstain. Arrows indicate positively stained macrophages. Original magnification, ×200. Scale bars: 10 μm.
Discussion
All forms of PAH are characterized by severe pulmonary vascular remodeling that leads to increased vascular resistance and ultimately right heart failure. The molecular mechanisms underlying the remodeling process and especially early pathogenic changes in the EC compartment remain elusive. Herein, we show that down-regulation of GATA-6 in ECs represents a key pathological event during development of PAH. GATA-6 is reduced in ECs, as well as smooth muscle cells of both occluded and nonoccluded pulmonary vessels of both IPAH and SSc-PAH in vivo, suggesting that reduction of GATA-6 is not a consequence of late stage vessel remodeling, but occurs before vessel occlusion and may reflect an early phase of EC activation and/or dysfunction during PAH. This notion is supported by observations from the two animal models of PAH, the MCT rat model and the chronic hypoxia mouse model, that show rapid reduction of the GATA-6 mRNA levels in the lungs after injury (at 3 days). Regulatory pathways that are involved in GATA-6 down-regulation remain to be elucidated.
Characterization of Gata6-KO mice revealed that GATA-6 is particularly important for pulmonary hemodynamics because a loss of GATA-6 alone was sufficient to raise PA pressure, but not systemic blood pressure. This may be due, in part, to enhanced vasoconstriction caused by changes in the levels of regulators of vascular tone (eNOS and ET-1). The eNOS null mice develop mild PAH under normoxic conditions and severe PAH under slightly hypoxic conditions.29 Impaired bioavailability of active nitric oxide is a major underlying feature of most clinical and experimental forms of PAH.30 Furthermore, ET-1, as well as endothelin-1 receptor type A, was significantly increased after down-regulation of GATA-6 by siRNA in cultured HPAECs and in Gata6-KO mice. ET-1 is a potent vasoconstrictor, a mitogen for pulmonary VSMCs, and a fibrogenic mediator.31 Plasma levels of ET-1 are elevated in PAH and are inversely proportional to the levels of pulmonary blood flow and cardiac output.32,33 Moreover, a recent study has linked EDNRA polymorphism to an increased susceptibility to PAH.34
Evidence suggests that inflammatory events may contribute to the pathogenesis of various forms of PAH, including IPAH and SSc-PAH.35,36 It has also been suggested that inflammatory pathways and autoimmunity play a more prominent role in SSc-PAH versus IPAH and may contribute to the differential response to therapy between the two syndromes.37 This study identified CX3CL1 (fractalkine) as one of the GATA-6 direct target genes. To our knowledge, this is the first demonstration that GATA-6 functions as a transcriptional repressor of the CX3CL1 gene. Elevated levels of fractalkine have been observed in inflammatory lung diseases including chronic obstructive pulmonary disease and PAH.38 Fractalkine was also found in the top 20 most upregulated genes in SSc-PAH by a recent microarray analysis.10 In human PAH, ECs were found to be the main source of CX3CL1 in the PA.39 In addition to recruiting inflammatory cells, CX3CL1 has been shown to act as a mitogenic agent on VSMCs in atherosclerosis40 and as an inducer of angiogenesis via stimulation of HIF-1α/vascular endothelial growth factor-A axis,41 suggesting that fractalkine may regulate various pathological aspects of PAH. Interestingly, although Gata-6 expression was reduced in hypoxia in Wt mice, we also observed concomitant reduction of Cx3cl1, suggesting that hypoxia-driven pathways supersede the effect of Gata-6 down-regulation. These findings corroborate an earlier in vitro study that demonstrated hypoxia-mediated inhibition of interferon-γ-induced Cx3cl1 in ECs.42
In addition to its effect on fractalkine expression, GATA-6 may regulate cytokine release by directly controlling expression of FLAP, which activates 5-lipooxygenase. It is possible that 5-lipooxygenase plays an early role in triggering a pro-inflammatory environment because its activation and translocation to the nuclear membrane are required for the production of leukotrienes, which are known to induce cytokine release.43 Both 5-lipooxygenase and FLAP have been shown to be upregulated in PAs of IPAH patients, and inhibition of FLAP in hypoxic rats ameliorates pulmonary hypertension and vascular reactivity.44,45
A reduction of Gata-6 alone significantly altered the PA pressure in mice, and increased vascular remodeling and infiltration of inflammatory cells under basal conditions. Perivascular inflammation is present in other animal models of PAH and in human PAH, however, the role of inflammatory cells in the disease process is not yet clear.46 It is important to note that in our model, Gata-6 was deleted from ECs only, whereas in patients with PAH, both endothelial and smooth muscle cells were deficient for GATA-6. Also, GATA-6 may have a distinct role in endothelial and smooth muscle cells, and its absence in both cell types would likely result in a more severe phenotype. As shown herein, Gata-6 expression in ECs was gradually decreased after treatment of mice with chronic hypoxia, suggesting that hypoxic conditions may be in part responsible for the changes in Gata-6 levels in patients with PAH. Further studies are needed to determine whether GATA-6 is also regulated by hypoxia in smooth muscle cells.
