Abstract
Implantation of a biomaterial into the body elicits a host foreign body response, during which polymorphonuclear leukocytes (PMNs) and then monocytes (MCs) are recruited to the site of implantation. MCs and MC-derived macrophages are central players in this response because they secrete proinflammatory and/or pro–wound-healing cytokines and growth factors that influence subsequent healing events. Although mechanisms of MC response to biomaterials are often studied in in vitro monoculture models, few studies have investigated how biomaterials modulate PMN-MC paracrine and juxtacrine interactions. To address this, we cultured human blood-derived MCs alone or in the presence of autologous PMN-conditioned medium (PCM) on poly(ethylene glycol) hydrogels, poly(dimethyl siloxane), and tissue culture polystyrene. We also directly co-cultured autologous PMNs and MCs on these biomaterials. PCM increased MC adhesion/viability and expression of IL-1β and tumor necrosis factor-α in a biomaterial- and time-dependent manner when compared with MCs that were not cultured in PCM. There were also biomaterial- and time-dependent differences in cell adhesion/viability, apoptosis, and expression of IL-6 and IL-8 in the PMN-MC direct co-cultures when compared with the sums of these activities in PMN and MC monocultures. In conclusion, these data suggest that biomaterials selectively modulate PMN-MC paracrine and juxtacrine interactions to influence MC and/or PMN adhesion/viability, apoptosis, and cytokine expression.
The increasing demand for biomaterial implants for a wide variety of medical applications warrants a deeper understanding of the host foreign body response and the development of pathologically/physiologically relevant models to assess material biocompatibility. Biomaterials elicit a complex sequence of biological events, which include inflammatory and wound-healing responses.1,2 Injury induced in vascularized tissue during implantation initiates an inflammatory response, resulting in the extravasation of polymorphonuclear leukocytes (PMNs; neutrophils) to the implant site. In addition to phagocytizing invading microorganisms and foreign matters, activated PMNs secrete products that influence monocyte (MC)/macrophage recruitment, activation, and function.3–18 Furthermore, MCs/macrophages phagocytize apoptotic PMNs, which can also lead to the regulation of MC/macrophage function.19–24 MCs and MC-derived macrophages at the biomaterial surface secrete proinflammatory and/or pro–wound-healing cytokines and growth factors that influence subsequent healing events and can contribute to the eventual fate of the implanted biomaterial.25
Mechanisms of MC response to biomaterials are often studied in in vitro monoculture models. Although these models provide important insights, they do not directly account for PMN-MC interactions that are present in vivo. We sought to determine how biomaterials modulate PMN-MC paracrine and juxtacrine interactions to influence downstream MC activation. PMN response to poly(ethylene glycol) (PEG) hydrogels, poly(dimethyl siloxane) (PDMS), and tissue culture polystyrene (TCPS) was first determined to understand how these biomaterials affect PMN adhesion, viability, necrosis, apoptosis, secondary necrosis, and secretion of soluble mediators critical to MC recruitment and modulation [eg, macrophage inflammatory protein (MIP)-1β and human neutrophil peptides (HNP1-3)]. We then cultured MCs on the biomaterials in monoculture or in the presence of autologous PMN-conditioned medium (PCM) to determine how soluble mediators from biomaterial-activated PMNs influence MC viability, necrosis, apoptosis, secondary necrosis, and cytokine expression. Furthermore, we directly co-cultured autologous PMNs and MCs to determine how the biomaterials mediate PMN-MC paracrine and juxtacrine interactions. We compared mean cell activities (eg, adherent cell density, caspase 3/7 activity, and cytokine concentration) of the three donors between conditions (ie, MC cultures with PCM priming compared with MC cultures without PCM priming or PMN-MC co-cultures compared with the sums of PMN and MC monocultures). In addition, to account for donor variability, we identified instances in which all three donors had >1.8-fold differences in cell activity between conditions.
Materials and Methods
PEG Hydrogel, PDMS, and TCPS Preparation
Poly(ethylene glycol) diacrylate (mol. weight, 3350) was synthesized using established procedures.26 Poly(ethylene glycol) diacrylate products were characterized by proton NMR (Varian Unity-Inova 400 MHz; Varian Inc., Palo Alto, CA) and were routinely found to have >85% acrylation. PEG hydrogels were prepared as previously described.27 Briefly, 10 weight percent poly(ethylene glycol) diacrylate and 0.1 weight percent Irgacure 2959 (Ciba Specialty Chemicals, Tarrytown, NY) were dissolved in PBS (Cellgro, Manassas, VA) with mild heat. The solution was filtered through a 0.2-μm syringe filter, injected into Teflon molds, and clamped between two acid-washed glass coverslips. The assembly was placed under a UV light (UV CF1000 light-emitting diode, λmax = 365 nm; Clearstone Technologies, Minneapolis, MN), and the solution was polymerized for 3 minutes per side. The PEG hydrogel films were sterilized in 70% ethanol (Decon Labs, Inc., King of Prussia, PA) for at least 45 minutes and equilibrated overnight in Dulbecco's PBS (Cellgro). The swollen PEG hydrogel films were cut into disks with biopsy punches (Miltex, Inc., York, PA), placed into 96- or 48-well culture plates (Corning Inc., Corning, NY), and sterilized with 70% ethanol for 45 minutes. Disks with a 5- or 6-mm diameter were placed into 96-well culture plates, and disks with an 8-mm diameter were placed into 48-well culture plates to closely fit the area of the well.
PDMS (0.762 mm thickness; nonreinforced vulcanized gloss/gloss; Specialty Manufacturing Inc., Saginaw, MI) was cut into disks with biopsy punches and sonicated for 10 minutes in a 0.5 weight percent solution of sodium dodecyl sulfate (Amresco, Solon, OH). The PDMS disks were rinsed with deionized water, placed into 96- or 48-well culture plates, and sterilized with 70% ethanol for 45 minutes. After sterilization with 70% ethanol, PEG hydrogel and PDMS disks were washed at least three times with Dulbecco's PBS and equilibrated in RPMI 1640 basal medium (Cellgro) before seeding with cells. TCPS wells (Corning Inc.) were also equilibrated in RPMI 1640 medium before cell seeding.
Culture of Primary Human Blood–Derived PMNs and MCs
Peripheral whole blood was obtained from consenting healthy adult donors after protocol approval by the University of Wisconsin–Madison Institutional Review Board. Primary PMNs and MCs were isolated simultaneously using a gradient method, followed by dextran sedimentation and erythrocyte hypotonic lysis, as previously described.28 This isolation procedure routinely results in approximately 80% to 90% MC purity, with lymphocytes as the primary impurities. A small percentage of impurities (<1%; basophils and eosinophils) is also possible in the PMN isolation. For monoculture studies, PMNs and MCs were adjusted to 1 × 106 cells/mL (Supplemental Figure S1A) or 5 × 105 cells/mL (Supplemental Figure S1B) in RPMI 1640 medium supplemented with 10% autologous human serum and were statically seeded onto PEG hydrogel, PDMS, and TCPS surfaces in 48- or 96-well plates and cultured in a humidified atmosphere at 37°C with 5% CO2. Seeding densities were varied to allow for comparison with PMN-MC paracrine studies (1 × 106 cells/mL, approximately 5.3 × 103 cells/mm2) (Supplemental Figure S1A) and with PMN-MC direct co-culture studies (5 × 105 cells/mL, approximately 2.6 × 103 cells/mm2) (Supplemental Figure S1B), as well as to determine the influence of seeding density on the extent of cell response to biomaterials. For PMN-MC paracrine studies (Supplemental Figure S1A), culture medium from PMN monocultures was collected after 24 hours of culture and spun to obtain debris-free PCM. MCs were adjusted to 1 × 106 MCs/mL in RPMI 1640 medium supplemented with 10% autologous human serum, and 168 μL of the MC suspension was statically seeded onto PEG hydrogel, PDMS, and TCPS surfaces in 96-well plates (approximately 5.3 × 103 MCs/mm2). After 2 hours, the culture medium was replaced with a 2:3 mixture of autologous PCM to fresh culture medium. This ratio of autologous PCM to fresh culture medium was selected to replenish depleted nutrients and provide ample PCM for triplicate samples. PCM priming lasted for 24 hours, after which the MC cultures were replaced with fresh culture medium without PCM. For each donor, MC cultures with and without PCM priming were conducted simultaneously. For PMN-MC direct co-culture studies (Supplemental Figure S1B), PMNs were adjusted to 5 × 105 PMNs/mL in RPMI 1640 medium supplemented with 10% autologous human serum, and 168 μL of the PMN suspension was statically seeded onto PEG hydrogel, PDMS, and TCPS surfaces in 96-well plates (approximately 2.6 × 103 PMNs/mm2). After 2 hours, the culture medium was replaced with 168 μL fresh culture medium containing 5 × 105 autologous MCs/mL (approximately 2.6 × 103 MCs/mm2). The culture medium was replaced again 2 hours after the MCs were added to remove nonadherent cells. For each donor, PMN and MC monoculture studies were conducted simultaneously with the PMN-MC direct co-culture studies to serve as controls.
