Abstract
Atopic dermatitis is an inflammatory cutaneous disorder characterized by dry skin and relapsing eczematous skin lesions. Besides antibody production, the contribution of B cells to the pathogenesis of atopic dermatitis is unclear. In mice, repeated epicutaneous sensitization with ovalbumin induces inflamed skin lesions resembling human atopic dermatitis and therefore serves as an experimental model for this condition. To investigate the role of B cells in a murine model of atopic dermatitis, ovalbumin-sensitized allergic skin inflammation was assessed in mice lacking CD19. In ovalbumin-sensitized skin from CD19-deficient mice, the number of eosinophils and CD4+ T cells was reduced, and both epidermal and dermal thickening were decreased. Following in vitro stimulation with ovalbumin, CD19 deficiency significantly reduced the proliferation of CD4+, but not CD8+, T cells from spleen and draining lymph nodes. Furthermore, splenocytes and draining lymph node cells from ovalbumin-sensitized CD19-deficient mice secreted significantly less IL-4, IL-13, and IL-17 than ovalbumin-sensitized wild-type mice. These results suggest that CD19 expression in B cells plays a critical role in antigen-specific CD4+ T-cell proliferation and T helper 2 and 17 responses in a murine model of atopic dermatitis. Furthermore, the present findings may have implications for B-cell–targeted therapies for the treatment of atopic dermatitis.
Atopic dermatitis (AD) is one of the most common inflammatory cutaneous disorders, characterized by dry, itchy skin and relapsing eczematous skin lesions, which affects approximately 15% to 30% of children and 2% to 10% of adults.1 Histologically, AD is characterized by epidermal and dermal thickening with marked infiltration of activated T cells, eosinophils, and monocytes/macrophages within the dermis.1 Approximately 60% to 90% of patients with AD show increased serum total IgE against environmental and/or food allergens.2–4 In addition, the expression of T helper (Th) 2 cytokines, such as IL-4, IL-5, and IL-13, is increased in the acute skin lesions of AD,5,6 suggesting that Th2 cells play critical roles in disease development.
Skin barrier dysfunction is a critical feature of AD. Recent studies have shown that more than 10% of patients with AD have mutations in the filaggrin gene, which is important for skin barrier function.7,8 It has been hypothesized that a disrupted skin barrier facilitates antigen penetration and epicutaneous sensitization, leading to allergic skin inflammation in patients with AD.9 Moreover, IL-4 and IL-13 reduce filaggrin gene and protein expression in keratinocytes.10 Thus, a genetic and/or acquired defect in filaggrin is likely to play an important role in the development of AD. In mice, repeated epicutaneous sensitization of tape-stripped skin with ovalbumin (OVA), mimicking epicutaneous allergen exposure to epidermal barrier dysfunction, was found to induce the appearance of inflamed pruritic skin lesions at the application site, as well as local and systemic Th2 responses. Because of the resemblance of these lesions to human AD,11,12 this experimental method can serve as a convenient experimental model.
Historically, B cells have been considered to mediate humoral immune responses by differentiating into antibody (Ab)-secreting plasma cells.13 However, recent studies have revealed that B cells also serve as antigen-presenting cells,14 secrete a variety of cytokines,15 provide costimulatory signals, and promote T-cell activation.15,16 Moreover, IL-10–producing B cell subsets can inhibit innate and adaptive immune responses, inflammation, and autoimmunity, demonstrating the existence of regulatory B cells.13,17–19 Thus, in addition to Ab production, B cells have multiple diverse immune functions.
The fate and function of B cells are controlled by signal transduction through B-cell receptors, which are further modified by other cell-surface molecules, including CD19, CD21, CD22, CD40, CD72, and Fcγ receptor IIb.20 CD19 is a general rheostat that defines signaling thresholds critical for humoral immune responses and autoimmunity.21 CD19 is a B-cell–specific cell-surface molecule of the Ig superfamily expressed by early pre-B cells in humans and mice until plasma cell differentiation.22,23 Human CD19 and mouse CD19 are functionally equivalent in vivo.22 B cells from CD19-deficient (CD19−/−) mice are hyporesponsive to a variety of transmembrane signals, including B-cell receptor ligation.22 CD20, a B-cell–specific cell-surface molecule involved in the regulation of B-cell activation and Ca2+ transport, is initially expressed by pre-B cells in humans and mice with continued expression until plasma cell differentiation.24,25 Although the role of B cells, besides Ab production, in the pathogenesis of AD remains unclear, B-cell depletion in humans with the chimeric human anti-CD20 monoclonal antibody (mAb) rituximab results in an improvement of AD,26,27 suggesting that B cells play important roles in the development of this condition. Therefore, in the present study, we examined the importance of B cells in an OVA-sensitized allergic skin inflammation model using CD19−/− and wild-type (WT) mice.
Materials and Methods
Mice
WT C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). CD19−/− (C57BL/6 × 129) mice were generated as described previously28 and backcrossed for 7 to 12 generations onto the C57BL/6 background before use in this study. Lack of cell-surface CD19 expression was verified by two-color immunofluorescence staining with flow cytometric analysis. All mice were bred in a specific pathogen–free barrier facility and used at 8 to 12 weeks of age. All studies were approved by the Committee on Animal Experimentation (University of Tokyo, Japan).
Epicutaneous Sensitization
Epicutaneous sensitization of mice was performed as described previously.12 Briefly, the dorsal skin of anesthetized mice was shaved and tape-stripped six times. Next, 100 μg of OVA (Grade V; Sigma-Aldrich, St. Louis, MO) in 100 μL of PBS or 100 μL of PBS alone was placed on a patch of 1 × 1-cm sterile gauze, which was secured to the dorsal skin with a transparent bio-occlusive dressing (Tegaderm; 3M Health Care, St. Paul, MN). Each mouse had a total of three 1-week exposures to the patch separated from each other by 2-week intervals.
