Abstract
We investigated TNF-α and IL-1β regulation of ADAMTS-4 expression in nucleus pulposus (NP) cells and its role in aggrecan degradation. Real-time quantitative RT-PCR, Western blotting, and transient transfections with rat NP cells and lentiviral silencing with human NP cells were performed to determine the roles of MAPK and NF-κB in cytokine-mediated ADAMTS-4 expression and function. ADAMTS4 expression and promoter activity increased in NP cells after TNF-α and IL-1β treatment. Treatment of cells with MAPK and NF-κB inhibitors abolished the inductive effect of the cytokines on ADAMTS4 mRNA and protein expression. Although ERK1, p38α, p38β2, and p38γ were involved in induction, ERK2 and p38δ played no role in TNF-α–dependent promoter activity. The inductive effect of p65 on ADAMTS4 promoter was confirmed through gain and loss-of-function studies. Cotransfection of p50 completely blocked p65-mediated induction. Lentiviral transduction with shRNA plasmids shp65, shp52, shIKK-α, and shIKK-β significantly decreased TNF-α–dependent increase in ADAMTS-4 and -5 levels and aggrecan degradation. Silencing of either ADAMTS-4 or -5 resulted in reduction in TNF-α–dependent aggrecan degradation in NP cells. By controlling activation of MAPK and NF-κB signaling, TNF-α and IL-1β modulate expression of ADAMTS-4 in NP cells. To our knowledge, this is the first study to show nonredundant contribution of both ADAMTS-4 and ADAMTS-5 to aggrecan degradation in human NP cells in vitro.
The intervertebral disk is a unique tissue that that permits rotation, as well as flexion and extension of the spine. It consists of a gel-like nucleus pulposus (NP) surrounded circumferentially by a fibrocartilagenous annulus fibrosus. Cells of the NP are derived from the notochord,1 an embryonic tissue with limited blood supply. In common with chondrocytes, NP cells secrete a complex extracellular matrix that contains fibrillar collagens and the proteoglycan aggrecan. Assembly of these macromolecules provides a robust hydrodynamic system that accommodates applied biomechanical forces to the spine.2–4
Intervertebral disk degeneration is characterized by increased expression of catabolic enzymes, decreased proteoglycan synthesis, and an overall shift toward synthesis of a fibrotic matrix. When this occurs, the water-binding capacity of the tissue is compromised, resulting in a failure to resist compressive forces and a reduction in disk height.5,6 Although a great deal is known about importance of proteoglycan secretion and function, the molecular mechanisms controlling aggrecan turnover in cells of the normal and the degenerated disk are not well understood. It has been reported that during disk degeneration and herniation, in addition to infiltrating immune cells, resident NP and annulus fibrosus cells produce high levels of the cytokines TNF-α and IL-1β.7,8 These cytokines stimulate production of NGF, BDNF, and VEGF, molecules associated with nerve ingrowth and angiogenesis by NP cells.9 Moreover, both cytokines up-regulate expression by NP cells of catabolic matrix metalloproteinases (MMPs)3 and two major aggrecanases, A disintegrin and metalloproteinase with thrombospondin motifs 4 (ADAMTS-4) and 5 (ADAMTS-5).7,10–12 Among several members of the ADAMTS family that cleave aggrecan in vitro, ADAMTS-4 (aggrecanase-1) and ADAMTS-5 (aggrecanase-2) are the most likely to play a role in aggrecan degradation and subsequent disk degeneration as in the pathogenesis of osteoarthritis.13,14 ADAMTS-4 and -5 produce fragments of aggrecan usually found in synovial fluid and cartilage by cleaving the protein following Glu373, Glu1545, Glu1714, Glu1819, and Glu1919.13,15,16
Unlike cartilage, in the NP both ADAMTS-4 and ADAMTS-5 expression is elevated in human degenerative disk disease.17–19 Surprisingly, despite the importance of these aggrecanases in the pathogenesis of osteoarthritis and disk disease, only a few studies have investigated regulation of ADAMTS transcription in NP cells,10,11,17,18 and none have used promoter analysis. A clue to the mechanism lies in the findings that, in NP cells, NF-κB may contribute to TNF-α regulation of ADAMTS-4 and ADAMTS-5 expression,11 and that TNF-α and IL-1β also modulate ADAMTS-5 enzymatic activity through syndecan-4.19 These observations beg the question of how TNF-α and IL-1β control the expression of ADAMTS-4 and-5 and what is their relative contribution in NP cells in terms of aggrecan degradation. Although the present study addressed both ADAMTS-4 and -5, the mechanistic aspects of transcriptional control were studied using a 3.5-kb human ADAMTS4 promoter fragment. Here, we show for the first time that TNF-α and IL-1β control ADAMTS4 transcription in MAPK- and NF-κB–dependent fashion. Importantly, our results show that ADAMTS-4 and ADAMTS-5 are nonredundant and that both play a role in the cytokine-dependent degradation of aggrecan in human NP cells. A therapeutic strategy could conceivably target these enzymes for the structural preservation of the intervertebral disk.
