Abstract
Inflammation stimulates new lymphatic vessel growth (inflammatory lymphangiogenesis). One key question is how recurrent inflammation, a common clinical condition, regulates lymphatic vessel remodeling. We show here that recurrent inflammation accelerated the development a functional lymphatic vessel network. This observation suggests a novel program of lymphangiogenesis and identifies a property of lymphatic vessel memory in response to recurrent inflammation. A brief episode of initial inflammation regressed lymphatic vessels, and a significant increase in CD11b+ macrophages were associated with the development of lymphatic vessel memory. These vessels had major differences in the structure and the spatial distribution of specialized lymphatic vessel features. Surprisingly, we found that the lymphatic vessel memory response did not depend on the vascular endothelial growth factor C or A pathway, indicating that different molecular pathways regulate inflammatory lymphangiogenesis and lymphatic vessel memory. These findings uncover a priming mechanism to facilitate a rapid lymphatic vessel memory response: a potential important component of peripheral host defense.
The lymphatic vasculature is one component of the inflammatory response that is remarkably understudied. The lymphatic system can be broadly classified into the lymphoid tissue (tonsils, lymph nodes, and spleen) and the conduit system or lymphatic vasculature. The focus of these studies is the lymphatic capillaries which literally are the most peripheral extension of the immune system and reside intimately in the diseased tissue. Aside from the classic functions of the lymphatic vasculature described many years ago (transport of extracellular fluid, cells, antigens, and lipid), surprisingly little is known about the normal physiology of this system and how it regulates inflammation and wound recovery. Multiple lines of evidence have shown that the lymphatic vasculature proliferates in response to inflammatory conditions, suggesting an active, perhaps essential, role during the inflammatory response.1–6 Inflammatory lymphangiogenesis is thought to be a physiological mechanism that develops to meet the increased demands of fluid, antigen, and cellular transport during an inflammatory response. New lymphatic vessel growth has been associated with beneficial effects in several different preclinical models of acute or chronic inflammatory disease.3,7–9 It is well recognized that vascular endothelial growth factor (VEGF)-C-VEGF receptor (VEGFR)-3 and VEGF-A-VEGFR-2 pathways are important in inflammatory lymphangiogenesis.10 The most accepted model of inflammatory lymphangiogenesis is that vessels sprout and elongate from pre-existing lymphatic vessels. In contrast, is some evidence is available that circulating endothelial progenitors or macrophages differentiate into lymphatic endothelial cells to comprise newly synthesized lymphatic vessels.11,12
Clinically, two general outcomes occur after an initial episode of inflammatory disease: wound recovery or recurrent inflammation. We developed a mouse model of wound recovery and recurrent inflammation to simulate these clinical outcomes and to study the lymphatic vasculature during these conditions. We recently demonstrated that lymphatic vessel regression developed during wound recovery in the cornea.13 Fragmented lymphatic vessels that persisted over time were visualized in wound recovery conditions.
In contrast to wound recovery, recurrent inflammation is a common clinical outcome after an initial episode of inflammation. We studied the effects of recurrent inflammation in corneal tissue recovered from an initial inflammatory response. This approach is different from earlier studies in that it features wound recovery followed by recurrent inflammation rather than an acute or chronic unrelenting pathogen or tumor-based inflammatory stimuli.3,4,7,14 We induced recurrent inflammation in recovered corneal tissue by placing a subsequent suture in the cornea (re-suturing). Here, we show that one feature of recurrent inflammation was the accelerated localized development of a functional lymphatic vessel network. The rapid kinetics and memory response were reminiscent of an immunological memory response; for this reason we describe this process as lymphatic vessel memory. This response appeared to stimulate the anastomosis of fragmented lymphatic vessels. Unlike inflammatory lymphangiogenesis induced by initial inflammation, we showed that lymphatic vessel memory was independent of the VEGF-C and VEGF-A pathways. Thus, these studies reveal a novel program of lymphatic vessel memory.
Materials and Methods
Mouse Model of Recurrent Corneal Inflammation
All animal protocols were approved by Boys Town National Research Hospital Institutional Animal Care and Use Committee in accordance with NIH guidelines. 129/SV mice were purchased from Charles River (Wilmington, MA). Groups of six to eight sex-matched 129/SV mice aged 6 to 10 weeks were used in the experimental studies. Corneal sutures were placed to induce initial inflammation. Wound recovery was stimulated by suture removal followed by a 14-day period. A subsequent suture was placed in wound recovered cornea to induce recurrent inflammation. Surgical techniques, tissue fixation conditions, antibodies for immunofluorescence, and H&E staining were described previously.13 Twenty-micron sections were cut from frozen eyes and mounted on glass slides. Corneal thickness was measured in the center of the cornea with the use of ImageJ (NIH, Bethesda, MD). We quantified infiltrating major histocompatibility complex (MHC) class II-positive leukocytes by counting the number of MHC class II-positive cells in the center corneal field at ×200.