Analysis of Cell Adhesion, Viability, Necrosis, Apoptosis, and Secondary Necrosis
At each predetermined time point, samples were washed once with Dulbecco's PBS to remove nonadherent cells and stained with a solution containing 4 μmol/L green-fluorescent calcein AM (to indicate intracellular esterase activity) and 2 μmol/L red-fluorescent ethidium homodimer-1 (to indicate loss of plasma membrane integrity) for 45 minutes at 37°C (reagents from LIVE/DEAD Viability/Cytotoxicity Kit; Molecular Probes, Eugene, OR). Two or three representative fields per well of a 96- or 48-well plate, respectively, were imaged with a digital camera attached to an inverted fluorescent microscope (Nikon Eclipse TE 300; Nikon Instruments Inc., Melville, NY), and viable (green) and necrotic (red) cells were counted in an area of 0.62 mm2 per field to determine adherent viable and necrotic cell densities (cells/mm2). As an indicator of apoptosis in adherent cells, intracellular caspase 3/7 activities were measured using the CaspaseGlo 3/7 luminescent assay (Promega Corporation, Madison, WI) at each predetermined time point. Culture medium was aspirated from each well of a white-walled 96-well plate (Corning Inc.) and replaced with 50 μL fresh culture medium and 50 μL CaspaseGlo 3/7 Reagent. The contents of the plate were mixed on a plate shaker for 30 seconds, and the plate was incubated at room temperature for 1 hour to lyse adherent cells. Luminescence was measured on a plate reader (FLUOstar OPTIMA; BMG Labtech, Cary, NC). During secondary necrosis, disruption of plasma membrane integrity results in the release of caspase-3/7 into the cell culture supernatant.29 Therefore, at each predetermined time point, secondary necrosis was also assessed by collecting 50 μL of cell culture supernatant, adding 50 μL CaspaseGlo 3/7 Reagent and following the CaspaseGlo 3/7 assay previously described.
Quantification of Selected Soluble Factors
Supernatants from PMN monocultures (seeded at a density of approximately 5.3 × 103 PMNs/mm2) were collected after 2, 24, and 72 hours and centrifuged at 2000 × g for 5 minutes to remove cells and cellular debris. MIP-1β was measured using an enzyme-linked immunosorbent assay (ELISA) kit (Invitrogen, Camarillo, CA), according to manufacturer's instructions. Supernatants were diluted 2000-fold, and HNP1-3 (α-defensins) were also measured using an ELISA kit (Hycult Biotech Inc., Plymouth Meeting, PA), according to manufacturer's instructions. MIP-1β is a C-C chemokine, secreted by activated PMNs, that chemoattracts MCs and CD4+ T lymphocytes.30–32 HNP1-3s are antimicrobial peptides stored in PMN granules that are released on cellular activation.33,34 In addition to having antimicrobial activity, HNPs have potent chemotactic activity for MCs and can modify cytokine expression in activated MCs.3,7
Supernatants from MC cultures with and without PCM priming were collected after 2, 98, and 170 hours (Supplemental Figure S1A). Supernatants from PMN-MC direct co-cultures were collected 48, 96, and 168 hours after MCs were added, and supernatants from PMN and MC monocultures were collected 48, 96, and 168 hours after MCs were added in the simultaneously conducted PMN-MC direct co-cultures (Supplemental Figure S1B). All supernatants were centrifuged at 2000 × g for 5 minutes to remove cells and cellular debris. Soluble IL-1β, IL-6, IL-8, IL-10, and tumor necrosis factor (TNF)-α concentrations were quantified as markers of MC proinflammatory or pro–wound-healing propensity using a Bio-Plex bead-based assay (Bio-Rad Laboratories, Hercules, CA). Data were analyzed using Bio-Plex Manager software, version 6.0, with the standard curve optimization function. Approximately one third of the IL-8 concentrations was extrapolated above the standard curve by Bio-Plex Manager software, version 6.0, and is reported. Cytokine concentrations extrapolated below the lowest concentration of the standard curve are also reported, and cytokine concentrations that were out of range below the standard curve are reported as the value halfway between the lowest extrapolated concentration and 0. IL-6, IL-8, and TNF-α are accepted as proinflammatory/anti–wound-healing markers, IL-1β as a proinflammatory/pro–wound-healing marker, and IL-10 as an anti-inflammatory/anti–wound-healing marker.25
Statistical Analysis
Culture conditions were established in duplicate or triplicate for each of the three independent experiments using the different donors (n = 3). To avoid introducing additional variables, we used autologous PCM for the PMN-MC paracrine studies and autologous PMNs and MCs in the PMN-MC direct co-culture studies. Results represent means ± SD unless otherwise noted. Statistical analysis of mean PMN or MC activities (eg, adherent cell density, caspase 3/7 activity, and cytokine concentration) was performed using one-way analysis of variance combined with Bonferroni's multiple comparison post tests (GraphPad Prism, San Diego, CA). Values of P ≤ 0.05 were considered statistically significant. In addition, to account for donor variability, we identified instances in which all three donors had >1.8-fold differences in cell activity between culture conditions (ie, MC cultures with PCM priming compared with MC cultures without PCM priming or PMN-MC co-cultures compared with the sums of PMN and MC monocultures). Differential expression is often selected using a fold-change cutoff between 1.8 and 3.0.35
Results
PMN-Biomaterial Interactions: Cell Adhesion, Viability, Necrosis, Apoptosis, Secondary Necrosis, and Soluble MIP-1β and HNP1-3 Concentrations
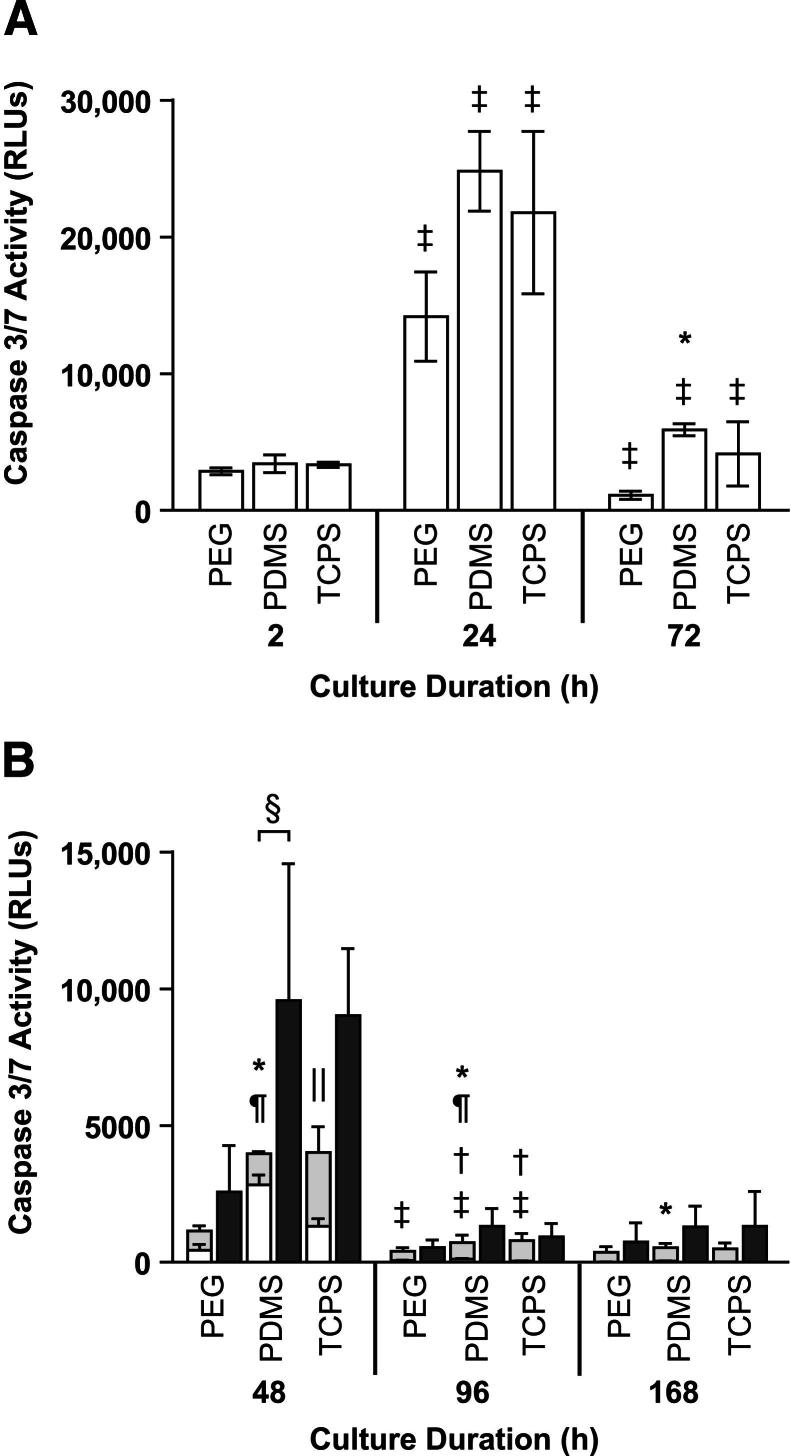
In PMN monocultures, the densities of viable adherent PMNs decreased between 2 and 72 hours, with the largest decreases occurring between 24 and 72 hours (Figure 1). Between 2 and 24 hours, the densities of necrotic adherent PMNs on PEG hydrogels increased, whereas the densities of necrotic adherent PMNs on PDMS and TCPS decreased (Figure 1). There were no significant differences in viable or necrotic adherent PMN densities among the surfaces at 2, 24, or 72 hours. In the PMN monocultures performed to 168 hours with a lower seeding density, few PMNs remained adherent after 48 hours (Figure 2), and most of those adherent PMNs were necrotic (Supplemental Figure S2). Cell death may contribute to the observed decrease in adherent cell densities; therefore, we measured the caspase 3/7 activities of adherent PMNs and of supernatants from PMN monocultures as markers of apoptosis and secondary necrosis, respectively. The caspase 3/7 activities of adherent PMNs on all surfaces increased transiently between 2 and 24 hours (P ≤ 0.01) before decreasing between 24 and 72 hours (P ≤ 0.01) (Figure 3A). The caspase 3/7 activities of supernatants from PMN monocultures on all surfaces increased between 2 and 24 hours (P ≤ 0.05) (Supplemental Figure S3A). On all surfaces, the caspase 3/7 activities of adherent PMNs (Figure 3B) and of supernatants from PMN monocultures (Supplemental Figure S3B) decreased significantly between 48 and 96 hours (P ≤ 0.05) and remained low at 168 hours. At 72 hours, the caspase 3/7 activities of adherent PMNs were significantly higher on PDMS than on PEG hydrogels (P = 0.01 to P = 0.05) (Figure 3A). At 48, 96, and 168 hours, the caspase 3/7 activities of adherent PMNs were significantly higher on PDMS than on PEG hydrogels (P ≤ 0.05) (Figure 3B). At 48 and 96 hours, the caspase 3/7 activities of adherent PMNs on PDMS were also significantly higher than those of adherent PMNs on TCPS (P = 0.001 to P = 0.01). In summary, in PMN monocultures, adherent PMN densities decreased between 2 and 168 hours. During this time, the caspase 3/7 activities of adherent PMNs and of supernatants from PMN monocultures increased initially and attenuated, peaking at approximately 24 to 48 hours. At later time points (48, 72, 96, and 168 hours), the caspase 3/7 activities of adherent PMNs were generally higher on PDMS than on PEG hydrogels or TCPS.