Materials and Methods
Reagents and Plasmids
The 3.5-kb (−3109 to +406 bp) human ADAMTS4 promoter in pβ-gal-Basic vector was a kind gift from Dr. K. Thirunavukkarasu (Lilly Research Labs, Indianapolis, IN).20 The insert was recloned in basic pGL3 using XhoI and HindIII digestion. pCMX-IκBM (catalog no. 12330), and RelA/p65 (catalog no. 20012), p50 (catalog no. 20018) developed by Dr. Inder Verma and psPAX2 (catalog no. 12260) and pMD2G (catalog no. 12259) developed by Dr. Didier Trono were obtained from the Addgene repository (Cambridge, MA). Plasmids DN-p38α, DN-p38β2, DN-p38γ, and DN-p38δ were kindly provided by Jiahui Han (Scripps Research Institute, La Jolla, CA); plasmids ERK-1K71R and ERK-2K52R, by Melanie Cobb (University of Texas Southwestern Medical Center, Dallas, TX); plasmids pLKO.1shADAMTS-4 and pLKO.1shADAMTS-5, by Dr. Mike Baker (University of Sheffield, Sheffield, UK); and plasmids shp65, shp52, shIKK-α, and shIKK-β in lentiviral FSVsi vector that coexpresses yellow fluorescent protein (YFP), by Dr. Andree Yeremian (University of Lleida, Lleida, Spain). The vector pRL-TK (Promega, Madison, WI) containing the Renilla luciferase gene was used as an internal transfection control.
The amount of transfected plasmid, the pretransfection period after seeding, and the post-transfection period before harvesting were optimized for NP cells with pSV β-galactosidase plasmid (Promega).21 Wild-type and p65 null cells were a kind gift from Dr. Denis Guttridge (Ohio State University, Columbus, OH). Antibody that recognizes ADAMTS-dependent aggrecan degradation in interglobular domain (anti-NITEGE) was a gift from Dr. Peter Roughley (Shriners Hospital for Children, Montreal, QC, Canada). Antibodies against ADAMTS-4, -5, and ADAMTS-generated aggrecan neoepitope ARGSVIL were obtained from Abcam (Cambridge, MA). P-p38, p38, p52, P-p65, p65, IKK-α, IKK-β, P-ERK, ERK, P-JNK, and JNK antibodies were obtained from Cell Signaling Technology (Danvers, MA). β-Tubulin was obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA) and GAPDH from Novus Biologicals (Littleton, CO). TNF-α and IL-1β were purchased from PeproTech (Rocky Hill, NJ).
Isolation of NP Cells and Cytokine Treatments
Rat and human NP cells were isolated using a method reported by Risbud et al.21 NP tissue from lumbar disks of three or four rats was pooled for each isolation. NP cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) and 10% fetal bovine serum (FBS) supplemented with antibiotics and used within the first three passages. To investigate the effect of cytokines, cells were treated with 5 to 20 ng/mL IL-1β and 25 to 100 ng/mL TNF-α for 24 hours in serum-free medium.
Human Tissue Collection and Grading
Both lumbar and cervical disk tissues were collected as surgical waste from individuals undergoing elective spinal surgical procedures. Consistent with Thomas Jefferson University’s Institutional Review Board guidelines, informed consent for sample collection was obtained from each patient. Assessment of the disease state was performed using Pfirrmann grading.22 This scheme uses T2-weighted magnetic resonance imaging with image analysis by three independent observers. Patient age, spinal level, and grade of NP tissues used for cell isolation are listed in Supplemental Table S1.
RT-qPCR Analysis
After treatment, total RNA was extracted from NP cells (5 × 105 cells per plate) using RNeasy mini spin columns (Qiagen, Valencia, CA). Before elution from the column, RNA was treated with RNase-free DNase I. Two micrograms of total DNA-free RNA was used to synthesize cDNA, using a SuperScipt III cDNA synthesis kit (Life Technologies–Invitrogen, Carlsbad, CA). Reactions were set up in triplicate in 96-well plates using 1 μL cDNA with Fast SYBR Green PCR Master Mix (Life Technologies–Applied Biosystems, Foster City, CA) to which gene-specific forward and reverse PCR primers were added. Each set of samples included a template-free control. PCR reactions were performed in a StepOnePlus real-time PCR system (Life Technologies–Applied Biosystems) according to the manufacturer’s instructions. Expression of the gene of interest was first normalized to the housekeeping gene hypoxanthine phosphoribosyltransferase 1 (Hprt1), with data expressed as relative to the corresponding control group. All of the primers were synthesized by Integrated DNA Technologies (Coralville, IA) (Table 1).
Table 1.