Histological Analysis
Mice were sacrificed 1 day after removal of the patch after the third sensitization (day 50). Skin samples were removed, and segments were fixed in 10% buffered formalin. After paraffin embedding, sections (5 μm thick) were cut and stained with H&E for eosinophil counting and with toluidine blue for mast cell counting. For immunohistochemistry, paraffin-embedded tissues were cut into 6-μm-thick sections, deparaffinized in xylene, and then dehydrated in PBS. Deparaffinized sections were treated with endogenous peroxidase blocking reagent (Dako, Glostrup, Denmark) and proteinase K (Dako) for 6 minutes at room temperature. Sections were then incubated with rat mAb specific to mouse CD4 (#2H9; ReliaTech, Wolfenbüttel, Germany), CD8 (D-9; Santa Cruz Biotechnology, Santa Cruz, CA), and B220 (RA3-6B2; BD Biosciences, San Jose, CA). Rat IgG (Southern Biotechnology Associates, Birmingham, AL) was used as a control for nonspecific staining. Sections were then incubated sequentially (20 minutes at 37°C) with a biotinylated rabbit anti-rat IgG secondary Ab followed by a horseradish peroxidase–conjugated avidin–biotin complex (Vectastain ABC kit; Vector Laboratories, Burlingame, CA). Sections were developed with 3,3′-diaminobenzidine tetrahydrochloride and hydrogen peroxide, and counterstained with methyl green. Stained cells were counted in 10 random grids under high magnification (×400) using a light microscope. Each section was examined independently by two investigators (K.Y. and M.K.) in a blinded manner.
Serum Ab Determination
Mice were bled and serum samples were collected on day 50 (1 day after the end of epicutaneous sensitization). All serum samples were stored at −70°C until use. Serum levels of OVA-specific IgG1, IgG2a, and IgE Abs were measured with a specific enzyme-linked immunosorbent assay (ELISA) kit (Alpha Diagnostic International, San Antonio, TX), according to the manufacturer’s protocol. Each sample was tested in duplicate.
Abs and Immunofluorescence Analysis
Anti-mouse mAbs against B220 (RA3-6B2), CD19 (1D3), CD5 (53-7.3), CD1d (1B1), CD4 (H129.19), CD8 (53-6.7), and CD25 (PC61) were obtained from BD Biosciences. For intracellular staining, mAbs against FoxP3 (FJK-16s; eBiosciences, San Diego, CA) and the Cytofix/Cytoperm kit (BD Biosciences) were used. Single-cell suspensions of the spleen and draining lymph nodes (pooled bilateral axial and inguinal lymph nodes) were prepared by gentle dissection. Peritoneal cavity leukocytes were isolated with 10 mL of cold (4°C) PBS injected into the peritoneum of sacrificed mice followed by gentle massage of the abdomen. Viable cells were counted using a hemocytometer, with relative lymphocyte percentages determined by flow cytometry. Single-cell leukocyte suspensions were stained on ice using predetermined optimal concentrations of each Ab for 20 to 60 minutes and fixed as previously described.23 Cells with the light scatter properties of lymphocytes were analyzed by immunofluorescence staining and a FACSVerse flow cytometer (BD Biosciences). Background staining was determined using unreactive isotype-matched control mAbs (Caltag Laboratories, San Francisco, CA) with gates positioned to exclude ≥98% of unreactive cells.
Lymphocyte Subset Isolation
Magnetic-activated cell sorting technology (Miltenyi Biotec, Bergisch Gladbach, Germany) was used to purify lymphocyte populations according to the manufacturer’s instructions. B220 mAb-coated microbeads and CD4+ and CD8+ T-cell isolation kits (Miltenyi Biotec) were used to purify B cells, CD4+ T cells, and CD8+ T cells, respectively. When necessary, the cells were enriched a second time using a fresh magnetic-activated cell sorting column to obtain purities >95%.
In Vitro T-Cell Proliferation Assays
On day 50, 3 × 105 purified CD4+ or CD8+ T cells harvested from the spleen or draining lymph nodes were cultured in 96-well plates in 200 μL of complete medium (RPMI 1640 containing 10% fetal calf serum, 200 μg/mL penicillin, 200 U/mL streptomycin, 4 mmol/L l-glutamine, and 5 × 10−5 mol/L 2-mercaptoethanol; all from Invitrogen, Carlsbad, CA) with 1.5 × 105 mitomycin C (Sigma-Aldrich)-treated splenic B cells and 200 μg/mL OVA. 5-Bromo-2′-deoxyuridine (BrdU; cell proliferation BrdU ELISA; Roche, Indianapolis, IN) was added during the final 2 hours of 4-day cultures. BrdU incorporation was then assessed by measuring absorbance at 450 nm.
Analysis of in Vitro Cytokine Synthesis
Single-cell suspensions of the spleen and draining lymph nodes were prepared in complete medium. Cells were cultured in complete medium at 2 × 106/mL in 24-well plates in the presence of 200 μg/mL OVA. Supernatant was collected after 96 hours of culture. The levels of IL-4, IL-10, IL-13, IL-17, and interferon (IFN)-γ in the supernatants were determined by ELISA according to the manufacturer instructions (R&D Systems, Minneapolis, MN).
Adoptive Transfer of B Cells
Splenic B cells were purified using CD19 mAb-coated microbeads (Miltenyi Biotech). The cells were enriched a second time using a fresh magnetic-activated cell sorting column to obtain purities >95%. Then, 2 × 107 CD19+ B cells from naive WT mice were transferred intravenously into CD19−/− mice. Two days later, the recipient mice were epicutaneously sensitized with OVA to induce allergic skin inflammation.
Statistical Analysis
All data are expressed as means ± SEM. The U-test was used for determining the level of significance of differences in sample means, and the Bonferroni test was used for multiple comparisons.
