Abstract
Benign prostate hyperplasia (BPH) is a major cause of lower urinary tract symptoms, with an increased volume of transitional zone and associated with increased stromal cells. It is known that androgen/androgen receptor (AR) signaling plays a key role in development of BPH, and that blockade of this signaling decreases BPH volume and can relieve lower urinary tract symptoms, but the mechanisms of androgen/AR signaling in BPH development remain unclear, and the effectiveness of current drugs for treating BPH is still limited. The detailed mechanisms of androgen/AR signaling need to be clarified, and new therapies are needed for better treatment of BPH patients. This review focuses on roles of AR in epithelial and stromal cells in BPH development. In epithelial cells, AR may contribute to BPH development via epithelial cell–stromal cell interaction with alterations of epithelial–mesenchymal transition, leading to proliferation of stromal cells. Data from several mouse models with selective knockout of AR in stromal smooth-muscle cells and/or fibroblasts indicate that the AR in stromal cells can also promote BPH development. In prostatic inflammation, AR roles in infiltrating macrophages and epithelial and stromal cells have been linked to BPH development, which has led to discovery of new therapeutic targets. For example, targeting AR with the novel AR degradation enhancer, ASC-J9 offers a potential therapeutic approach against BPH development.
Benign prostatic hyperplasia (BPH) is the most common male benign proliferative disease, and approximately 8 million patients are estimated to visit physicians with the diagnosis of primary or secondary BPH.1 The incidence of gross enlargement of the prostate gland has been reported as 40% in 70-year-old men, and microscopic foci of the prostate gland are present in up to 80% of these men.2 BPH patients often have lower urinary tract symptoms, and need to be treated with surgery or medication. Although transurethral resection of the prostate is the most common surgical treatment for BPH worldwide, the procedure can lead to complications (eg, bleeding, urethral stricture, incontinence) and may have limitations for people of advanced age.3–5 Although α-blockers are frequently prescribed for treatment of BPH and have a quick onset of action, within 3 to 5 days,6 these drugs alone fail to shrink BPH volume and are often insufficient to eliminate symptoms.7 In contrast, 5-α reductase inhibitors (5-ARIs), which suppress testosterone conversion into dihydrotestosterone (DHT), have greater efficacy in reducing BPH volume, and clinical data indicate that the combination of 5-ARIs with α-blockers leads to the best symptomatic response to date.8,9
The above clinical data suggest that androgen/androgen receptor (AR) signaling plays key roles in development of BPH and that targeting androgen/AR signaling could be a major therapeutic approach for BPH. Nevertheless, the detailed mechanisms of androgen/AR signaling, and especially the pathogenic roles of AR in BPH,10 are still unclear.
In this review, we focus on the roles of AR in promoting prostate stromal cell growth11 and prostate epithelial cell growth with increased epithelial–mesenchymal transition (EMT).12 We also discuss how AR regulates development of BPH through the inflammatory environment with macrophage infiltration, and address potential new therapeutic approaches, such as targeting AR with the newly identified AR degradation enhancer ASC-J9 and/or targeting specific inflammatory cytokines downstream of AR.
Clinical Evidence of Androgen/AR Roles in Development of BPH
Basic Evidence for Androgen/AR Roles Supporting Clinical Effects
The hormone dependency of development of BPH is well documented, and androgen concentration or AR activity in BPH patients has been a subject of study since 1895.13 One study in the 1980s found little difference in AR expression in prostate tissues isolated from either BPH patients or unaffected control subjects.14 However, the serum concentration of testosterone and DHT may change with age, with reduced serum testosterone in healthy old men, relative to that in healthy younger men. In contrast, DHT levels are elevated, and serum DHT in BPH patients is significantly higher than that in unaffected men at a similar age.15,16 Similar conclusions were also obtained recently in a large cross-sectional study that included 505 men aged 40 to 79 years (mean, 58 years); higher serum DHT levels and DHT/testosterone ratios were associated with larger prostate volume and higher prevalence of BPH.17
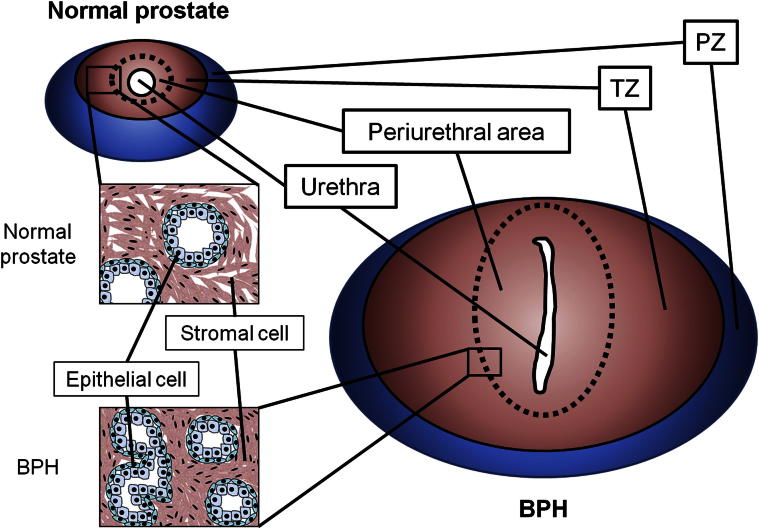
Tang et al18 found no difference in AR expression between the peripheral zone (PZ) and the transitional zone (TZ) in BPH. In other studies, although nucleic AR expression was detected in both epithelial and stromal cells of hyperplastic nodules, higher nuclear AR expression was detected in prostate epithelial cells than in stromal cells.19,20 Others found higher 5-α reductase activity in stromal cells than in epithelial cells, with AR distributed evenly between epithelial and stromal cells.21,22 Importantly, the primitive BPH nodules found in the periurethral area of the TZ had higher concentrations of androgens and higher nuclear AR expression than those in other prostate regions. These findings suggest that androgen/AR signaling may play important roles in promoting the proliferation of epithelial and stromal cells in the periurethral area of the TZ, thus leading to development of BPH with urinary obstruction (Figure 1).23
Figure 1.
Schematic of prostate structure. Most cases of prostate cancer occur in the peripheral zone (PZ), but BPH is caused by enlargement of the transitional zone (TZ), especially in the periurethral area. The urethra penetrates the periurethral area, sandwiched between left and right adenoma and flattened in BPH.