Sequences of Primers Used in RT-qPCR
| Target | Primer sequence |
|---|---|
| HPRT1 | |
| Forward | 5′-AGTCCCAGCGTCGTGATTAGTGAT-3′ |
| Reverse | 5′-GAGCAAGTCTTTCAGTCCTGTCCA-3′ |
| ADAMTS4 | |
| Forward | 5′-ACAATGGCTATGGACACTGCCTCT-3′ |
| Reverse | 5′-TGTGGACAATGGCTTGAGTCAGGA-3′ |
| ADAMTS5 | |
| Forward | 5′-GTCCAAATGCACTTCAGCCACGAT-3′ |
| Reverse | 5′-AATGTCAAGTTGCACTGCTGGGTG-3′ |
Protein Extraction and Western Blotting
NP cells (1 × 106 cells per plate) were placed on ice immediately after treatment and washed with ice-cold Hanks’ balanced salt solution. All of the wash buffers and the final resuspension buffer included 1× protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN), 5 mmol/L NaF, and 200 μmol/L Na3VO4. Conditioned medium was collected and concentrated using centrifugal filter units (EMD Millipore, Billerica, MA). For detecting aggrecan neoepitopes, protein lysates were pretreated with 0.1 U/mL chondroitinase ABC (Sigma-Aldrich, St. Louis, MO) for 1 to 6 hours at 37°C. Proteins were resolved on 8% to 12% SDS-PAGE gels and were transferred by electroblotting to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA). The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline–Tween (50 mmol/L Tris, pH 7.6, 150 mmol/L NaCl, 0.1% tween 20) and incubated overnight at 4°C in 3% nonfat dry milk in Tris-buffered saline–Tween with the specific antibodies all at a dilution of 1:1000. Immunolabeling was detected using Amersham ECL reagent (GE Healthcare, Little Chalfont, UK).
Transfections and Dual-Luciferase Reporter Assay
NP cells were transferred to 48-well plates at a density of 2 × 104 cells per well, at 1 day before transfection. To investigate the effect of NF-κB on ADAMTS4 promoter activity, cells were cotransfected with 50 to 200 ng of p65, p50, or both p65 and p50 with or without appropriate backbone vector and 175 ng ADAMTS4 reporter and 175 ng pRL-TK plasmid. To investigate the effects of p38 and ERK signaling, cells were transfected with 50 to 150 ng of dominant-negative p38 (DN-p38) or DN-ERK plasmids. In some wells, cells were treated with the inhibitors for NF-κB (10 μmol/L sm-7368), p38 (10 μmol/L SB203580), ERK (10 μmol/L PD98059), or JNK (10 μmol/L SP600125) (all Calbiochem, from EMD Millipore). In some experiments, cells were transfected with 250 ng of ADAMTS4 reporter plasmids with 250 ng pRL-TK plasmid. Lipofectamine 2000 (Life Technologies–Invitrogen) was used as a transfection reagent. For each transfection, plasmids were premixed with the transfection reagent. At 48 hours after transfection, the cells were harvested and a dual-luciferase reporter assay system (Promega) was used for sequential measurements of firefly and Renilla luciferase activities. Quantification of luciferase activities and calculation of relative ratios were performed using a luminometer (TD-20/20; Turner Designs, Sunnyvale, CA). At least three independent transfections were performed, and all analyses were performed in triplicate.
Lentiviral Particle Production and Viral Transduction
HEK 293T human embryonic kidney cells (1.3 × 106 cells per plate) were seeded in 10-cm plates in DMEM with 10% heat-inactivated FBS, at 2 days before transfection. Cells were transfected with 2.5 μg of shRNA control sequence or gene-specific shRNA plasmids, along with 1.875 μg psPAX2 (a packaging vector) and 0.625 μg pMD2.G (an envelope vector). After 16 hours, the transfection medium was removed and replaced with DMEM with 5% heat-inactivated FBS and penicillin–streptomycin. Lentiviral particles were harvested at 48 and 60 hours after transfection. Human NP cells (1 × 106 cells per plate) were plated in DMEM with 5% heat-inactivated FBS, at 1 day before transduction. Cells in 10-cm plates were transduced with 5 mL of medium containing viral particles, along with 6 μg/mL polybrene. After 24 hours, the medium was removed and replaced with DMEM with 5% heat-inactivated FBS. Cells were harvested for protein extraction at 5 days after viral transduction.
Statistical Analysis
All experiments were repeated independently three times. Data are presented as means ± SEM. Differences between groups were analyzed by Student’s t-test and analysis of variance. P < 0.05 was considered significant.
Results
Expression of ADAMTS-4 Is Regulated by TNF-α and IL-1β in NP Cells
Expression of ADAMTS-4 in mature rat tissues was studied using real-time PCR and Western blot analysis. Compared with Hprt1, the basal expression of ADAMTS4 mRNA in healthy NP and in annulus fibrosus tissue is very low (Figure 1, A and B). NP tissue shows weak ADAMTS-4 bands at approximately 58 and 73 kDa (Figure 1B). To explore the premise that cytokines concerned with disk degeneration regulate ADAMTS-4 expression, rat NP cells were treated with TNF-α and IL-1β, and expression of ADAMTS-4 was analyzed. Treatment with both TNF-α and IL-1β resulted in dose-dependent increase in ADAMTS4 mRNA levels (Figure 1, C and D). In addition, we measured the level of ADAMTS-4 protein in conditioned medium of treated NP cells by Western blot analysis. Cytokine treatment significantly increased ADAMTS-4 protein expression in both rat NP cells (Figure 1, E and F) and human NP cells (Supplemental Figure S1A). To investigate whether the regulation of expression is at the transcriptional level, we measured the activity of a 3.5-kb ADAMTS4 promoter (Figure 1G) after cytokine treatment. Both cytokines significantly increased the promoter activity (Figure 1H).
Figure 1.