In SSc, endothelial injury resulting in the structural changes in the microvasculature and autoimmunity are the earliest manifestations of the disease.47 Although PAH develops only in a subset of patients, survival of SSc-PAH patients is very poor as compared to IPAH.9 In addition to pulmonary vessels, we have also observed decreased levels of GATA-6 in the dermis of the majority of SSc patients (Ghatnekar and Trojanowska, unpublished data) supporting the notion that chronic systemic changes in the vasculature occurring in various organs may be the principal reason for the differences in survival between these two diseases. In conclusion, we describe a novel model of PAH characterized by EC injury and persistent inflammation that may provide new insights into the role of endothelial-immune axis during development of PAH. We believe that this new model will be particularly informative in dissecting the mechanisms involved in SSc-PAH.
Footnotes
Supported by NIH grants RO1 AR42334 (M.T.) and T32 AR 050958 (A.G.), and the Entelligence Young Investigators Award from Actelion Pharmaceuticals US, Inc. (A.G.).
Current address of E.W., The Heart Institute, Cincinnati Children's Hospital Medical Center, Cincinnati, OH; of Y.A., Department of Dermatology, University of Tokyo, Tokyo, Japan.
Supplemental Data
Gata-6 regulates genes involved in vessel remodeling in vivo. Lung samples from Gata6-KO (KO) mice and control Wt mice subjected to hypoxic conditions for 2 months were assayed by RT-qPCR to determine mRNA levels of Gata-6 target genes relative to control Wt mice housed in normoxic conditions. *P < 0.05, **P < 0.01. n = 3 to 5 per group.
GATA-6 is decreased in the established monocrotaline rat model of PAH. Male Sprague-Dawley rats were dosed with either a single injection of 60 mg/kg, s.c., body mass monocrotaline (Mct) or saline (Ctl). Rats were euthanized at 20 days postinjection and the lungs were fixed overnight in 4% paraformaldehyde. Samples were subjected to H&E staining (A), Masson's staining (B) and immunostaining for α-SMA using the standard avidin-biotin-peroxidase methodology (C). D: The ventricles were excised and weighed. The weight ratio of the right ventricle to the left ventricle plus septum (RV/[LV + Septum]) was calculated as indices of RV hypertrophy. ∗P < 0.01 (n = 5 to 6 per group). E: Left panel: Mct-treated rats were euthanized at 1, 3, 5, 10, and 20 days postinjection and lung samples were assayed by RT-qPCR to determine mRNA levels of GATA-6 for five controls (saline-injected) and five MCT-treated rats. Right panel: Lung samples from rats euthanized at 20 days postinjection were assayed by immunoprecipitation, followed by immunoblot analysis to determine protein levels of GATA-6. Pre-cleared lysate (10 μL input) was subjected to immunoblotting for β-actin as a loading control. One representative blot is shown for five controls and five monocrotaline-treated rats. Band intensities were quantified by densitometric analysis. *P < 0.05, **P < 0.01. Original magnification, ×400 (B); ×200 (C). Scale bars: 50 μm (B); 100 μm (C). M. Pulmonary Artery.