Clinical Effects of Antiandrogens and 5-ARIs on BPH
Castrated BPH patients showed a marked decrease of prostatic volume after 2 to 3 months, and this volume reduction correlated well with a relief of lower urinary tract symptoms.24,25 A double-blind, randomized trial for BPH patients with the antiandrogen flutamide (750 mg/day for 6 months) showed significantly decreased prostate volume by 35%, compared with controls, within 6 months.26 However, these androgen-deprivation therapies with surgical or medical castration or with antiandrogens are no longer used as a standard treatment because of adverse reactions27,28 and have been replaced by the 5-ARIs, which have better efficacy in suppressing development of BPH. A study of BPH patients treated for 1 year with finasteride, a 5-ARI for 5-α reductase type 2, showed a significant decrease in the size of the periurethral area, as well as total prostate gland size, suggesting that suppression of testosterone conversion into DHT could lead to a significant decrease in the size of the prostate.29 The efficacy and safety profiles of finasteride in BPH patients over a 12-month period also showed a 74% reduction in serum DHT levels, a 21% reduction in prostate volume, and a significant improvement in urinary obstructive symptoms.30,31 However, finasteride treatment led to a 55% decrease of DHT in epithelial cells of the TZ, with little effect on stromal cells,32 even though 5-α reductase type 2 is expressed in both stromal and epithelial cells in the prostate. In contrast, another 5-ARI, dutasteride, inhibiting both type 2 and type 1 of 5-α reductases (the latter expressed predominantly in epithelial cells33), showed a greater decrease in DHT (98.4 ± 1.2%) than did finasteride (70.8 ± 18.3%).34
Taken together, these clinical studies suggest that androgen/AR may contribute to the increase of prostate volume, and 5-ARIs with suppression of testosterone conversion into DHT are effective medicines for reducing BPH size.
Limitations of Current 5-ARIs Targeting Testosterone Conversion into DHT for Treatment of BPH
Although 5-ARIs have been shown to reduce the volume of BPH, these may have some limitations. The Combination of Avodart and Tamsulosin (CombAT) study, with 4844 BPH patients, showed that combined therapy with both dutasteride and the α-blocker tamsulosin was significantly superior to either monotherapy in reducing the relative risk of BPH progression and symptoms.35 Later analysis, however, found that combined therapy was better than dutasteride monotherapy in men with prostate volumes of ≥30 to <58 mL, but not in men with a prostate volume of <30 or ≥58 mL.36 The benefit of combination therapy is thus greatest only in patients with larger BPH volumes, and α-blockers alone may be sufficient to alleviate lower urinary tract symptoms in patients with smaller BPH volumes; however, transurethral resection of the prostate may be needed for patients with the largest BPH volumes (approximately 60 mL).6 Importantly, 5-ARIs have been reported to produce significant adverse sexual reactions, including decreased libido, erectile dysfunction, or ejaculation problems.37–42 In addition, 5-ARIs may not be able to suppress completely the intraprostatic DHT in BPH patients, and the remaining DHT may still be able to transactivate AR and lead to development of BPH.
AR Roles in Promoting Prostate Epithelial Cell Growth with Enhanced EMT in Development of BPH
Epithelial AR Influences Cell Growth
Bello et al,43 using the human prostate epithelial cell line RWPE-1, found that addition of the synthetic androgen mibolerone increased AR expression level and enhanced cell growth, and Silva et al44 found that DHT can promote human primary prostate epithelial cell growth. These in vitro cell-line data suggest that androgen/AR signaling may play positive roles in promoting prostate epithelial cell proliferation.
Wu et al45 generated conditional knockout AR mice that lack AR only in prostate epithelial cells (pes-ARKO) and found that the pes-ARKO mice developed larger but less differentiated prostate with increased cell death, compared with wild-type littermates. Further analyses of each individual cell type within the epithelium in pes-ARKO mice revealed that loss of epithelial AR increased the proportion of CK5+ basal epithelial cells but decreased the proportion of CK8+ luminal epithelial cells, suggesting that luminal epithelial AR may play survival roles and that basal epithelial AR may play suppressor roles in maintaining homeostasis of prostate epithelial cells.45 It would be interesting to learn whether AR in epithelial cells of human BPH has roles similar to those in the normal prostate.
Epithelial Cells Contribute to Development of BPH via Epithelial Cell–Stromal Cell Interaction
Clinical studies showing better efficacy of dutasteride than finasteride in suppressing BPH33,34 suggest that prostate epithelial cells may play positive roles in promoting development of BPH. In two studies from the 1990s, epithelial cell content was significantly greater in the TZ than in the PZ,46 and epithelial cells were only 9.0% of BPH cells.47 However, epithelial cells may still play important roles in enhancing stromal cell growth via epithelial cell–stromal cell interaction. In coculture of primary human BPH stromal fibroblasts and epithelial cells, cell growth of cocultured cells was significantly increased, compared to separate culture,48 suggesting that epithelial cells may have a supportive role in the growth of stromal cells in development of BPH. On the other hand, stromal androgen/AR signaling may be able to enhance the expression and/or release of growth factors that act on epithelial cells.49,50 Thus, prostate epithelial and stromal cells may each support proliferation of the other cell type through growth factors in a paracrine manner.