Figure 1.
Viable (grey bars) and necrotic (white bars) adherent cell densities in PMN monocultures (seeded at approximately 5.3 × 103 PMNs/mm2) on PEG hydrogels, PDMS, and TCPS at 2, 24, and 72 hours. Results represent means + SD (n = 3).
Figure 2.
Total (viable and necrotic) adherent cell densities in PMN monocultures (white bars, seeded at approximately 2.6 × 103 PMNs/mm2), MC monocultures (light grey bars, seeded at approximately 2.6 × 103 MCs/mm2), and PMN-MC direct co-cultures (dark grey bars, seeded at approximately 2.6 × 103 PMNs/mm2 and approximately 2.6 × 103 MCs/mm2) on PEG hydrogels, PDMS, and TCPS at 48, 96, and 168 hours. Results represent means + SD (n = 3). ∗P ≤ 0.05, significant difference from PEG hydrogels and TCPS in MC monocultures at the same time point.
Figure 3.
Apoptosis in PMN monocultures. A: Apoptosis in PMN monocultures (white bars, seeded at approximately 5.3 × 103 PMNs/mm2), as measured by luminescence because of the caspase 3/7 activities of PMNs adherent on PEG hydrogels, PDMS, and TCPS at 2, 24, and 72 hours. B: Apoptosis in PMN monocultures (light grey bars, seeded at approximately 2.6 × 103 PMNs/mm2), MC monocultures (dark grey bars, seeded at approximately 2.6 × 103 MCs/mm2), and PMN-MC direct co-cultures (seeded at approximately 2.6 × 103 PMNs/mm2 and approximately 2.6 × 103 MCs/mm2), as measured by luminescence due to the caspase 3/7 activities of PMNs and/or MCs adherent on PEG hydrogels, PDMS, and TCPS at 48, 96, and 168 hours. Results represent means ± SD (n = 3). ∗P = 0.01 to P = 0.05, significant difference from PEG hydrogels in PMN monocultures at the same time point; †P ≤ 0.05, significant difference from previous time point in MC monocultures; ‡P ≤ 0.05, significant difference from previous time point in PMN monocultures; §P = 0.01 to P = 0.05, significant difference for PMNs and MCs in direct co-culture compared with the sum of PMNs and MCs in monoculture; ¶P ≤ 0.05, significant difference from TCPS in PMN monocultures at the same time point; ‖P = 0.01 to P = 0.05, significant difference from PEG hydrogels and PDMS in MC monocultures at the same time point. RLUs, relative luminescence units.
MIP-1β concentrations in supernatants from PMN monocultures on all surfaces were higher at 24 hours than at 2 or 72 hours, ranging from 52 ± 41 to 85 ± 63 pg/mL at 24 hours (Figure 4A). At 2 hours, supernatants from PMN monocultures on PEG hydrogels had significantly higher HNP1-3 concentrations than those from PMN monocultures on PDMS or TCPS (P = 0.001 to P = 0.01) (Figure 4B). HNP1-3 concentrations in supernatants on PDMS and TCPS increased between 24 and 72 hours (P ≤ 0.05), resulting in comparable concentrations for all surfaces by 72 hours (ranging from 662 ± 156 to 969 ± 184 ng/mL).
Figure 4.

MIP-1β and HNP1-3 concentrations in supernatants from PMN monocultures. MIP-1β (A) and HNP1-3 (B) concentrations in supernatants from PMN monocultures (seeded at approximately 5.3 × 103 PMNs/mm2) on PEG hydrogels (dark grey bars), PDMS (light grey bars), and TCPS (white bars) at 2, 24, and 72 hours. Results represent means ± SD (n = 3). ∗P = 0.001 to P = 0.01, significant difference between surfaces; †P ≤ 0.05, significant difference from previous time point.
MC-Biomaterial Interactions: Cell Adhesion, Viability, Necrosis, Apoptosis, Secondary Necrosis, and Soluble Cytokine Concentrations
In MC monocultures, the densities of viable adherent MCs decreased between 2 and 170 hours on all surfaces, with a statistically significant decrease between 2 and 98 hours on TCPS (P = 0.001 to P = 0.01) (Figure 5). A decrease in viable adherent MC densities was also observed between 48 and 168 hours in MC monocultures with lower seeding density on all surfaces (Supplemental Figure S2A). There were no significant differences in viable adherent MC densities among surfaces at any of the time points investigated (Figure 5 and Supplemental Figure S2A). In contrast, there were differences in necrotic adherent MC densities among surfaces. At 2 and 98 hours, necrotic adherent MC densities were significantly higher on PDMS than on PEG hydrogels or TCPS (P ≤ 0.01) (Figure 5). Similarly, at 48 and 168 hours, necrotic adherent MC densities were significantly higher on PDMS than on PEG hydrogels or TCPS (P ≤ 0.05) (Supplemental Figure S2B). These higher densities of necrotic adherent MCs were reflected in the total (viable and necrotic) adherent MC densities, which were significantly higher on PDMS than on PEG hydrogels or TCPS at 48 and 168 hours (P ≤ 0.05) (Figure 2).
Figure 5.

Viable (grey bars) and necrotic (white bars) adherent cell densities in MC monocultures (seeded at approximately 5.3 × 103 MCs/mm2) on PEG hydrogels, PDMS, and TCPS at 2, 98, and 170 hours. Results represent means + SD (n = 3). ∗P ≤ 0.01, significant difference in necrotic adherent cell densities from PEG hydrogels and TCPS at the same time point; †P = 0.001 to P = 0.01, significant difference in viable adherent cell densities from previous time point.
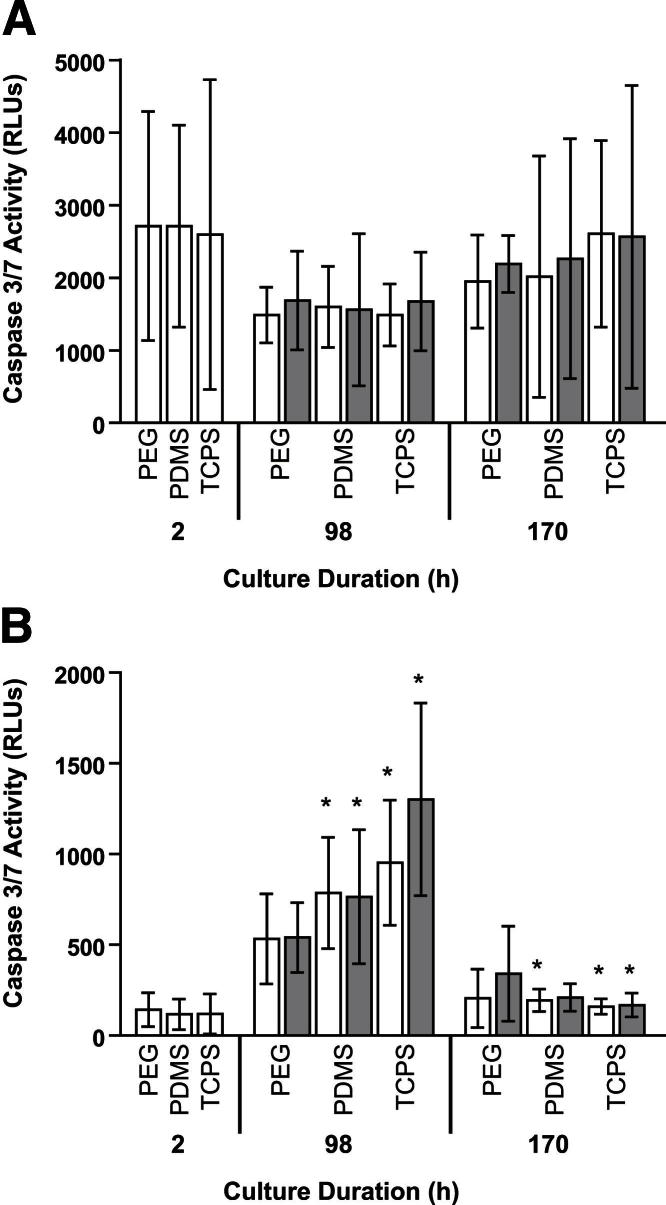
In MC monocultures, the caspase 3/7 activities of adherent MCs were similar at 2, 98, and 170 hours among all surfaces, ranging from 1487 ± 426 relative luminescence units (RLUs) (98 hours, TCPS) to 2711 ± 1392 RLUs (2 hours, PDMS) (Figure 6A). In MC monocultures with lower seeding density, the caspase 3/7 activities of MCs adherent on PDMS and TCPS decreased significantly between 48 and 96 hours (P = 0.01 to P = 0.05) (Figure 3B). In addition, the caspase 3/7 activities of supernatants from MC monocultures on PDMS and TCPS changed over time, increasing between 2 and 98 hours (P ≤ 0.05), before decreasing between 98 and 170 hours (P ≤ 0.05) (Figure 6B). There was also a decrease in the caspase 3/7 activities of supernatants from MC monocultures with lower seeding density on all surfaces between 48 and 168 hours, with a significant decrease between 48 and 96 hours on PEG hydrogels (P = 0.01 to P = 0.05) (Supplemental Figure S3B). In contrast to our observations in PMN monocultures, in which the caspase 3/7 activities of adherent PMNs were generally higher on PDMS than on PEG hydrogels or TCPS, there was only one instance of a significant difference in caspase 3/7 activities of adherent MCs (or of supernatants from MC monocultures) among surfaces: at 48 hours, the caspase 3/7 activities of MCs adherent on TCPS were significantly higher than those of MCs adherent on PEG hydrogels or PDMS (P = 0.01 to P = 0.05) (Figure 3B). Thus, in monoculture, there was a difference in PMN and MC regulation of apoptosis in response to biomaterials, particularly PDMS.
