Abstract
Brain metastases occur in more than one-third of metastatic breast cancer patients whose tumors overexpress HER2 or are triple negative. Brain colonization of cancer cells occurs in a unique environment, containing microglia, oligodendrocytes, astrocytes, and neurons. Although a neuroinflammatory response has been documented in brain metastasis, its contribution to cancer progression and therapy remains poorly understood. Using an experimental brain metastasis model, we characterized the brain metastatic microenvironment of brain tropic, HER2-transfected MDA-MB-231 human breast carcinoma cells (231-BR-HER2). A previously unidentified subpopulation of metastasis-associated astrocytes expressing phosphorylated platelet-derived growth factor receptor β (at tyrosine 751; p751-PDGFRβ) was identified around perivascular brain micrometastases. p751-PDGFRβ+ astrocytes were also identified in human brain metastases from eight craniotomy specimens and in primary cultures of astrocyte-enriched glial cells. Previously, we reported that pazopanib, a multispecific tyrosine kinase inhibitor, prevented the outgrowth of 231-BR-HER2 large brain metastases by 73%. Here, we evaluated the effect of pazopanib on the brain neuroinflammatory microenvironment. Pazopanib treatment resulted in 70% (P = 0.023) decrease of the p751-PDGFRβ+ astrocyte population, at the lowest dose of 30 mg/kg, twice daily. Collectively, the data identify a subpopulation of activated astrocytes in the subclinical perivascular stage of brain metastases and show that they are inhibitable by pazopanib, suggesting its potential to prevent the development of brain micrometastases in breast cancer patients.
Breast cancer is the second most common cause of brain metastasis after lung cancer, occurring in 10% to 15% of advanced patients and in approximately 30% of autopsies.1,2 Risk factors for the development of brain metastases include young patient age, large primary tumors, multiple positive lymph nodes, and hormone receptor negativity.3 In addition, the incidence of brain metastasis appears to be increasing because of the introduction of more sensitive diagnostic methods and improved therapies, the latter particularly in patients with HER2-overexpressing metastatic breast cancer.4 The standard of care for brain metastases is palliative, and in most cases chemotherapy is ineffective.5,6 New drugs that are both brain permeable and prevent specific pathogenic mechanisms of the brain metastasis process have been identified in preclinical experiments but await appropriate clinical trials.7–11
The cancer microenvironment is of crucial importance for a complete understanding of the disease12–15 because it is the interface between cancer cells and pathophysiology of the patient.16 The brain represents a unique microenvironment for epithelial cancers that remains to be further investigated. Salient features include the blood-brain barrier that surrounds the vasculature and protects the brain from unwanted substances and leukocyte infiltration, and a rich cellular milieu, including neurons, pericytes, and glial cells. Because the brain is critical for both cognitive and physical function, microenvironmental changes during cancer metastasis may adversely affect the patient. A better understanding of the brain microenvironment during metastasis may contribute to development of more effective therapeutics.
Relatively little is known about the microenvironment of brain metastases of breast or other cancers. Most of our information comes from experimental models of brain metastasis in which brain tropic lines are introduced into the circulation of mice via the left cardiac ventricle or carotid artery and then colonize the brain over a several-week period.17–20 In the 231-BR model system, cancer cells extravasate the circulatory system and bind to the surrounding basement membrane through β1 integrin; in this microenvironment, cancer cells move and proliferate along the outside of the blood vessels.21 During the subclinical stage of the brain metastasis process, where injury is subtle but consistent, a continuous neuroinflammatory response involves activation of astrocytes and microglia, identified by expression of glial fibrillary acidic protein (GFAP) and F4/80 or CD11b/CD45, respectively.21–23 This neuroinflammatory response is also observed in clinical samples from resected human brain metastases in which reactive astrocytes and microglia both surround and infiltrate the metastatic lesion, validating experimental observations.22 In coculture experiments, glial cells increased the number of colonies formed in soft agar by 231-BR cells by fivefold,22 and astrocytes also increased cancer cell proliferation and up-regulated the expression of survival genes,24,25 suggesting mechanistic contributions of microenvironmental cells to brain metastasis.
Consistent with what has been reported for other organs, platelet-derived growth factor (PDGF)-B is also a key protective factor in noncancerous brain damage,26,27 contributing to blood-brain barrier stability, angiogenesis, and vascular remodeling through the activation of PDGF receptor β (PDGFRβ)-expressing brain pericytes and neuroglial progenitor cells.28,29 During cancer progression PDGFRβ expression has long been associated with tumor-associated stromagenic and angiogenic activities.30 However, its role during brain metastasis development is unknown.
In this article, we characterize the neuroinflammatory microenvironment of a breast cancer experimental brain metastasis model system (231-BR cells transfected with HER2; 231-BR-HER2) and identify a novel subpopulation of metastasis-activated astrocytes that express an active (phosphorylated) form of PDGFRβ (p-PDGFRβ). The existence of this novel subset of astrocytes was confirmed in resected specimens of human brain metastasis from five patients with HER-2–overexpressing breast cancer, two patients with lung cancer, and one patient with colorectal cancer. Importantly, we demonstrate that primary cultured human astrocytes expressed (activated) p-PDGFRβ in response to tumor-derived soluble factors. We previously reported that pazopanib, an inhibitor of vascular endothelial growth factor receptors, PDGFRs, c-kit,31 and B-Raf19,32 prevented brain metastasis formation in the 231-BR-HER2 model by 73%, targeting B-Raf activation in the tumor cells.19 Herein, we show that pazopanib also inhibited the activation of p-PDGFRβ–expressing astrocytes in the experimental brain metastasis model and in tumor-activated astrocytes in vitro. Our results indicate that brain-permeable drugs can target both tumor cells and the neuroinflammatory microenvironment in the brain metastatic process.
Materials and Methods
Drugs
Pazopanib was provided by GlaxoSmithKline (London, United Kingdom) through a Material Collaborative Research and Development Agreement with NIH. For in vitro experiments, pazopanib was reconstituted in dimethyl sulfoxide (DMSO) and stored at −80°C.
Cell Lines and in Vitro Culture Conditions
The human MDA-MB-231-BR brain-tropic line (231-BR) transfected with HER2 (231-BR-HER2) was previously described.17 Conditioned medium from 231-BR-HER2 cells was prepared as follows: 5 × 106 subconfluent-growing 231-BR-HER2 cancer cells were incubated in 10 mL of serum-free RPMI 1640 medium, in a 75-cm2 flask, for 12 hours. Supernatant fluids were then collected, centrifuged at 2500 × g for 3 minutes, supplemented with 50% fresh serum-free medium, and run through a 0.22-μm filter before being used. A human immortalized astrocyte line33 was kindly provided by Drs. Jayne Stommel (National Cancer Institute, Bethesda, MD) and Russell O. Pieper (University of California, San Francisco, CA) and maintained in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum.
Tumor Tissue
All tumor specimens used in this study were obtained from patients with cancer undergoing medically indicated neurosurgical treatment after written consent in accordance with the appropriate clinical protocol. Tissue collection was approved by the Institutional Review Board/Comité Ético de Investigación Clínica of the Hospital of Madrid Scientific Foundation (Madrid, Spain). In total, eight tissue samples from brain metastases (five from breast cancer, two from lung cancer, and one from rectal cancer) were used. The diagnosis was histopathologically verified, and their clinicopathological information is reported as Supplemental Table S1. The material obtained was snap-frozen in liquid nitrogen and kept at −80°C. Five micron-thick tissue sections were cut, dried for 5 minutes, and then fixed in ice-cold acetone for 10 minutes. The sections were then dried for 15 minutes and stored at −20°C. Before staining, the sections were thawed and rinsed in phosphate buffered saline (PBS).
Isolation and Primary Culture of Human Glial Cells
Brain tissue from a tumor-unaffected area obtained during the neurosurgical procedure of brain tumor removal was disaggregated mechanically by cutting the tumor into small pieces and enzymatically with Accumax (Millipore, Billerica, MA). Dissociated cells were resuspended in Neurobasal media (Life Technologies, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum, l-glutamine, antibiotics (100 U/mL penicillin; 0.1 mg/mL streptomycin), and plated into 25-cm2 culture flasks. During the initial establishment of cultures, 2.5 μg/mL Fungizone (Gibco-Invitrogen, Carlsbad, CA) was also added to the culture medium. The culture medium was replaced every 4 days. Primary cultures were grown to confluence (70% to 90%), and further astrocyte enrichment of the glial cell culture was obtained by placing the flask on a shaker for 12 to 14 hours at 250 rpm; mainly astrocytes remained adhered to the flask. The medium with detached neurons and glial cells was removed. Astrocyte-enriched glial cell cultures were checked for existing cell phenotypes with the use of the following markers: Olig2, neuron/glial antigen 2 (NG2), GFAP, CD11b/CD18.