Gata-6 deficiency induces hemodynamic changes in mice. Pulmonary acceleration time (PAT) and PAT as a fraction of ejection time (PAT/ET) were measured in mice housed in normoxic (baseline) and hypoxic conditions for 2 months (see Materials and Methods). *P < 0.05 versus baseline; †P < 0.05 versus Wt (n = 4 per group).
Cardiac valve histology is normal in Gata6 conditional knockout mice in both normoxic and hypoxic conditions. The histology of the cardiac valves was analyzed by comparison of H&E stained serial tissue sections. Wild-type (WT) normoxia (A–D), Gata6 conditional knockout (Gata6 CKO) normoxia (E–H), Wt hypoxia (I–L), Gata6 CKO hypoxia (M–P), pulmonary valve (A, E, I, and M), aortic valve (B, F, J, and N), mitral valve (C, G, K, and O), and tricuspid valve (D, H, L, and P). Arrows indicate the respective valve leaflets.
References
- 1.Simonneau G., Robbins I.M., Beghetti M., Channick R.N., Delcroix M., Denton C.P., Elliott C.G., Gaine S.P., Gladwin M.T., Jing Z.C., Krowka M.J., Langleben D., Nakanishi N., Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Fisher M.R., Mathai S.C., Champion H.C., Girgis R.E., Housten-Harris T., Hummers L., Krishnan J.A., Wigley F., Hassoun P.M. Clinical differences between idiopathic and scleroderma-related pulmonary hypertension. Arthritis Rheum. 2006;54:3043–3050. doi: 10.1002/art.22069. [DOI] [PubMed] [Google Scholar]
- 3.Chan S.Y., Loscalzo J. Pathogenic mechanisms of pulmonary arterial hypertension. J Mol Cell Cardiol. 2008;44:14–30. doi: 10.1016/j.yjmcc.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The International PPH Consortium. Lane K.B., Machado R.D., Pauciulo M.W., Thomson J.R., Phillips J.A., 3rd, Loyd J.E., Nichols W.C., Trembath R.C. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 5.Mahmoud M., Borthwick G.M., Hislop A.A., Arthur H.M. Endoglin and activin receptor-like-kinase 1 are co-expressed in the distal vessels of the lung: implications for two familial vascular dysplasias, HHT and PAH. Lab Invest. 2009;89:15–25. doi: 10.1038/labinvest.2008.112. [DOI] [PubMed] [Google Scholar]
- 6.Hong K.H., Lee Y.J., Lee E., Park S.O., Han C., Beppu H., Li E., Raizada M.K., Bloch K.D., Oh S.P. Genetic ablation of the BMPR2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension. Circulation. 2008;118:722–730. doi: 10.1161/CIRCULATIONAHA.107.736801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morse J., Barst R., Horn E., Cuervo N., Deng Z., Knowles J. Pulmonary hypertension in scleroderma spectrum of disease: lack of bone morphogenetic protein receptor 2 mutations. J Rheumatol. 2002;29:2379–2381. [PubMed] [Google Scholar]
- 8.Selva-O'Callaghan A., Balada E., Serrano-Acedo S., Simeon Aznar C.P., Ordi-Ros J. Mutations of activin-receptor-like kinase 1 (ALK-1) are not found in patients with pulmonary hypertension and underlying connective tissue disease. Clin Rheumatol. 2007;26:947–949. doi: 10.1007/s10067-006-0388-x. [DOI] [PubMed] [Google Scholar]