Results
Decreased Severity of OVA-Sensitized Allergic Skin Inflammation in CD19−/− Mice
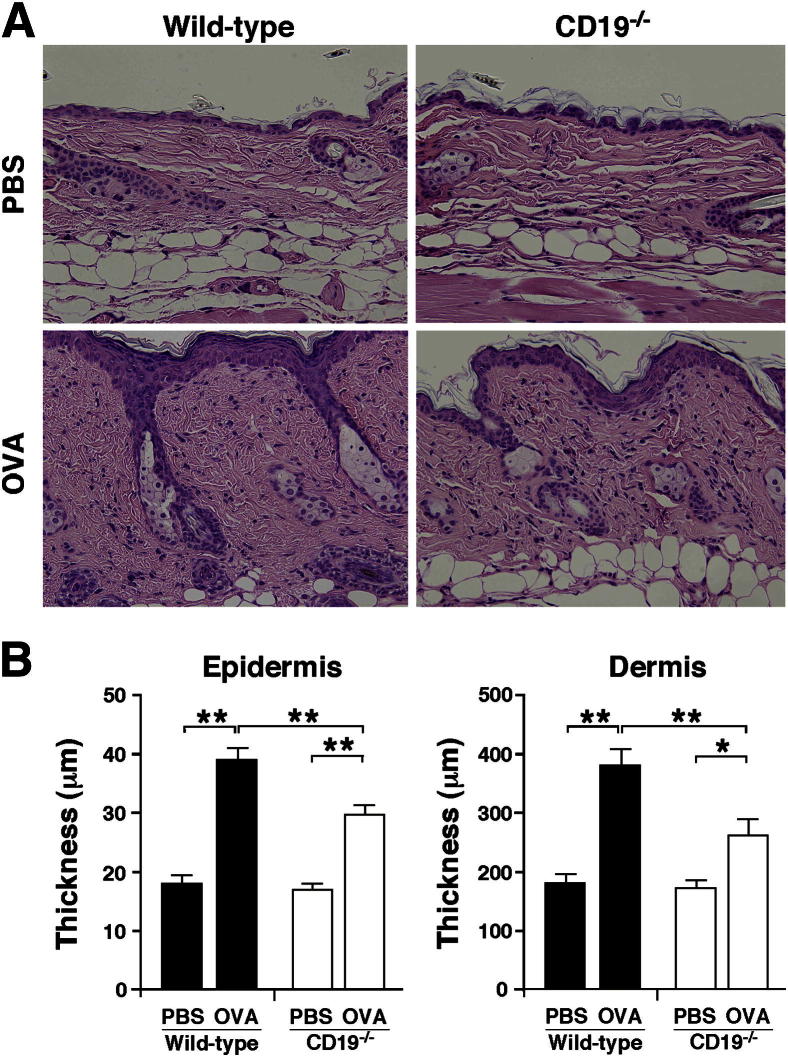
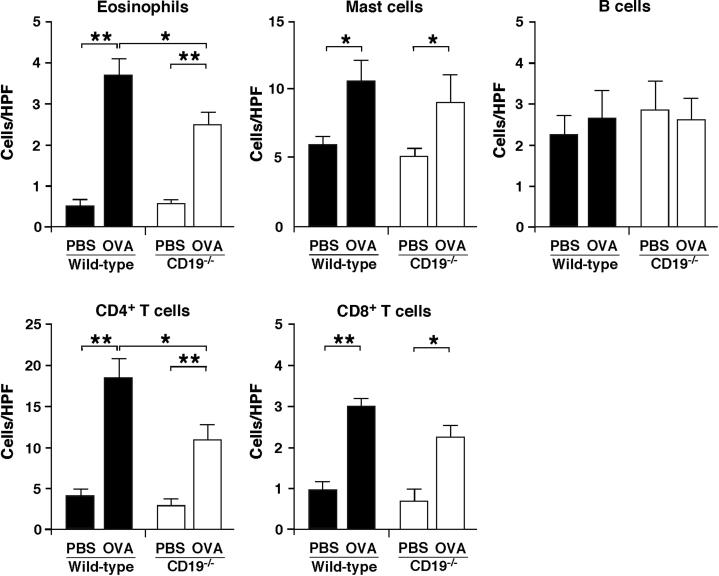
To assess whether CD19 expression played a role in the pathogenesis of OVA-sensitized allergic skin inflammation, we sensitized CD19−/− and WT mice with OVA epicutaneously over 7 weeks, and the site of repeated sensitization was histopathologically assessed. Epicutaneous sensitization with OVA induced thickening of the epidermis and dermis in both WT and CD19−/− mice, but to a lesser degree in CD19−/− mice (Figure 1). Furthermore, OVA sensitization significantly increased the numbers of eosinophils, mast cells, and CD4+ and CD8+ T cells in both WT and CD19−/− mice, but the numbers of eosinophils and CD4+ T cells were significantly lower in CD19−/− than in WT mice after sensitization with OVA (Figure 2). There were no significant differences in the numbers of mast cells, B cells, and CD8+ T cells between WT and CD19−/− mice. These results show that allergic skin inflammation was suppressed in CD19−/− mice compared with WT mice.
Figure 1.
Decreased allergic skin inflammation in CD19−/− mice. The shaved back skin of WT and CD19−/− mice was epicutaneously sensitized with PBS or OVA. A: Representative skin sections from WT and CD19−/− mice stained with H&E. Original magnification, ×200. B: Epidermal and dermal thickness in WT and CD19−/− mice. Values represent means ± SEM from n ≥ 5 mice per group. Significant differences between sample means are indicated as ∗P < 0.05, ∗∗P < 0.01. Similar results were obtained in at least two independent experiments.
Figure 2.
CD19 deficiency reduced inflammatory cell infiltration in allergic skin inflammation. The numbers of eosinophils, mast cells, B220+ B cells, CD4+ T cells, and CD8+ T cells per field of view were counted. Original magnification, ×400. Values represent means ± SEM from n ≥ 5 mice per group. Significant differences between sample means are indicated as ∗P < 0.05, ∗∗P < 0.01. Results represent one of two independent experiments with similar results. HPF, high-power field.
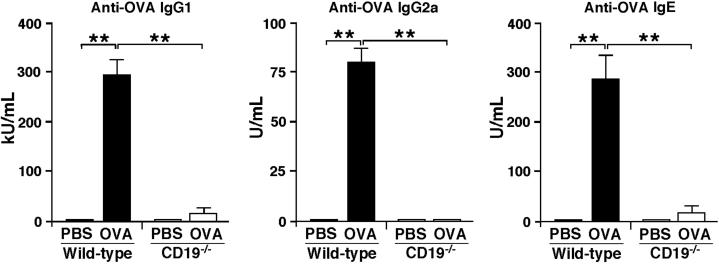
CD19 Deficiency Inhibits OVA-Specific Ab Production
The effect of CD19 deficiency on serum Ab responses was also assessed after repeated OVA sensitization. OVA-sensitized WT mice were able to mount OVA-specific IgG1, IgG2a, and IgE Ab responses following sensitization, whereas no OVA-specific Abs were detected in the serum of PBS-sensitized mice (Figure 3). By contrast, the serum levels of IgG1, IgG2a, and IgE anti-OVA Abs remained significantly lower in CD19−/− mice sensitized with OVA; levels in these animals were not significantly higher than those in WT mice sensitized with PBS. Thus, CD19 deficiency significantly attenuated OVA-specific Ab production in OVA-sensitized allergic skin inflammation.
Figure 3.
Impaired antigen-specific Ab production in CD19−/− mice. Serum OVA-specific IgG1, IgG2a, and IgE concentrations in OVA-sensitized WT and CD19−/− mice. Values represent means ± SEM from n ≥ 5 mice per group. Significant differences between sample means are indicated as ∗∗P < 0.01. Similar results were obtained in at least two independent experiments.