Cell Viability Assay
The human immortalized astrocytes were plated at a density of 2000 cells/well in 96-well plates and incubated overnight to allow cells to adhere. Astrocytes were maintained in 10% fetal bovine serum and treated with increasing concentrations of pazopanib (0.5, 1, 2, 4, 6, 8, and 10 μmol/L), or with DMSO as a control, for 120 hours. The number of viable cells was determined by adding MTT (Sigma-Aldrich, St. Louis, MO) at a final concentration of 0.5 mg/mL to each well for 4 hours. After incubation, MTT was dissolved in DMSO, and absorbance was measured at 570 nm. Results are representative of three independent experiments, each performed in quintuplicate.
Immunostaining of Cultured Glial Cells
Chamber slides (Lab-Tek, Thermo Scientific, Waltham, MA; ref. 154526) were coated with laminin at 10 μg/mL for 1 hour at 37°C. Both the human astrocyte-enriched glial cell primary culture and immortalized astrocytes were seeded in chamber slides at 0.5 × 104 cells per chamber for 48 hours to allow cells to adhere. Human immortalized astrocytes were serum-starved overnight and subsequently treated with DMSO or 5 μmol/L pazopanib for 24 hours, then retreated for an additional 24 hours. Human astrocyte-enriched glial cells were maintained in Neurobasal medium or incubated in tumor-activated medium with DMSO or pazopanib for 18 hours. Both type of astrocyte cultures were washed twice with PBS and fixed with 4% cold paraformaldehyde for 10 minutes. After two washes with PBS for 5 minutes, cells were incubated with blocking buffer (PBS plus 5% goat serum and 0.2% Triton X-100) for 1 hour, then with anti-PDGFRβ phosphotyrosine 751 (p751-PDGFRβ; dilution 1/50; Santa Cruz Biotechnology, Santa Cruz, CA) or rabbit anti-GFAP (dilution 1/300; Dako, Carpinteria, CA) in the same blocking buffer for 2 hours at room temperature. After three 5-minute washes with PBS, cells were incubated with Alexa 488-conjugated secondary antibody (Invitrogen) and 200 μg/mL DAPI for 1 hour at room temperature. Sections were mounted in Fluorescent Mounting Medium (Dako) and photographed with an Axioskop microscope with an Axiocam digital camera (Carl Zeiss Microimaging, Jena, Germany). AxioVision software version 4 was used for image acquisition and quantification.
Immunostaining of Brain Tissue from Mouse and from Patient Brain Metastatic Tissues
The mouse experiments were described previously and were conducted under an approved animal use agreement with the National Cancer Institute.19 For the tissues from the mouse model, immunostaining was performed on 10-μm thick sections of immediately frozen tissue in Optimal Cutting Temperature compound. Sections were fixed and permeabilized in ice-cold methanol or ice-cold acetone or were fixed with paraformaldehyde 4% (depending on the antibody), and the sections were preblocked in PBS containing goat serum, 5% and 0.2% Triton X-100 for 1 hour to permeabilize. Subsequently, the first and the secondary antibodies were incubated in the same PBS solution. The following antibodies were used: rat anti-mouse CD31 (dilution 1/500; BD Pharmingen, San Diego, CA), mouse anti-human cytokeratin MNF116 (dilution 1/50; Dako), rabbit anti-mouse NG2 (dilution 1/200l Millipore), rabbit anti-mouse Collagen type IV (dilution 1/500; Millipore), mouse anti-human desmin (dilution 1/50; Dako), rat anti-mouse CD11b (dilution 1/50; Millipore), rat anti-mouse CD45 (dilution 1/200; Millipore), rabbit anti-human GFAP ready to use (Dako), mouse anti-human GFAP (dilution 1/100; Millipore), rabbit anti-mouse PDGFRβ phosphotyrosine 751 (dilution 1/50; Santa Cruz Biotechnology and Cell Signaling Technology, Danvers, MA), and rabbit anti-mouse PDGFRβ phosphotyrosine 1021 (dilution 1/50; Santa Cruz Biotechnology). First antibodies were incubated for 2 hours at room temperature. Alexa 546-conjugated or Alexa 488-conjugated secondary antibody (Invitrogen) was used; 200 μg/mL DAPI was added to the secondary antibody mixture and incubated for 1 hour at room temperature. Sections were mounted in Fluorescent Mounting Medium (Dako) and photographed by using an Axioskop microscope with an Axiocam digital camera (Carl Zeiss Microimaging). AxioVision software version 4 was used for image acquisition and quantification. To quantify p751-PDGFRβ–expressing astrocytes and CD11b/CD45-expressing microglia, all lesions within one brain section per mouse, from five to seven mice per group, were analyzed. Two measures were performed: the number of positive cells per group of metastases and the percentage of area covered by the imunostaining.
Flow Cytometry on p751-PDGFRβ–Expressing Cells
Astrocyte-enriched glial cell cultures were incubated for 18 hours with either Neurobasal medium or 50%-diluted tumor-conditioned medium (from 231-BR-HER2 cells cultured in serum-free basal conditions and prepared as described in Cell Lines and in Vitro Culture Conditions.) in the presence of 5 μmol/L pazopanib or 0.025% DMSO (vehicle). Cells were then analyzed by flow cytometry with the use of a FACS Vantage SE flow cytometer (Becton Dickinson, Madrid, Spain) by using a wavelength analysis (green-fluorescein isothiocyanate, 530 nm) after excitation with 488-nm light. p751-PDGFRβ–expressing intensity was expressed as the logarithm of the specific green fluorescence. Data were represented as average intensity values for live cells. Dead cells (<10%) were excluded from all of the analysis with the use of Viaprobe (Becton Dickinson).
Statistical Analysis
We performed either a two-factor factorial or weighted mixed model analysis of variance on the data as appropriate. For the ratio of p751-PDGFRB to DAPI intensity data, we performed a two-factor (experiment, treatment) analysis of variance (ANOVA) on transformed data (4√y as indicated by the Box-Cox transformation). The experiment by treatment interaction effect was dropped from the model if P > 0.05. For the percentage of cells expressing p751-PDGFRB data, we compared distributions with the use of the exact version of the Wilcoxon rank sum test. For the number of p751-PDGFR+ astrocytes and percentage of p751-PDGFR+ astrocytes coverage data, we performed a weighted mixed model ANOVA on transformed data [√y and log10(y), respectively, per Box-Cox transformation]. The total surface area was used as the weight for each observation, mice were specified as the random effect, and dose was the fixed effect. ANOVA-based two-tailed t-test P values were adjusted with Dunnett methods. For the number of CD11b/CD45+ microglia and percentage of CD11b/CD45+ microglia coverage data, we used the same methods as described in this paragraph for the p751-PDGFR astrocytes data. For the percentage of GFAP+ astrocyte coverage data, we compared distributions with the use of the exact version of the Wilcoxon rank sum test. For all ANOVAs, residuals were examined for normality and homogeneity and were partitioned if found to be heterogeneous.
Results
Architectural and Microenvironmental Features of the Brain Colonization Process of 231-BR-HER2 Breast Cancer Cells
Brain micrometastases were studied on day 21 after left cardiac ventricular injection of 231-BR-HER2 cells. In Figure 1, A and B, 231-BR-HER2 cells were stained with anti-human cytokeratin antibody (green) to verify the human origin of cytokeratin-expressing perivascular cells, and the brain microvasculature was stained with anti-murine CD31 antibody (red). Metastatic cancer cells almost exclusively located and grew around brain microvasculature from gray matter but some deposition also occurred at the interface of gray and white matter as well. Interestingly, micrometastases did not develop as pushing-type growth nodules. Instead, they formed a widespread microvascular ensheathment that progressively expanded as a layer around and along brain microvessels, without any evidence of parenchymal infiltration and neoangiogenesis, which altered normal brain microvascular architecture.
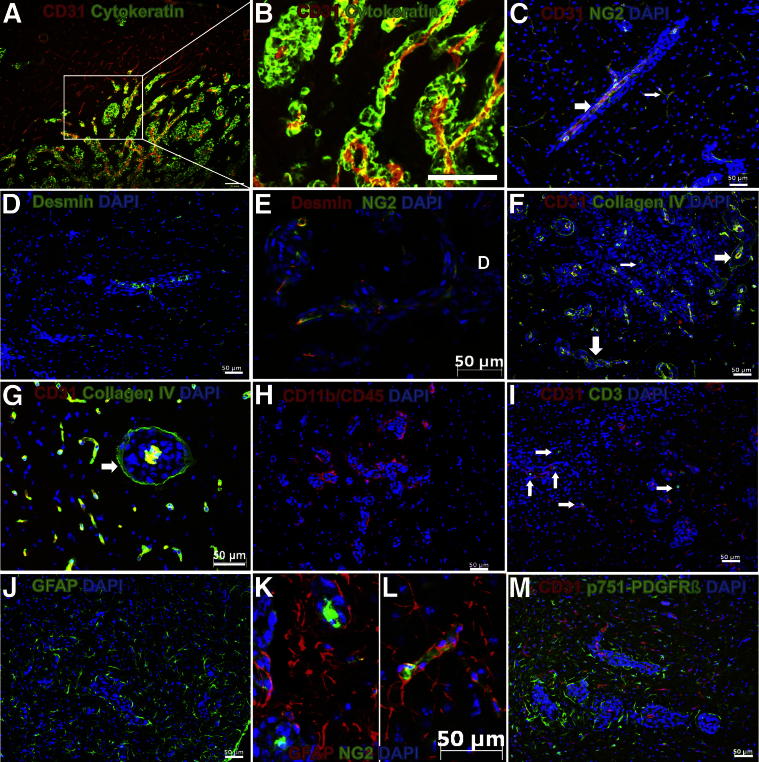
Figure 1.