Figure 6.
PCM does not significantly influence apoptosis (A) or secondary necrosis (B) in MC cultures (seeded at approximately 5.3 × 103 MCs/mm2). MC cultures on PEG hydrogels, PDMS, and TCPS were primed with PCM from 2 to 26 hours. In MC cultures with (grey bars) or without (white bars) PCM priming apoptosis and secondary necrosis were assessed by measuring caspase 3/7 activities of adherent MCs or of supernatants from MC cultures, respectively. Results represent means ± SD (n = 3). ∗P ≤ 0.05, significant difference from previous time point.
In MC monocultures on all surfaces, soluble IL-1β and IL-6 concentrations increased between 2 and 98 hours and decreased between 98 and 170 hours (Figure 7A and Supplemental Figure S4A). There were also decreases in IL-1β and IL-6 concentrations between 48 and 168 hours in MC monocultures with lower seeding density on all surfaces (Supplemental Figure S5, A and B). IL-1β and IL-6 concentrations were higher in MC monocultures on PEG hydrogels than in those on PDMS or TCPS at all time points (Figure 7A and Supplemental Figures S4A and S5, A and B). IL-8 expression in MC monocultures increased between 2 and 98 hours on all surfaces and decreased between 98 and 170 hours on PDMS and TCPS (Figure 7B). On all surfaces, IL-8 concentrations decreased between 48 and 168 hours in MC monocultures with lower seeding density (Supplemental Figure S5C). IL-8 concentrations were greater in MC monocultures on PEG hydrogels than in those on PDMS or TCPS at all time points, except at 48 hours, when IL-8 expression was comparable on PEG hydrogels and PDMS (4 ± 2 × 104 pg/mL on PEG hydrogels and 4 ± 1 × 104 pg/mL on PDMS) (Figure 7B and Supplemental Figure S5C). There were no consistent increases or decreases in concentrations of IL-10 (Figure 7C and Supplemental Figure S5D) or TNF-α (Supplemental Figures S4B and S5E) between time points in MC monocultures. TNF-α concentrations were greater in MC monocultures on PEG hydrogels than in those on PDMS or TCPS at all time points (Supplemental Figures S4B and S5E). IL-10 concentrations were greater in MC monocultures on PEG hydrogels than in those on PDMS or TCPS at intermediate time points (48, 96, and 98 hours) but similar on all surfaces at earlier and later time points (2, 168, and 170 hours) (Figure 7C and Supplemental Figure S5D). In general, MCs cultured on PEG hydrogels expressed higher concentrations of IL-1β, IL-6, IL-8, IL-10, and TNF-α than MCs cultured on PDMS or TCPS.
Figure 7.
Soluble IL-1β, IL-8, and IL-10 concentrations in MC cultures. Soluble IL-1β (A), IL-8 (B), and IL-10 (C) concentrations in MC cultures (seeded at approximately 5.3 × 103 MCs/mm2) on PEG hydrogels, PDMS, and TCPS at 2, 98, and 170 hours, with (grey bars) or without (white bars) PCM priming from 2 to 26 hours of culture. Results represent means ± SD (n = 3). Values are shown in parentheses if the bar is not visible. ∗P ≤ 0.05, significant difference from TCPS at the same time point; †P ≤ 0.05, significant difference from PDMS at the same time point; ‡P ≤ 0.05, significant difference from previous time point.
PMN-MC Paracrine Interactions Selectively Increase MC Adhesion/Viability and IL-1β and TNF-α Expression But Not Apoptosis or Necrosis
MCs were primed with PCM from 2 to 26 hours to determine how soluble mediators secreted from biomaterial-activated PMNs influence MCs. Compared with concurrent MC monocultures without PCM priming, at 170 hours, the densities of total (viable and necrotic) adherent MCs on PEG hydrogels were significantly higher when MCs were primed with PCM (P = 0.01 to P = 0.05) (Figure 8). This increase in total adherent MC densities reflected a significant increase in viable adherent MC densities on PEG hydrogels in MC cultures with PCM priming compared with MC monocultures without PCM priming (P = 0.01 to P = 0.05); there were no significant differences in necrotic adherent densities with PCM priming (data not shown). Compared with the caspase 3/7 activities of MC monocultures, PCM priming did not significantly influence the caspase 3/7 activities of adherent MCs (Figure 6A) or of supernatants from MC cultures (Figure 6B) at 98 or 170 hours. Furthermore, PCM priming did not significantly alter the concentrations of IL-1β, IL-6, IL-8, IL-10, or TNF-α in MC cultures at 98 or 170 hours when compared with MC monocultures without PCM priming (Figure 7 and Supplemental Figure S4).
Figure 8.
PCM modulates MC adhesion in MC cultures (seeded at approximately 5.3 × 103 MCs/mm2) on PEG hydrogels. MC cultures on PEG hydrogels, PDMS, and TCPS were primed with PCM from 2 to 26 hours. Total (viable and necrotic) adherent cell densities were measured in MC cultures (seeded at approximately 5.3 × 103 MCs/mm2), with (grey bars) or without (white bars) PCM priming. Results represent means ± SD (n = 3). ∗P = 0.01 to P = 0.05, significant difference with PCM priming; †P ≤ 0.01, significant difference from previous time point.
In addition to comparing the mean MC activities (eg, adherent MC density, caspase 3/7 activity, and cytokine concentration) of the three donors with and without PCM priming (using one-way analysis of variance combined with Bonferroni's multiple comparison post tests), to account for donor variability, we compared each data set for the three donors individually and identified instances in which all three donors had >1.8-fold differences in MC activity with or without PCM priming (Table 1). In MC cultures with PCM priming on PEG hydrogels at 170 hours, the densities of viable adherent MCs and the densities of total (viable and necrotic) adherent MCs were 2.9- to 4.9-fold and 2.6- to 4.2-fold higher, respectively, than these densities in MC cultures without PCM priming. Although these increases in adherent MC densities with PCM priming on PEG hydrogels were identified as statistically significant (P = 0.01 to P = 0.05) using one-way analysis of variance combined with Bonferroni's multiple comparison post tests, there were other changes in MC activity with PCM priming listed in Table 1 that were not identified as statistically significant using the one-way analysis of variance method. For example, on PDMS at 170 hours, viable adherent MC densities in MC cultures with PCM priming were 1.9- to 4.9-fold higher than those without PCM priming. In addition, on PDMS at 98 hours, IL-1β and TNF-α concentrations were 2.5- to 3.3-fold and 1.8- to 5.9-fold higher, respectively, in MC cultures with PCM priming than in those without PCM priming. Thus, when comparing within a single donor, the impact of biomaterial-mediated PMN-MC paracrine regulation on MC activity was more pronounced.
Table 1.
Instances in Which All Three Donors Had >1.8-Fold Differences in MC Activity with or without PCM Priming
| MC activity | Culture time (hours) | Biomaterial surface | PCM priming (+/−) | Donor 1 | Donor 2 | Donor 3 |
|---|---|---|---|---|---|---|
| Viable adherent MCs/mm2 | 170 | PEG hydrogel | − | 207 | 241 | 82 |
| 170 | PEG hydrogel∗ | + | 888 | 1058 | 235 | |
| Fold-change with PCM priming | 4.3 | 4.4 | 2.9 | |||
| 170 | PDMS | − | 116 | 90 | 16 | |
| 170 | PDMS | + | 222 | 217 | 80 | |
| Fold-change with PCM priming | 1.9 | 2.4 | 4.9 | |||
| Total (viable and necrotic) adherent MCs/mm2 | 170 | PEG hydrogel | − | 226 | 272 | 103 |
| 170 | PEG hydrogel∗ | + | 950 | 1111 | 270 | |
| Fold-change with PCM priming | 4.2 | 4.1 | 2.6 | |||
| IL-1β (pg/mL)† | 98 | PDMS | − | 20 | 18 | 18 |
| 98 | PDMS | + | 51 | 60 | 56 | |
| Fold-change with PCM priming | 2.5 | 3.3 | 3.1 | |||
| TNF-α (pg/mL) | 98 | PDMS | − | 62 | 37 | 9 |
| 98 | PDMS | + | 173 | 218 | 17 | |
| Fold-change with PCM priming | 2.8 | 5.9 | 1.8 | |||
P = 0.01 to P = 0.05, significant difference in the mean of the three donors with PCM priming based on one-way analysis of variance combined with Bonferroni's multiple comparison post tests.