- 9.Le Pavec J., Humbert M., Mouthon L., Hassoun P.M. Systemic sclerosis-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;181:1285–1293. doi: 10.1164/rccm.200909-1331PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu E., Shi H., Jordan R.M., Lyons-Weiler J., Pilewski J.M., Feghali-Bostwick C.A. Lung tissues in patients with systemic sclerosis have gene expression patterns unique to pulmonary fibrosis and pulmonary hypertension. Arthritis Rheum. 2011;63:783–794. doi: 10.1002/art.30159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molkentin J.D. The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J Biol Chem. 2000;275:38949–38952. doi: 10.1074/jbc.R000029200. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki E., Evans T., Lowry J., Truong L., Bell D.W., Testa J.R., Walsh K. The human GATA-6 gene: structure, chromosomal location, and regulation of expression by tissue-specific and mitogen-responsive signals. Genomics. 1996;38:283–290. doi: 10.1006/geno.1996.0630. [DOI] [PubMed] [Google Scholar]
- 13.Nishida W., Nakamura M., Mori S., Takahashi M., Ohkawa Y., Tadokoro S., Yoshida K., Hiwada K., Hayashi K., Sobue K. A triad of serum response factor and the GATA and NK families governs the transcription of smooth and cardiac muscle genes. J Biol Chem. 2002;277:7308–7317. doi: 10.1074/jbc.M111824200. [DOI] [PubMed] [Google Scholar]
- 14.Wada H., Hasegawa K., Morimoto T., Kakita T., Yanazume T., Sasayama S. A p300 protein as a coactivator of GATA-6 in the transcription of the smooth muscle-myosin heavy chain gene. J Biol Chem. 2000;275:25330–25335. doi: 10.1074/jbc.M000828200. [DOI] [PubMed] [Google Scholar]
- 15.Mano T., Luo Z., Malendowicz S.L., Evans T., Walsh K. Reversal of GATA-6 downregulation promotes smooth muscle differentiation and inhibits intimal hyperplasia in balloon-injured rat carotid artery. Circ Res. 1999;84:647–654. doi: 10.1161/01.res.84.6.647. [DOI] [PubMed] [Google Scholar]
- 16.Ghatnekar A., Trojanowska M. GATA-6 is a novel transcriptional repressor of the human Tenascin-C gene expression in fibroblasts. Biochim Biophys Acta. 2008;1779:145–151. doi: 10.1016/j.bbagrm.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cowan K.N., Jones P.L., Rabinovitch M. Elastase and matrix metalloproteinase inhibitors induce regression, and tenascin-C antisense prevents progression, of vascular disease. J Clin Invest. 2000;105:21–34. doi: 10.1172/JCI6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubo M., Umemoto S., Fujii K., Itoh S., Tanaka M., Kawahara S., Matsuzaki M. Effects of angiotensin II type 1 receptor antagonist on smooth muscle cell phenotype in intramyocardial arteries from spontaneously hypertensive rats. Hypertens Res. 2004;27:685–693. doi: 10.1291/hypres.27.685. [DOI] [PubMed] [Google Scholar]
- 19.Liu B., Wang X.Q., Yu L., Zhou T.F., Wang X.M., Liu H.M. Simvastatin restores down-regulated GATA-6 expression in pulmonary hypertensive rats. Exp Lung Res. 2009;35:411–426. doi: 10.1080/01902140902736819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Froese N., Kattih B., Breitbart A., Grund A., Geffers R., Molkentin J.D., Kispert A., Wollert K.C., Drexler H., Heineke J. GATA6 promotes angiogenic function and survival in endothelial cells by suppression of autocrine transforming growth factor beta/activin receptor-like kinase 5 signaling. J Biol Chem. 2011;286:5680–5690. doi: 10.1074/jbc.M110.176925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giusti B., Fibbi G., Margheri F., Serrati S., Rossi L., Poggi F., Lapini I., Magi A., Del Rosso A., Cinelli M., Guiducci S., Kahaleh B., Bazzichi L., Bombardieri S., Matucci-Cerinic M., Gensini G.F., Del Rosso M., Abbate R. A model of anti-angiogenesis: differential transcriptosome profiling of microvascular endothelial cells from diffuse systemic sclerosis patients. Arthritis Res Ther. 2006;8:R115. doi: 10.1186/ar2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wirrig E.E., Snarr B.S., Chintalapudi M.R., O'Neal J.L., Phelps A.L., Barth J.L., Fresco V.M., Kern C.B., Mjaatvedt C.H., Toole B.P., Hoffman S., Trusk T.C., Argraves W.S., Wessels A. Cartilage link protein 1 (Crtl1), an extracellular matrix component playing an important role in heart development. Dev Biol. 2007;310:291–303. doi: 10.1016/j.ydbio.2007.