Visualization and Morphometric Analysis of Corneal Lymphatic Vessels
These techniques were described previously.13 Briefly, we recorded immunofluorescence images from unsutured (noninflamed), sutured (initial inflammation), suture removed (wound recovered), and re-sutured (recurrent inflammation) corneas with the use of a Zeiss Axio Imager A.1 microscope (for epifluoresence) or a Zeiss LSM510 META confocal microscope. For each corneal epifluorescence image, we overlaid a customized rectangular grid adjacent to the constitutive limbal lymphatic vessels. The grid consisted of 250 square boxes. Each box measured 115 μm2. For lymphatic density measurements, we counted the number of grid squares occupied by a lymphatic vessel wall, lumen, or sprout. We expressed this data as number of occupied grid squares (relative area) ± SD. We quantified the number of lymphatic vessel sprouts, bulbous termini, or fragments within the grid. We defined a vessel sprout as a terminal conical projection with a length of at least 10 μm and bulbous termini with a rounded clubbed-shape. Data for lymphatic vessel plexus size were acquired from stacked confocal microscopy data (×400). We calculated the difference in microns between the superior and inferior z dimension planes in which the lymphatic vessels positive for lymphatic vessel endothelial hyaluronic acid receptor (LYVE)-1 were detected in corneal tissue with initial or recurrent inflammation.
Quantitative Analysis of MHC Class II-Positive Cells or CD11b+ Macrophages in the Cornea and Lymphatic Vessels
These techniques were described in detail previously.13 Whole mount cornea was immunostained with antibodies to LYVE-1, MHC class II, or CD11b (clone M1/70; BD Pharmingen, San Jose, CA) and the appropriate secondary antibodies. We counted the number of MHC class II or CD11b+ cells in corneal hemisections with the use of confocal microscopy. We quantified MHC class II-positive cells in contact or within LYVE-1-positive primary or secondary lymphatic vessels in projected images at ×400.
Quantitative RT-PCR
RNA was isolated from whole mouse unsutured cornea or corneas with initial inflammation, wound recovery, or recurrent inflammation conditions with the use of TRIzol Reagent per manufacturer instructions (Invitrogen, Grand Island, NY). Three or four mouse corneas were studied independently in each condition. Corneal RNA (300 ng) was reverse transcribed with Superscript III First Strand Synthesis System (Invitrogen). cDNA was amplified in triplicate reactions with the use of TaqMan Gene Expression Master Mix and TaqMan-based probes and primers (Applied Biosystems, Grand Island, NY). Data were normalized to glyceraldehyde 3-phosphate dehydrogenase. Mean fold induction of mRNA levels relative to unsutured cornea, using three replicates, was shown.
VEGFR-2-Fc and VEGFR-3-Fc Decoy Receptor Studies
After induction of initial or recurrent inflammation, Fc control protein, VEGFR-2-Fc and VEGFR-3-Fc, or VEGFR-3-Fc alone (R& D Systems, Minneapolis, MN) was injected into the subconjunctival space at 0, 2, and 4 days. Approximately 10 μL of 100 ng/μL decoy receptor or Fc control protein suspended in PBS + 0.1% bovine serum albumin, pH 7.4 were injected. After 7 days, corneal tissue was harvested, and the lymphatic vasculature was visualized and quantified.
Statistical Analysis
Dot plots representing data from individual corneas were shown in all figures. The broad horizontal bars were the means and the two-way error bars show means ± SD. A one-way analysis of variance was used followed by Dunnett multiple comparison test to compare the control group, denoted with asterisk, with the experimental groups. A two-tailed t-test was used to compare studies with two groups. Brackets between groups show a P value < 0.05.
Results
Corneal Model of Recurrent Inflammation
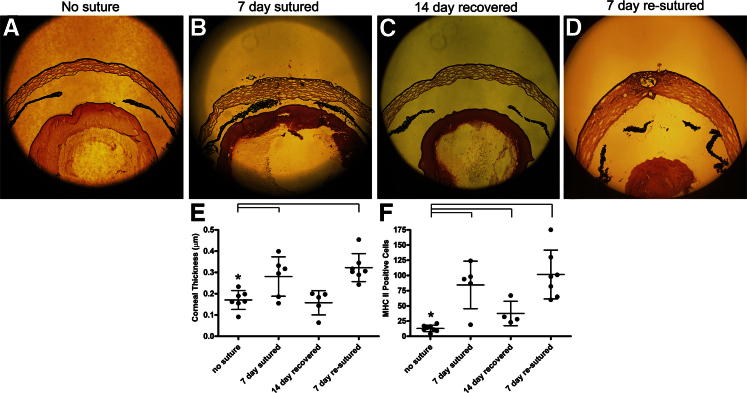
The present study was designed to investigate lymphatic vessel remodeling during recurrent inflammation. We stimulated recurrent inflammation by re-suturing wound recovered cornea (Figure 1). Increased corneal thickness, infiltrating inflammatory cells, and inflammatory cytokines (IL-6 and IL-1) were quantified in resutured cornea (Figure 2, A–F) (data not shown). These findings were consistent with a recurrent inflammatory response.
Figure 1.

Schematic of the corneal model of wound recovery and recurrent inflammation. Initial inflammation was induced by suture placement at day 0. Wound recovery was studied 14 days after suture removal. Recurrent inflammation was induced by placement of a subsequent suture.
Figure 2.
Corneal wound recovery and recurrent inflammation. Placement of a subsequent suture (re-sutured) in wound recovered cornea stimulated recurrent inflammation. Anterior globe images were obtained by thin-section H&E staining of unsutured (A), sutured (B), 14-day recovered (C), and re-sutured (D) corneas. E and F: Corneal thickness and the number of MHC class II-positive cells were increased in sutured and re-sutured cornea. Dots represent data from individual mice. Dot plots and means ± SD are shown. The data are representative of two independent experiments. Asterisk denotes the control group. Brackets indicate statistical significance P < 0.05. Original magnification: ×100 (A–D).