There were also >1.8-fold increases in IL-1β expression with PCM priming in MC cultures on PEG hydrogels and PDMS at 170 hours, but because some of the IL-1β values were extrapolated below the standard curve at 170 hours, these increases in IL-1β expression with PCM priming were not included in the table.
Cell Adhesion, Viability, Necrosis, Apoptosis, Secondary Necrosis, and Cytokine Expression of PMNs and MCs in Direct Co-Culture
We co-cultured autologous PMNs and MCs (seeded at a density of approximately 2.6 × 103 cells/mm2 each) and compared their activity in direct co-cultures with the sums of the activities of PMNs and MCs in monocultures (seeded at a density of approximately 2.6 × 103 cells/mm2). There were no significant differences in the viable, necrotic, or total adherent cell densities in the PMN-MC direct co-cultures compared with the sums of these adherent cell densities in the PMN and MC monocultures at 48, 96, or 168 hours (Figure 2 and Supplemental Figure S2). However, at 48 hours on all surfaces, the caspase 3/7 activities of adherent PMNs and MCs in direct co-cultures were higher than the sums of the caspase 3/7 activities of adherent PMNs and MCs in monocultures (1.1- to 3.3-fold higher on PEG hydrogels, 1.3- to 3.5-fold higher on PDMS, and 1.7- to 2.8-fold higher on TCPS); these differences were statistically significant on PDMS (P = 0.01 to P = 0.05) (Figure 3B). There were no significant differences between the caspase 3/7 activities of supernatants from PMN-MC direct co-cultures and the sums of the caspase 3/7 activities of supernatants from the PMN and MC monocultures at 48, 96, or 168 hours (Supplemental Figure S3B). Furthermore, at 48, 96, or 168 hours, there were no significant differences between the concentrations of IL-1β, IL-6, IL-8, IL-10, or TNF-α in the PMN-MC direct co-cultures and the sums of the concentrations of these cytokines in the PMN and MC monocultures (Supplemental Figure S5).
To account for donor variability, we compared each data set in the PMN-MC direct co-cultures with the sums of the PMN and MC monocultures for each of the three donors individually and identified instances in which all three donors had >1.8-fold differences in cell activity between the two conditions (ie, the PMN-MC direct co-cultures and the sums of the PMN and MC monocultures) (Table 2). For example, at 48 hours, the densities of viable adherent cells in the PMN-MC direct co-cultures were 1.8- to 2.4-fold higher on PDMS and 1.9- to 4.1-fold higher on TCPS than the sums of the densities of viable adherent cells in the PMN and MC monocultures. In contrast, on PEG hydrogels at 168 hours, the viable adherent cell densities in the PMN-MC direct co-cultures were 1.9- to 17.6-fold lower than the sums of viable adherent cell densities in the PMN and MC monocultures. On TCPS at 48 hours, IL-6 concentrations in the PMN-MC direct co-cultures were 1.9- to 17.9-fold lower than the sums of IL-6 concentrations in the PMN and MC monocultures (Table 2). Last, on TCPS at 168 hours, IL-8 concentrations in PMN-MC direct co-cultures were 1.8- to 2.6-fold higher than the sums of IL-8 concentrations in the PMN and MC monocultures (Table 2). Thus, when comparing within a single donor, the impact of biomaterial modulation on PMN-MC juxtacrine and paracrine interactions was more pronounced.
Table 2.
Instances in Which All Three Donors Had >1.8-Fold Differences in Cell Activity between the PMN-MC Direct Co-Culture and the Sum of PMN and MC Monocultures
| Cell activity | Culture time (hours) | Biomaterial surface | Direct co-culture (PMN-MC) or sum of PMN and MC monocultures (PMN + MC) | Donor 1 | Donor 2 | Donor 3 |
|---|---|---|---|---|---|---|
| Viable adherent cells/mm2 | 48 | PDMS | PMN + MC | 412 | 48 | 185 |
| 48 | PDMS | PMN-MC | 833 | 116 | 340 | |
| Fold-change with direct co-culture (PMN-MC) | 2.0 | 2.4 | 1.8 | |||
| 48 | TCPS | PMN + MC | 187 | 88 | 66 | |
| 48 | TCPS | PMN-MC | 349 | 173 | 270 | |
| Fold-change with direct co-culture (PMN-MC) | 1.9 | 2.0 | 4.1 | |||
| 168 | PEG hydrogel | PMN + MC | 44 | 46 | 41 | |
| 168 | PEG hydrogel | PMN-MC | 22 | 3 | 22 | |
| Fold-change with direct co-culture (PMN-MC) | 0.50 | 0.06 | 0.53 | |||
| IL-6 (pg/mL)∗ | 48 | TCPS | PMN + MC | 14 | 797 | 146 |
| 48 | TCPS | PMN-MC | 7 | 44 | 78 | |
| Fold-change with direct co-culture (PMN-MC) | 0.47 | 0.06 | 0.54 | |||
| IL-8 (pg/mL) | 168 | TCPS | PMN + MC | 795 | 897 | 353 |
| 168 | TCPS | PMN-MC | 1436 | 2253 | 919 | |
| Fold-change with direct co-culture (PMN-MC) | 1.8 | 2.5 | 2.6 | |||
There were also >1.8-fold decreases in IL-6 expression between the PMN-MC direct co-culture and the sum of the PMN and MC monocultures on TCPS at 96 hours, but because some of the IL-6 values were extrapolated below the standard curve at 96 hours, these decreases in IL-6 expression were not included in this table.
Discussion
Despite the importance of PMN-MC interactions in MC recruitment, activation, and function, few studies have investigated how biomaterials modulate these interactions. Furthermore, existing (non–biomaterial-focused) data regarding PMN-MC interactions show contrasting trends. Treatment of MCs with PMN granule proteins has been shown to enhance proinflammatory cytokine release from lipopolysaccharide (LPS)–, phorbol 12-myristate 13-acetate–, or Staphylococcus aureus–treated MCs,4,6,7 whereas phagocytosis of apoptotic PMNs or treatment with ectosomes released from PMNs has been shown to reduce the release of proinflammatory cytokines from zymosan- or LPS-treated MCs or MC-derived macrophages.8,19,21 The objective of this study was to determine how biomaterials modulate PMN-MC interactions. To better dissect how biomaterials influence PMN-MC paracrine and juxtacrine interactions, experiments consisted of PMN-MC paracrine studies, in which MCs were primed with autologous PCM and PMN-MC direct co-cultures.
In the PMN-MC paracrine studies, at 170 hours, viable adherent MC densities in MC cultures with PCM priming were 2.9- to 4.4-fold higher on PEG hydrogels and 1.9- to 4.9-fold higher on PDMS than those without PCM priming. Because MC adhesion is mediated through interactions of cell surface integrin receptors and proteins adsorbed on the biomaterial, PCM priming may modulate the quantities and identities of proteins adsorbed onto PEG hydrogels and PDMS and/or the integrin receptor repertoire displayed by MCs through outside-in signaling. Changes in integrin receptor expression that occur during MC-to-macrophage differentiation (generally after 7 to 10 days of in vitro culture36) may explain why there were no increases in adherent MC densities on PEG hydrogels or PDMS with PCM priming at 98 hours, despite observing increases at 170 hours (7 days).
In addition, with PCM priming, MCs cultured on PDMS had 2.5- to 3.3-fold and 1.8- to 5.9-fold higher concentrations of proinflammatory cytokines IL-1β and TNF-α, respectively, at 98 hours compared with the concentrations of these cytokines in MC monocultures without PCM priming. These findings are in agreement with other studies that demonstrated that PMN granule proteins enhance the release of proinflammatory cytokines from activated MCs. For example, treatment with HNPs increased expression of IL-1β and TNF-α in MCs activated with S. aureus or phorbol 12-myristate 13-acetate.7 In our study, treatment of MCs with HNPs from the PCM was likely not the major cause of the increase in IL-1β and TNF-α in MCs cultured on PDMS; in fact, PCM used to treat MC cultures on PEG hydrogels contained more HNPs (768 ± 468 ng/mL HNP1-3) than PCM used to treat MC cultures on PDMS (218 ± 128 ng/mL HNP1-3), yet we did not see an increase in IL-1β and TNF-α in MCs cultured on PEG hydrogels. In a study by Heinzelmann et al,6 treatment with another PMN granule protein, heparin-binding protein (alias azurocidin and CAP37), induced the release of TNF-α in resting MCs and enhanced the release of TNF-α and prostaglandin E2 from LPS-activated MCs. However, in some studies, treatment of MCs with HNPs or heparin-binding protein alone (without an activating agent, such as S. aureus, phorbol 12-myristate 13-acetate, or LPS) did not enhance their cytokine release,4,7 suggesting that MC modulation by PMN granule proteins may require a secondary activating signal. Thus, insufficient biomaterial activation of MCs may explain why we did not observe increases in the concentrations of IL-1β and TNF-α in MC cultures with PCM priming at other time points or on other surfaces. Furthermore, differences in the concentrations of PMN granule proteins used to treat MCs (ie, concentrations of isolated PMN granule proteins versus concentrations of PMN granule proteins in PCM) or the presence of serum proteins in the culture medium, which inactivate certain PMN granule proteins, such as HNPs,37 may contribute to differences in the results of our study compared with others.