Characterization of the microenvironment of the 231-BR-HER2 brain micrometastases. Brain sections were immunostained with different markers to analyze the microenvironmental structure around micrometastases. A and B: Metastatic 231-BR-HER2 cells (human cytokeratin; green) grew along brain microvessels (CD31, red). B: Higher magnification of boxed area in A. C–M: Clusters of cells stained with DAPI (blue) underwent 231-BR-HER2 micrometastases. Individual dots are neuronal nuclei. C: Brain microvessel (CD31, red) coverage by NG2-expressing pericytes (green), surrounded by 231-BR-HER2 cancer cells (large white arrow). The thin white arrow indicates NG2-covered blood vessels in normal brain area. D: Micrometastases grew along desmin-expressing pericytes (green). E: NG2-expressing pericytes (green) and desmin-expressing pericytes (red) showed the same localization pattern. F and G: Collagen type IV-expressing basement membranes (green) around both brain microvessels (CD31, red) and perivascular cancer cells, forming the inner and outer limits (large white arrows) of brain micrometastases. The thin white arrow indicates residual collagen IV. H: CD11b and CD45 double-staining for reactive microglia/macrophages (red) located around metastases. I: Some CD3-expressing lymphocytes (green; white arrows) were observed near micrometastatic clusters (CD31, red). J: Hypertrophic reactive GFAP-expressing astrocytes (green). K: 231-BR-HER2 metastases (DAPI) grew around pericytes from brain microvessel (NG2, green) and was surrounded by GFAP-expressing astrocytes (red). L: Microvessel-associated NG2-expressing pericytes (green) surrounded by GFAP-expressing astrocytes (red). M: p751-PDGFRβ–expressing cells localized only around micrometastases (cluster of DAPI-stained cells). Brain microvessels were visualized with anti-CD31 antibody (red). Scale bars: 200 μm (A and B); 50 μm (C–J, L, and M).
In Figure 1, C–M, DAPI was used to study the perivascular growth pattern of 231-BR-HER2 cells in combination with markers for the main components of the normal blood-brain barrier, including endothelial cells (anti-CD31), basement membrane (anti-collagen IV), pericytes, and progenitor glial cells (anti-NG2), smooth muscle cells (anti-desmin) and astrocyte branching and feet (anti-GFAP). As shown in Figure 1, C–E, perivascular development of metastatic cells remarkably altered the blood-brain barrier structure. NG2 (nerve/glia antigen, chondroitin sulfate proteoglycan), which is expressed by immature pericytes34 and oligodentrocyte progenitors35 was associated with CD31-expressing vessels, and their number remarkably increased at microvascular segments surrounded by cancer cells. Moreover, as shown in Figure 1C, brain microvessels costained by NG2 and CD31 were smaller and thinner in tumor-unaffected areas compared with intrametastatic microvascular segments. The muscle cell-specific class III intermediate filament34 desmin, usually used as a marker of mature pericytes, appeared to follow the same distribution as the CD31 and NG2 staining (Figure 1D). Some of the NG2-expressing pericytes also coexpressed desmin (Figure 1E), confirming the mesenchymal nature of those cells.
Collagen IV distribution (Figure 1, F and G, and Supplemental Figure S1) in this brain metastasis model was colocalized with CD31-expressing vessels from both tumor-unaffected and -metastasized areas, suggesting that basement membrane collagen was preserved around any brain capillary. However, collagen IV also formed a well-defined layer around perivascular metastases, in the limit between cancer cells and normal brain parenchyma (Figure 1, F and G). Therefore, collagen IV delineated the cancer cell territory between an inner layer at intrametastatic capillaries and an outer layer around metastatic tissue between cancer and normal brain parenchyma. In larger metastases, residual collagen IV can be observed (Figure 1F and Supplemental Figure S1).
Consistent with our previous observations,22 we also detected CD11b-CD45 coexpressing cells around, but not inside, micrometastases, indicating reactive microglia recruitment to the vicinity of cancer cells (Figure 1H). However, few single CD3+ lymphocytes were observed (Figure 1I), suggesting limited lymphocyte infiltration in the metastasized brain at this early stage.
Another remarkable glial cell type that surrounded, but not infiltrated, metastatic tissue was represented by GFAP-expressing cells (Figure 1J). These cells were most probably of astrocyte nature and also delineated an outer layer of tumor cell growth from the microvessels (Figure 1K). Compared with highly stellated phenotype of astrocytes from tumor-unaffected brain areas (Figure 1L), perimetastatic astrocytes were low stellated (Figure 1K). These GFAP-expressing cells were NG2 negative and unrelated to axonal fibers, discarding their oligodendroglial nature.
A Subpopulation of Brain Metastasis Microenvironment-Associated Astrocytes Express Activated PDGFRβ
With the use of an antibody to p751-PDGFRβ we noticed a previously undescribed population of neuroinflammatory cells with a structure indicative of astrocytes, but positive staining for this activated growth factor receptor (Figures 1M and 2A). To confirm the astrocytic lineage of these cells, dual staining with anti–p751-PDGFRβ and GFAP was conducted (Figure 2B). In addition, the p751-PDGFRβ–expressing astrocytic cells were negative for microglial and pericyte markers in other coimmunoflourescence studies (Figure 2, C and D, respectively). To eliminate the possibility that astrocytic p751-PDGFRβ expression was an artifact of an antibody cross reactivity, the staining obtained with the p751-PDGFRβ antibody initially used (Figure 2E) was compared with the staining obtained with two other antibodies: an independent polyclonal antibody against the same phosphotyrosine 751 of PDGFRβ (Figure 2F) and a polyclonal antibody against the phosphotyrosine 1021 of PDGFRβ (Figure 2G). The three antibodies gave the same pattern of staining, exclusively in the vicinity of metastatic cells, further supporting that phosphorylated PDGFRβ expression was a specific feature of the astrocyte neuroinflammatory response around perivascular brain micrometastases.
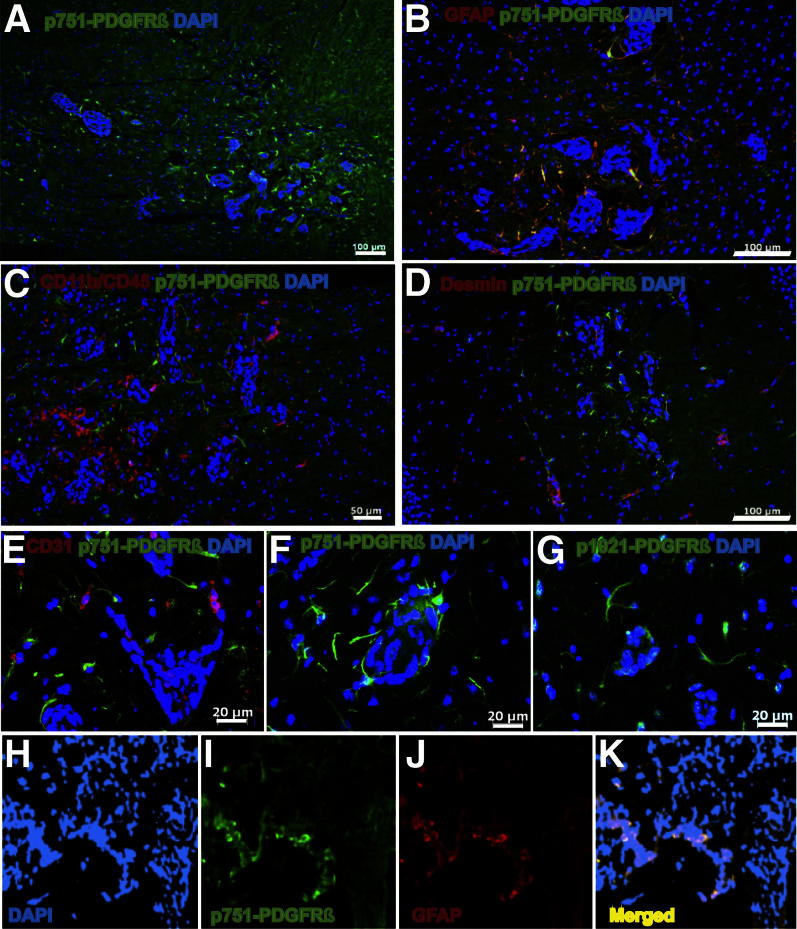
Figure 2.