Expression and cytokine dependency of ADAMTS-4 in rat NP cells. A: RT-qPCR analysis shows ADAMTS-4 was expressed at a very low level in adult rat NP and annulus fibrosus (AF) tissues. B: Western blot analysis of ADAMTS4 expression in adult disk tissues reveals bands at 58 and 73 kDa, representing mature/processed protein. C and D: RT-qPCR analysis of ADAMTS4 expression by rat NP cells treated with the cytokines TNF-α (C) and IL-1β (D) for 24 hours. There was a dose-dependent increase in ADAMTS4 mRNA expression by the cytokine treatment. E and F: Western blot analysis of NP cells indicates increased expression of ADAMTS-4 after TNF-α and IL-1β treatment. G: Schematic of ADAMTS4 promoter constructs, showing important transcription factor and regulatory elements. H: Treatment of NP cells with TNF-α and IL-1β resulted in significant induction of ADAMTS4 promoter activity. Data are expressed as means ± SEM from three independent experiments. ∗P < 0.05. Ctrl, control.
TNF-α and IL-1β Promote ADAMTS-4 and -5 Expression through Activation of MAPK and NF-κB Signaling
To determine whether MAPK and/or NF-κB signaling is required for the cytokine-dependent induction of ADAMTS-4 in rat NP cells, we first evaluated activation of these signaling pathways after treatment with TNF-α and IL-1β. After treatment with TNF-α (Figure 2A) or IL-1β (Figure 2B), there was a rapid increase in P-p65 protein levels. Activation was maximal at 5 to 30 minutes and then declined rapidly. As expected, there was no appreciable change in the level of total p65 during the treatment period. We also examined levels of the phosphorylated MAPK isoforms P-p38, P-ERK1/2, and P-JNK. Again, there was a rapid increase in all three isoforms, among which ERK exhibited more sustained levels of phosphorylation. To ascertain whether the cytokine-induced expression of ADAMTS-4 and -5 requires NF-κB and/or MAPK signaling, rat NP cells were pretreated with pathway-specific inhibitors. Pretreatment caused a significant suppression in TNF-α and IL-1β induction of both ADAMTS4 and ADAMTS5 mRNA levels (Figure 2, C–F). Similarly, a pronounced decrease in cytokine-mediated increase in levels of ADAMTS-4 protein (58 and 73 kDa) was seen in the presence of MAPK and NF-κB pathway inhibitors (Figure 2, G and H).
Figure 2.
Modulation of cytokine-dependent expression of ADAMTS-4 and -5 expression by NF-κB and MAPK signaling in rat NP cells. A and B: Western blot analysis of NF-κB and MAPK signaling proteins after treatment of NP cells with TNF-α (A) and IL-1β (B). Cytokine treatment induced phosphorylation of p65, p38, ERK, JNK within the first 15 minutes. No appreciable change was observed in expression of p65, p38, ERK, and JNK. C–F: RT-qPCR analysis of ADAMTS4 and ADAMTS5 expression by NP cells after treatment with TNF-α (C and E) or IL-1β (D and F) for 24 hours with or without inhibitors for NF-κB (SM7368, 10 μmol/L), p38 (SB203580, 10 μmol/L), ERK (PD98059, 10 μmol/L), and JNK (SP600125, 10 μmol/L). Inhibition of NF-κB signaling and MAPK signaling resulted in a significant blocking of cytokine-dependent induction in ADAMTS4 and ADAMTS5 mRNA expression. G and H: Western blot analysis indicates that treatment with NF-κB and MAPK inhibitors completely abolished ADAMTS-4 protein induction (the bands are indicated by asterisk) by TNF-α (G) and IL-1β (H). Data are expressed as means ± SEM from three independent experiments. ∗P < 0.05.
MAPK and C/EBP-β Control ADAMTS4 Promoter Activity in NP Cells
To investigate the mechanism of MAPK regulation of ADAMTS-4 expression, we transfected rat NP cells with dominant-negative (DN) DN-p38α, DN-p38β2, DN-p38γ, DN-p38δ, or DN-ERK1 or DN-ERK2 expression plasmids and measured ADAMTS4 promoter activity. DN-p38δ (Supplemental Figure S1B) and DN-ERK2 (Supplemental Figure S1, C and D) did not suppress promoter activity. In contrast, cytokine-dependent induction in ADAMTS4 promoter activity was significantly suppressed by cotransfection with DN-P38α (Figure 3, A and B), p38β2 (Figure 3C), p38γ (Figure 3D), and DN-ERK1 (Figure 3, E and F). Because CCAAT enhancer-binding protein β (C/EBP-β; alias LAP2) has been shown to control IL-1β– and TNF-α–dependent transcription in chondrocytes,23 we investigated whether a similar regulatory system exists in cells of the NP. We transfected rat NP cells with liver-enriched inhibitory protein (LIP), a functional LAP antagonist, and measured cytokine-dependent ADAMTS4 promoter activity. Surprisingly, suppression of C/EBP-β function resulted in further induction of the promoter activity by TNF-α (Figure 3G). On the other hand, cotransfection with LAP2 resulted in suppression of the basal promoter activity (Figure 3H).
Figure 3.