Lymphatic Vessel Memory
Using whole corneal mounts and immunofluorescence microscopy, we demonstrated an accelerated process of lymphangiogenesis during a recurrent inflammatory response. Initial inflammation induced inflammatory lymphangiogenesis that sprouted from the pre-existing limbal lymphatic vessel and was readily visualized after 3 days (Figure 3, A–D). After a 14-day period of wound recovery and lymphatic vessel regression, recurrent inflammation induced the alignment of regressed lymphatic vessels at day 1. A mature-appearing lymphatic vessel network was visualized at day 3 and continued to develop until day 7 (Figure 3, E–H). One interpretation of these results is that recurrent inflammation reactivated regressed lymphatic vessels to quickly assemble a lymphatic vessel network. The kinetics of this response were rapid and reminiscent of immunological memory response. Hence, we described this response as lymphatic vessel memory. Compared with inflammatory lymphangiogenesis stimulated with initial inflammation, the lymphatic vessel memory response was dense and had a number of other distinct structural features. A similar number of sprouts were quantified in both conditions; however, the zone of sprouting was more distal (Figure 3I). Some of the memory vessels had a complex bizarre structure and appeared to form a syncytium with frequent anastomosis that formed loop structures. We visualized an oak leaf pattern of LYVE-1 staining in lymphatic memory vessel stalks that extended from the limbal lymphatic vessels. This staining pattern was consistent with mature constitutively expressed lymphatic vessels.15 We quantified bulbous tips rather than lymphatic sprouts proximally and observed few new sprouts emerging from the limbus (Figure 3, G–I). Although most lymphatic fragments seemed to anastomose and form the secondary lymphangiogenic network, some lymphatic fragments persisted within the corneal stroma. These fragments had the unusual characteristics of nonpolarized filopodia, intense LYVE-1 expression, and the tendency to accumulate MHC class II leukocytes (Figure 4, A–D). Not only was the density (measured in the x and y dimensions) increased, but also the space occupied by the lymphatic memory vessels in the z IF increased several times relative to inflammatory lymphangiogenesis (Figure 4E). This finding suggested that these lymphatic vessels may have increased exposure to components of the extracellular matrix. These structural observations distinguished inflammatory lymphangiogenesis from lymphatic vessel memory responses.
Figure 3.
The time course and structural characteristics of lymphatic vessel memory during recurrent inflammation. The time course of inflammatory lymphangiogenesis was visualized in healthy unsutured cornea (A) or corneas with sutures placed for 1 (B), 3 (C), or 7 (D) days. Corneal recovery and lymphatic vessel regression was induced in other groups of mice (E). Accelerated lymphangiogenesis was visualized in recovered corneas with recurrent inflammation induced by a subsequent suture placement for 1 (F), 3 (G), or 7 (H) days. These vessels displayed complex distal sprouting that inosculated to form sheet-like structures (arrowhead, H), sprout suppression proximally (arrow, H), and little new limbal sprouting. Scale bar = 100 μm. An increase in lymphatic vessel density and distal tip sprouting were identified in recurrent inflammation. I: The number of lymphatic vessel bulbous tips and fragments per corneal field decreased during recurrent inflammation. Dots represent data from individual mice. Dot plots and means ± SD are shown. Data from more than four independent experiments with similar results is shown. D, days.
Figure 4.
Recurrent inflammation stimulated unusual, but not uncommon, lymphatic vessel structure. Lymphatic vessels were stained with antibodies to LYVE-1 (red) or CD31 (green). Lymphatic vessel fragments were visualized with intense LYVE-1 staining and expression of nonpolarized filopodia (A) or features of bulbous termini and sprout formation (B) in corneas with recurrent inflammation. Some distal sprouts had complex abundant filopodia and sprout development along stalk structures (C). MHC class II-positive cells (white) were visualized within bulbous termini (arrowhead) and in some cases adjacent to a sprouting vessel (D). A–D: Images were obtained with confocal microscopy. E: A statistically significant increase was detected in the z dimension (corneal depth) in secondary lymphangiogenesis. Dots represent data from individual mice. Dot plots and means ± SD are shown. Asterisk denotes the control group. Brackets indicate statistical significance P < 0.05. Scale bar = 50 μm (A–D).
The duration of initial inflammation required to stimulate lymphatic vessel memory was investigated. For these experiments we varied the duration of initial inflammation (suture placement for 1, 3, 5, or 7 days) and held constant for the duration of wound recovery (14 days) and recurrent inflammation (7 days). We did not visualize lymphatic vessels during wound recovery conditions after initial inflammation stimulated by suture placement for 1 day. In contrast, regressed lymphatic vessels were visualized in wound recovery conditions after initial inflammation stimulated by suture placement for 3, 5, or 7 days (Figure 5, A–D). We induced recurrent inflammation in similarly treated groups of mice. Initial inflammation for 1 day was not adequate to stimulate lymphatic vessel memory. Lymphatic vessel memory responses were visualized and quantified in mice with initial inflammation for 3, 5, and 7 days (Figure 5, E–I). The density and structural characteristics of the lymphatic vessel memory responses were remarkably similar, despite the variable duration of the initial inflammation episode.
Figure 5.