Biomaterial modulation of PMN-MC interactions in the direct co-cultures had different effects on cell activity compared with biomaterial modulation of PMN-MC paracrine interactions in the MC cultures with PCM priming. At 48 hours, the densities of viable adherent cells in the PMN-MC direct co-cultures were 1.8- to 2.4-fold higher on PDMS and 1.9- to 4.1-fold higher on TCPS than the sums of the densities of viable adherent cells in the PMN and MC monocultures, suggesting that PMN-MC interactions extended the viability and adhesion of PMNs and/or MCs. In agreement, Kirk et al38 demonstrated that, at 2, 3, and 4 days of culture, the presence of MCs/macrophages in direct co-culture with PMNs enhanced PMN survival compared with PMNs in monoculture. Kirk et al38 also showed that there was a decrease in adherent MC/macrophage densities at 7 and 10 days in direct PMN-MC/macrophage co-cultures compared with adherent MC/macrophage densities in MC/macrophage monocultures. Similarly, in our study, on PEG hydrogels at 168 hours, viable adherent cell densities in direct PMN-MC co-cultures were 1.9- to 17.6-fold lower than the sums of viable adherent cell densities in the PMN and MC monocultures.
Because phagocytosis of apoptotic PMNs by MCs was possible in the PMN-MC direct co-cultures, we hypothesized that there may be a decrease in the expression of proinflammatory cytokines in PMN-MC co-cultures, as observed in other studies in which MCs phagocytized apoptotic PMNs.19,21 However, there was only a single decrease in the expression of a proinflammatory cytokine: on TCPS at 48 hours, IL-6 concentrations in PMN-MC direct co-cultures were 1.9- to 17.9-fold lower than the sums of IL-6 concentrations in the PMN and MC monocultures. Furthermore, on TCPS at 168 hours, IL-8 concentrations in PMN-MC direct co-cultures were 1.8- to 2.6-fold higher than the sums of IL-8 concentrations in the PMN and MC monocultures. In other studies, LPS or zymosan activation of MCs/macrophages enhanced the reduction in the release of proinflammatory cytokines from MCs/macrophages after phagocytosis of apoptotic PMNs.19,21 Therefore, there may not have been sufficient biomaterial activation of MCs in PMN-MC direct co-cultures to observe a noticeable reduction in proinflammatory cytokine expression. Another possibility is that there was a balance between proinflammatory signals (from MC uptake of PMN granule proteins) and anti-inflammatory signals (from MC uptake of PMN ectosomes or MC phagocytosis of PMNs), resulting in minimal changes in cytokine concentrations in the PMN-MC direct co-cultures compared with the sums of cytokine concentrations in the PMN and MC monocultures.
In conclusion, our data suggest that biomaterials selectively modulate PMN-MC paracrine and juxtacrine interactions to influence MC and/or PMN viability/adhesion, apoptosis, and expression of cytokines. This was demonstrated by biomaterial-dependent differences in MC activity in MC cultures with PCM priming (compared with MC activity in MC monocultures without PCM priming) and biomaterial-dependent differences in cell activity in the PMN-MC direct co-cultures (compared with the sums of cell activities in the PMN and MC monocultures). Data from experiments in which MCs were primed with PCM suggest that PMN-MC paracrine interactions increase MC viability/adhesion on PEG hydrogels and PDMS at 170 hours and enhance the release of proinflammatory cytokines IL-1β and TNF-α on PDMS at 98 hours. Data from PMN-MC direct co-culture experiments suggest that PMN-MC paracrine and/or juxtacrine interactions increase PMN and/or MC viability/adhesion on PDMS and TCPS at 48 hours, decrease PMN and/or MC viability/adhesion on PEG hydrogels at 168 hours, decrease PMN and/or MC expression of IL-6 on TCPS at 48 hours, and increase PMN and/or MC expression of IL-8 on TCPS at 168 hours. There is limited literature as to how biomaterials influence PMN-MC interactions, and these initial observations allow for the formulation of hypothesis-driven mechanistic studies. Future studies are needed to determine how biomaterials modulate the release of PMN secretion products and how biomaterials modulate PMN-MC paracrine and juxtacrine interactions in the presence of an additional activating agent, such as LPS. Studies are also needed to compare the PCM priming and PMN-MC direct co-culture in vitro models with in vivo biomaterial implantation outcomes to determine whether these in vitro models are more predictive of in vivo outcomes than traditional MC monocultures. Knowledge gained from such studies may eventually allow for the design of biomaterials to regulate PMN-MC interactions to promote a favorable wound-healing response after biomaterial implantation.
Acknowledgments
We thank David A. Cantu for his assistance with phlebotomy procedures and the University of Wisconsin Carbone Comprehensive Cancer Center for use of the Bio-Plex suspension array system to complete this research.
Footnotes
Supported by NIH grant EB6613 and the Department of Defense through a National Defense Science and Engineering Graduate fellowship (H.C.C.).
Supplemental Data
Study design diagram. A: In the PMN monoculture studies, PMNs were seeded at a density of approximately 5.3 × 103 PMNs/mm2 on PEG hydrogels, PDMS, and TCPS in 48- or 96-well plates. Three wells of PMN monocultures (per donor) were terminated at 2, 24, and 72 hours, and soluble MIP-1β and HNP1-3 concentrations were quantified. Cell adhesion, viability, necrosis, apoptosis, and secondary necrosis were also quantified. PCM was collected at 24 hours for use in the PMN-MC paracrine studies. In the PMN-MC paracrine studies, MCs were seeded at a density of approximately 5.3 × 103 MCs/mm2 on PEG hydrogels, PDMS, and TCPS in 96-well plates. At 2 hours, the culture medium was replaced with a 2:3 mixture of autologous PCM and fresh culture medium. PCM priming lasted for 24 hours, after which the MC cultures were replaced with fresh culture medium. Three wells of MC cultures (per donor) were terminated at 98 and 170 hours, and cell adhesion, viability, necrosis, apoptosis, and secondary necrosis were quantified. In addition, soluble IL-1β, IL-6, IL-8, IL-10, and TNF-α concentrations were quantified as markers of MC proinflammatory or pro–wound-healing propensity. MC monoculture studies were conducted simultaneously with the PMN-MC paracrine studies. In the MC monoculture studies, MCs were seeded at a density of approximately 5.3 × 103 MCs/mm2 on PEG hydrogels, PDMS, and TCPS in 96-well plates. Three wells of MC cultures (per donor) were terminated at 2, 98, and 170 hours, and cell adhesion, viability, necrosis, apoptosis, and secondary necrosis were quantified. In addition, soluble IL-1β, IL-6, IL-8, IL-10, and TNF-α concentrations were quantified. B: In the PMN-MC direct co-culture studies, PMNs were seeded at a density of approximately 2.6 × 103 PMNs/mm2 on PEG hydrogels, PDMS, and TCPS in 96-well plates. At 2 hours, the culture medium was replaced with fresh culture medium containing MCs for a seeding density of approximately 2.6 × 103 MCs/mm2. Two hours later, the culture medium was replaced again to remove nonadherent cells. Two wells of PMN-MC direct co-cultures (per donor) were terminated at 48, 96, and 168 hours (after the MCs were added), and cell adhesion, viability, necrosis, apoptosis, and secondary necrosis were quantified. In addition, soluble IL-1β, IL-6, IL-8, IL-10, and TNF-α concentrations were quantified. PMN and MC monoculture studies were conducted simultaneously with the PMN-MC direct co-culture studies. In the PMN monoculture studies, PMNs were seeded at a density of approximately 2.6 × 103 PMNs/mm2 on PEG hydrogels, PDMS, and TCPS in 96-well plates. To replicate the culture media changes in the simultaneously conducted PMN-MC direct co-cultures studies, the culture medium in the PMN monoculture studies was replaced after 2 hours and again 2 hours later. Two wells of PMN monocultures (per donor) were terminated at 48, 96, and 168 hours (after the MCs were added in the simultaneously conducted PMN-MC direct co-culture and MC monoculture studies). At each time point, cell adhesion, viability, necrosis, apoptosis, and secondary necrosis were quantified, as were concentrations of soluble IL-1β, IL-6, IL-8, IL-10, and TNF-α. In the MC monoculture studies, MCs were seeded at a density of approximately 2.6 × 103 MCs/mm2 on PEG hydrogels, PDMS, and TCPS in 96-well plates. After 2 hours, the culture medium was replaced. Two wells of MC monocultures (per donor) were terminated at 48, 96, and 168 hours (after the MCs were added). At each time point, cell adhesion, viability, necrosis, apoptosis, and secondary necrosis were quantified, as were concentrations of soluble IL-1β, IL-6, IL-8, IL-10, and TNF-α.
Viable and adherent cell densities in PMN monocultures. Viable (A) and necrotic (B) adherent cell densities in PMN monocultures (white bars, seeded at approximately 2.6 × 103 PMNs/mm2), MC monocultures (light grey bars, seeded at approximately 2.6 × 103 MCs/mm2), and PMN-MC direct co-cultures (dark grey bars, seeded at approximately 2.6 × 103 PMNs/mm2 and approximately 2.6 × 103 MCs/mm2) on PEG hydrogels, PDMS, and TCPS at 48, 96, and 168 hours. Results represent means + SD (n = 3). *P ≤ 0.05, significant difference from PEG hydrogels and TCPS in MC monocultures at the same time point; †P ≤ 0.05, significant difference from TCPS in PMN-MC direct co-cultures at the same time point; ‡P ≤ 0.05, significant difference from PDMS in PMN-MC direct co-cultures at the same time point.