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shirasaki F., Makhluf H.A., LeRoy C., Watson D.K., Trojanowska M. Ets transcription factors cooperate with Sp1 to activate the human tenascin-C promoter. Oncogene. 1999;18:7755–7764. doi: 10.1038/sj.onc.1203360. [DOI] [PubMed] [Google Scholar]
- 24.Nakerakanti S.S., Kapanadze B., Yamasaki M., Markiewicz M., Trojanowska M. Fli1 and Ets1 have distinct roles in connective tissue growth factor/CCN2 gene regulation and induction of the profibrotic gene program. J Biol Chem. 2006;281:25259–25269. doi: 10.1074/jbc.M600466200. [DOI] [PubMed] [Google Scholar]
- 25.Summer R., Fiack C.A., Ikeda Y., Sato K., Dwyer D., Ouchi N., Fine A., Farber H.W., Walsh K. Adiponectin deficiency: a model of pulmonary hypertension associated with pulmonary vascular disease. Am J Physiol Lung Cell Mol Physiol. 2009;297:L432–L438. doi: 10.1152/ajplung.90599.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steudel W., Ichinose F., Huang P.L., Hurford W.E., Jones R.C., Bevan J.A., Fishman M.C., Zapol W.M. Pulmonary vasoconstriction and hypertension in mice with targeted disruption of the endothelial nitric oxide synthase (NOS 3) gene. Circ Res. 1997;81:34–41. doi: 10.1161/01.res.81.1.34. [DOI] [PubMed] [Google Scholar]
- 27.Morrell N.W., Adnot S., Archer S.L., Dupuis J., Jones P.L., MacLean M.R., McMurtry I.F., Stenmark K.R., Thistlethwaite P.A., Weissmann N., Yuan J.X., Weir E.K. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S20–S31. doi: 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thibault H.B., Kurtz B., Raher M.J., Shaik R.S., Waxman A., Derumeaux G., Halpern E.F., Bloch K.D., Scherrer-Crosbie M. Noninvasive assessment of murine pulmonary arterial pressure: validation and application to models of pulmonary hypertension. Circ Cardiovasc Imaging. 2010;3:157–163. doi: 10.1161/CIRCIMAGING.109.887109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fagan K.A., Fouty B.W., Tyler R.C., Morris K.G., Jr., Hepler L.K., Sato K., LeCras T.D., Abman S.H., Weinberger H.D., Huang P.L., McMurtry I.F., Rodman D.M. The pulmonary circulation of homozygous or heterozygous eNOS-null mice is hyperresponsive to mild hypoxia. J ClinI Invest. 1999;103:291–299. doi: 10.1172/JCI3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giaid A., Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333:214–221. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 31.Shao D., Park J.E., Wort S.J. The role of endothelin-1 in the pathogenesis of pulmonary arterial hypertension. Pharmacol Res. 2011;63:504–511. doi: 10.1016/j.phrs.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Allen S.W., Chatfield B.A., Koppenhafer S.A., Schaffer M.S., Wolfe R.R., Abman S.H. Circulating immunoreactive endothelin-1 in children with pulmonary hypertension. Association with acute hypoxic pulmonary vasoreactivity. Am Rev Respir Dis. 1993;148:519–522. doi: 10.1164/ajrccm/148.2.519. [DOI] [PubMed] [Google Scholar]