The Effects of Repeated OVA Sensitization on the Numbers of Regulatory B Cells and T Cells
The effects of CD19 deficiency on the numbers of CD4+CD25+FoxP3+ regulatory T cells in the spleen and draining lymph nodes, peritoneal CD5+B220+ B1-a cells, and splenic CD1dhiCD5+ regulatory B cells (B10 cells)19 were also assessed by flow cytometry after repeated OVA sensitization. Without OVA sensitization, CD19−/− mice had significantly reduced B220+ B cells in the spleen and peritoneal cavity compared with WT mice (P < 0.01), whereas B220+ B-cell numbers in the draining lymph nodes were comparable between WT and CD19−/− mice (Table 1). The numbers of B220+ B cells in the spleen, draining lymph nodes, and peritoneal cavity increased during OVA-sensitized allergic skin inflammation in both WT and CD19−/− mice, although these changes were not significant. The numbers of regulatory T cells in the spleen and draining lymph nodes were significantly increased during OVA-sensitized allergic skin inflammation in both WT and CD19−/− mice (P < 0.05 and P < 0.01, respectively). The numbers of regulatory T cells in the spleen and draining lymph nodes were comparable in OVA-sensitized WT and CD19−/− mice. The numbers of peritoneal B-1a cells were significantly decreased in CD19−/− mice compared with WT mice, as previously described,23 but there was no effect on these peritoneal B-1a cell numbers after OVA sensitization of either WT or CD19−/− mice. Moreover, OVA sensitization did not affect splenic regulatory B-cell numbers in WT mice, whereas no significant numbers of regulatory B cells were observed in CD19−/− mice, regardless of OVA sensitization as described.19 Thus, repeated epicutaneous OVA sensitization affected the numbers of regulatory T cells, which did not correlate with the observed decreased disease severity in CD19−/− mice.
Table 1.
Cell Numbers in Wild-Type and CD19−/− Mice
| Tissue | Subset | Cell number (× 10−5) |
|||
|---|---|---|---|---|---|
| Wild-type PBS | CD19−/− PBS | Wild-type OVA | CD19−/− OVA | ||
| Spleen | B220+ | 499 ± 45 | 235 ± 32∗∗ | 532 ± 58 | 262 ± 39∗∗ |
| CD1d hiCD5+B220+ | 16 ± 3 | 0.2 ± 0.1∗∗ | 20 ± 4 | 0.3 ± 0.1∗∗ | |
| CD4+CD25+FoxP3+ | 15 ± 2 | 14 ± 3 | 21 ± 3∗ | 20 ± 4∗ | |
| Draining lymph node | B220+ | 11 ± 2 | 12 ± 3 | 14 ± 3 | 14 ± 2 |
| CD4+CD25+FoxP3+ | 2.5 ± 0.4 | 2.3 ± 0.6 | 4.4 ± 0.8∗∗ | 4.9 ± 1.0∗∗ | |
| Peritoneal cavity | B220+ | 12 ± 1.8 | 2.5 ± 0.4∗∗ | 11 ± 2.2 | 2.3 ± 0.1∗∗ |
| CD5+B220+ | 1.5 ± 0.4 | 0.09 ± 0.02∗∗ | 1.6 ± 0.3 | 0.08 ± 0.03∗∗ | |
Data are expressed as means ± SEM of at least four mice.
∗P < 0.05, ∗∗P < 0.01 versus PBS-sensitized wild-type mice.
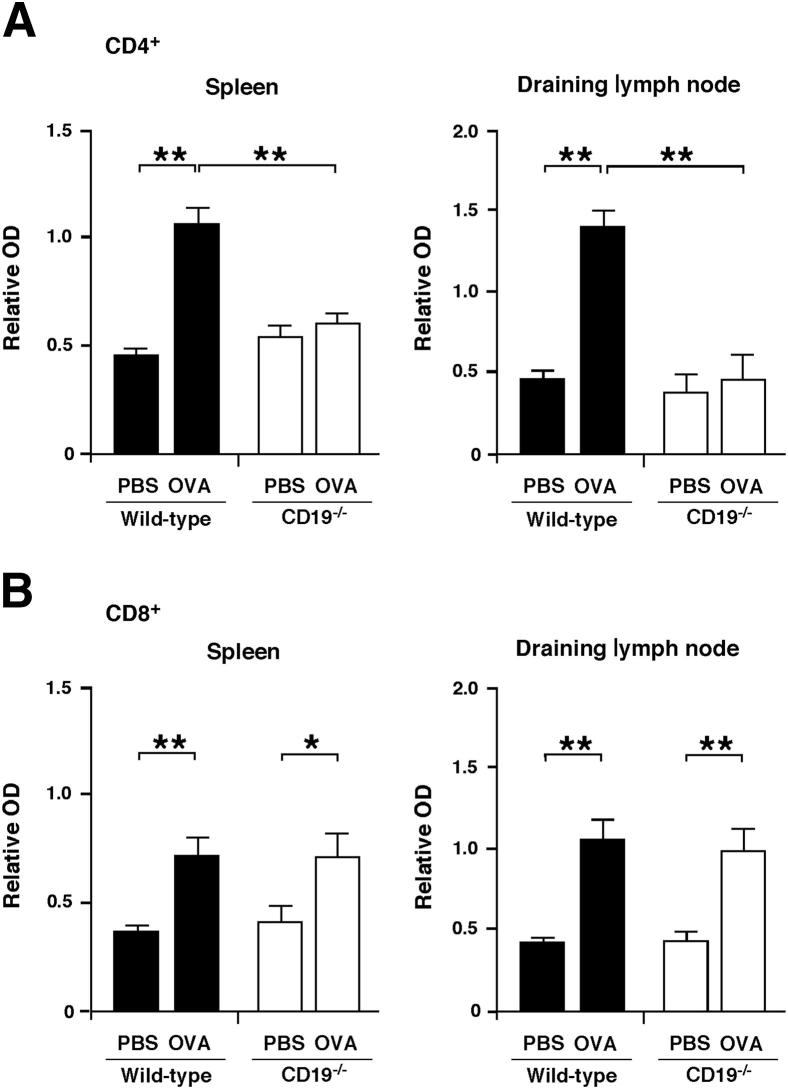
CD19 Deficiency Attenuates OVA-Specific CD4+ T-Cell Proliferation
The effects of CD19 deficiency on OVA-specific CD4+ and CD8+ T cell responses was evaluated after repeated OVA sensitization. Spleen and draining lymph node CD4+ and CD8+ T cells were purified after OVA sensitization, and OVA-specific T-cell proliferation was quantified in vitro using purified mitomycin C–treated B cells from WT mice sensitized with OVA as antigen-presenting cells in the presence of PBS or OVA. Spleen and draining lymph node CD4+ T-cell recall responses to OVA in OVA-sensitized CD19−/− mice were reduced by 40% (P < 0.01) and 66% (P < 0.01) compared to OVA-sensitized WT mice, respectively (Figure 4A). By contrast, OVA-specific CD8+ T-cell proliferation was equivalent in WT and CD19−/− mice (Figure 4B). Thus, CD19 deficiency impaired the expansion of antigen-specific CD4+ T cells, but not CD8+ T cells, following OVA sensitization.