MAPK signaling controls ADAMTS4 promoter activity in rat NP cells. A and B: Cotransfection of rat NP cells with DN-p38α abolished TNF-α–dependent (A) and IL-1β–dependent (B) induction in ADAMTS4 promoter activity. C and D: IL-1β–dependent increase in ADAMTS4 promoter activity was blocked by DN-p38β2 (C) and DN-p38γ (D). E and F: DN-ERK1 suppressed induction in ADAMTS4 promoter activity by TNF-α (C) and IL-1β (D) treatment. For IL-1β treatment, inhibition was seen only at the highest dose (150 ng). G: NP cells were cotransfected with LIP and ADAMTS4 promoter, and luciferase activity was measured after TNF-α treatment. Addition of LIP further increased TNF-α–dependent ADAMTS-4 reporter activity. H:ADAMTS4 promoter activity was measured after cotransfection with LAP2. LAP2 suppressed ADAMTS4 promoter activity. Data are expressed as means ± SEM from three independent experiments. ∗P < 0.05.
NF-κB Signaling Controls ADAMTS4 Promoter Activity in the NP Cells
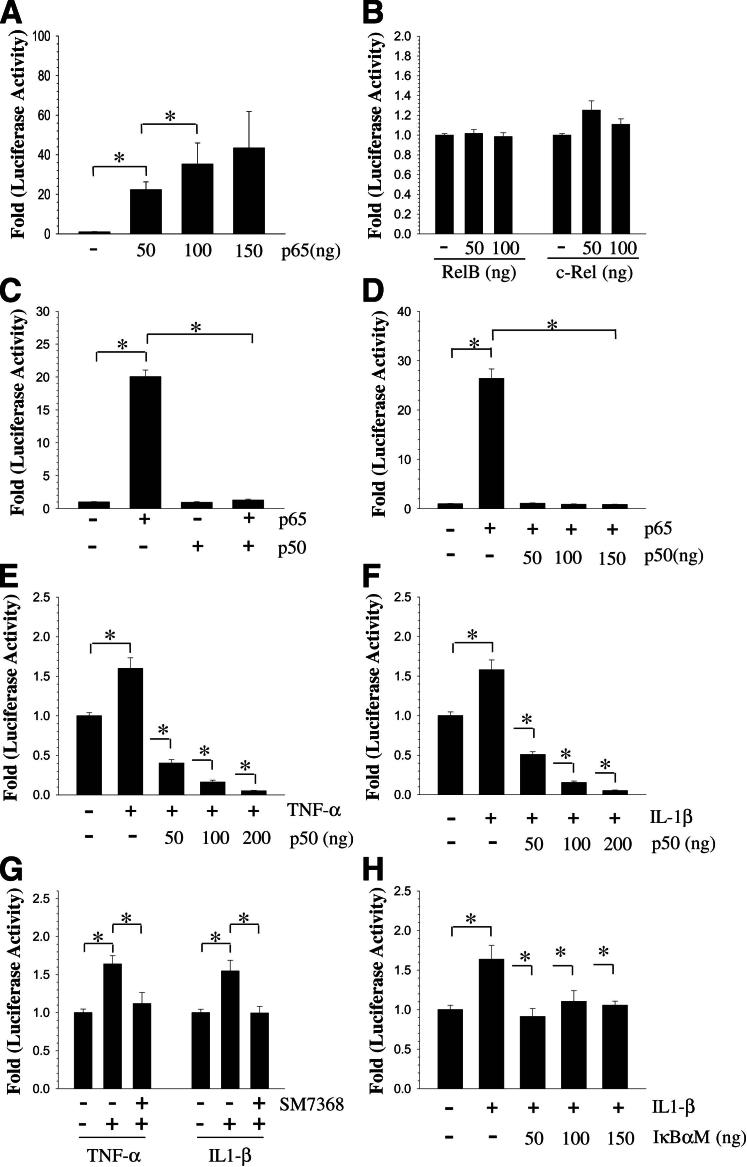
To investigate the role of NF-κB in the transcriptional regulation of ADAMTS4, we first used JASPAR database analysis (performed January 2012)24 (http://jaspar.genereg.net) for evidence of putative NF-κB binding motifs in the promoter. Sequence analysis revealed four putative binding sites. JASPAR analysis provides a quantitative score for each potential binding site, based on the probability of observing each nucleotide at each position of the binding motif compared to the consensus sequences of known binding sites. The raw score is normalized to a range of 0-1 to provide a relative score.24 The sequence, the location in the promoter relative to transcription start site, and relative score was as follows: GGCAAGTCCC, −159 relative to −150 bp, score 0.90; GGGGATTCTC, −1042 relative to −1033 bp, score 0.88; GGGATTCTCC, −1043 relative to −1034 bp, score 0.88; and GGGGATTTCC, −1394 relative to −1385 bp, score 0.98. We then examined the effect of overexpression of NF-κB subunits on ADAMTS4 promoter activity in rat NP cells. Cotransfection with p65 resulted in a dose-dependent increase in ADAMTS4 promoter activity (Figure 4A). On the other hand, neither the RelB nor the c-Rel subunit influenced ADAMTS4 promoter activity (Figure 4B). Although p50 alone had no effect on ADAMTS4 promoter activity, it blocked the inductive effect of p65 even at a low dose (Figure 4, C and D). Notably, p50 completely suppressed the inductive effect of both cytokines on the ADAMTS4 promoter (Figure 4, E and F).