A brief episode of initial inflammation and the presence of lymphatic vessels were associated with lymphatic vessel memory. Regressed lymphatic vessels were visualized in conditions of wound recovery after initial inflammation, in some cases for 1 day (A), and all cases for 3 (B), 5 (C), or 7 (D) days. Recurrent inflammation was stimulated in similarly treated groups of mice. Some mice with initial inflammation for 1 day (E) and all mice with initial inflammation for 3 to 7 days (F–H) developed a lymphatic vessel memory response. Scale bar = 100 μm. I: Similar increases in lymphatic vessel memory density were visualized in mice stimulated with 3 to 7 days of initial inflammation. Dots represent data from individual mice. Dot plots and means ± SD are shown. Data shown was representative from two independent experiments with similar results. Black squares represent wound recovery and white squares represent recurrent inflammation after wound recovery. D, days.
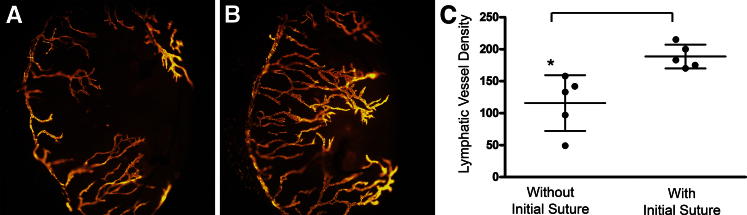
We investigated whether lymphatic vessel memory was a spatially restricted or global corneal property after an initial inflammatory response. Initial inflammation was stimulated by placing two sutures for 7 days in the temporal one-half of the cornea. After wound recovery we placed two black sutures in the temporal corneal hemisphere (previously inflamed) and two blue sutures in the nasal hemisphere. The different color sutures allowed us to distinguish between the hemispheres during the analysis. Inflammatory lymphangiogenesis was visualized and quantified in the corneal nasal hemisphere with an initial suture. Dense structurally distinct responses consistent with a lymphatic vessel memory response were detected in the temporal hemispheres with initial suture-induced inflammation. A statically significant difference was observed in lymphatic vessel density between the hemispheres (Figure 6, A–C). These findings indicated a lymphatic vessel memory property of spatial restriction.
Figure 6.
Lymphatic vessel memory was spatially restricted after an initial inflammatory response. Initial inflammation was stimulated in the temporal one-half of the cornea with two sutures for 7 days. After suture removal and wound recovery, two sutures were placed in the nasal hemisphere (A) and two sutures were placed in the temporal hemisphere (B). C: A statically significant difference in lymphatic vessel density was observed between the hemispheres. Dots represent data from individual mice. Dot plots and means ± SD are shown. Data shown was representative from two independent experiments with similar results. Brackets between groups indicate a statistically significant difference. Asterisk denotes the control group. Brackets indicate statistical significance P < 0.05.
Leukocyte trafficking is one well-accepted function of lymphatic vessels.16,17 We visualized and quantified similar numbers of MHC class II-positive cells within the corneal stroma, and similar numbers of MHC class II-positive cells were visualized interfacing or migrating within lymphatic vessels stimulated with initial or recurrent inflammation (Figure 7, A–F). This data indicated that lymphatic memory vessels function to traffic MHC class II-positive cells.
Figure 7.
Similar numbers of MHC class II-positive cells were visualized and quantified in primaryz and secondary lymphatic vessels. Similar numbers of MHC class II-positive cells were visualized interfaced or within primary lymphatic vessels (A and B) and secondary lymphatic vessels (C and D). B and D: Shown are orthogonal images of a single z dimension image that shows LYVE-1-positive lymphatic vessels (red) around MHC class II-positive leukocytes (green), top and far right panels. Similar numbers of MHC class II-positive cells were quantified in corneal tissue with primary or secondary lymphangiogenesis (E) and interfaced or within primary or secondary lymphatic vessels (F). E and F: Dots represent data from individual mice. Dot plots and means ± SD are shown. The data are representative of two independent experiments. Asterisk denotes the control group. Brackets indicate statistical significance P < 0.05.
Candidate Cellular and Molecular Mechanisms that Regulate Lymphatic Vessel Memory
The rapid expansion of a functional lymphatic vessel memory response during recurrent inflammation suggested that recovered tissue was endowed with a priming or memory mechanism to facilitate these processes. CD11b+ macrophages have been described to regulate inflammatory lymphangiogenesis. The two most well-accepted mechanisms are nonexclusive. Several lines of evidence have indicated that the macrophages express VEGF-C, a well-characterized lymphangiogenic growth factor. Macrophages have been reported to differentiate into lymphatic endothelial cells. We used three-color immunofluorescent confocal microscopy to visualize and localize CD11b+ macrophages during corneal wound recovery and recurrent inflammation. We visualized and quantified a significant increase of CD11b+ macrophages during initial inflammation. Few CD11b+ macrophages were identified in conditions of wound recovery, and this cell population increased during recurrent inflammation (Figure 8, A–E). The absolute number of CD11b+ macrophages was greater than the MHC class II population (Figure 8F), and few if any CD11b+ macrophages were MHC class II positive. Unlike the MHC class II population, the CD11b+ cells were not detected within the lumen of the lymphatic vessels.
Figure 8.