Figure 4.
CD19 deficiency inhibits OVA-specific CD4+ T-cell proliferation. CD4+ (A) or CD8+ (B) T cells were purified from spleen or draining lymph nodes and cultured with mitomycin C-treated B cells from OVA-sensitized WT mice in the presence of PBS or OVA. Values represent means ± SEM BrdU incorporation from triplicate cultures. All data are representative of two independent experiments; n ≥ 5 mice per group. Significant differences between sample means are indicated as ∗P < 0.05, ∗∗P < 0.01. OD, optical density.
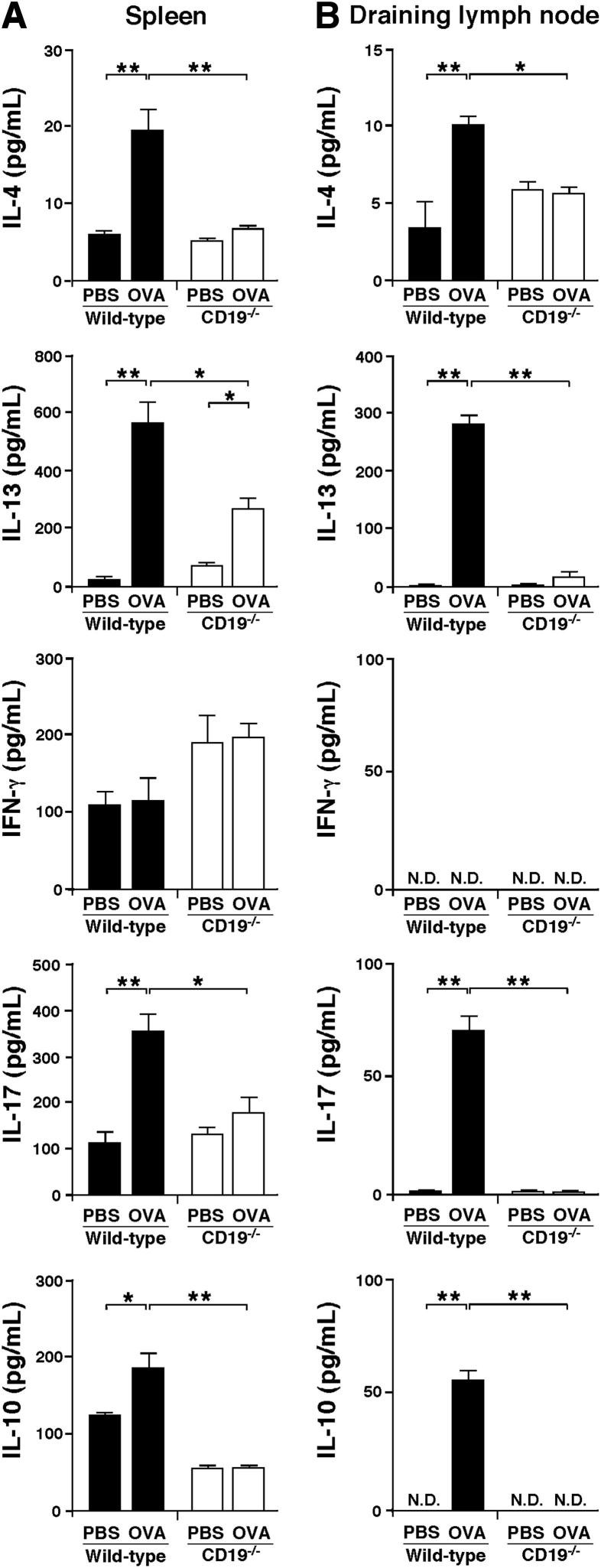
Th2 and Th17 Cytokine Production Is Decreased in CD19−/− Mice
Because CD19 regulates OVA-specific T-cell proliferation, it is possible that CD19 also affects cytokine production in response to OVA stimulation. Therefore, we stimulated OVA-sensitized splenocytes and draining lymph node cells with PBS or OVA in vitro and, using ELISA, examined whether the loss of CD19 affected cytokine secretion. OVA-sensitized WT splenocytes secreted more IL-4, IL-13, IL-17, and IL-10 than PBS-sensitized WT splenocytes (Figure 5A). OVA-sensitized CD19−/− splenocytes exhibited reduced secretion of IL-4, IL-13, IL-17, and IL-10 compared to OVA-sensitized WT splenocytes. Similarly, WT draining lymph node cells treated with OVA showed increased IL-4, IL-13, IL-17, and IL-10 production compared to cells treated with PBS, whereas draining lymph node cells from OVA-sensitized CD19−/− mice secreted significantly less IL-4, IL-13, IL-17, and IL-10 relative to those from OVA-sensitized WT mice (Figure 5B). Thus, CD19 deficiency decreased Th2 and Th17 cytokine secretion in an OVA-sensitized model of allergic skin inflammation.
Figure 5.
CD19 deficiency suppresses Th2 and Th17 cytokine production. IL-4, IL-13, IFN-γ, IL-17, and IL-10 secretion by splenocytes (A) and draining lymph node cells (B) from OVA-sensitized WT and CD19−/− mice after in vitro PBS or OVA stimulation. Values represent means ± SEM from n ≥ 5 mice per group. Significant differences between sample means are indicated as ∗P < 0.05, ∗∗P < 0.01. Similar results were obtained in at least two independent experiments. N.D., not detected.