Epithelial Cells Contribute to Increased Stromal Cell Population via EMT and So Contribute to Development of BPH
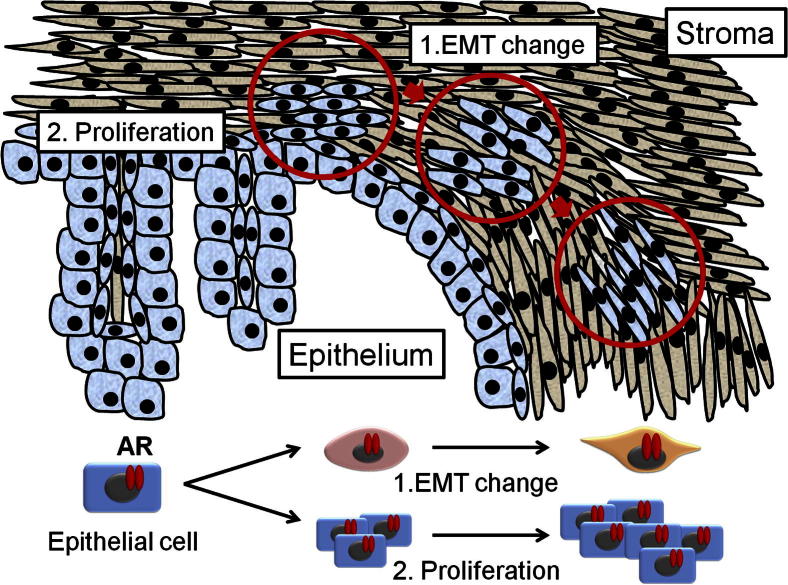
EMT is a physiological process in which epithelial cells acquire the motile characteristics of mesenchymal (stromal) cells.51 EMT is characterized by decrease of epithelial markers (eg, E-cadherin) and increase of mesenchymal and transcriptional factors (eg, N-cadherin, vimentin, and Snail).52 EMT is associated mainly with cancer progression, including migration, invasion, and metastasis. Cancer cells undergoing EMT may acquire invasive properties, entering the surrounding stromal cells and creating a favorable microenvironment for cancer progression and metastasis.53 Interestingly, Alonso-Magdalena et al12 found that EMT may also contribute to development of BPH. Although they found little evidence of proliferation in clinical human BPH stromal cells, they found that 0.7% of basal cells and 0.4% of luminal cells were positive for the proliferation markers Ki-67 and PCNA in the epithelium of some ducts.12 Importantly, they found that regions of the ductal epithelium had lost their polarization and expressed little E-cadherin.12 Others reported that Snail2/Slug is the important transcriptional factor for TGF-β1–induced EMT of prostate epithelial cells in BPH.54 These results suggest that EMT in epithelial cells may also promote development of BPH (Figure 2).
Figure 2.
Schematic of EMT and proliferation in development of BPH. Epithelial cells (both basal and luminal) undergo EMT, changing their shape over time to a more spindle-like form (arrows and circles), and proliferate.
AR Promotes Development of BPH by Influencing Infiltrating Immune Cell Migration toward Prostate Epithelial Cells
Inflammation with infiltrating macrophages influences epithelial cells and may contribute to development of BPH. A study investigating the association between immune inflammation and AR expression in human BPH specimens from prostatectomy showed that the total prostate volume was significantly higher in specimens with infiltrated inflammatory cells including B or T lymphocytes than in those without infiltrated inflammatory cells, suggesting that the immune inflammatory process may also contribute to development of BPH.55 A recent study by Lu et al56 also showed higher expression of the human macrophage marker CD68 in human BPH specimens, further confirming that infiltrated immune cells may be able to contribute to development of BPH.
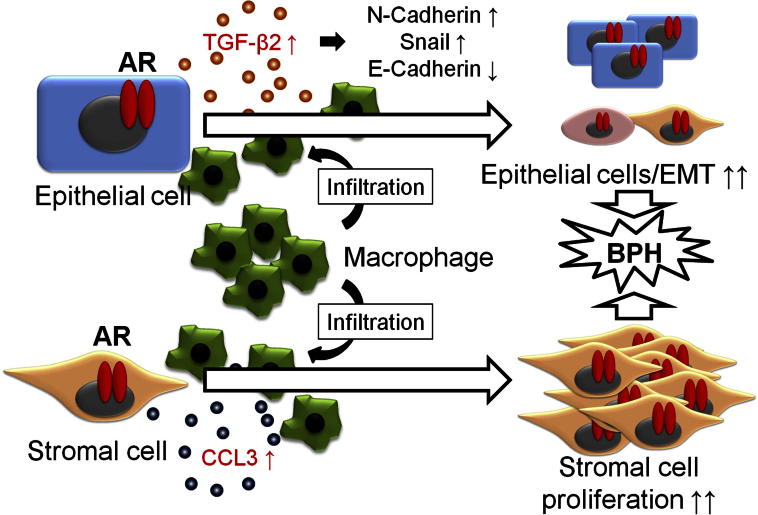
Using coculture of human BPH-1 epithelial cells with human monocyte/macrophage THP-1 cells, Lu et al56 found that BPH-1 cells have the capacity to recruit more THP-1 cells. They found that recruited THP-1 cells subsequently enhanced BPH-1 cell growth in a three-dimensional culture system, with increase of EMT in BPH-1 cells, and they demonstrated that increased TGF-β2 expression in BPH-1 cells and the addition of TGF-β2 neutralizing antibody suppressed THP-1 macrophages-mediated cell growth and EMT in BPH-1 cells. Importantly, they found that addition of AR in BPH-1 cells induced EMT gene expression in BPH-1 cells and promoted THP-1 macrophage migration during coculture with THP-1 cells. Addition of the AR degradation enhancer ASC-J9 also suppressed THP-1 macrophage-mediated cell growth in stably transfected AR BPH-1 cells.56 Similar data were also obtained when human BPH-1/THP-1 cells were replaced with mouse epithelial mPrE cells and mouse macrophage RAW264.7 cells in their coculture system (Figure 3).56
Figure 3.
AR of epithelial cells attracts macrophages and interaction between epithelial cells, and macrophage infiltration increases secretion of TGF-β2 from epithelial cells. TGF-β2 promotes EMT of epithelial cells and finally forms spheres in three-dimensional culture. AR of stromal cells attracts macrophages and interaction between stromal cells and infiltrated macrophages increases CCL3 secretion from both stromal cells and infiltrated macrophages. This interaction induces the proliferation of stromal cells.
Taken together, these results demonstrate that epithelial AR may be able to enhance epithelial cell growth and EMT induction by influencing recruitment of infiltrating immune cells, which could then enhance development of BPH.
AR Roles in Prostate Stromal Cell Growth That Promote Development of BPH
Stromal AR Contributes to Development of BPH
It is well known that prostate cancer occurs predominantly in the PZ, whereas BPH typically develops in the TZ.57 A study in the 1990s reported that BPH is comprised of 88.4% stromal cells and 9.0% epithelial cells,47 and it is therefore reasonable to think that stromal cells may play a major role in development of BPH. Jiang et al57 found differences between stromal cells in the TZ and those in the PZ with regard to AR expression, DHT response, and cytokine expressions, and they demonstrated that stromal cells in the PZ, but not the TZ, are more supportive for prostate cancer epithelial cell growth; however, little is known about whether stromal cells in the TZ also contribute to development of BPH.57 In other studies, DHT concentration and IGF-II activity were highest in the periurethral area of the TZ in BPH,58 and basic fibroblast growth factor (FGF) expression in stromal cells, but not in epithelial cells, was reduced in BPH patients after finasteride treatment.59 These results indicate that stromal cells in the TZ may play an important role in development of BPH via modulation of androgen/AR signaling and expression of growth factors and cytokines.