Identification of metastasis-associated astrocytes expressing p751-PDGFRβ. A: p751-PDGFRβ–expressing cells are located near 231-BR-HER2 experimental micrometastases. B: Brain tissue sections were costained with GFAP (red) to detect reactive astrocytes with p751-PDGFRβ (green). C: p751-PDGFRβ–expressing cells (green) were distinct from reactive microglia (CD11b/CD45, red). D: p751-PDGFRβ+ cells (green) were distinct from desmin-expressing cells (red). E: p751-PDGFRβ–expressing cells (green) (antibody from Santa Cruz Biotechnology) and GFAP-expressing astrocytes (red). F: p751-PDGFRβ–expressing cells (green) (antibody from Cell Signaling Technology). G: p1021-PDGFRβ–expressing cells (green). H–K: Immunostaining of a brain metastasis tissue section from a biopsy of a patient with stage IV breast cancer. Same antibodies were used as above. DAPI staining in blue (H). Some p751-PDGFRβ–expressing cells in green (I) and GFAP-expressing cells in red (J) are colocalized in yellow (K). Scale bars: 100 μm (A, B, and D); 50 μm (C); 20 μm (E–G); 40 μm (H–K).
We next asked whether a subpopulation of p751-PDGFRβ+ astrocytes is found in human brain metastases. Sections from eight craniotomy specimens were stained with p751-PDGFRβ and GFAP antibodies. Five brain metastatic tissues were from patients with stage IV HER-2–overexpressing breast cancer, with estrogen receptor– and progesterone receptor–negative invasive ductal breast cancers. To determine whether the presence of this p751-PDGFRβ+ astrocyte population was generalizable to different brain metastases, craniotomy specimens from two patients with small cell lung adenocarcinoma and one with a colorectal adenocarcinoma were also stained and analyzed (Supplemental Table S1). In all cases, metastatic lesions showed undefined boundaries and tended to invade the surrounding parenchyma as single cancer cells. An extensive GFAP staining around metastatic lesions was also observed in all cases, which was indicative of reactive gliosis. In addition, some GFAP-expressing cells also expressed p751-PDGFRβ, particularly near intrametastatic microvessels. A representative brain metastasis from breast cancer is shown in Figure 2, H–K. Most GFAP-expressing astrocytes that decorated the tumor cells coexpressed p751-PDGFRβ. The data indicate that, in both an experimental model system and human craniotomy specimens, PDGFRβ activation is a feature of tumor-activated astrocytes.
Pazopanib Inhibits PDGFRβ Phosphorylation in Human Astrocytes in Vitro and ex Vivo
We previously reported that pazopanib, an inhibitor of vascular endothelial growth factor receptor, PDGFR, c-kit, and B-Raf activities, prevented the formation of experimental brain metastases by 231-BR-HER2 cells by 73% and of MCF7-HER2-BR3 cells by 55%.19 The major target for pazopanib in the tumor cells appeared to be B-Raf, based on a reduction in tumor cell pErk but not microvessel density in vivo. Given the presence of activated PDGFRβ in the astrocytic neuroinflammatory response, we asked whether pazopanib modulated PDGFR activation in these cells.
In vitro studies were conducted with human immortalized astrocytes (immortalized by expression of the protein E6 and E7 form the human papillomavirus33). These cells uniformly expressed p751-PDGFRβ under basal culture conditions. The addition of 5 μmol/L pazopanib to the culture medium for 48 hours decreased p751-PDGFRβ by 71.2% (difference between medians) compared with DMSO control culture (P < 0.0001) (Figure 3, A–C). Using MTT viability assays, the concentration that inhibited 50% of pazopanib activity was 9 μmol/L after 120 hours of incubation (data not shown). This concentration compared favorably to its concentration that inhibited 50% of tumor cells in vitro.32 The data suggest that pazopanib can deactivate and inhibit the proliferation of activated PDGFRβ-expressing astrocytes.
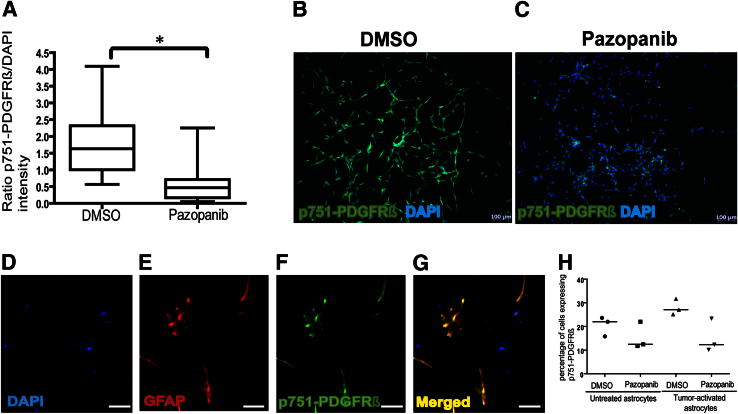
Figure 3.
Pazopanib inhibited PDGFRβ phosphorylation from human immortalized astrocytes and tumor-activated primary cultured human glial in vitro. A: Microfluorimetric image analysis of pazopanib effect on p751-PDGFRβ immunostaining (in green) by immortalized human astrocytes cultured in basal conditions overnight and treated with 5 μmol/L pazopanib (added every 24 hours) or DMSO for 48 hours. p751-PDGFRβ staining intensity was quantified and normalized to DAPI staining. Data represent average values from five or seven images per well from three independent experiments (n = 17 per group). Box-and-whisker plots of the raw data are shown, but the statistical analysis was performed on transformed data. ∗P < 0.0001. B and C: Representative images of p751-PDGFRβ staining after DMSO (B) or pazopanib (C) treatments. D–G: Identification of PDGFRβ phosphorylation in GFAP-expressing cells from a primary culture of astrocyte-enriched human glial cells. Glial cells were isolated by mechanical cell separation, enzyme digestion, and differential plating, from a tumor-unaffected area in human brain tissue obtained by neurosurgery from a patient with a metastatic brain tumor. Same antibodies were used as above. DAPI staining in blue (D). Some p751-PDGFRβ–expressing cells in green (E) and GFAP-expressing cells in red (F) are colocalized in yellow (G). H: Same enriched glial cell cultures were exposed to basal medium (untreated astrocytes) or the conditioned medium from serum-free and basal condition-cultured 231-BR-HER2 cells (tumor-activated astrocytes) for 18 hours in the presence of 5 μmol/L pazopanib or DMSO. The number of cells expressing p751-PDGFRβ was quantified by flow cytometry after immunostaining with the same antibody as above. Three independent experiments, using primary culture from three different patients (n = 3 per group), are shown in the dot plots. The bars represent the median. Scale bars: 100 μm (B and C); 10 μm (D–G).
To extend these observations past an immortalized astrocyte cell line, short-term primary cultures of astrocyte-enriched glial cells were prepared from fresh craniotomy specimens from five different patients. Figure 3, D–G, presents representative immunofluorescence staining of these cultures, showing colocalization of p751-PDGFRβ and GFAP. PDGFRβ activation was stable through short-term culture. Medium from 231-BR-HER2 cell cultures was harvested as a tumor-conditioned medium, mimicking the brain metastasis microenvironment, and added to the primary astrocyte cultures. Four different conditions were performed: incubation with Neurobasal medium containing pazopanib or DMSO, or incubation with tumor-conditioned medium containing either pazopanib or DMSO, for 18 hours (Figure 3H). The tumor-conditioned medium increased the median number of p751-PDGFRβ–expressing primary astrocytes by 23.2% compared with Neurobasal medium culture (Figure 3H). The data outline a tumor cell–microenvironmental interaction in the brain where tumor-derived soluble factors induced p751-PDGFRβ–expressing astrocytes. Pazopanib decreased the basal level of p751-PDGFRβ expression in normal medium from a median of 21.98% to 12.47%, or a 43.4% decrease. A greater decrease was observed in tumor-activated cultures, with pazopanib inducing 54.6% reduction in the intensity of p751-PDGFRβ.