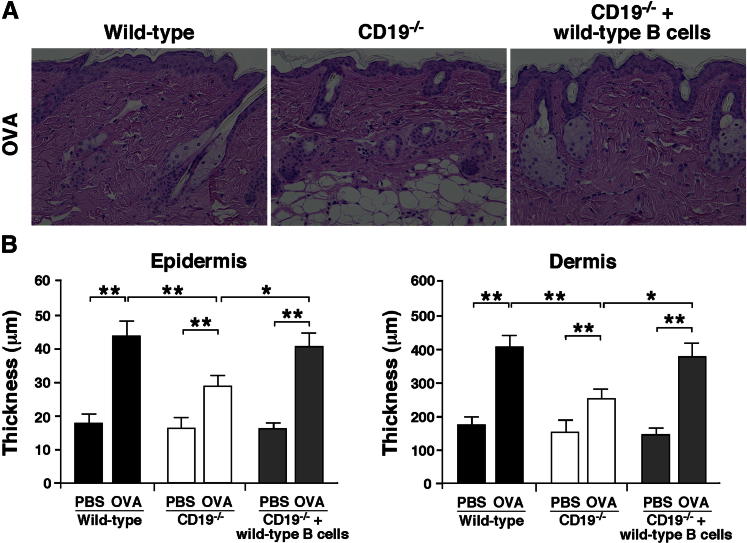
Adoptive Transfer of CD19+ B Cells Restored the Severity of Allergic Skin Inflammation in CD19−/− Mice
Genetic deficiency of CD19 may affect normal T-cell development and impair allergic skin inflammation. Therefore, we next assessed whether CD19 expression in B cells was responsible in vivo for allergic skin inflammation. B cells were purified from the spleen of naive WT mice and transferred to CD19−/− mice before OVA sensitization (2 × 107 cells, >95% CD19+). Transferring WT B cells into CD19−/− mice significantly enhanced thickening of the epidermis and dermis (P < 0.05 for both) (Figure 6). The CD19−/− mice that received WT B cells developed allergic skin inflammation of the same severity as that in WT mice. Therefore, reduced allergic skin inflammation of CD19−/− mice is enhanced to normal levels when spleen B cells from WT mice are transferred, indicating the pathogenic role of CD19 expression in B cells.
Figure 6.
Adoptive transfer of WT B cells in allergic skin inflammation. WT splenic B cells were purified from naive WT mice and transferred into CD19−/− mice. Two days later, the recipient mice were epicutaneously sensitized with OVA to induce allergic skin inflammation. A: Representative skin sections stained with H&E from WT, CD19−/−, and WT B-cell–transferred CD19−/− mice. Original magnification, ×200. B: Epidermal and dermal thickness in WT, CD19−/−, and WT B-cell–transferred CD19−/− mice. Values represent means ± SEM from n ≥ 4 mice per group. Significant differences between sample means are indicated as ∗P < 0.05, ∗∗P < 0.01.
Discussion
Previous studies have shown that B-cell depletion with CD20 mAb reduces CD4+ T-cell activation during immune responses to low-dose, but not high-dose, antigens.29 B-cell depletion also suppresses both arthritogenic collagen-specific CD4+ T-cell proliferation in murine collagen-induced arthritis and B-cell–specific CD4+ T-cell proliferation in pancreatic lymph nodes of NOD mice.29,30 Consistently, the results of the present study showed that CD19 expression in B cells is required for antigen-specific CD4+ T-cell activation following epicutaneous sensitization. Therefore, it is most likely that B cells contribute to antigen-specific CD4+ T-cell activation and expansion during allergic skin inflammation. Moreover, antigen-specific B cells are considered to be essential for Th2 cell development.31 B cells control parasite infection by promoting Th2 cell development in mice.32,33 Of note, the results of the present study show that CD19 expression in B cells promoted, not only IL-4 and IL-13, but also IL-17 production. It has been reported that the numbers of Th17 cells are increased in patients with asthma and acute AD.34,35 In addition, B-cell–depletion therapy with CD20 mAb inhibits Th17 responses in patients with rheumatoid arthritis,36 suggesting the potential role of B cells in Th17 responses. Thus, B cells are likely to enhance both Th2 and Th17 responses, thereby regulating allergic inflammatory responses. By contrast, IL-17 expression in patients with AD is significantly decreased compared with those with psoriasis, a representative Th17-dominant disease, although it is slightly increased compared with healthy individuals.37 Furthermore, we have examined IL-17 mRNA expression using real-time PCR and found that expression in the inflamed skin was not affected by OVA sensitization in either WT or CD19−/− mice (data not shown). Therefore, the role of Th17 cells in allergic diseases remains largely unclear. Additional studies will therefore be required to clarify the role of Th17 responses in AD.
B cells play both positive effector and negative regulatory roles during immune responses.13 CD19 deficiency may either decrease or increase inflammation depending on the disease model. It has previously been demonstrated that CD19 deficiency is beneficial in mouse models of systemic sclerosis,38 rheumatoid arthritis,39 and pulmonary fibrosis,40 whereas CD19 deficiency enhances inflammation in the contact hypersensitivity response,19 an experimental autoimmune encephalomyelitis model of human multiple sclerosis,41 and a dextran sulfate sodium–induced colitis model of human ulcerative colitis.18 Because CD19−/− mice have few, if any, CD1dhiCD5+ regulatory B10 cells,19 CD19 deficiency may lead to worsening of the inflammatory response in diseases that have a predominance of regulatory B cells relative to effector B cells. Our experimental findings revealed that the splenic B10 cell subset did not have a critical role in the inhibition of OVA-sensitized allergic skin inflammation. Consistently, rituximab-induced B-cell depletion in humans led to the improvement of AD,26,27 suggesting the predominance of effector B cells relative to regulatory B cells in this condition. However, it is also possible that the balance between opposing positive and negative regulatory B-cell functions changes during the course of disease in AD. In mice, B-cell depletion before experimental autoimmune encephalomyelitis induction causes a deterioration in disease symptoms, whereas B-cell depletion after the development of experimental autoimmune encephalomyelitis symptoms inhibits pathogenic T-cell expansion and decreases disease severity.42 Further studies are needed to determine the contribution of each B-cell subset to the pathogenesis of AD.
The absence of CD19 from birth affects normal B-cell development, and the number of B220+ B cells was found to be significantly reduced in CD19−/− mice.22,43 The proliferative capacity of CD19−/− B cells to mitogens is also lower than WT B cells.22 Therefore, suppressed allergic skin inflammation in CD19−/− mice is likely due to the decreased B-cell numbers and/or impaired proliferation capacity of B cells, resulting in impaired allergic skin inflammation. Moreover, it was reported that peritoneal B-1a cells are also decreased in CD19−/− mice compared with WT mice,23 although it has also been shown that peritoneal B-1a cells promote Th17 cell differentiation in mice.44 Thus, reduced peritoneal B-1a cells may decrease IL-17 secretion in CD19−/− mice. Future studies may determine the precise mechanisms by which B-cell CD19 expression is involved in allergic skin inflammation.