Yu et al60 established the stromal smooth-muscle selective AR knockout (SM-ARKO) mouse model and found decreased epithelial cell proliferation, with little change of apoptosis and differentiation. They also found decreased IGF-I expression in the prostates of SM-ARKO mice and primary PS-1 stromal cells with AR knockdown, suggesting that AR in stromal smooth-muscle cells may also contribute to development of BPH via regulation of IGF-I signaling.
Yu et al61 also established the stromal fibroblast selective AR knockout (FSP-ARKO) mouse model, and found that loss of AR in the stromal fibroblasts decreased proliferation of epithelial cells with increased apoptosis, which may involve the suppression of IGF-I and FGFs.
Lai et al62 established the double stromal AR knockout (dARKO) mouse model with selectively deleted AR in both stromal fibroblasts and smooth-muscle cells, and found that the size of the anterior prostate lobes was significantly reduced, compared with those from wild-type littermate controls. Decreased proliferation and increased apoptosis of the prostate epithelium in the dARKO mouse anterior prostate was also observed. Lai et al62 further confirmed these phenotypic changes with newly established immortalized prostate stromal cells (PrSC) from wild-type and dARKO mice. Mechanism dissection studies on the PrSCs from these mice again suggested that IGF-I may play key roles in mediating positive roles of stromal AR in normal prostate growth.
The above in vivo mouse models showing stromal AR can promote normal prostate growth were recently further extended to a prolactin transgenic (Pb-PRL tg) mouse BPH model. Mice with loss of AR in both stromal fibroblasts and smooth-muscle cells (dARKO/Pb-PRL tg) developed smaller prostates with a lower proliferative index and exhibited better urination function and normal bladder volume, compared with wild-type/Pb-PRL tg mice (Lai et al, unpublished data). Mechanism dissection suggested that prolactin-induced hyperplastic prostate growth involved the epithelial-stromal interaction through epithelial autonomous prolactin/prolactin receptor action to regulate granulocyte colony-stimulating factor and granulocyte/macrophage colony stimulating factor production in a paracrine manner to facilitate stromal cell growth by elevating STAT3 activity (Lai et al, unpublished data). The results indicated that stromal AR could modulate such epithelial–stromal interacting signals, resulting in promotion of hyperplastic prostate growth (Lai et al, unpublished data). Furthermore, therapeutic approaches targeting stromal AR with ASC-J9 reduced prostate size and suppressed stromal cell proliferation (Lai et al, unpublished data).
Taken together, results from various stromal ARKO mouse models suggest that stromal AR may play crucial roles in promoting stromal cell growth in both normal prostate and in development of BPH.
Stromal AR Promotes Development of BPH by Influencing Infiltrating Macrophage Migration toward Prostate Stromal Cells
The potential influence of inflammatory infiltrating cells on stromal cell growth during development of BPH has also been addressed.63,64 Wang et al65 found that the number of infiltrated macrophages increased in stroma of human BPH specimens, and that mouse stromal mPrSC cells had the capacity to recruit more infiltrating macrophage RAW264.7 cells in a coculture system. The consequent increase of macrophage infiltration toward stromal cells could promote the proliferation of stromal mPrSC cells.65
Mechanism dissection further revealed a remarkable increase of expression of the cytokine CCL3 in both mPrSC stromal cells and RAW264.7 macrophages in a coculture system, and addition of neutralizing CCL3 antibody resulted in a significant reduction of the migration of macrophage RAW264.7 cells toward stromal cells and a significant reduction of macrophage-enhanced stromal mPrSC cell proliferation during coculture.65
These in vitro coculture data were further confirmed using an in vivo prolactin-induced BPH mouse model in a study showing increased macrophage numbers and CCL3 expression, compared with those from wild-type littermate controls.65 Importantly, targeting stromal AR via deletion of the stromal fibromuscular AR in the prolactin-induced BPH model reduced the number of infiltrated macrophages and reduced CCL3 expression in prostate tissues. These findings confirm in vitro coculture data and support the key role of prostate stromal AR in controlling CCL3 expression, which could affect macrophage infiltration and prostate stromal cell growth during development of BPH.65 Moreover, human clinical immunohistochemical analysis also revealed higher expression of CCL3 in human BPH prostates, compared with normal prostates.65
Taken together, the various findings described above lead to the conclusion that stromal AR may promote development of BPH by enhancing the recruitment of infiltrating macrophages with increased CCL3 expression, thereby increasing stromal cell growth (Figure 3).65
Targeting AR with ASC-J9 as a New Therapeutic Approach to Treatment of BPH
The conclusion that AR in both stromal and epithelial cells may play key roles in promoting development of BPH suggests that targeting AR could provide a potential therapeutic approach for BPH. Alternatively, targeting AR-regulated downstream genes (eg, TGFB2 or CCL3) could provide a potential therapeutic approach. Indeed, Lu et al56 and Wang et al65 reported that treatment with ASC-J9 suppressed epithelial and stromal cell growth, respectively. Importantly, injection of ASC-J9 into prolactin-induced BPH mice also led to suppression of development of BPH. Early studies have demonstrated well the efficacy of ASC-J9 in treating several AR-related diseases, including prostate cancer,66–68 liver cancer,69,70 and bladder cancer,71 as well as promoting wound healing,72 with little change in serum testosterone concentration and less toxicity or adverse effects in sexual function or fertility.66–73 ASC-J9 thus has potential as a candidate drug targeting AR for treatment of BPH. Further in vivo or clinical studies of this agent for treatment of BPH may be expected.
Conclusions
Androgen/AR signaling plays key roles in enhancing cell growth in both stromal and epithelial cells, thus promoting development of BPH. Although BPH is comprised largely of stromal cells, epithelial cells may still play an important role in development of BPH via epithelial cell–stromal cell interaction or EMT. One such mechanism with positive roles of AR involves increased recruitment of infiltrating macrophages, with modulation of some cytokines. Targeting AR may therefore provide a reasonable therapeutic approach for treatment of BPH. ASC-J9, which offers improved efficacy in targeting AR and reduction in number or severity of adverse effects, has the potential to become a next-generation therapy for BPH patients.