Prevention of 231-BR-HER2 Brain Metastasis Formation Is Associated with Pazaponib Inhibition of p751-PDGFRβ+ Astrocytes in Vivo
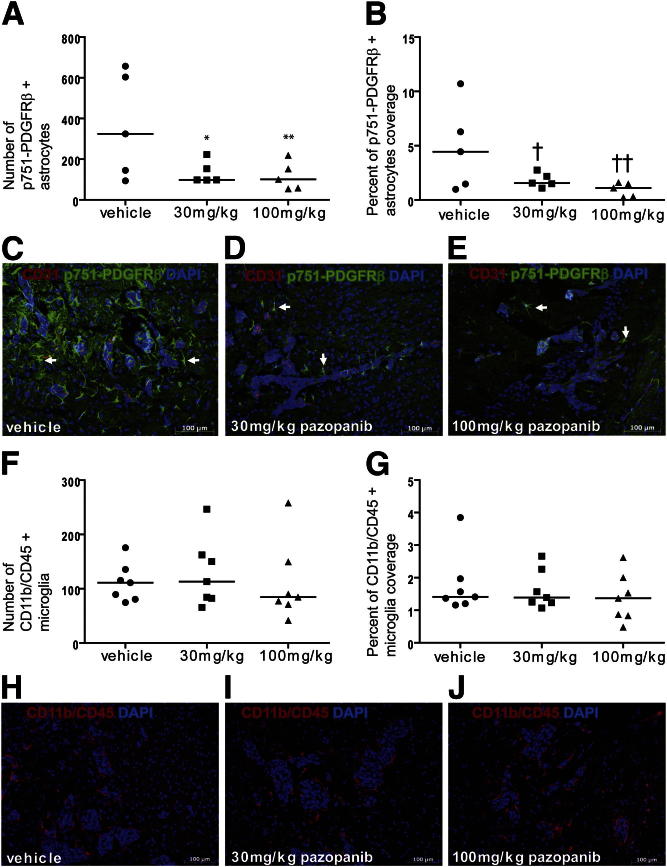
In previously reported studies, the 231-BR-HER2 cell line was injected into the left cardiac ventricle of immunocompromised mice, and, beginning 3 days after injection, mice were treated twice daily by oral gavage for 21 days with vehicle or with 30 or 100 mg/kg pazopanib. To determine the effect of pazopanib on the neuroinflammatory response, sections of brains from five mice per treatment group were examined for p751-PDGFRβ–expressing astrocytes (Figure 4). Pazopanib significantly inhibited the number of p751-PDGFRβ–expressing astrocytes from a median of 324.6 positive cells in vehicle-treated mice to 98.1 and 101.3, producing 69.8% and 68.8% decrease at 30 and 100 mg/kg, respectively (P = 0.023 and P = 0.022, respectively) (Figure 4A). The surface area covered by p751-PDGFRβ+ astrocytes was also measured by image analysis, and pazopanib treatment resulted in a 64.9% and 75.2% decline in the percentage of p751-PDGFRβ–expressing astrocytes, at 30 and 100 mg/kg, respectively [a median 4.44% of the area covered by p751-PDGFRβ+ astrocytes in vehicle-treated mice compared with 1.56% (P = 0.13) and 1.10% (P = 0.070) for 30 and 100 mg/kg pazopanib, respectively]. Figure 4, C–E, shows representative images of p751-PDGFRβ–expressing astrocytes in the vicinity of metastases at the three different treatments (vehicle, 30 mg/kg, and 100 mg/kg, respectively). Serial tissue sections from the same mice used to quantify p751-PDGFRβ–expressing astrocytes were immunostained with a GFAP antibody to evaluate the effect of pazopanib on the general population of astrocytes. Virtually all of the astrocytes expressed GFAP, but an increase in expression was observed in the astrocytes near the metastases. Pazopanib induced only a minor decrease in the number for GFAP+ astrocytes (Supplemental Figure S2). Because the GFAP-overexpressing astrocyte population was not significantly decreased by pazopanib, this observation suggests that pazopanib might not kill astrocytes but rather prevent their activation. As a control, microglial activation was analyzed and quantified in the same manner. Pazopanib treatment did not result in any effect on the reactive microglia (Figure 4, F–J), confirming its specificity to metastasis-associated astrocytes. The median numbers of CD11b/CD45+ microglia cells per section were 111, 113, and 84.7 for vehicle, 30-mg/kg, and 100-mg/kg pazopanib treatment groups, respectively (Figure 4F). Collectively, the data establish the existence of a previously unknown PDGFRβ p751-expressing population of astrocytes in the cancer metastasis neuroinflammatory response in the brain, which is inhibitable by pazopanib.
Figure 4.
Pazopanib induces a decrease in the p751-PDGFRβ–expressing astrocyte population but does not alter reactive microglia. Brains, from necropsies of athymic nude mouse that received 231-BR-HER2 cells and vehicle or 30 mg/kg or 100 mg/kg pazopanib for 21 days, were fixed, sectioned, and stained. A: Number of p751-PDGFRβ+ astrocytes per high power field. ∗P = 0.023 for vehicle-treated versus 30 mg/kg; ∗∗P = 0.022 for vehicle-treated versus 100 mg/kg. B: Percentage of area occupied by p751-PDGFRβ+ astrocytes. †P = 0.13 for vehicle-treated versus 30 mg/kg; ††P = 0.070 for vehicle-treated versus 100 mg/kg. Data in A and B were both determined by photographic analysis of p751-PDGFRβ staining. Raw data are presented, but statistical analysis was performed on square root transformed data in A and on log10 transformed data in B. Each dot represents one mouse and is the average of up to nine groups of metastases. C–E: Representative images of p751-PDGFRβ and CD31 staining: vehicle (C), 30 mg/kg pazopanib (D), and 100 mg/kg pazopanib (E). Arrows indicate representative p751-PDGFRβ+ astrocytes. F: Number of CD11b/CD45+ microglia/macrophages per high power field. G: Percentage of area occupied by CD11b/CD45+ microglia/macrophages. Data in F and G were both determined by photographic analysis of CD11b/CD45 staining, and data were transformed for ANOVA. Each dot represents one mouse and is the average of up to three groups of metastases. H–J: Representative images of CD11b/CD45+ staining: vehicle (H), 30 mg/kg pazopanib (I), 100 mg/kg pazopanib (J). Scale bars: 100 μm (C–E and H–J).
Discussion
The brain metastatic process is known to alter the surrounding microenvironment, changing gene expression,36 producing a neuroinflammatory response,22,23,37 and increasing the permeability of the blood-brain barrier to a limited extent.38–41 However, the molecular characteristics of these changes, their functional contributions to brain metastatic colonization, and their effect on therapeutic efficacy remain to be fully understood. With the use of the 231-BR-HER2 experimental brain metastasis model system, we find that perivascular cancer cell growth involved a remarkable perturbation of the neurovascular unit, including increased density of NG2/desmin coexpressing pericytes in specific brain microvessels ensheathed by metastatic cells and perimetastatic accumulation of collagen IV in 40% of lesions and of reactive glial cells in virtually all metastases. Reactive glia included microglia coexpressing CD11b/CD45 and astrocytes expressing GFAP. An increase in pericyte coverage is generally consistent with our previous unanticipated observation that more permeable experimental brain metastases contained vessels decorated with greater numbers of pericytes.39 In addition to type IV collagen, perimetastatic deposition of laminin has also been reported in brain metastases,42 suggesting the deposition of at least elements of a basement membrane. Type IV collagen ensheathment of metastases was also heterogeneously reported in a limited study of human tissues.43
Herein, we report that a population of astrocytes in the neuroinflammatory response to 231-BR-HER2 brain metastases expressed p-PDGFRβ. The p-PDGFRβ+ neuroinflammatory cells coexpressed GFAP but not NG2, desmin, or CD11b/CD45. This finding was confirmed in eight human craniotomy specimens and in short-term cultures of primary craniotomy-derived human astrocytes. PDGF and its α and β receptors are well known in the central nervous system but are mainly characterized in pericyte function in normal and diseased states29,44,45 and in gliomas.46 A population of oligodendroglia and their precursors (which can give rise to astrocyte populations) have been reported to be PRGDFRα+47,48 and PDGFRβ+.49 Although we cannot formally rule out that the cells identified in the metastasis-associated neuroinflammatory response are oligodendroglia, their predominance in the gray matter and lack of NG2 positivity argues against this presumption. The literature about astrocyte expression of PDGFRs is limited and unclear. In human brain specimens from patients with ischemic stroke, PDGRFR mRNA detected by in situ hybridization was robust in the penumbral astrocytes, although the subtype of receptor was not determined50; in a mouse staining study, PDGFRβ was undetectable on normal brain astrocytes.29 Astrocytes and their precursor cells respond to PDGF with a variety of proliferative and invasive phenotypes,51–53 although it is impossible to determine whether these responses are direct or indirect through other cell types present. Together with our data, we hypothesize that p-PDGFRβ expression by astrocytes is a hallmark of their aberrant activation in disease states. Epidermal growth factor receptor has also been found to be induced in optic nerve astrocytes after injury,54,55 suggesting that additional molecular alterations may occur as well.