In conclusion, the current findings demonstrate that CD19 expression in B cells plays a pathogenic role in OVA-sensitized skin inflammation by enhancing antigen-specific CD4+ T-cell expansion and Th2 and Th17 responses. Currently, patients with AD are treated with corticosteroids and immunosuppressive drugs, but these therapies can cause severe side effects such as infection and may fail in some severe cases. The therapeutic use of B-cell–targeted therapies that effectively eliminate B cells or simply reduce B-cell hyperactivity may hold promise for the treatment of allergic skin diseases such as AD. Examining the effects of B-cell depletion in a murine model of OVA-sensitized skin inflammation with an intact immune system using mouse anti-mouse CD20 mAb can provide a preclinical test for B-cell–based immunotherapy for AD in humans.
Acknowledgments
We thank Tamami Kaga and Yoshiko Ito for technical assistance.
Footnotes
Supported by grants from the NIH (AI56363), Southeastern Regional Center of Excellence for Emerging Infections and Biodefense (U54 AI057157), and the Lymphoma Research Foundation (T.F.T.).
Disclosure: T.F.T. is a paid consultant to MedImmune and Angelica Therapeutics.
References
- 1.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 2.Ponyai G., Hidvegi B., Nemeth I., Sas A., Temesvari E., Karpati S. Contact and aeroallergens in adulthood atopic dermatitis. J Eur Acad Dermatol Venereol. 2008;22:1346–1355. doi: 10.1111/j.1468-3083.2008.02886.x. [DOI] [PubMed] [Google Scholar]
- 3.Mori T., Ishida K., Mukumoto S., Yamada Y., Imokawa G., Kabashima K., Kobayashi M., Bito T., Nakamura M., Ogasawara K., Tokura Y. Comparison of skin barrier function and sensory nerve electric current perception threshold between IgE-high extrinsic and IgE-normal intrinsic types of atopic dermatitis. Br J Dermatol. 2010;162:83–90. doi: 10.1111/j.1365-2133.2009.09440.x. [DOI] [PubMed] [Google Scholar]
- 4.Ott H., Stanzel S., Ocklenburg C., Merk H.F., Baron J.M., Lehmann S. Total serum IgE as a parameter to differentiate between intrinsic and extrinsic atopic dermatitis in children. Acta Derm Venereol. 2009;89:257–261. doi: 10.2340/00015555-0627. [DOI] [PubMed] [Google Scholar]
- 5.Hamid Q., Boguniewicz M., Leung D.Y. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest. 1994;94:870–876. doi: 10.1172/JCI117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamid Q., Naseer T., Minshall E.M., Song Y.L., Boguniewicz M., Leung D.Y. In vivo expression of IL-12 and IL-13 in atopic dermatitis. J Allergy Clin Immunol. 1996;98:225–231. doi: 10.1016/s0091-6749(96)70246-4. [DOI] [PubMed] [Google Scholar]
- 7.Palmer C.N., Irvine A.D., Terron-Kwiatkowski A., Zhao Y., Liao H., Lee S.P., Goudie D.R., Sandilands A., Campbell L.E., Smith F.J., O’Regan G.M., Watson R.M., Cecil J.E., Bale S.J., Compton J.G., DiGiovanna J.J., Fleckman P., Lewis-Jones S., Arseculeratne G., Sergeant A., Munro C.S., El Houate B., McElreavey K., Halkjaer L.B., Bisgaard H., Mukhopadhyay S., McLean W.H. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 8.Akiyama M. FLG mutations in ichthyosis vulgaris and atopic eczema: spectrum of mutations and population genetics. Br J Dermatol. 2010;162:472–477. doi: 10.1111/j.1365-2133.2009.09582.x. [DOI] [PubMed] [Google Scholar]
- 9.Barnes K.C. An update on the genetics of atopic dermatitis: scratching the surface in 2009. J Allergy Clin Immunol. 2010;125:16–31. doi: 10.1016/j.jaci.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howell M.D., Kim B.E., Gao P., Grant A.V., Boguniewicz M., Debenedetto A., Schneider L., Beck L.A., Barnes K.C., Leung D.Y. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2007;120:150–155. doi: 10.1016/j.jaci.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spergel J.M., Mizoguchi E., Oettgen H., Bhan A.K., Geha R.S. Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J Clin Invest. 1999;103:1103–1111. doi: 10.1172/JCI5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spergel J.M., Mizoguchi E., Brewer J.P., Martin T.R., Bhan A.K., Geha R.S. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614–1622. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanaba K., Bouaziz J.D., Matsushita T., Magro C.M., St Clair E.W., Tedder T.F. B-lymphocyte contributions to human autoimmune disease. Immunol Rev. 2008;223:284–299. doi: 10.1111/j.1600-065X.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 14.Kurt-Jones E.A., Liano D., HayGlass K.A., Benacerraf B., Sy M.S., Abbas A.K. The role of antigen-presenting B cells in T cell priming in vivo. Studies of B cell-deficient mice. J Immunol. 1988;140:3773–3778. [PubMed] [Google Scholar]
- 15.Harris D.P., Haynes L., Sayles P.C., Duso D.K., Eaton S.M., Lepak N.M., Johnson L.L., Swain S.L., Lund F.E. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1:475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 16.Linton P.J., Bautista B., Biederman E., Bradley E.S., Harbertson J., Kondrack R.M., Padrick R.C., Bradley L.M. Costimulation via OX40L expressed by B cells is sufficient to determine the extent of primary CD4 cell expansion and Th2 cytokine secretion in vivo. J Exp Med. 2003;197:875–883. doi: 10.1084/jem.20021290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouaziz J.D., Yanaba K., Tedder T.F. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224:201–214. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 18.Yanaba K., Yoshizaki A., Asano Y., Kadono T., Tedder T.F., Sato S. IL-10-producing regulatory B10 cells inhibit intestinal injury in a mouse model. Am J Pathol. 2011;178:735–743. doi: 10.1016/j.ajpath.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanaba K., Bouaziz J.-D., Haas K.M., Poe J.C., Fujimoto M., Tedder T.F. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Tsubata T. Co-receptors on B lymphocytes. Curr Opin Immunol. 1999;11:249–255. doi: 10.1016/s0952-7915(99)80041-7. [DOI] [PubMed] [Google Scholar]