Footnotes
Supported by NIH grant CA156700, the George H. Whipple Professorship Endowment, the Taiwan Department of Health Clinical Trial, and Research Center of Excellence grant DOH99-TD-B-111-004 (China Medical University, Taichung, Taiwan).
Disclosures: ASC-J9 was patented by the University of Rochester, the University of North Carolina, and AndroScience Corporation, and then was licensed to AndroScience. Both the University of Rochester and C.C. own equity in and receive royalties from AndroScience Corporation.
References
- 1.Wei J.T., Calhoun E., Jacobsen S.J. Urologic diseases in America project: benign prostatic hyperplasia. J Urol. 2005;173:1256–1261. doi: 10.1097/01.ju.0000155709.37840.fe. [DOI] [PubMed] [Google Scholar]
- 2.Ekman P. BPH epidemiology and risk factors. Prostate Suppl. 1989;2:23–31. doi: 10.1002/pros.2990150505. [DOI] [PubMed] [Google Scholar]
- 3.Ahyai S.A., Gilling P., Kaplan S.A., Kuntz R.M., Madersbacher S., Montorsi F., Speakman M.J., Stief C.G. Meta-analysis of functional outcomes and complications following transurethral procedures for lower urinary tract symptoms resulting from benign prostatic enlargement. Eur Urol. 2010;58:384–397. doi: 10.1016/j.eururo.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Smith R.D., Patel A. Transurethral resection of the prostate revisited and updated. Curr Opin Urol. 2011;21:36–41. doi: 10.1097/MOU.0b013e3283411455. [DOI] [PubMed] [Google Scholar]
- 5.Kavanagh L.E., Jack G.S., Lawrentschuk N. Prevention and management of TURP-related hemorrhage. Nat Rev Urol. 2011;8:504–514. doi: 10.1038/nrurol.2011.106. [DOI] [PubMed] [Google Scholar]
- 6.Kapoor A. Benign prostatic hyperplasia (BPH) management in the primary care setting. Can J Urol. 2012;19(Suppl 1):10–17. [PubMed] [Google Scholar]
- 7.Lepor H., Kazzazi A., Djavan B. alpha-Blockers for benign prostatic hyperplasia: the new era. Curr Opin Urol. 2012;22:7–15. doi: 10.1097/MOU.0b013e32834d9bfd. [DOI] [PubMed] [Google Scholar]
- 8.Emberton M., Fitzpatrick J.M., Rees J. Risk stratification for benign prostatic hyperplasia (BPH) treatment. BJU Int. 2011;107:876–880. doi: 10.1111/j.1464-410X.2010.10041.x. [DOI] [PubMed] [Google Scholar]
- 9.Barkin J. Benign prostatic hyperplasia and lower urinary tract symptoms: evidence and approaches for best case management. Can J Urol. 2011;18(Suppl):14–19. [PubMed] [Google Scholar]
- 10.van der Sluis T.M., Meuleman E.J., van Moorselaar R.J., Bui H.N., Blankenstein M.A., Heijboer A.C., Vis A.N. Intraprostatic testosterone and dihydrotestosterone. Part II: concentrations after androgen hormonal manipulation in men with benign prostatic hyperplasia and prostate cancer. BJU Int. 2012;109:183–188. doi: 10.1111/j.1464-410X.2011.10652.x. [DOI] [PubMed] [Google Scholar]
- 11.Bierhoff E., Vogel J., Benz M., Giefer T., Wernert N., Pfeifer U. Stromal nodules in benign prostatic hyperplasia. Eur Urol. 1996;29:345–354. doi: 10.1159/000473774. [DOI] [PubMed] [Google Scholar]
- 12.Alonso-Magdalena P., Brössner C., Reiner A., Cheng G., Sugiyama N., Warner M., Gustafsson J.A. A role for epithelial-mesenchymal transition in the etiology of benign prostatic hyperplasia. Proc Natl Acad Sci USA. 2009;106:2859–2863. doi: 10.1073/pnas.0812666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White JW. I. The results of double castration in hypertrophy of the prostate. Ann Surg. 1895;22:1–80. doi: 10.1097/00000658-189507000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elhilali M., Lehoux J.G., Carmel M., Madarnas P., Mongeau C., Beauchesne C., Tétreault L., Bénard B. Nuclear androgen receptors of human prostatic tissue–a quantitative histological study. Arch Androl. 1983;10:21–27. doi: 10.3109/01485018308990165. [DOI] [PubMed] [Google Scholar]
- 15.Horton R., Hsieh P., Barberia J., Pages L., Cosgrove M. Altered blood androgens in elderly men with prostate hyperplasia. J Clin Endocrinol Metab. 1975;41:793–796. doi: 10.1210/jcem-41-4-793. [DOI] [PubMed] [Google Scholar]
- 16.Hammond G.L., Kontturi M., Vihko P., Vihko R. Serum steroids in normal males and patients with prostatic diseases. Clin Endocrinol (Oxf) 1978;9:113–121. doi: 10.1111/j.1365-2265.1978.tb02189.x. [DOI] [PubMed] [Google Scholar]
- 17.Liao C.H., Li H.Y., Chung S.D., Chiang H.S., Yu H.J. Significant association between serum dihydrotestosterone level and prostate volume among Taiwanese men aged 40–79 years. Aging Male. 2012;15:28–33. doi: 10.3109/13685538.2010.550660. [DOI] [PubMed] [Google Scholar]
- 18.Tang J., Yang J.C., Zhang Y., Liu X., Zhang L., Wang Z., Li J., Luo Y., Xu J., Shi H. Does benign prostatic hyperplasia originate from the peripheral zone of the prostate? A preliminary study. BJU Int. 2007;100:1091–1096. doi: 10.1111/j.1464-410X.2007.07081.x. [DOI] [PubMed] [Google Scholar]
- 19.Kyprianou N., Davies P. Association states of androgen receptors in nuclei of human benign hypertrophic prostate. Prostate. 1986;8:363–380. doi: 10.1002/pros.2990080408. [DOI] [PubMed] [Google Scholar]
- 20.Peters C.A., Barrack E.R. Androgen receptor localization in the human prostate: demonstration of heterogeneity using a new method of steroid receptor autoradiography. J Steroid Biochem. 1987;27:533–541. doi: 10.1016/0022-4731(87)90351-7. [DOI] [PubMed] [Google Scholar]