Figure 4.
NF-κB regulation of ADAMTS-4 expression. A: Rat NP cells were transfected with RelA/p65, and ADAMTS4 promoter activity was measured. There was a dose-dependent increase in promoter activity up to 100 ng of p65. B: Cotransfection with RelB and c-Rel had no effect on ADAMTS4 promoter activity in rat NP cells. C: Rat NP cells were cotransfected with RelA/p65 and/or p50, and promoter activity was measured. When p65 and p50 were added together, p50 significantly blocked p65-mediated induction in promoter activity. D: Rat NP cells were cotransfected with p65 alone and with increasing doses of p50. Even at 50 ng, p50 completely blocked p65-mediated induction of promoter activity. E and F: Rat NP cells cotransfected with p50, and ADAMTS4 promoter activity was measured after TNF-α (E) and IL-1β (F) treatment. Cytokine-mediated induction in promoter activity was completely blocked by p50. G: TNF-α– and IL-1β–mediated induction in promoter activity was completely blocked by the NF-κB inhibitor SM7368. H: Cotransfection of cells with DN-NF-κB/IκBαM resulted in a significant inhibition of IL-1β–dependent ADAMTS4 promoter activity. Data are expressed as means ± SEM from three independent experiments. ∗P < 0.05.
To confirm that ADAMTS4 promoter activity is responsive to NF-κB signaling, we performed loss-of-function studies. When cells were treated with the NF-κB inhibitor SM7368 (Figure 4G) or cotransfected with DN-NF-κB/IκBαM (Figure 4H), cytokine-mediated induction in ADAMTS4 promoter activity was completely abolished. Specificity of the NF-κB inhibitors SM7368 and IκBαM was validated by measuring the activity of a well characterized NF-κB responsive reporter (Supplemental Figure S2A). To further validate the role of p65/RelA and to determine whether there is cell type specificity, we measured ADAMTS4 promoter activity in RelA null and wild-type mouse embryonic fibroblasts. Only in wild-type cells was the promoter activity cytokine inducible (Supplemental Figure S2, B and C).
NF-κB Signaling Controls TNF-α–Dependent ADAMTS-4 and -5 Expression and Aggrecan Degradation in NP Cells
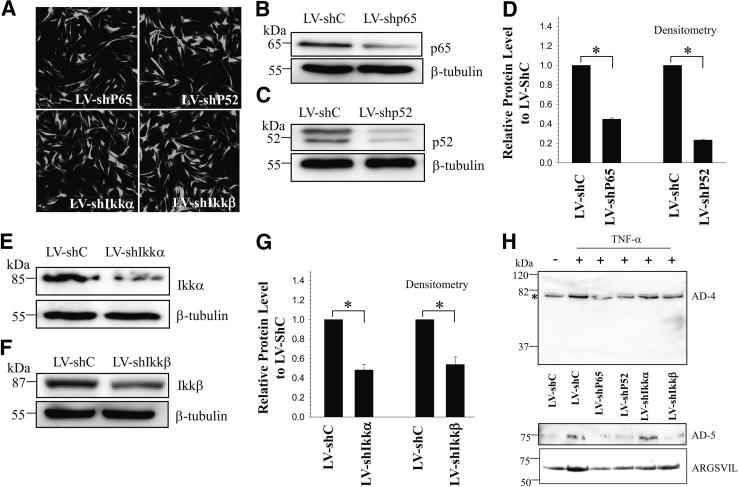
Given that IL-1β and TNF-α regulated ADAMTS-4 expression using similar signaling pathways, we performed lentiviral-mediated gene silencing studies using TNF-α as a representative cytokine. We first silenced the expression of individual NF-κB signaling components and then measured ADAMTS-4 expression in human NP cells. There was robust YFP expression by the virally transduced cells (Figure 5A), indicating high levels of transduction efficiency and transgene expression. In cells transduced with plasmids shp65 and shp52, there was a significant decrease in the protein levels of p65 and p52, respectively, compared with cells transduced with control shRNA (Figure 5, B–D). Similarly, transduction of human NP cells with plasmids shIKK-α and shIKK-β resulted in decreased levels of IKK-α and IKK-β protein, respectively (Figure 5, E–G). Importantly, suppression of individual NF-κB signaling components significantly blocked the inductive effect of TNF-α on the expression of ADAMTS-4 and -5 protein levels, as well as aggrecan degradation as measured by neoepitope generation (Figure 5H).
Figure 5.
Regulation of ADAMTS-4 and -5 expression by NF-κB. A: Immunofluorescence detection of YFP in human NP cells transduced with lentivirus coexpressing YFP and NF-κB pathway–specific shRNAs (LV-shp65, LV-shp52, LV-shIKKα, LV-shIKKβ) show high transduction efficiency. B–G: Western blot analysis of cells transduced with control lentivirus LV-shC and LV-shp65 (B), LV-shp52 (C), LV-shIKKα (E), and LV-shIKKβ (F). Expression of p65, p52, IKK-α, and IKK-β was suppressed by corresponding shRNAs, compared with cells transduced with a lentivirus expressing control shRNA. Densitometric analysis of p65 and p52 in cells transduced with LV-shp65 and LV-shp52 (D) and of IKK-α and IKK-β in cells transduced with LV-shIKKα and LV-shIKKβ (G). H: Western blot analysis of ADAMTS-4 (AD-4), ADAMTS-5 (AD-5), and aggrecan neoepitope (ARGSVIL) in human NP cells infected with LV-shC and LV-shp65, LV-shp52, LV-shIKKα, and LV-shIKKβ after TNF-α treatment. Note that the TNF-α–dependent increase in ADAMTS-4 (the band is indicated by an asterisk), ADAMTS-5, and aggrecan neoepitope (ARGSVIL) levels is significantly blocked by suppression of components of the NF-κB signaling pathway. Data are expressed as means ± SEM from three independent experiments. ∗P < 0.05. Original magnification, ×20.