- 21.Tedder T.F., Inaoki M., Sato S. The CD19/21 complex regulates signal transduction thresholds governing humoral immunity and autoimmunity. Immunity. 1997;6:107–118. doi: 10.1016/s1074-7613(00)80418-5. [DOI] [PubMed] [Google Scholar]
- 22.Sato S., Steeber D.A., Jansen P.J., Tedder T.F. CD19 expression levels regulate B lymphocyte development: human CD19 restores normal function in mice lacking endogenous CD19. J Immunol. 1997;158:4662–4669. [PubMed] [Google Scholar]
- 23.Sato S., Ono N., Steeber D.A., Pisetsky D.S., Tedder T.F. CD19 regulates B lymphocyte signaling thresholds critical for the development of B-1 lineage cells and autoimmunity. J Immunol. 1996;157:4371–4378. [PubMed] [Google Scholar]
- 24.Stashenko P., Nadler L.M., Hardy R., Schlossman S.F. Characterization of a human B lymphocyte-specific antigen. J Immunol. 1980;125:1678–1685. [PubMed] [Google Scholar]
- 25.Uchida J., Lee Y., Hasegawa M., Liang Y., Bradney A., Oliver J.A., Bowen K., Steeber D.A., Haas K.M., Poe J.C., Tedder T.F. Mouse CD20 expression and function. Int Immunol. 2004;16:119–129. doi: 10.1093/intimm/dxh009. [DOI] [PubMed] [Google Scholar]
- 26.Ponte P., Lopes M.J. Apparent safe use of single dose rituximab for recalcitrant atopic dermatitis in the first trimester of a twin pregnancy. J Am Acad Dermatol. 2010;63:355–356. doi: 10.1016/j.jaad.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Simon D., Hosli S., Kostylina G., Yawalkar N., Simon H.U. Anti-CD20 (rituximab) treatment improves atopic eczema. J Allergy Clin Immunol. 2008;121:122–128. doi: 10.1016/j.jaci.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Engel P., Zhou L.-J., Ord D.C., Sato S., Koller B., Tedder T.F. Abnormal B lymphocyte development, activation and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity. 1995;3:39–50. doi: 10.1016/1074-7613(95)90157-4. [DOI] [PubMed] [Google Scholar]
- 29.Bouaziz J.D., Yanaba K., Venturi G.M., Wang Y., Tisch R.M., Poe J.C., Tedder T.F. therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci U S A. 2007;104:20882–20887. doi: 10.1073/pnas.0709205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yanaba K., Hamaguchi Y., Venturi G.M., Steeber D.A., St. Clair E.W., Tedder T.F. B cell depletion delays collagen-induced arthritis in mice: arthritis induction requires synergy between humoral and cell-mediated immunity. J Immunol. 2007;179:1369–1380. doi: 10.4049/jimmunol.179.2.1369. [DOI] [PubMed] [Google Scholar]
- 31.Macaulay A.E., DeKruyff R.H., Goodnow C.C., Umetsu D.T. Antigen-specific B cells preferentially induce CD4+ T cells to produce IL-4. J Immunol. 1997;158:4171–4179. [PubMed] [Google Scholar]
- 32.Liu Q., Liu Z., Rozo C.T., Hamed H.A., Alem F., Urban J.F., Jr., Gause W.C. The role of B cells in the development of CD4 effector T cells during a polarized Th2 immune response. J Immunol. 2007;179:3821–3830. doi: 10.4049/jimmunol.179.6.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leon B., Ballesteros-Tato A., Browning J.L., Dunn R., Randall T.D., Lund F.E. Regulation of TH2 development by CXCR5+ dendritic cells and lymphotoxin-expressing B cells. Nat Immunol. 2012;13:681–690. doi: 10.1038/ni.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Souwer Y., Szegedi K., Kapsenberg M.L., de Jong E.C. IL-17 and IL-22 in atopic allergic disease. Curr Opin Immunol. 2010;22:821–826. doi: 10.1016/j.coi.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 35.Koga C., Kabashima K., Shiraishi N., Kobayashi M., Tokura Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J Invest Dermatol. 2008;128:2625–2630. doi: 10.1038/jid.2008.111. [DOI] [PubMed] [Google Scholar]
- 36.van de Veerdonk F.L., Lauwerys B., Marijnissen R.J., Timmermans K., Di Padova F., Koenders M.I., Gutierrez-Roelens I., Durez P., Netea M.G., van der Meer J.W., van den Berg W.B., Joosten L.A. The anti-CD20 antibody rituximab reduces the Th17 cell response. Arthritis Rheum. 2011;63:1507–1516. doi: 10.1002/art.30314. [DOI] [PubMed] [Google Scholar]
- 37.Guttman-Yassky E., Lowes M.A., Fuentes-Duculan J., Zaba L.C., Cardinale I., Nograles K.E., Khatcherian A., Novitskaya I., Carucci J.A., Bergman R., Krueger J.G. Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J Immunol. 2008;181:7420–7427. doi: 10.4049/jimmunol.181.10.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saito E., Fujimoto M., Hasegawa M., Komura K., Hamaguchi Y., Kaburagi Y., Nagaoka T., Takehara K., Tedder T.F., Sato S. CD19-dependent B lymphocyte signaling thresholds influence skin fibrosis and autoimmunity in the tight-skin mouse. J Clin Invest. 2002;109:1453–1462. doi: 10.1172/JCI15078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Del Nagro C.J., Kolla R.V., Rickert R.C. A critical role for complement C3d and the B cell coreceptor (CD19/CD21) complex in the initiation of inflammatory arthritis. J Immunol. 2005;175:5379–5389. doi: 10.4049/jimmunol.175.8.5379. [DOI] [PubMed] [Google Scholar]
- 40.Komura K., Yanaba K., Horikawa M., Ogawa F., Fujimoto M., Tedder T.F., Sato S. CD19 regulates the development of bleomycin-induced pulmonary fibrosis in a mouse model. Arthritis Rheum. 2008;58:3574–3584. doi: 10.1002/art.23995. [DOI] [PubMed] [Google Scholar]
- 41.Matsushita T., Fujimoto M., Hasegawa M., Komura K., Takehara K., Tedder T.F., Sato S. Inhibitory role of CD19 in the progression of experimental autoimmune encephalomyelitis by regulating cytokine response. Am J Pathol. 2006;168:812–821. doi: 10.2353/ajpath.2006.050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsushita T., Yanaba K., Bouaziz J.D., Fujimoto M., Tedder T.F. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haas K.M., Poe J.C., Steeber D.A., Tedder T.F. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Zhong X., Gao W., Degauque N., Bai C., Lu Y., Kenny J., Oukka M., Strom T.B., Rothstein T.L. Reciprocal generation of Th1/Th17 and Treg cells by B1 and B2 B cells. Eur J Immunol. 2007;37:2400–2404. doi: 10.1002/eji.200737296. [DOI] [PubMed] [Google Scholar]