- 21.Krieg M., Bartsch W., Thomsen M., Voigt K.D. Androgens and estrogens: their interaction with stroma and epithelium of human benign prostatic hyperplasia and normal prostate. J Steroid Biochem. 1983;19:155–161. [PubMed] [Google Scholar]
- 22.Tunn S., Hochstrate H., Grunwald I., Flüchter S.H., Krieg M. Effect of aging on kinetic parameters of 5 alpha-reductase in epithelium and stroma of normal and hyperplastic human prostate. J Clin Endocrinol Metab. 1988;67:979–985. doi: 10.1210/jcem-67-5-979. [DOI] [PubMed] [Google Scholar]
- 23.Monti S., Di Silverio F., Toscano V., Martini C., Lanzara S., Varasano P.A., Sciarra F. Androgen concentrations and their receptors in the periurethral region are higher than those of the subcapsular zone in benign prostatic hyperplasia (BPH) J Androl. 1998;19:428–433. [PubMed] [Google Scholar]
- 24.Schroeder F.H., Westerhof M., Bosch R.J., Kurth K.H. Benign prostatic hyperplasia treated by castration or the LH-RH analogue buserelin: a report on 6 cases. Eur Urol. 1986;12:318–321. doi: 10.1159/000472646. [DOI] [PubMed] [Google Scholar]
- 25.Bianchi S., Gravina G., Podestà A., Barletta D., Franchi F., Kicovic P., Luisi M. Treatment of complicated benign prostatic hyperplasia with LHRH-analogues in aged patients. Int J Androl. 1989;12:104–109. doi: 10.1111/j.1365-2605.1989.tb01292.x. [DOI] [PubMed] [Google Scholar]
- 26.Stone N.N., Clejan S.J. Response of prostate volume, prostate-specific antigen, and testosterone to flutamide in men with benign prostatic hyperplasia. J Androl. 1991;12:376–380. [PubMed] [Google Scholar]
- 27.Jønler M., Riehmann M., Bruskewitz R.C. Benign prostatic hyperplasia. Current pharmacological treatment. Drugs. 1994;47:66–81. doi: 10.2165/00003495-199447010-00005. [DOI] [PubMed] [Google Scholar]
- 28.Wysowski D.K., Freiman J.P., Tourtelot J.B., Horton M.L., 3rd Fatal and nonfatal hepatotoxicity associated with flutamide. Ann Intern Med. 1993;118:860–864. doi: 10.7326/0003-4819-118-11-199306010-00006. [DOI] [PubMed] [Google Scholar]
- 29.Tempany C.M., Partin A.W., Zerhouni E.A., Zinreich S.J., Walsh P.C. The influence of finasteride on the volume of the peripheral and periurethral zones of the prostate in men with benign prostatic hyperplasia. Prostate. 1993;22:39–42. doi: 10.1002/pros.2990220106. [DOI] [PubMed] [Google Scholar]
- 30.Finasteride (MK-906) in the treatment of benign prostatic hyperplasia. The Finasteride Study Group. Prostate. 1993;22:291–299. doi: 10.1002/pros.2990220403. [DOI] [PubMed] [Google Scholar]
- 31.Grino P., Stoner E. Finasteride for the treatment and control of benign prostatic hyperplasia: summary of phase III controlled studies. The Finasteride Study Group. Eur Urol. 1994;25(Suppl 1):24–28. doi: 10.1159/000475328. [DOI] [PubMed] [Google Scholar]
- 32.Marks L.S., Partin A.W., Gormley G.J., Dorey F.J., Shery E.D., Garris J.B., Subong E.N., Stoner E., deKernion J.B. Prostate tissue composition and response to finasteride in men with symptomatic benign prostatic hyperplasia. J Urol. 1997;157:2171–2178. [PubMed] [Google Scholar]
- 33.Shirakawa T., Okada H., Acharya B., Zhang Z., Hinata N., Wada Y., Uji T., Kamidono S., Gotoh A. Messenger RNA levels and enzyme activities of 5 alpha-reductase types 1 and 2 in human benign prostatic hyperplasia (BPH) tissue. Prostate. 2004;58:33–40. doi: 10.1002/pros.10313. [DOI] [PubMed] [Google Scholar]
- 34.Clark R.V., Hermann D.J., Cunningham G.R., Wilson T.H., Morrill B.B., Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab. 2004;89:2179–2184. doi: 10.1210/jc.2003-030330. [DOI] [PubMed] [Google Scholar]
- 35.Roehrborn C.G., Siami P., Barkin J., Damião R., Major-Walker K., Nandy I., Morrill B.B., Gagnier R.P., Montorsi F., CombAT Study Group The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol. 2010;57:123–131. doi: 10.1016/j.eururo.2009.09.035. [Erratum appeared in Eur Urol 2010, 58:801] [DOI] [PubMed] [Google Scholar]
- 36.Montorsi F., Roehrborn C., Garcia-Penit J., Borre M., Roeleveld T.A., Alimi J.C., Gagnier P., Wilson T.H. The effects of dutasteride or tamsulosin alone and in combination on storage and voiding symptoms in men with lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia (BPH): 4-year data from the Combination of Avodart and Tamsulosin (CombAT) study. BJU Int. 2011;107:1426–1431. doi: 10.1111/j.1464-410X.2011.10129.x. [DOI] [PubMed] [Google Scholar]
- 37.Traish A.M., Hassani J., Guay A.T., Zitzmann M., Hansen M.L. Adverse side effects of 5alpha-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and depression in a subset of patients. J Sex Med. 2011;8:872–884. doi: 10.1111/j.1743-6109.2010.02157.x. [DOI] [PubMed] [Google Scholar]
- 38.Kirby R.S., Roehrborn C., Boyle P., Bartsch G., Jardin A., Cary M.M., Sweeney M., Grossman E.B., Prospective European Doxazosin and Combination Therapy Study Investigators Efficacy and tolerability of doxazosin and finasteride, alone or in combination, in treatment of symptomatic benign prostatic hyperplasia: the Prospective European Doxazosin and Combination Therapy (PREDICT) trial. Urology. 2003;61:119–126. doi: 10.1016/s0090-4295(02)02114-3. [DOI] [PubMed] [Google Scholar]
- 39.Byrnes C.A., Morton A.S., Liss C.L., Lippert M.C., Gillenwater J.Y. Efficacy, tolerability, and effect on health-related quality of life of finasteride versus placebo in men with symptomatic benign prostatic hyperplasia: a community based study. CUSP Investigators. Community based study of Proscar. Clin Ther. 1995;17:956–969. doi: 10.1016/0149-2918(95)80073-5. [DOI] [PubMed] [Google Scholar]