Our experiments suggest the exciting hypothesis that p-PDGFRβ expression by activated astrocytes in brain metastasis contributes to functional phenotypes. In short-term primary cultures, astrocyte p-PDGFRβ expression was inducible by tumor-derived soluble factors, suggesting that it results from a tumor–microenvironment cross talk similar to that previously characterized for bone metastasis.56 Pazopanib is a drug with inhibitory activity to vascular endothelial growth factor receptor, PDGFR, c-kit, and B-Raf, approved by the Food and Drug Administration. It inhibited p-PDGFRβ+ human immortalized astrocyte proliferation by 50% at concentrations comparable with that of many tumor cell lines.32,57 In vitro, pazopanib was able to decrease p-PDGFRβ expression in astrocytes both in normal culture medium and tumor-activated medium. Our previous work showed the preventive effect of pazopanib on metastasis outgrowth, directly targeting the tumor cells. Combining these two studies, pazopanib's potent efficacy is most likely because of its ability to directly attack the metastatic process on two fronts: the tumor cells and the microenvironment. Therefore, the decrease in p-PDGFRβ+ astrocytes might be the result of both a direct block of the PDGFRβ expressed by the astrocytes and a decrease in tumor-secreted factors subsequent to the metastatic outgrowth prevention effect. An inherent limitation in our studies is that pazopanib, as a multikinase inhibitor, could modify astrocyte activation through other, currently undetectable pathways. Astrocytes have been reported to serve multiple functions in brain metastasis, including the production of heparanase,58 nitric oxide,59 and other cytokines24; the stimulation of tumor cell proliferation60 and chemotherapeutic resistance25; and the activation of microglia to further amplify the neuroinflammatory response.61 It will be of interest to determine whether the astrocytic PDGFRβ pathway mediates these or other additional phenotypes.
The cancer microenvironment is the interface between cancer cells and pathophysiology of the patient, and a potentially important trend in cancer research is that functional pathways in the tumor microenvironment may represent valuable therapeutic targets for more effective cancer treatment. The recent success of targeting cytotoxic T lymphocyte antigen 4 in T lymphocytes in the tumor microenvironment in metastatic melanoma points to potential clinical success.62 For brain metastasis, several potential therapeutic targets have been identified in the microenvironment, including the heparanase, plasminogen activator, and metalloproteinase systems.63–65 Herein, we demonstrate that pazopanib treatment of mice significantly reduced the percentage of p-PDGFRβ+ astrocytes in the brain in vivo at two doses. In these same experiments, the development of large brain metastases was significantly prevented. Of the different chemotherapeutic and molecular drugs that we have tested to date in the 231-BR model system, pazopanib has been the most efficacious in prevention of metastatic colonization.9,10,66,67 We hypothesize that pazopanib activity resulted from both tumor and microenvironmental effects. Although the brain metastasis process in this animal model might have limitations for a direct clinical translation, our results show that the newly identified subpopulation of activated p-PDGFRβ+ astrocytes is playing a key role in the mechanism of brain micrometastasis progression. However, this was inhibited by pazopanib, suggesting it is a specific drug able to prevent further development of subclinical perivascular micrometastases.
Footnotes
Supported by the intramural program of the National Cancer Institute (P.S.S.), the Department of Defense Breast Cancer Research Program grant W81XWH-062-0033 (P.S.S.), GlaxoSmithKline (P.S.S.), the Carlos III Health Institute (FIS, Madrid) grant ADE09/90041 (F.V.-V.), and the Burdinola Professorship on Molecular Medicine (F.V.-V.).
Disclosure: Pazopanib was provided by GlaxoSmithKline through a Material Collaborative Research and Development Agreement with NIH.
Current address of T.A., University of Michigan Medical Scientist Training Program, Ann Arbor, MI.
Contributor Information
Brunilde Gril, Email: grilbrun@mail.nih.gov.
Fernando Vidal-Vanaclocha, Email: fernando.vidalvanaclocha@ceu.es.
Supplementary Data
Type IV collagen pattern of expression in the microenvironment of the 231-BR-HER2 brain micrometastases. A–E: Metastatic 231-BR-HER2 cells grew around blood vessels (CD31, red; C, D, and E) which are surrounded by collagen IV (green; B, D, and E). The thin elongated micrometastases are encapsulated in a collagen IV layer (large white arrows), whereas in the more round one, only residual collagen IV can be seen in the center (thin white arrow). F: The serial section was stained with human cytokeratin antibody (green) to confirm the presence of metastases. Scale bars: 50 μm (E); 100 μm (F).
Pazopanib induces a trend in GFAP-expressing astrocyte population decrease. Brains, from necropsies of athymic nude mice that received 231-BR-HER2 cells and either vehicle or 100 mg/kg pazopanib for 21 days, were fixed, sectioned, and stained. A: Percentage of area occupied by GFAP+ astrocytes. P = 0.0556. The bars represent the median. Data in A were determined by photographic analysis of GFAP staining. Each dot represents one mouse that is the average of up to nine groups of metastases. B–E: Two representative images of GFAP staining (green) for each treatment: vehicle (B and C), 100 mg/kg pazopanib (D and E). Scale bars: 200 μm (B–E).
References
- 1.Barnholtz-Sloan J.S., Sloan A.E., Davis F.G., Vigneau F.D., Lai P., Sawaya R.E. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22:2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 2.Stemmler H.J., Heinemann V. Central nervous system metastases in HER-2-overexpressing metastatic breast cancer: a treatment challenge. Oncologist. 2008;13:739–750. doi: 10.1634/theoncologist.2008-0052. [DOI] [PubMed] [Google Scholar]
- 3.Gril B., Evans L., Palmieri D., Steeg P.S. Translational research in brain metastasis is identifying molecular pathways that may lead to the development of new therapeutic strategies. Eur J Cancer. 2010;46:1204–1210. doi: 10.1016/j.ejca.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin N.U., Winer E.P. Brain metastases: the HER2 paradigm. Clin Cancer Res. 2007;13:1648–1655. doi: 10.1158/1078-0432.CCR-06-2478. [DOI] [PubMed] [Google Scholar]
- 5.Steeg P.S., Camphausen K.A., Smith Q.R. Brain metastases as preventive and therapeutic targets. Nat Rev Cancer. 2011;11:352–363. doi: 10.1038/nrc3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eichler A.F., Chung E., Kodack D.P., Loeffler J.S., Fukumura D., Jain R.K. The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol. 2011;8:344–356. doi: 10.1038/nrclinonc.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steeg P.S. Perspective: the right trials. Nature. 2012;485:S58–S59. doi: 10.1038/485S58a. [DOI] [PubMed] [Google Scholar]
- 8.Palmieri D., Fitzgerald D., Shreeve S.M., Hua E., Bronder J.L., Weil R.J., Davis S., Stark A.M., Merino M.J., Kurek R., Mehdorn H.M., Davis G., Steinberg S.M., Meltzer P.S., Aldape K., Steeg P.S. Analyses of resected human brain metastases of breast cancer reveal the association between up-regulation of hexokinase 2 and poor prognosis. Mol Cancer Res. 2009;7:1438–1445. doi: 10.1158/1541-7786.MCR-09-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmieri D., Lockman P.R., Thomas F.C., Hua E., Herring J., Hargrave E., Johnson M., Flores N., Qian Y., Vega-Valle E., Taskar K.S., Rudraraju V., Mittapalli R.K., Gaasch J.A., Bohn K.A., Thorsheim H.R., Liewehr D.J., Davis S., Reilly J.F., Walker R., Bronder J.L., Feigenbaum L., Steinberg S.M., Camphausen K., Meltzer P.S., Richon V.M., Smith Q.R., Steeg P.S. Vorinostat inhibits brain metastatic colonization in a model of triple-negative breast cancer and induces DNA double-strand breaks. Clin Cancer Res. 2009;15:6148–6157. doi: 10.1158/1078-0432.CCR-09-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gril B., Palmieri D., Bronder J.L., Herring J.M., Vega-Valle E., Feigenbaum L., Liewehr D.J., Steinberg S.M., Merino M.J., Rubin S.D., Steeg P.S. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J Natl Cancer Inst. 2008;100:1092–1103. doi: 10.1093/jnci/djn216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao H., Cui K., Nie F., Wang L., Brandl M.B., Jin G., Li F., Mao Y., Xue Z., Rodriguez A., Chang J., Wong S.T. The effect of mTOR inhibition alone or combined with MEK inhibitors on brain metastasis: an in vivo analysis in triple-negative breast cancer models. Breast Cancer Res Treat. 2012;131:425–436. doi: 10.1007/s10549-011-1420-7. [DOI] [PubMed] [Google Scholar]