CD11b+ macrophages accumulation during corneal inflammation. Three-color confocal immunofluorescence microscopy was performed to visualize and quantify CD11b+ macrophages (green), lymphatic vessels (red), and MHC class II-positive cells (white) in conditions of initial inflammation (suture placement for 7 days; A), wound recovery for 14 days (B), or recurrent inflammation for 1 (C) or 3 (D) days. Increased numbers of CD11b+ macrophages were detected in conditions of initial or recurrent inflammation, and few cells were visualized in conditions of wound recovery (E). Compared with CD11b+ cells substantially fewer MHC class II cells were quantified (F). Increased numbers of MHC class II-positive cells were quantified in initial inflammatory conditions, and similar numbers were detected during wound recovery and recurrent inflammation. Little to no MHC class II expression was detected in the CD11b+ macrophage population. Unlike MHC class II-positive cells, CD11b+ cells were not visualized trafficking within the lymphatic vessels in any conditions. E and F: Dot plots and means ± SD are shown. The data are representative of two independent experiments. Asterisk denotes the control group. Brackets indicate statistical significance P < 0.05.
A number of genes known to be involved in developmental or inflammatory lymphangiogenesis were examined (Figure 9). mRNA levels of the prolymphangiogenic cytokines, VEGF-C and VEGF-A, increased during wound recovery and recurrent inflammation. VEGFR-3 mRNA levels did not change. mRNA levels of the prolymphangiogenic protease matrix metalloprotease 10 were increased during inflammatory conditions,18 and levels returned to baseline during wound recovery. The level of sex determining region Y-box 18 mRNA increased during recurrent inflammation. Prospero homeobox 1 and LYVE1 mRNA levels did not change. Chicken ovalbumin upstream promoter transcription factor 2 mRNA levels were decreased during initial and recurrent inflammation. Notch homolog 1 mRNA levels were decreased in conditions of wound recovery and recurrent inflammation, and no significant changes were detected in Delta-like ligand 4. These results identified VEGF-A and VEGF-C as candidate factors to facilitate the lymphatic vessel memory response.
Figure 9.
Increased mRNA levels of VEGF-C and VEGF-A during wound recovery and recurrent inflammation. VEGF-C, VEGF-A, VEGFR-3, matrix metalloprotease (MMP)-10, sex determining region Y-box 18 (Sox-18), prospero homeobox 1 (Prox1), LYVE1, chicken ovalbumin upstream promoter transcription factor 2 (CoupTFII), Notch homolog 1 (Notch 1), and Delta-like ligand 4 (DLL4) mRNA levels were quantified in unsutured corneal tissue and conditions of initial inflammation, wound recovery, and recurrent inflammation. Dots represent data from individual mice. The dot plots and means ± SD are mean fold mRNA levels normalized to glyceraldehyde 3-phosphate dehydrogenase. Asterisk denotes the control group. Brackets indicate statistical significance P < 0.05.
Lymphatic Vessel Memory Is Independent of VEGF-C and VEGF-A
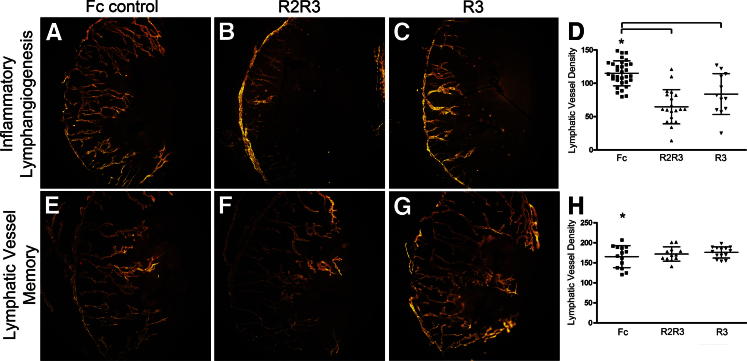
It is well established that VEGF-C and VEGF-A regulate lymphangiogenesis. We explored whether lymphatic vessel memory involved the VEGF-C and VEGF-A pathways with the use of VEGFR-2-Fc (R2) and VEGFR-3-Fc (R3) decoy receptors. We stimulated inflammatory lymphangiogenesis with initial inflammation and treated mice with serial subconjunctival injection of control Fc protein, a combination of R2R3 decoy receptors, or R3 decoy receptor alone. We visualized and quantified inhibition of inflammatory lymphangiogenesis in groups of mice treated with the R2R3 decoy receptor combination or the R3 decoy receptor alone. These results were statistically significant. We stimulated lymphatic vessel memory with recurrent inflammation and treated groups of mice with serial subconjunctival injection of control Fc protein, a combination of R2R3 decoy receptors, or the R3 decoy receptor alone. No difference was observed in structural features and density in the lymphatic vessel memory responses, despite R2R3 and R3 decoy receptor administration (Figure 10, A–H).
Figure 10.
Lymphatic vessel memory was independent of VEGF-A and VEGF-C pathways. Inflammatory lymphangiogenesis stimulated with initial inflammation was visualized after injection of Fc control (A). Inflammatory lymphangiogenesis was inhibited after injection of decoy receptors VEGFR-2-Fc and VEGFR-3-Fc (R2R3) (B) or VEGFR-3-Fc (R3) (C). Similar programs of lymphatic vessel memory were detected in corneas injected with Fc control (E), VEGFR-2-Fc and VEGFR-3-Fc (F), and VEGFR-3-Fc (G). D and H: Dots represent data from individual mice. The dot plots and means ± SD are pooled data from three independent experiments. Asterisk denotes the control group. Brackets indicate statistical significance P < 0.05.