Both ADAMTS-4 and ADAMTS-5 Contribute to TNF-α–Induced Aggrecan Degradation in NP Cells
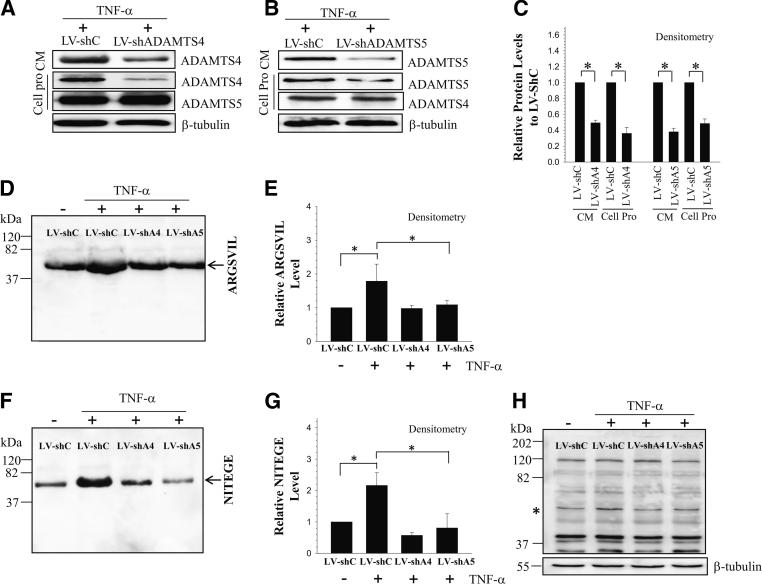
We examined the effect of silencing of ADAMTS-4 and ADAMTS-5 expression on aggrecan degradation in human NP cells. ADAMTS protein levels in the silenced NP cells or the conditioned medium were significantly reduced (Figure 6, A–C). Furthermore, there was no compensatory increase in either ADAMTS-4 or -5 when the other ADAMTS was silenced. We then used neoepitope-specific antibodies anti-ARGSVIL and anti-NITEGE to measure aggrecan degradation in ADAMTS silenced cells. Suppression of ADAMTS-4 and -5 expression resulted in a significant inhibition in TNF-α–mediated aggrecan degradation in NP cells (Figure 6, D and F). Densitometric analysis validated these findings (Figure 6, E and G). As expected, cells transduced with control shRNA exhibited an increase in aggrecan neoepitope generation after TNF-α treatment (Figure 6, D and F). The level of cell-associated versus pericellular matrix aggrecan degradation was also evaluated. In the cell-associated protein fraction, compared with control cells, silencing of ADAMTS-4 and ADAMTS-5 blocked aggrecan degradation after TNF-α treatment.
Figure 6.
ADAMTS-4 and ADAMTS-5 promote aggrecan degradation in human NP cells. A and B: Western blot analysis of human NP cells infected with control lentivirus (LV-shC) and lentivirus expressing shRNA ADAMTS-4 (LV-shADAMTS4) (A) and shRNA ADAMTS-5 (LV-shADAMTS5) (B) plasmids. Compared with control cells, expression of ADAMTS-4 and ADAMTS-5 was suppressed by shRNA shADAMTS-4 and shADAMTS-5 in both the conditioned medium and cell protein. C: Densitometric analysis of multiple blots from the experiment presented in panels A and B. D and E: Western blot (D) and corresponding densitometric analysis (E) of aggrecan neoepitope (ARGSVIL) in conditioned medium of cells treated with TNF-α. The level of ARGSVIL was significantly reduced after cytokine treatment in cells transduced with LV-shADAMTS-4 (LV-shA4) and LV-shADAMTS-4 (LV-shA5), compared with control (LV-shC). F and G: Western blot (F) and corresponding densitometric analysis (G) of aggrecan neoepitope (NITEGE) in concentrated conditioned medium of cells treated with TNF-α. The level of NITEGE was significantly reduced after TNF-α treatment in ADAMTS-4 and ADAMTS-5 silenced NP cells. H: Western blot analysis of NITEGE in cell-associated protein (the band is indicated by an asterisk) shows a significant reduction in levels after cytokine treatment in ADAMTS-4 and ADAMTS-5 silenced cells, compared with controls. Data are expressed as means ± SEM from three independent experiments. ∗P < 0.05. Cell Pro, cell protein; CM, conditioned medium.