- 12.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:99–101. [PubMed] [Google Scholar]
- 13.Condeelis J., Pollard J.W. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Kalluri R., Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 15.Fidler I. The pathogenesis of cancer metastasis: the “seed and soil” hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 16.Vidal-Vanaclocha F. The liver prometastatic reaction of cancer patients: implications for microenvironment-dependent colon cancer gene regulation. Cancer Microenviron. 2011;4:163–180. doi: 10.1007/s12307-011-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmieri D., Bronder J.L., Herring J.M., Yoneda T., Weil R.J., Stark A.M., Kurek R., Vega-Valle E., Feigenbaum L., Halverson D., Vortmeyer A.O., Steinberg S.M., Aldape K., Steeg P.S. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007;67:4190–4198. doi: 10.1158/0008-5472.CAN-06-3316. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald D.P., Subramanian P., Deshpande M., Graves C., Gordon I., Qian Y., Snitkovsky Y., Liewehr D.J., Steinberg S.M., Paltan-Ortiz J.D., Herman M.M., Camphausen K., Palmieri D., Becerra S.P., Steeg P.S. Opposing effects of pigment epithelium-derived factor on breast cancer cell versus neuronal survival: implication for brain metastasis and metastasis-induced brain damage. Cancer Res. 2012;72:144–153. doi: 10.1158/0008-5472.CAN-11-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gril B., Palmieri D., Qian Y., Smart D., Ileva L., Liewehr D.J., Steinberg S.M., Steeg P.S. Pazopanib reveals a role for tumor cell B-Raf in the prevention of HER2+ breast cancer brain metastasis. Clin Cancer Res. 2011;17:142–153. doi: 10.1158/1078-0432.CCR-10-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oskarsson T., Acharyya S., Zhang X.H., Vanharanta S., Tavazoie S.F., Morris P.G., Downey R.J., Manova-Todorova K., Brogi E., Massague J. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med. 2011;17:867–874. doi: 10.1038/nm.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carbonell W.S., Ansorge O., Sibson N., Muschel R. The vascular basement membrane as “soil” in brain metastasis. PLoS One. 2009;4:e5857. doi: 10.1371/journal.pone.0005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzgerald D., Palmieri D., Hua E., Hargrave E., Herring J., Qian Y., Vega-Valle E., Weil R., Stark A., Vortmeyer A., Steeg P. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin Exp Metast. 2008;25:799–810. doi: 10.1007/s10585-008-9193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorger M., Felding-Habermann B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am J Pathol. 2010;176:2958–2971. doi: 10.2353/ajpath.2010.090838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seike T., Fujita K., Yamakawa Y., Kido M.A., Takiguchi S., Teramoto N., Iguchi H., Noda M. Interaction between lung cancer cells and astrocytes via specific inflammatory cytokines in the microenvironment of brain metastasis. Clin Exp Metastasis. 2011;28:13–25. doi: 10.1007/s10585-010-9354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S.J., Kim J.S., Park E.S., Lee J.S., Lin Q., Langley R.R., Maya M., He J., Kim S.W., Weihua Z., Balasubramanian K., Fan D., Mills G.B., Hung M.C., Fidler I.J. Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia. 2011;13:286–298. doi: 10.1593/neo.11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norazit A., Nguyen M.N., Dickson C.G., Tuxworth G., Goss B., Mackay-Sim A., Meedeniya A.C. Vascular endothelial growth factor and platelet derived growth factor modulates the glial response to a cortical stab injury. Neuroscience. 2011;192:652–660. doi: 10.1016/j.neuroscience.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 27.Fisher M. Pericyte signaling in the neurovascular unit. Stroke. 2009;40:S13–S15. doi: 10.1161/STROKEAHA.108.533117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valenzuela C.F., Kazlauskas A., Weiner J.L. Roles of platelet-derived growth factor in the developing and mature nervous systems. Brain Res Brain Res Rev. 1997;24:77–89. doi: 10.1016/s0165-0173(97)00012-x. [DOI] [PubMed] [Google Scholar]
- 29.Winkler E.A., Bell R.D., Zlokovic B.V. Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol Neurodegener. 2010;5:32. doi: 10.1186/1750-1326-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ostman A., Heldin C.H. PDGF receptors as targets in tumor treatment. Adv Cancer Res. 2007;97:247–274. doi: 10.1016/S0065-230X(06)97011-0. [DOI] [PubMed] [Google Scholar]
- 31.Kumar R., Knick V.B., Rudolph S.K., Johnson J.H., Crosby R.M., Crouthamel M.C., Hopper T.M., Miller C.G., Harrington L.E., Onori J.A., Mullin R.J., Gilmer T.M., Truesdale A.T., Epperly A.H., Boloor A., Stafford J.A., Luttrell D.K., Cheung M. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther. 2007;6:2012–2021. doi: 10.1158/1535-7163.MCT-07-0193. [DOI] [PubMed] [Google Scholar]
- 32.Gril B., Palmieri D., Qian Y., Anwar T., Ileva L., Bernardo M., Choyke P., Liewehr D.J., Steinberg S.M., Steeg P.S. The B-Raf status of tumor cells may be a significant determinant of both antitumor and anti-angiogenic effects of pazopanib in xenograft tumor models. PLoS One. 2011;6:e256k25. doi: 10.1371/journal.pone.0025625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonoda Y., Ozawa T., Hirose Y., Aldape K.D., McMahon M., Berger M.S., Pieper R.O. Formation of intracranial tumors by genetically modified human astrocytes defines four pathways critical in the development of human anaplastic astrocytoma. Cancer Res. 2001;61:4956–4960. [PubMed] [Google Scholar]
- 34.Diaz-Flores L., Gutierrez R., Madrid J.F., Varela H., Valladares F., Acosta E., Martin-Vasallo P., Diaz-Flores L., Jr. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909–969. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- 35.Kang S.H., Fukaya M., Yang J.K., Rothstein J.D., Bergles D.E. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park E., Kim S., Kim S., Yoon S.L., Leem S.H., Kim S.B., Kim S., Park Y.Y., Cheong J.H., Woo H., Mills G., Fidler I., Lee J.S. Cross-species hybridization of microarrays for studying tumor transcriptome of brain metastasis. Proc Natl Acad Sci U S A. 2011;108:17456–17461. doi: 10.1073/pnas.1114210108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang M., Olsson Y. Reactions of astrocytes and microglial cells around hematogenous metastases of the human brain. Expression of endothelin-like immunoreactivity in reactive astrocytes and activation of microglial cells. J Neurol Sci. 1995;134:26–32. doi: 10.1016/0022-510x(95)00227-9. [DOI] [PubMed] [Google Scholar]
- 38.Gerstner E., Fine R. Increased permeability of the blood-brain barrier to chemotherapy in metastatic brain tumors: establishing a treatment paradigm. J Clin Oncol. 2007;25:2306–2312. doi: 10.1200/JCO.2006.10.0677. [DOI] [PubMed] [Google Scholar]
- 39.Lockman P., Mittapalli R., Taskar K., Rudraruju V., Gril B., Bohn K., Adkins C., Thomas F., Thorsheim H., Gaasch J., Huang S., Palmieri D., Steeg P., Smith Q. Heterogeneous blood-brain barrier permeability determines drug efficacy in mouse brain metastases of breast cancer. Clin Cancer Res. 2010;16:5662–5678. doi: 10.1158/1078-0432.CCR-10-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Percy D.B., Ribot E.J., Chen Y., McFadden C., Simedrea C., Steeg P.S., Chambers A.F., Foster P.J. In vivo characterization of changing blood-tumor barrier permeability in a mouse model of breast cancer metastasis: a complementary magnetic resonance imaging approach. Invest Radiol. 2011;46:718–725. doi: 10.1097/RLI.0b013e318226c427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deeken J.F., Loscher W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13:1663–1674. doi: 10.1158/1078-0432.CCR-06-2854. [DOI] [PubMed] [Google Scholar]
- 42.Castronovo V., Bracke M., Mareel M., Reznik M., Foidart J. Absence of laminin deposition in breast-cancer and metastases except to the brain. Pathol Res Pract. 1991;187:201–208. doi: 10.1016/S0344-0338(11)80772-7. [DOI] [PubMed] [Google Scholar]