Discussion
The main finding presented here is that recurrent inflammation stimulated an accelerated structurally distinct program of lymphangiogenesis that we refer to as lymphatic vessel memory. We showed that in contrast to inflammatory lymphangiogenesis, lymphatic vessel memory was not dependent on the VEGF-C or VEGF-A pathway.
Wound Recovery and Lymphatic Vessel Regression
We designed experiments to simulate the two primary clinical outcomes of inflammatory disease: wound recovery or recurrent inflammation. One of the strengths of the corneal model is the ability to induce and objectively quantify wound recovery and recurrent inflammation. Many pathogen- and tumor-based models establish chronic unrelenting inflammatory responses that make it difficult to induce wound recovery or serial episodes of inflammation. We recently established that lymphatic vessel regression developed during corneal wound recovery.13 These observations lead us to consider whether lymphatic vessel regression was reversible.
Recurrent Inflammation and Lymphatic Vessel Memory
Here, we show that recurrent inflammation stimulated the rapid development of a functional lymphatic vessel memory network. Unlike inflammatory lymphangiogenesis, lymphatic vessel memory was not dependent on the VEGF-C or VEGF-A pathway. Several models of inflammatory lymphangiogenesis are described: vessel sprouting and stalk elongation from pre-existing lymphatic vessels,6 transdifferentiation of macrophages,11 or the incorporation of circulating endothelial progenitors.19 Although there may be some common mechanisms, lymphatic vessel memory appears to represent a distinct type of lymphangiogenesis. The rapid development of the lymphatic vessel memory response suggested a priming mechanism that likely developed during initial inflammation or wound recovery. The structure of the lymphatic vessel memory response is unique. It appears to involve the activation and anastomosis of regressed lymphatic vessels and mechanisms to facilitate tip-to-tip guidance, fragment migration, and vessel fusion, none of which have been described previously. The specific role of the CD11b+ macrophages in lymphatic vessel memory is unclear. Several lines of evidence support a role for macrophages during inflammatory lymphangiogenesis.11,20,21 Here, we show that the number of CD11b+ macrophages increased significantly during initial inflammation and inflammatory lymphangiogenesis, decreased during wound recovery, and increased dramatically during the lymphatic vessel memory response stimulated by recurrent inflammation. Surprisingly, no detectable MHC class II-positive CD11b+ macrophages were identified during inflammatory conditions. Corneal CD11b+ macrophages have been described to express low levels of MHC class II,22 and our results may represent a technologic limitation of MHC class II detection with the use of whole mount immunostaining protocols. At least one report has described that macrophages function to couple blood vessels during vascular remodeling.23 A similar mechanism may facilitate lymphatic vessel fragment anastomosis during a lymphatic vessel memory response. CD11b+ macrophages have been described to facilitate the resolution of inflammation.24 It remains unclear whether the macrophage population represents a specific component of the priming mechanism, an active participant in the resolution of inflammation, or an essential constituent of the lymphatic vessel memory network.
Features of lymphatic vessel specialization such as sprouting, bulbous tip formation, and stalk development are presumably related to lymphatic vessel function. We observed spatial restriction and spatial regulation of specialized lymphatic vessel features during lymphatic vessel memory responses. For example, the number of lymphatic vessel sprouts was similar; however, the sprouting was more distal in lymphatic vessel memory responses than in inflammatory lymphangiogenesis. Interestingly, few new sprouts developed from the pre-existing limbal lymphatic vessels, and we did not visualize lymphatic vessel sprouts close to the limbal vessels in these conditions. The Notch-Delta pathway may be involved in lymphatic vessel memory because this pathway was described to suppress lymphangiogenic sprouts and to promote tip-to-stalk transformation.25–29 The results of the RT-qPCR studies show that Delta-like ligand 4 mRNA levels were similar in corneal wound recovery and recurrent inflammation and that Notch 1 mRNA levels were decreased in corneal tissue during wound recovery and recurrent inflammation. Given the number of components and the complexity of the Notch pathways, a more comprehensive investigation will be required to determine the precise role of the Notch pathways in lymphatic vessel memory. Lymphatic vessels stimulated by recurrent inflammation were functional because they had the capacity to transport MHC class II-positive leukocytes. Although the mechanisms are not well appreciated, there is little doubt that leukocyte migration via the lymphatic vessels contributes to immunity.16,30 Lymphatic vessel memory may represent an important peripheral component of host defense. The rapid assembly of a secondary lymphatic vessel network may facilitate efficient antigen and leukocyte transport to regional lymph nodes, sites of intense immunological activity.
It is unclear whether lymphatic vessel memory is a process that is unique to the cornea or represents a systemic process. In both corneal and noncorneal models, lymphatic vessels have been identified to persist after the resolution of the inflammatory response.3,4 It is possible that these persistent lymphatic vessels are conditioned to respond to recurrent inflammation with a memory response.