Secondary necrosis in PMN monocultures. A: Secondary necrosis in PMN monocultures (white bars, seeded at approximately 5.3 × 103 PMNs/mm2), as measured by luminescence due to the caspase 3/7 activities of supernatants from PMN monocultures on PEG hydrogels, PDMS, and TCPS at 2, 24, and 72 hours. B: Secondary necrosis in PMN monocultures (white bars, seeded at approximately 2.6 × 103 PMNs/mm2), MC monocultures (light grey bars, seeded at approximately 2.6 × 103 MCs/mm2), and PMN-MC direct co-cultures (dark grey bars, seeded at approximately 2.6 × 103 PMNs/mm2 and approximately 2.6 × 103 MCs/mm2), as measured by luminescence due to the caspase 3/7 activities of supernatants from PMN or MC monocultures or direct PMN-MC co-cultures on PEG hydrogels, PDMS, and TCPS at 48, 96, and 168 hours. Results represent means ± SD (n = 3). *P = 0.001 to P = 0.01, significant difference between surfaces; †P = 0.01 to P = 0.05, significant difference from previous time point in MC monocultures; ‡P ≤ 0.05, significant difference from previous time point in PMN monocultures.
Soluble IL-6 and TNF-α concentrations in MC cultures. Soluble IL-6 (A) and TNF-α (B) concentrations in MC cultures (seeded at approximately 5.3 × 103 MCs/mm2) on PEG hydrogels, PDMS, and TCPS at 2, 98, and 170 hours, with (grey bars) or without (white bars*) PCM priming from 2 to 26 hours of culture. Results represent means ± SD (n = 3). Values are shown in parentheses if the bar is not visible. *P ≤ 0.05, significant difference from previous time point.
Soluble IL-1β, IL-6, IL-8, IL-10, and TNF-α concentrations in PMN monocultures. Soluble IL-1β (A), IL-6 (B), IL-8 (C), IL-10 (D), and TNF-α (E) concentrations in PMN monocultures (white bars, seeded at approximately 2.6 × 103 PMNs/mm2), MC monocultures (light grey bars, seeded at approximately 2.6 × 103 MCs/mm2), and PMN-MC direct co-cultures (dark grey bars, seeded at approximately 2.6 × 103 PMNs/mm2 and approximately 2.6 × 103 MCs/mm2) on PEG hydrogels, PDMS, and TCPS at 48, 96, and 168 hours. Results represent means + SD (log scale; n = 3). *P = 0.01 to P = 0.05, significant difference from previous time point.
References
- 1.Anderson J.M. Inflammatory response to implants. ASAIO Trans. 1988;34:101–107. doi: 10.1097/00002480-198804000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JM, Rodriguez A, Chang DT: Foreign body reaction to biomaterials. 2008, 20:86–100 [DOI] [PMC free article] [PubMed]
- 3.Territo M.C., Ganz T., Selsted M.E., Lehrer R. Monocyte-chemotactic activity of defensins from human neutrophils. J Clin Invest. 1989;84:2017–2020. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasmussen P.B., Bjørn S., Hastrup S., Nielsen P.F., Norris K., Thim L., Wiberg F.C., Flodgaard H. Characterization of recombinant human HBP/CAP37/azurocidin, a pleiotropic mediator of inflammation-enhancing LPS-induced cytokine release from monocytes. FEBS Lett. 1996;390:109–112. doi: 10.1016/0014-5793(96)00639-4. [DOI] [PubMed] [Google Scholar]
- 5.Chertov O., Ueda H., Xu L.L., Tani K., Murphy W.J., Wang J.M., Howard O.M.Z., Sayers T.J., Oppenheim J.J. Identification of human neutrophil-derived cathepsin G and azurocidin/CAP37 as chemoattractants for mononuclear cells and neutrophils. J Exp Med. 1997;186:739–747. doi: 10.1084/jem.186.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heinzelmann M., Mercer-Jones M.A., Flodgaard H., Miller F.N. Heparin-binding protein (CAP37) is internalized in monocytes and increases LPS-induced monocyte activation. J Immunol. 1998;160:5530–5536. [PubMed] [Google Scholar]
- 7.Chaly Y.V., Paleolog E.M., Kolesnikova T.S., Tikhonov I.I., Petratchenko E.V., Voitenok N.N. Neutrophil alpha-defensin human neutrophil peptide modulates cytokine production in human monocytes and adhesion molecule expression in endothelial cells. Eur Cytokine Netw. 2000;11:257–266. [PubMed] [Google Scholar]
- 8.Gasser O., Schifferli J.A. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood. 2004;104:2543–2548. doi: 10.1182/blood-2004-01-0361. [DOI] [PubMed] [Google Scholar]
- 9.Soehnlein O., Xie X., Ulbrich H., Kenne E., Rotzius P., Flodgaard H., Eriksson E.E., Lindbom L. Neutrophil-derived heparin-binding protein (HBP/CAP37) deposited on endothelium enhances monocyte arrest under flow conditions. J Immunol. 2005;174:6399–6405. doi: 10.4049/jimmunol.174.10.6399. [DOI] [PubMed] [Google Scholar]
- 10.Janardhan K.S., Sandhu S.K., Singh B. Neutrophil depletion inhibits early and late monocyte/macrophage increase in lung inflammation. Front Biosci. 2006;11:1569–1576. doi: 10.2741/1904. [DOI] [PubMed] [Google Scholar]
- 11.Soehnlein O., Zernecke A., Eriksson E.E., Rothfuchs A.G., Pham C.T., Herwald H., Bidzhekov K., Rottenberg M.E., Weber C., Lindbom L. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 2008;112:1461–1471. doi: 10.1182/blood-2008-02-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soehnlein O., Kai-Larsen Y., Frithiof R., Sorensen O.E., Kenne E., Scharffetter-Kochanek K., Eriksson E.E., Herwald H., Agerberth B., Lindbom L. Neutrophil primary granule proteins HBP and HNP1–3 boost bacterial phagocytosis by human and murine macrophages. J Clin Invest. 2008;118:3491–3502. doi: 10.1172/JCI35740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soehnlein O., Kenne E., Rotzius P., Eriksson E.E., Lindbom L. Neutrophil secretion products regulate anti-bacterial activity in monocytes and macrophages. Clin Exp Immunol. 2008;151:139–145. doi: 10.1111/j.1365-2249.2007.03532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eken C., Martin P.J., Sadallah S., Treves S., Schaller M., Schifferli J.A. Ectosomes released by polymorphonuclear neutrophils induce a MerTK-dependent anti-inflammatory pathway in macrophages. J Biol Chem. 2010;285:39914–39921. doi: 10.1074/jbc.M110.126748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soehnlein O., Weber C., Lindbom L. Neutrophil granule proteins tune monocytic cell function. Trends Immunol. 2009;30:538–546. doi: 10.1016/j.it.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Soehnlein O., Lindbom L., Weber C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood. 2009;114:4613–4623. doi: 10.1182/blood-2009-06-221630. [DOI] [PubMed] [Google Scholar]
- 17.Soehnlein O., Zernecke A., Weber C. Neutrophils launch monocyte extravasation by release of granule proteins. Thromb Haemost. 2009;102:198–205. doi: 10.1160/TH08-11-0720. [DOI] [PubMed] [Google Scholar]
- 18.Soehnlein O., Lindbom L. Neutrophil-derived azurocidin alarms the immune system. J Leukoc Biol. 2009;85:344–351. doi: 10.1189/jlb.0808495. [DOI] [PubMed] [Google Scholar]
- 19.Fadok V.A., Bratton D.L., Konowal A., Freed P.W., Westcott J.Y., Henson P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrne A., Reen D.J. Lipopolysaccharide induces rapid production of IL-10 by monocytes in the presence of apoptotic neutrophils. J Immunol. 2002;168:1968–1977. doi: 10.4049/jimmunol.168.4.1968. [DOI] [PubMed] [Google Scholar]
- 21.Mikołajczyk T.P., Skrzeczyńska-Moncznik J.E., Zarębski M.A., Marewicz E.A., Wiśniewska A.M., Dzięba M., Dobrucki J.W., Pryjma J.R. Interaction of human peripheral blood monocytes with apoptotic polymorphonuclear cells. Immunology. 2009;128:103–113. doi: 10.1111/j.1365-2567.2009.03087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serhan C.N., Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 23.Soehnlein O., Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 24.Silva M.T. Macrophage phagocytosis of neutrophils at inflammatory/infectious foci: a cooperative mechanism in the control of infection and infectious inflammation. J Leukoc Biol. 2011;89:675–683. doi: 10.1189/jlb.0910536. [DOI] [PubMed] [Google Scholar]
- 25.Brodbeck W.G., Voskerician G., Ziats N.P., Nakayama Y., Matsuda T., Anderson J.M. In vivo leukocyte cytokine mRNA responses to biomaterials are dependent on surface chemistry. J Biomed Mater Res A. 2003;64:320–329. doi: 10.1002/jbm.a.10425. [DOI] [PubMed] [Google Scholar]