- 40.Lepor H., Williford W.O., Barry M.J., Brawer M.K., Dixon C.M., Gormley G., Haakenson C., Machi M., Narayan P., Padley R.J. The efficacy of terazosin, finasteride, or both in benign prostatic hyperplasia. Veterans Affairs Cooperative Studies Benign Prostatic Hyperplasia Study Group. N Engl J Med. 1996;335:533–539. doi: 10.1056/NEJM199608223350801. [DOI] [PubMed] [Google Scholar]
- 41.Marberger M.J. Long-term effects of finasteride in patients with benign prostatic hyperplasia: a double-blind, placebo-controlled, multicenter study. PROWESS Study Group. Urology. 1998;51:677–686. doi: 10.1016/s0090-4295(98)00094-6. [DOI] [PubMed] [Google Scholar]
- 42.Roehrborn C.G., Siami P., Barkin J., Damião R., Major-Walker K., Morrill B., Montorsi F., CombAT Study Group The effects of dutasteride, tamsulosin and combination therapy on lower urinary tract symptoms in men with benign prostatic hyperplasia and prostatic enlargement: 2-year results from the CombAT study. J Urol. 2008;179:616–621. doi: 10.1016/j.juro.2007.09.084. [Erratum appeared in J Urol 2008, 180:1191] discussion 621. [DOI] [PubMed] [Google Scholar]
- 43.Bello D., Webber M.M., Kleinman H.K., Wartinger D.D., Rhim J.S. Androgen responsive adult human prostatic epithelial cell lines immortalized by human papillomavirus 18. Carcinogenesis. 1997;18:1215–1223. doi: 10.1093/carcin/18.6.1215. [DOI] [PubMed] [Google Scholar]
- 44.Silva I.S., Morsch D.M., Urnauer L., Spritzer P.M. Androgen-induced cell growth and c-myc expression in human non-transformed epithelial prostatic cells in primary culture. Endocr Res. 2001;27:153–169. doi: 10.1081/erc-100107177. [DOI] [PubMed] [Google Scholar]
- 45.Wu C.T., Altuwaijri S., Ricke W.A., Huang S.P., Yeh S., Zhang C., Niu Y., Tsai M.Y., Chang C. Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc Natl Acad Sci USA. 2007;104:12679–12684. doi: 10.1073/pnas.0704940104. [Erratum appeared in Proc Natl Acad Sci U S A 2007, 104:17240] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feneley M.R., Puddefoot J.R., Xia S., Sowter C., Slavin G., Kirby R.S., Vinson G.P. Zonal biochemical and morphological characteristics in BPH. Br J Urol. 1995;75:608–613. doi: 10.1111/j.1464-410x.1995.tb07418.x. [DOI] [PubMed] [Google Scholar]
- 47.Svindland A., Eri L.M., Tveter K.J. Morphometry of benign prostatic hyperplasia during androgen suppressive therapy. Relationships among epithelial content, PSA density, and clinical outcome. Scand J Urol Nephrol Suppl. 1996;179:113–117. [PubMed] [Google Scholar]
- 48.Bayne C.W., Donnelly F., Chapman K., Bollina P., Buck C., Habib F. A novel coculture model for benign prostatic hyperplasia expressing both isoforms of 5 alpha-reductase. J Clin Endocrinol Metab. 1998;83:206–213. doi: 10.1210/jcem.83.1.4486. [Erratum appeared in J Clin Endocrinol Metab 1998, 83:910] [DOI] [PubMed] [Google Scholar]
- 49.Peehl D.M., Rubin J.S. Keratinocyte growth factor: an androgen-regulated mediator of stromal-epithelial interactions in the prostate. World J Urol. 1995;13:312–317. doi: 10.1007/BF00185975. [DOI] [PubMed] [Google Scholar]
- 50.Nakano K., Fukabori Y., Itoh N., Lu W., Kan M., McKeehan W.L., Yamanaka H. Androgen-stimulated human prostate epithelial growth mediated by stromal-derived fibroblast growth factor-10. Endocr J. 1999;46:405–413. doi: 10.1507/endocrj.46.405. [DOI] [PubMed] [Google Scholar]
- 51.Nisticò P., Bissell M.J., Radisky D.C. Epithelial-mesenchymal transition: general principles and pathological relevance with special emphasis on the role of matrix metalloproteinases. Cold Spring Harb Perspect Biol. 2012;4(2) doi: 10.1101/cshperspect.a011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu K., Bonavida B. The activated NF-kappaB-Snail-RKIP circuitry in cancer regulates both the metastatic cascade and resistance to apoptosis by cytotoxic drugs. Crit Rev Immunol. 2009;29:241–254. doi: 10.1615/critrevimmunol.v29.i3.40. [DOI] [PubMed] [Google Scholar]
- 53.Iwatsuki M., Mimori K., Yokobori T., Ishi H., Beppu T., Nakamori S., Baba H., Mori M. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 2010;101:293–299. doi: 10.1111/j.1349-7006.2009.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slabáková E., Pernicová Z., Slavíčková E., Staršíchová A., Kozubík A., Souček K. TGF-beta1-induced EMT of non-transformed prostate hyperplasia cells is characterized by early induction of SNAI2/Slug. Prostate. 2011;71:1332–1343. doi: 10.1002/pros.21350. [DOI] [PubMed] [Google Scholar]
- 55.Wu Z.L., Yuan Y., Geng H., Xia S.J. Influence of immune inflammation on androgen receptor expression in benign prostatic hyperplasia tissue. Asian J Androl. 2012;14:316–319. doi: 10.1038/aja.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu T., Lin W.J., Izumi K., Wang X., Xu D., Fang L.Y., Li L., Jiang Q., Jin J., Chang C. Targeting androgen receptor to suppress macrophage-induced EMT and benign prostatic hyperplasia (BPH) development. Mol Endocrinol. 2012;26:1707–1715. doi: 10.1210/me.2012-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang Q., Han B.M., Zhao F.J., Hong Y., Xia S.J. The differential effects of prostate stromal cells derived from different zones on prostate cancer epithelial cells under the action of sex hormones. Asian J Androl. 2011;13:798–805. doi: 10.1038/aja.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Monti S., Di Silverio F., Iraci R., Martini C., Lanzara S., Falasca P., Poggi M., Stigliano A., Sciarra F., Toscano V. Regional variations of insulin-like growth factor I (IGF-I), IGF-II, and receptor type I in benign prostatic hyperplasia tissue and their correlation with intraprostatic androgens. J Clin Endocrinol Metab. 2001;86:1700–1706. doi: 10.1210/jcem.86.4.7413. [DOI] [PubMed] [Google Scholar]