Lymphatic Vessel Memory Is Independent of VEGF-C and VEGF-A
Many lines of evidence have indicated a critical role for VEGF-C and VEGF-A during lymphangiogenesis.10,31–33 We identified higher levels of VEGF-C and VEGF-A mRNA in conditions of wound recovery and recurrent inflammation. This finding was consistent with a role for these cytokines in lymphatic vessel memory. With the use of VEGFR-2 and R3 decoy receptors, we inhibited inflammatory lymphangiogenesis but not lymphatic vessel memory. This finding suggests that mechanisms other than the VEGF-A or VEGF-C pathway regulate lymphatic vessel memory. Long-lasting VEGFR-2 and R3 signaling effects or the inability of the decoy receptors to sequester cytokines in a different microenvironment are two alternative interpretations of this result. Despite the inhibition of the VEGF-C and VEGF-A pathways, the limbal lymphatic vessels persisted in both conditions. This result was similar to other investigations that described the persistence of pre-existing lymphatic vessels despite VEGF-C or VEGF-A inhibition.34,35 Together these findings support a model of lymphatic vessel network maturation in which initial inflammatory lymphangiogenesis is regulated by VEGF-C or VEGF-A signals, and lymphatic vessel memory responses are regulated by different mechanisms.
Clinical Significance
Many corneal and possibly noncorneal conditions of recurrent inflammation may involve pathological processes that stimulate lymphatic vessel memory pathways. Directed VEGF-C or VEGF-A pathway manipulation may not provide the anticipated clinical benefits because these pathways do not appear to regulate lymphatic vessel memory. Identification of the factors that prime and regulate lymphatic vessel memory will be important in future studies. Understanding how lymphatic vessel memory responses regulate recurrent inflammatory disease will shape clinical paradigms and future medical therapies.
Footnotes
Supported by the State of Nebraska Tobacco Settlement and the NIH: National Center for Research Resources grant 5P20RR018788-09 and the National Institute of General Medical Sciences grant 8 P20 GM103471-09, and grant R01EY021571. This investigation is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute for General Medical Science or NIH.
References
- 1.Pullinger B.D., Florey H.W. Proliferation of lymphatics in inflammation. J Pathol Bact. 1937;45:157–170. [Google Scholar]
- 2.Yamashita M., Iwama N., Date F., Chiba R., Ebina M., Miki H., Yamauchi K., Sawai T., Nose M., Sato S., Takahashi T., Ono M. Characterization of lymphangiogenesis in various stages of idiopathic diffuse alveolar damage. Hum Pathol. 2009;4:542–551. doi: 10.1016/j.humpath.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 3.Baluk P., Tammela T., Ator E., Lyubynska N., Achen M.G., Hicklin D.J., Jeltsch M., Petrova T.V., Pytowski B., Stacker S.A., Yla-Herttuala S., Jackson D.G., Alitalo K., McDonald D.M. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wuest T.R., Carr D.J. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J Exp Med. 2010;207:101–115. doi: 10.1084/jem.20091385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim H., Kataru R.P., Koh G.Y. Regulation and implications of inflammatory lymphangiogenesis. Trends Immunol. 2012;33:350–356. doi: 10.1016/j.it.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17:1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 7.Huggenberger R., Siddiqui S.S., Brander D., Ullmann S., Zimmermann K., Antsiferova M., Werner S., Alitalo K., Detmar M. An important role of lymphatic vessel activation in limiting acute inflammation. Blood. 2011;117:4667–4678. doi: 10.1182/blood-2010-10-316356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo R., Zhou Q., Proulx S.T., Wood R., Ji R.C., Ritchlin C.T., Pytowski B., Zhu Z., Wang Y.J., Schwarz E.M., Xing L. Inhibition of lymphangiogenesis and lymphatic drainage via vascular endothelial growth factor receptor 3 blockade increases the severity of inflammation in a mouse model of chronic inflammatory arthritis. Arthritis Rheum. 2009;60:2666–2676. doi: 10.1002/art.24764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huggenberger R., Ullmann S., Proulx S.T., Pytowski B., Alitalo K., Detmar M. Stimulation of lymphangiogenesis via VEGFR-3 inhibits chronic skin inflammation. J Exp Med. 2010;207:2255–2269. doi: 10.1084/jem.20100559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tammela T., Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 11.Maruyama K., Ii M., Cursiefen C., Jackson D.G., Keino H., Tomita M., van Rooijen N., Takenaka H., D’Amore P.A., Stein-Streilein J., Losordo D.W., Streilein J.W. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b+ positive macrophages. J Clin Invest. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerjaschki D., Huttary N., Raab I., Regele H., Bojarski-Nagy K., Bartel G., Krober S.M., Greinix H., Rosenmaier A., Karlhofer F., Wick N., Mazal P.R. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med. 2006;12:230–234. doi: 10.1038/nm1340. [DOI] [PubMed] [Google Scholar]
- 13.Kelley P.M., Steele M.M., Tempero R.M. Regressed lymphatic vessels develop during corneal repair. Lab Invest. 2011;91:1643–1651. doi: 10.1038/labinvest.2011.121. [DOI] [PubMed] [Google Scholar]