- 26.Xu K., Fu Y., Chung W., Zheng X., Cui Y., Hsu I.C., Kao W.J. Thiol-ene-based biological/synthetic hybrid biomatrix for 3-D living cell culture. Acta Biomater. 2012;8:2504–2516. doi: 10.1016/j.actbio.2012.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt D., Joyce E.J., Kao W.J. Fetal bovine serum xenoproteins modulate human monocyte adhesion and protein release on biomaterials in vitro. Acta Biomater. 2011;7:515–525. doi: 10.1016/j.actbio.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waldeck H., Wang X., Joyce E., Kao W.J. Active leukocyte detachment and apoptosis/necrosis on PEG hydrogels and the implication in the host inflammatory response. Biomaterials. 2012;33:29–37. doi: 10.1016/j.biomaterials.2011.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krysko D.V., Vanden Berghe T., D'Herde K., Vandenabeele P. Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods. 2008;44:205–221. doi: 10.1016/j.ymeth.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Schall T.J., Bacon K., Camp R.D., Kaspari J.W., Goeddel D.V. Human macrophage inflammatory protein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populations of lymphocytes. J Exp Med. 1993;177:1821–1826. doi: 10.1084/jem.177.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taub D.D., Conlon K., Lloyd A.R., Oppenheim J.J., Kelvin D.J. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science. 1993;260:355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 32.Uguccioni M., D'Apuzzo M., Loetscher M., Dewald B., Baggiolini M. Actions of the chemotactic cytokines MCP-1, MCP-2, MCP-3, RANTES, MIP-1α and MIP-1β on human monocytes. Eur J Immunol. 1995;25:64–68. doi: 10.1002/eji.1830250113. [DOI] [PubMed] [Google Scholar]
- 33.Ganz T. Extracellular release of antimicrobial defensins by human polymorphonuclear leukocytes. Infect Immun. 1987;55:568–571. doi: 10.1128/iai.55.3.568-571.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ganz T., Selsted M.E., Lehrer R.I. Defensins. Eur J Haematol. 1990;44:1–8. doi: 10.1111/j.1600-0609.1990.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 35.Mutch D.M., Berger A., Mansourian R., Rytz A., Roberts M.A. The limit fold change model: a practical approach for selecting differentially expressed genes from microarray data. BMC Bioinformatics. 2002;3:17. doi: 10.1186/1471-2105-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nares S., Wahl S.M. Monocytes and macrophages. In: Lotze M.T., Thomson A.W., editors. Measuring Immunity. Academic Press; London: 2005. pp. 299–311. [Google Scholar]
- 37.Panyutich A.V., Hiemstra P.S., Van Wetering S., Ganz T. Human neutrophil defensin and serpins form complexes and inactivate each other. Am J Respir Cell Mol Biol. 1995;12:351–357. doi: 10.1165/ajrcmb.12.3.7873202. [DOI] [PubMed] [Google Scholar]
- 38.Kirk J.T., McNally A.K., Anderson J.M. Polymorphonuclear leukocyte inhibition of monocytes/macrophages in the foreign body reaction. J Biomed Mater Res A. 2010;94:683–687. doi: 10.1002/jbm.a.32682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study design diagram. A: In the PMN monoculture studies, PMNs were seeded at a density of approximately 5.3 × 103 PMNs/mm2 on PEG hydrogels, PDMS, and TCPS in 48- or 96-well plates. Three wells of PMN monocultures (per donor) were terminated at 2, 24, and 72 hours, and soluble MIP-1β and HNP1-3 concentrations were quantified. Cell adhesion, viability, necrosis, apoptosis, and secondary necrosis were also quantified. PCM was collected at 24 hours for use in the PMN-MC paracrine studies. In the PMN-MC paracrine studies, MCs were seeded at a density of approximately 5.3 × 103 MCs/mm2 on PEG hydrogels, PDMS, and TCPS in 96-well plates. At 2 hours, the culture medium was replaced with a 2:3 mixture of autologous PCM and fresh culture medium. PCM priming lasted for 24 hours, after which the MC cultures were replaced with fresh culture medium. Three wells of MC cultures (per donor) were terminated at 98 and 170 hours, and cell adhesion, viability, necrosis, apoptosis, and secondary necrosis were quantified. In addition, soluble IL-1β, IL-6, IL-8, IL-10, and TNF-α concentrations were quantified as markers of MC proinflammatory or pro–wound-healing propensity. MC monoculture studies were conducted simultaneously with the PMN-MC paracrine studies. In the MC monoculture studies, MCs were seeded at a density of approximately 5.3 × 103 MCs/mm2 on PEG hydrogels, PDMS, and TCPS in 96-well plates. Three wells of MC cultures (per donor) were terminated at 2, 98, and 170 hours, and cell adhesion, viability, necrosis, apoptosis, and secondary necrosis were quantified. In addition, soluble IL-1β, IL-6, IL-8, IL-10, and TNF-α concentrations were quantified. B: In the PMN-MC direct co-culture studies, PMNs were seeded at a density of approximately 2.6 × 103 PMNs/mm2 on PEG hydrogels, PDMS, and TCPS in 96-well plates. At 2 hours, the culture medium was replaced with fresh culture medium containing MCs for a seeding density of approximately 2.6 × 103 MCs/mm2. Two hours later, the culture medium was replaced again to remove nonadherent cells. Two wells of PMN-MC direct co-cultures (per donor) were terminated at 48, 96, and 168 hours (after the MCs were added), and cell adhesion, viability, necrosis, apoptosis, and secondary necrosis were quantified. In addition, soluble IL-1β, IL-6, IL-8, IL-10, and TNF-α concentrations were quantified. PMN and MC monoculture studies were conducted simultaneously with the PMN-MC direct co-culture studies. In the PMN monoculture studies, PMNs were seeded at a density of approximately 2.6 × 103 PMNs/mm2 on PEG hydrogels, PDMS, and TCPS in 96-well plates. To replicate the culture media changes in the simultaneously conducted PMN-MC direct co-cultures studies, the culture medium in the PMN monoculture studies was replaced after 2 hours and again 2 hours later. Two wells of PMN monocultures (per donor) were terminated at 48, 96, and 168 hours (after the MCs were added in the simultaneously conducted PMN-MC direct co-culture and MC monoculture studies). At each time point, cell adhesion, viability, necrosis, apoptosis, and secondary necrosis were quantified, as were concentrations of soluble IL-1β, IL-6, IL-8, IL-10, and TNF-α. In the MC monoculture studies, MCs were seeded at a density of approximately 2.6 × 103 MCs/mm2 on PEG hydrogels, PDMS, and TCPS in 96-well plates. After 2 hours, the culture medium was replaced. Two wells of MC monocultures (per donor) were terminated at 48, 96, and 168 hours (after the MCs were added). At each time point, cell adhesion, viability, necrosis, apoptosis, and secondary necrosis were quantified, as were concentrations of soluble IL-1β, IL-6, IL-8, IL-10, and TNF-α.
Viable and adherent cell densities in PMN monocultures. Viable (A) and necrotic (B) adherent cell densities in PMN monocultures (white bars, seeded at approximately 2.6 × 103 PMNs/mm2), MC monocultures (light grey bars, seeded at approximately 2.6 × 103 MCs/mm2), and PMN-MC direct co-cultures (dark grey bars, seeded at approximately 2.6 × 103 PMNs/mm2 and approximately 2.6 × 103 MCs/mm2) on PEG hydrogels, PDMS, and TCPS at 48, 96, and 168 hours. Results represent means + SD (n = 3). *P ≤ 0.05, significant difference from PEG hydrogels and TCPS in MC monocultures at the same time point; †P ≤ 0.05, significant difference from TCPS in PMN-MC direct co-cultures at the same time point; ‡P ≤ 0.05, significant difference from PDMS in PMN-MC direct co-cultures at the same time point.
Secondary necrosis in PMN monocultures. A: Secondary necrosis in PMN monocultures (white bars, seeded at approximately 5.3 × 103 PMNs/mm2), as measured by luminescence due to the caspase 3/7 activities of supernatants from PMN monocultures on PEG hydrogels, PDMS, and TCPS at 2, 24, and 72 hours. B: Secondary necrosis in PMN monocultures (white bars, seeded at approximately 2.6 × 103 PMNs/mm2), MC monocultures (light grey bars, seeded at approximately 2.6 × 103 MCs/mm2), and PMN-MC direct co-cultures (dark grey bars, seeded at approximately 2.6 × 103 PMNs/mm2 and approximately 2.6 × 103 MCs/mm2), as measured by luminescence due to the caspase 3/7 activities of supernatants from PMN or MC monocultures or direct PMN-MC co-cultures on PEG hydrogels, PDMS, and TCPS at 48, 96, and 168 hours. Results represent means ± SD (n = 3). *P = 0.001 to P = 0.01, significant difference between surfaces; †P = 0.01 to P = 0.05, significant difference from previous time point in MC monocultures; ‡P ≤ 0.05, significant difference from previous time point in PMN monocultures.
Soluble IL-6 and TNF-α concentrations in MC cultures. Soluble IL-6 (A) and TNF-α (B) concentrations in MC cultures (seeded at approximately 5.3 × 103 MCs/mm2) on PEG hydrogels, PDMS, and TCPS at 2, 98, and 170 hours, with (grey bars) or without (white bars*) PCM priming from 2 to 26 hours of culture. Results represent means ± SD (n = 3). Values are shown in parentheses if the bar is not visible. *P ≤ 0.05, significant difference from previous time point.
Soluble IL-1β, IL-6, IL-8, IL-10, and TNF-α concentrations in PMN monocultures. Soluble IL-1β (A), IL-6 (B), IL-8 (C), IL-10 (D), and TNF-α (E) concentrations in PMN monocultures (white bars, seeded at approximately 2.6 × 103 PMNs/mm2), MC monocultures (light grey bars, seeded at approximately 2.6 × 103 MCs/mm2), and PMN-MC direct co-cultures (dark grey bars, seeded at approximately 2.6 × 103 PMNs/mm2 and approximately 2.6 × 103 MCs/mm2) on PEG hydrogels, PDMS, and TCPS at 48, 96, and 168 hours. Results represent means + SD (log scale; n = 3). *P = 0.01 to P = 0.05, significant difference from previous time point.