- 43.McArdle J., Muller H., Roff B., Murphy W. Basal lamina redevelopment in tumours metastatic to the brain: an immunoperoxidase study using an antibody to type-IV collagen. Int J Cancer. 1984;34:633–638. doi: 10.1002/ijc.2910340508. [DOI] [PubMed] [Google Scholar]
- 44.Arimura K., Ago T., Kamouchi M., Nakamura K., Ishitsuka K., Kuroda J., Sugimori H., Ooboshi H., Sasaki T., Kitazono T. PDGF receptor beta signaling in pericytes following ischemic brain injury. Curr Neurovasc Res. 2012;9:1–9. doi: 10.2174/156720212799297100. [DOI] [PubMed] [Google Scholar]
- 45.Song N., Huang Y., Shi H., Yuan S., Ding Y., Song X., Fu Y., Luo Y. Overexpression of platelet-derived growth factor-BB increases tumor pericyte content via stromal-derived factor-1alpha/CXCR4 axis. Cancer Res. 2009;69:6057–6064. doi: 10.1158/0008-5472.CAN-08-2007. [DOI] [PubMed] [Google Scholar]
- 46.Dai C., Celestino J.C., Okada Y., Louis D.N., Fuller G.N., Holland E.C. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001;15:1913–1925. doi: 10.1101/gad.903001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson H.C., Scolding N.J., Raine C.S. Co-expression of PDGF alpha receptor and NG2 by oligodendrocyte precursors in human CNS and multiple sclerosis lesions. J Neuroimmunol. 2006;176:162–173. doi: 10.1016/j.jneuroim.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 48.Maeda Y., Solanky M., Menonna J., Chapin J., Li W., Dowling P. Platelet-derived growth factor-alpha receptor-positive oligodendroglia are frequent in multiple sclerosis lesions. Ann Neurol. 2001;49:776–785. doi: 10.1002/ana.1015. [DOI] [PubMed] [Google Scholar]
- 49.Grinspan J.B., Reddy U.R., Stern J.L., Hardy M., Williams M., Baird L., Pleasure D. Oligodendroglia express PDGF beta-receptor protein and are stimulated to proliferate by PDGF. Ann N Y Acad Sci. 1990;605:71–80. doi: 10.1111/j.1749-6632.1990.tb42382.x. [DOI] [PubMed] [Google Scholar]
- 50.Krupinski J., Issa R., Bujny T., Slevin M., Kumar P., Kumar S., Kaluza J. A putative role for platelet-derived growth factor in angiogenesis and neuroprotection after ischemic stroke in humans. Stroke. 1997;28:564–573. doi: 10.1161/01.str.28.3.564. [DOI] [PubMed] [Google Scholar]
- 51.Nakamura-Ishizu A., Kurihara T., Okuno Y., Ozawa Y., Kishi K., Goda N., Tsubota K., Okano H., Suda T., Kubota Y. The formation of an angiogenic astrocyte template is regulated by the neuroretina in a HIF-1-dependent manner. Dev Biol. 2012;363:106–114. doi: 10.1016/j.ydbio.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 52.Wang H.H., Hsieh H.L., Yang C.M. Calmodulin kinase II-dependent transactivation of PDGF receptors mediates astrocytic MMP-9 expression and cell motility induced by lipoteichoic acid. J Neuroinflammation. 2010;7:84. doi: 10.1186/1742-2094-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nazarenko I., Hedren A., Sjodin H., Orrego A., Andrae J., Afink G.B., Nister M., Lindstrom M.S. Brain abnormalities and glioma-like lesions in mice overexpressing the long isoform of PDGF-A in astrocytic cells. PLoS One. 2011;6:e18303. doi: 10.1371/journal.pone.0018303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang X., Neufeld A. Activation of the epidermal growth factor receptor in optic nerve astrocytes leads to early and transient induction of cyclooxygenase-2. Invest Opthamol Vis Sci. 2005;46:2035–2041. doi: 10.1167/iovs.04-1473. [DOI] [PubMed] [Google Scholar]
- 55.Liu B., Chen H., Johns T., Neufeld A. Epidermal growth factor receptor activation: an upstream signal for transition of quiescent astrocytes into reactive astrocytes after injury. J Neurosc. 2006;26:7532–7540. doi: 10.1523/JNEUROSCI.1004-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mundy G. Metastasis to the bone: causes, consequences and therapeutic opportunities. Nat Cancer Rev. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 57.Olaussen K.A., Commo F., Tailler M., Lacroix L., Vitale I., Raza S.Q., Richon C., Dessen P., Lazar V., Soria J.C., Kroemer G. Synergistic proapoptotic effects of the two tyrosine kinase inhibitors pazopanib and lapatinib on multiple carcinoma cell lines. Oncogene. 2009;28:4249–4260. doi: 10.1038/onc.2009.277. [DOI] [PubMed] [Google Scholar]
- 58.Marchetti D., Li J., Shen R. Astrocytes contribute to the brain-metastatic specificity of melanoma cells by producing heparanase. Cancer Res. 2000;60:4767–4770. [PubMed] [Google Scholar]
- 59.Samdani A.F., Kuchner E.B., Rhines L., Adamson D.C., Lawson C., Tyler B., Brem H., Dawson V.L., Dawson T.M. Astroglia induce cytotoxic effects on brain tumors via a nitric oxide-dependent pathway both in vitro and in vivo. Neurosurgery. 2004;54:1231–1237. doi: 10.1227/01.neu.0000119576.76193.b8. discussion 1237–1238. [DOI] [PubMed] [Google Scholar]
- 60.Sierra A., Price J., Garcia-Ramirez M., Mendez O., Lopez L., Fabra A. Astrocyte derived cytokines contribute to the metastatic brain specificity of breast cancer cells. Lab Invest. 1997;77:357–368. [PubMed] [Google Scholar]
- 61.Zhang M., Olsson Y. Reactions of astrocytes and microglial cells around hematogenous metastases of the human brain. Expression of endothelin-like immunoreactivity in reactive astrocytes and action of microglial cells. J Neurol Sci. 1995;134:26–32. doi: 10.1016/0022-510x(95)00227-9. [DOI] [PubMed] [Google Scholar]
- 62.Prieto P.A., Yang J.C., Sherry R.M., Hughes M.S., Kammula U.S., White D.E., Levy C.L., Rosenberg S.A., Phan G.Q. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18:2039–2047. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kruger A., Sanchez-Sweatman O.H., Martin D.C., Fata J.E., Ho A.T., Orr F.W., Ruther U., Khokha R. Host TIMP-1 overexpression confers resistance to experimental brain metastasis of a fibrosarcoma cell line. Oncogene. 1998;16:2419–2423. doi: 10.1038/sj.onc.1201774. [DOI] [PubMed] [Google Scholar]
- 64.Maillard C., Bouquet C., Petitjean M., Mesdagt M., Frau E., Jost M., Masset A., Opolon P., Beermann F., Abitbol M., Foidart J., Perricaudet M., Noel A. Reduction of brain metastases in plasminogen activator inhibitor-1 deficient mice with transgenic ocular tumors. Carcinogenesis. 2008;29:2236–2242. doi: 10.1093/carcin/bgn204. [DOI] [PubMed] [Google Scholar]
- 65.Marchetti D., Nicolson G. Human haparanase: a molecular determinant of brain metastasis. Advan Enzyme Regul. 2001;41:343–359. doi: 10.1016/s0065-2571(00)00016-9. [DOI] [PubMed] [Google Scholar]
- 66.Fitzgerald D.P., Emerson D.L., Qian Y., Anwar T., Liewehr D.J., Steinberg S.M., Silberman S., Palmieri D., Steeg P.S. TPI-287, a new taxane family member, reduces the brain metastatic colonization of breast cancer cells. Mol Cancer Ther. 2012;11:1959–1967. doi: 10.1158/1535-7163.MCT-12-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qian Y., Hua E., Bisht K., Woditschka S., Skordos K.W., Liewehr D.J., Steinberg S.M., Brogi E., Akram M.M., Killian J.K., Edelman D.C., Pineda M., Scurci S., Degenhardt Y.Y., Laquerre S., Lampkin T.A., Meltzer P.S., Camphausen K., Steeg P.S., Palmieri D. Inhibition of Polo-like kinase 1 prevents the growth of metastatic breast cancer cells in the brain. Clin Experimental Metastasis. 2011;28:899–908. doi: 10.1007/s10585-011-9421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Type IV collagen pattern of expression in the microenvironment of the 231-BR-HER2 brain micrometastases. A–E: Metastatic 231-BR-HER2 cells grew around blood vessels (CD31, red; C, D, and E) which are surrounded by collagen IV (green; B, D, and E). The thin elongated micrometastases are encapsulated in a collagen IV layer (large white arrows), whereas in the more round one, only residual collagen IV can be seen in the center (thin white arrow). F: The serial section was stained with human cytokeratin antibody (green) to confirm the presence of metastases. Scale bars: 50 μm (E); 100 μm (F).
Pazopanib induces a trend in GFAP-expressing astrocyte population decrease. Brains, from necropsies of athymic nude mice that received 231-BR-HER2 cells and either vehicle or 100 mg/kg pazopanib for 21 days, were fixed, sectioned, and stained. A: Percentage of area occupied by GFAP+ astrocytes. P = 0.0556. The bars represent the median. Data in A were determined by photographic analysis of GFAP staining. Each dot represents one mouse that is the average of up to nine groups of metastases. B–E: Two representative images of GFAP staining (green) for each treatment: vehicle (B and C), 100 mg/kg pazopanib (D and E). Scale bars: 200 μm (B–E).