- 59.Sáez C., González-Baena A.C., Japón M.A., Giráldez J., Segura D.I., Rodríguez-Vallejo J.M., González-Esteban J., Miranda G., Torrubia F. Expression of basic fibroblast growth factor and its receptors FGFR1 and FGFR2 in human benign prostatic hyperplasia treated with finasteride. Prostate. 1999;40:83–88. doi: 10.1002/(sici)1097-0045(19990701)40:2<83::aid-pros3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 60.Yu S., Zhang C., Lin C.C., Niu Y., Lai K.P., Chang H.C., Yeh S.D., Chang C., Yeh S. Altered prostate epithelial development and IGF-1 signal in mice lacking the androgen receptor in stromal smooth muscle cells. Prostate. 2011;71:517–524. doi: 10.1002/pros.21264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu S., Yeh C.R., Niu Y., Chang H.C., Tsai Y.C., Moses H.L., Shyr C.R., Chang C., Yeh S. Altered prostate epithelial development in mice lacking the androgen receptor in stromal fibroblasts. Prostate. 2012;72:437–449. doi: 10.1002/pros.21445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lai K.P., Yamashita S., Vitkus S., Shyr C.R., Yeh S., Chang C. Suppressed prostate epithelial development with impaired branching morphogenesis in mice lacking stromal fibromuscular androgen receptor. Mol Endocrinol. 2012;26:52–66. doi: 10.1210/me.2011-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fibbi B., Penna G., Morelli A., Adorini L., Maggi M. Chronic inflammation in the pathogenesis of benign prostatic hyperplasia. Int J Androl. 2010;33:475–488. doi: 10.1111/j.1365-2605.2009.00972.x. [DOI] [PubMed] [Google Scholar]
- 64.Begley L.A., Kasina S., MacDonald J., Macoska J.A. The inflammatory microenvironment of the aging prostate facilitates cellular proliferation and hypertrophy. Cytokine. 2008;43:194–199. doi: 10.1016/j.cyto.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang X., Lin W.J., Izumi K., Jiang Q., Lai K.P., Xu D., Fang L.Y., Lu T., Li L., Xia S., Chang C. Increased infiltrated macrophages in benign prostatic hyperplasia (BPH): role of stromal androgen receptor in macrophage-induced prostate stromal cell proliferation. J Biol Chem. 2012;287:18376–18385. doi: 10.1074/jbc.M112.355164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamashita S., Lai K.P., Chuang K.L., Xu D., Miyamoto H., Tochigi T., Pang S.T., Li L., Arai Y., Kung H.J., Yeh S., Chang C. ASC-J9 suppresses castration-resistant prostate cancer growth through degradation of full-length and splice variant androgen receptors. Neoplasia. 2012;14:74–83. doi: 10.1593/neo.111436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lai K.P., Yamashita S., Huang C.K., Yeh S., Chang C. Loss of stromal androgen receptor leads to suppressed prostate tumourigenesis via modulation of pro-inflammatory cytokines/chemokines. EMBO Mol Med. 2012;4:791–807. doi: 10.1002/emmm.201101140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee S.O., Ma Z., Yeh C.R., Luo J., Lin T.H., Lai K.P., Yamashita S., Liang L., Tian J., Li L., Jiang Q., Huang C.K., Niu Y., Yeh S., Chang C. New therapy targeting differential androgen receptor signaling in prostate cancer stem/progenitor vs. non-stem/progenitor cells. J Mol Cell Biol. 2013;5:14–26. doi: 10.1093/jmcb/mjs042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu M.H., Ma W.L., Hsu C.L., Chen Y.L., Ou J.H., Ryan C.K., Hung Y.C., Yeh S., Chang C. Androgen receptor promotes hepatitis B virus-induced hepatocarcinogenesis through modulation of hepatitis B virus RNA transcription. Sci Transl Med. 2010;2:32ra35. doi: 10.1126/scitranslmed.3001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma W.L., Hsu C.L., Wu M.H., Wu C.T., Wu C.C., Lai J.J., Jou Y.S., Chen C.W., Yeh S., Chang C. Androgen receptor is a new potential therapeutic target for the treatment of hepatocellular carcinoma. Gastroenterology. 2008;135:947–955. doi: 10.1053/j.gastro.2008.05.046. 955.e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miyamoto H., Yang Z., Chen Y.T., Ishiguro H., Uemura H., Kubota Y., Nagashima Y., Chang Y.J., Hu Y.C., Tsai M.Y., Yeh S., Messing E.M., Chang C. Promotion of bladder cancer development and progression by androgen receptor signals. J Natl Cancer Inst. 2007;99:558–568. doi: 10.1093/jnci/djk113. [DOI] [PubMed] [Google Scholar]
- 72.Lai J.J., Lai K.P., Chuang K.H., Chang P., Yu I.C., Lin W.J., Chang C. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-alpha expression. J Clin Invest. 2009;119:3739–3751. doi: 10.1172/JCI39335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang Z., Chang Y.J., Yu I.C., Yeh S., Wu C.C., Miyamoto H., Merry D.E., Sobue G., Chen L.M., Chang S.S., Chang C. ASC-J9 ameliorates spinal and bulbar muscular atrophy phenotype via degradation of androgen receptor. Nat Med. 2007;13:348–353. doi: 10.1038/nm1547. [DOI] [PubMed] [Google Scholar]