- 33.Giaid A., Yanagisawa M., Langleben D., Michel R.P., Levy R., Shennib H., Kimura S., Masaki T., Duguid W.P., Stewart D.J. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 34.Calabro P., Limongelli G., Maddaloni V., Vizza C.D., D'Alto M., D'Alessandro R., Poscia R., Argiento P., Ziello B., Badagliacca R., Romeo E., Pacileo G., Russo M.G., Fedele F., Calabro R. Analysis of endothelin-1 and endothelin-1 receptor A gene polymorphisms in patients with pulmonary arterial hypertension. Intern Emerg Med. 2012;7:425–430. doi: 10.1007/s11739-011-0643-2. [DOI] [PubMed] [Google Scholar]
- 35.Crosswhite P., Sun Z. Nitric oxide, oxidative stress and inflammation in pulmonary arterial hypertension. J Hypertens. 2010;28:201–212. doi: 10.1097/HJH.0b013e328332bcdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hassoun P.M., Mouthon L., Barbera J.A., Eddahibi S., Flores S.C., Grimminger F., Jones P.L., Maitland M.L., Michelakis E.D., Morrell N.W., Newman J.H., Rabinovitch M., Schermuly R., Stenmark K.R., Voelkel N.F., Yuan J.X., Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54:S10–S19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Humbert M., Morrell N.W., Archer S.L., Stenmark K.R., MacLean M.R., Lang I.M., Christman B.W., Weir E.K., Eickelberg O., Voelkel N.F., Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J., Patel J.M. Role of the CX3CL1-CX3CR1 axis in chronic inflammatory lung diseases. Int J Clin Exp Med. 2010;3:233–244. [PMC free article] [PubMed] [Google Scholar]
- 39.Balabanian K., Foussat A., Dorfmuller P., Durand-Gasselin I., Capel F., Bouchet-Delbos L., Portier A., Marfaing-Koka A., Krzysiek R., Rimaniol A.C., Simonneau G., Emilie D., Humbert M. CX(3)C chemokine fractalkine in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002;165:1419–1425. doi: 10.1164/rccm.2106007. [DOI] [PubMed] [Google Scholar]
- 40.Chandrasekar B., Mummidi S., Perla R.P., Bysani S., Dulin N.O., Liu F., Melby P.C. Fractalkine (CX3CL1) stimulated by nuclear factor kappaB (NF-kappaB)-dependent inflammatory signals induces aortic smooth muscle cell proliferation through an autocrine pathway. Biochem J. 2003;373:547–558. doi: 10.1042/BJ20030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryu J., Lee C.W., Hong K.H., Shin J.A., Lim S.H., Park C.S., Shim J., Nam K.B., Choi K.J., Kim Y.H., Han K.H. Activation of fractalkine/CX3CR1 by vascular endothelial cells induces angiogenesis through VEGF-A/KDR and reverses hindlimb ischaemia. Cardiovasc Res. 2008;78:333–340. doi: 10.1093/cvr/cvm067. [DOI] [PubMed] [Google Scholar]
- 42.Yamashita K., Imaizumi T., Hatakeyama M., Tamo W., Kimura D., Kumagai M., Yoshida H., Satoh K. Effect of hypoxia on the expression of fractalkine in human endothelial cells. Tohoku J Exp Med. 2003;200:187–194. doi: 10.1620/tjem.200.187. [DOI] [PubMed] [Google Scholar]
- 43.Radmark O.P. The molecular biology and regulation of 5-lipoxygenase. Am J Respir Crit Care Med. 2000;161:S11–S15. doi: 10.1164/ajrccm.161.supplement_1.ltta-3. [DOI] [PubMed] [Google Scholar]
- 44.Voelkel N.F., Tuder R.M., Wade K., Hoper M., Lepley R.A., Goulet J.L., Koller B.H., Fitzpatrick F. Inhibition of 5-lipoxygenase-activating protein (FLAP) reduces pulmonary vascular reactivity and pulmonary hypertension in hypoxic rats. J Clin Invest. 1996;97:2491–2498. doi: 10.1172/JCI118696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wright L., Tuder R.M., Wang J., Cool C.D., Lepley R.A., Voelkel N.F. 5-Lipoxygenase and 5-lipoxygenase activating protein (FLAP) immunoreactivity in lungs from patients with primary pulmonary hypertension. Am J Respir Crit Care Med. 1998;157:219–229. doi: 10.1164/ajrccm.157.1.9704003. [DOI] [PubMed] [Google Scholar]