- 14.Achen M.G., Stacker S.A. Tumor lymphangiogenesis and metastatic spread-new players begin to emerge. Int J Cancer. 2006;119:1755–1760. doi: 10.1002/ijc.21899. [DOI] [PubMed] [Google Scholar]
- 15.Baluk P., Fuxe J., Hashizume H., Romano T., Lashnits E., Butz S., Vestweber D., Corada M., Molendini C., Dejana E., McDonald D.M. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Randolph G.J., Angeli V., Swartz M.A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez D., Vollmann E.H., von Andrian U.H. Mechanisms and consequences of dendritic cell migration. Immunity. 2008;29:325–342. doi: 10.1016/j.immuni.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steele M.M., Schieler A.M., Kelley P.M., Tempero R.M. beta1 integrin regulates MMP-10 dependant tubulogenesis in human lymphatic endothelial cells. Matrix Biol. 2011;30:218–224. doi: 10.1016/j.matbio.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Lee J.Y., Park C., Cho Y.P., Lee E., Kim H., Kim P., Yun S.H., Yoon Y.S. Podoplanin-expressing cells derived from bone marrow play a crucial role in postnatal lymphatic neovascularization. Circulation. 2010;122:1413–1425. doi: 10.1161/CIRCULATIONAHA.110.941468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim K.E., Koh Y.J., Jeon B.H., Jang C., Han J., Kataru R.P., Schwendener R.A., Kim J.M., Koh G.Y. Role of CD11b+ macrophages in intraperitoneal lipopolysaccharide-induced aberrant lymphangiogenesis and lymphatic function in the diaphragm. Am J Pathol. 2009;175:1733–1745. doi: 10.2353/ajpath.2009.090133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maruyama K., Asia J., Li M., Thorne T., Losordo D.W., D’Amore P.A. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol. 2007;170:1178–1191. doi: 10.2353/ajpath.2007.060018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brissette-Storkus C.S., Reynolds S.M., Lepisto A.J., Hendricks R.L. Identification of a novel macrophage population in the normal mouse corneal stroma. Invest Ophthalmol Vis Sci. 2002;43:2264–2271. [PMC free article] [PubMed] [Google Scholar]
- 23.Fantin A., Vieira J.M., Gestri G., Denti L., Schwartz Q., Prykhozhij S., Peri F., Wilson S.W., Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katuru R.P., Jung K., Jang C., Yang H., Schwendener R.A., Han S.H., Alitalo K., Koh G.Y. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009;133:5650–5659. doi: 10.1182/blood-2008-09-176776. [DOI] [PubMed] [Google Scholar]
- 25.Niessen K., Zhang G., Ridgway J.B., Chen H., Kolumam G., Siebel C.W., Yan M. The Notch1-Dll4 signaling pathway regulates mouse postnatal lymphatic development. Blood. 2011;118:1989–1997. doi: 10.1182/blood-2010-11-319129. [DOI] [PubMed] [Google Scholar]
- 26.Zheng W., Tammela T., Yamamoto M., Anisimov A., Holopainen T., Kaijalainen S., Karpanen T., Lehti K., Yla-Herttuala S., Alitalo K. Notch restricts lymphatic vessel sprouting induced by vascular endothelial growth factor. Blood. 2011;118:1154–1162. doi: 10.1182/blood-2010-11-317800. [DOI] [PubMed] [Google Scholar]
- 27.Lobov I.B., Renard R.A., Papadopoulos N., Gale N.W., Thurston G., Yancopoulos G.D., Wiegand S.J. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci U S A. 2007;104:3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lobov I.B., Cheung E., Wudali R., Cao J., Halasz G., Wei Y., Economides A., Lin H.C., Papadopoulos N., Yancopoulos G.D., Wiegand S.J. The Dll4/Notch pathway controls postangiogenic blood vessel remodeling and regression by modulating vasoconstriction and blood flow. Blood. 2011;117:6728–6737. doi: 10.1182/blood-2010-08-302067. [DOI] [PubMed] [Google Scholar]
- 29.Tammela T., Zarkada G., Nurmi H., Jakobsson L., Heinolainen K., Tvorogov D., Zheng W., Franco C.A., Murtomaki A., Aranda E., Miura N., Yla-Herttuala S., Fruttiger M., Makinen T., Eichmann A., Pollard J.W., Gerhardt H., Alitalo K. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nat Cell Biol. 2011;13:1202–1213. doi: 10.1038/ncb2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarez D., Vollmann E.H., von Andrian U.H. Mechanisms and consequences of dendritic cell migration. Immunity. 2008;29:325–342. doi: 10.1016/j.immuni.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cursiefen C., Chen L., Borges L.P., Jackson D., Cao J., Radziejewski C., D’Amore P.A., Dana M.R., Wiegand S.J., Streilein J.W. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung E.S., Saban D.R., Chauhan S.K., Dana R. Regulation of blood vessel versus lymphatic vessel growth in the cornea. Invest Ophthalmol Vis Sci. 2009;50:1613–1618. doi: 10.1167/iovs.08-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L., Hamrah P., Cursiefen C., Zhang Q., Pytowski B., Streilein J.W., Dana M.R. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004;10:813–815. doi: 10.1038/nm1078. [DOI] [PubMed] [Google Scholar]
- 34.Pytowski B., Goldman J., Persaud K., Wu Y., Witte L., Hicklin D.J., Skobe M., Boardman K.C., Swartz M.A. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J Natl Cancer Inst. 2005;97:14–21. doi: 10.1093/jnci/dji003. [DOI] [PubMed] [Google Scholar]
- 35.Karpanen T., Wirzenius M., Makinen T., Veikkola T., Haisma H.J., Achen M.G., Stacker S.A., Pytowski B., Yla-Herttuala S., Alitalo K. Lymphangiogenic growth factor responsiveness is modulated by postnatal lymphatic vessel maturation. Am J Pathol. 2006;169:708–718. doi: 10.2353/ajpath.2006.051200. [DOI] [PMC free article] [PubMed] [Google Scholar]