- 46.Price L.C., Wort S.J., Perros F., Dorfmuller P., Huertas A., Montani D., Cohen-Kaminsky S., Humbert M. Inflammation in pulmonary arterial hypertension. Chest. 2012;141:210–221. doi: 10.1378/chest.11-0793. [DOI] [PubMed] [Google Scholar]
- 47.Koenig M., Joyal F., Fritzler M.J., Roussin A., Abrahamowicz M., Boire G., Goulet J.R., Rich E., Grodzicky T., Raymond Y., Senecal J.L. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud's phenomenon to systemic sclerosis: a twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheum. 2008;58:3902–3912. doi: 10.1002/art.24038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gata-6 regulates genes involved in vessel remodeling in vivo. Lung samples from Gata6-KO (KO) mice and control Wt mice subjected to hypoxic conditions for 2 months were assayed by RT-qPCR to determine mRNA levels of Gata-6 target genes relative to control Wt mice housed in normoxic conditions. *P < 0.05, **P < 0.01. n = 3 to 5 per group.
GATA-6 is decreased in the established monocrotaline rat model of PAH. Male Sprague-Dawley rats were dosed with either a single injection of 60 mg/kg, s.c., body mass monocrotaline (Mct) or saline (Ctl). Rats were euthanized at 20 days postinjection and the lungs were fixed overnight in 4% paraformaldehyde. Samples were subjected to H&E staining (A), Masson's staining (B) and immunostaining for α-SMA using the standard avidin-biotin-peroxidase methodology (C). D: The ventricles were excised and weighed. The weight ratio of the right ventricle to the left ventricle plus septum (RV/[LV + Septum]) was calculated as indices of RV hypertrophy. ∗P < 0.01 (n = 5 to 6 per group). E: Left panel: Mct-treated rats were euthanized at 1, 3, 5, 10, and 20 days postinjection and lung samples were assayed by RT-qPCR to determine mRNA levels of GATA-6 for five controls (saline-injected) and five MCT-treated rats. Right panel: Lung samples from rats euthanized at 20 days postinjection were assayed by immunoprecipitation, followed by immunoblot analysis to determine protein levels of GATA-6. Pre-cleared lysate (10 μL input) was subjected to immunoblotting for β-actin as a loading control. One representative blot is shown for five controls and five monocrotaline-treated rats. Band intensities were quantified by densitometric analysis. *P < 0.05, **P < 0.01. Original magnification, ×400 (B); ×200 (C). Scale bars: 50 μm (B); 100 μm (C). M. Pulmonary Artery.
Gata-6 deficiency induces hemodynamic changes in mice. Pulmonary acceleration time (PAT) and PAT as a fraction of ejection time (PAT/ET) were measured in mice housed in normoxic (baseline) and hypoxic conditions for 2 months (see Materials and Methods). *P < 0.05 versus baseline; †P < 0.05 versus Wt (n = 4 per group).
Cardiac valve histology is normal in Gata6 conditional knockout mice in both normoxic and hypoxic conditions. The histology of the cardiac valves was analyzed by comparison of H&E stained serial tissue sections. Wild-type (WT) normoxia (A–D), Gata6 conditional knockout (Gata6 CKO) normoxia (E–H), Wt hypoxia (I–L), Gata6 CKO hypoxia (M–P), pulmonary valve (A, E, I, and M), aortic valve (B, F, J, and N), mitral valve (C, G, K, and O), and tricuspid valve (D, H, L, and P). Arrows indicate the respective valve leaflets.