Discussion
The experiments described here demonstrated for the first time that expression of ADAMTS-4, an important enzyme concerned with aggrecan degradation, is regulated by the inflammatory cytokines TNF-α and IL-1β through the MAPK and NF-κB signaling pathways in NP cells. A second major observation is that, by regulating ADAMTS-4 and -5 expression and activity, these inflammatory cytokines controlled aggrecan turnover. Importantly, both ADAMTS-4 and -5 were required for the cytokine-dependent aggrecan degradation in human NP cells, and their function appears to be nonredundant, suggesting a possible role in intervertebral disk pathologies.
According to our gene and protein expression studies, ADAMTS-4 is expressed in tissues of the intervertebral disk. The 73-kDa product on Western blot suggests that a mature/processed form of the protease is present in healthy discal tissues; the level of zymogen expression in the rat probably reflects the low level of matrix (aggrecan) turnover in the healthy disk. Although ADAMTS-4 is considered chiefly in terms of aggrecan catabolism, it is involved with a number of diverse physiological functions. Moreover, the mechanism of regulation of expression is incompletely understood. Conflicting data have been reported on the expression of ADAMTS-4 in chondrocytes and fibroblasts; some studies show that ADAMTS-4 is up-regulated by TNF-α and IL-1β,25–27 whereas others show that cytokine treatment does not affect ADAMTS-4 expression.28,29 In the present study, treatment of NP cells with TNF-α and IL-1β clearly induced ADAMTS-4 expression. Moreover, our promoter studies showed that regulation was at the transcript level. These results are consistent with previous reports that the inflammatory cytokines induce ADAMTS4 mRNA expression in the NP.10,11,19,30 Although the mechanism is unknown, there is some evidence to indicate that TNF-α–dependent ADAMTS-4 expression and aggrecanase activity in the NP may be regulated by ERK and NF-κB signaling.11 We confirmed that both cytokines promoted MAPK and p65/RelA activation and that these signaling pathways controlled ADAMTS-4 expression. Furthermore, mechanistic insights into regulation were forthcoming from gain and loss-of-function transfection studies that measured ADAMTS4 promoter activity. Our results clearly showed isoform specificity for both MAPK signaling pathways. Although p38α, p38β2, p38γ, and ERK1 positively controlled cytokine-dependent ADAMTS4 promoter activity, p38δ and ERK2 were not involved.
The presence of four putative NF-κB motifs in the ADAMTS4 promoter indicated functional involvement of this factor in controlling transcription. Mizui et al31 suggested that the region between −383 and +10 is required for full activity of the human ADAMTS4 promoter. Notably, the NF-κB motif with the second-highest relative score is contained within this region. In further support for the role of NF-κB in ADAMTS4 promoter regulation, our studies clearly indicated that the inductive effect is restricted to p65 and p52 and that subunits RelB and c-Rel do not play a regulatory role. Interestingly, p50 suppressed the inductive effect of p65 on ADAMTS4 promoter. The observation that the cytokine- and p65-dependent induction of ADAMTS-4 expression is suppressed by p50 is consistent with previous reports of repressive function of p50 homodimers in controlling expression of a number of genes, including CCL2, CXCL10, GMCSF, and MMP13,32–34 as well as the recent report from our research group that clearly identified p50 as a negative regulator of cytokine-dependent CCL3 transcription.35 On the other hand, our group has also shown that p65 and p50 act synergistically to induce the expression of the syndecan-4 gene (SDC4), one of the target genes of TNF-α and IL-1β in NP cells.19 These results highlight the importance of both context and target-gene specificity in the regulatory machinery of NP cells. It is not unreasonable to assume that formation of p50 homodimers and their binding to the κB motifs results in recruitment of transcriptional repressor such as HDAC1 to the ADAMTS4 promoter, thereby suppressing RelA/p65 response.32 A detailed investigation would be required to further elucidate the mechanism and significance of this interesting finding. The observation that RelA null cells failed to induce ADAMTS4 promoter activity, even when treated with cytokines, provides further validation of the importance of NF-κB signaling in promoter regulation. Moreover, the silencing studies that demonstrated inhibition of TNF-α–dependent ADAMTS-4 and -5 expression after suppression of several NF-κB signaling highlight the importance of this pathway in controlling ADAMTS-4 expression. Taken together, the results of these functional studies indicate that, by controlling the activity of both MAPK and NF-κB, especially RelA/p65 signaling, cytokines control the expression of ADAMTS-4 in NP cells.
Relevant to this discussion of control of ADAMTS4 transcription, Thirunavukkarasu et al20 showed that IL-1α and oncostatin induced ADAMTS4 promoter activity, possibly through NFATp and Runx2 in chondrocytic cells. Mizui et al31 showed that nuclear factor I (NFI) is involved in the negative regulation of the human ADAMTS4 promoter activity in chondrocytes; they speculated that Sp1 and AP2 sites located within −382 to +10 of the promoter may be required for its full activity. Because the ADAMTS4 promoter contains several CCAAT enhancer elements and because C/EBP-β is known to promote cytokine-dependent transcription in chondrocytes,36 it was also important to consider their possible regulatory roles. In contrast to its role in chondrocytes, our present data clearly identify C/EBP-β as a negative regulator of cytokine-dependent ADAMTS4 transcription in the NP, thus highlighting the unique cell-type-specific regulation of this gene in NP cells.
We and others have demonstrated increased expression of ADAMTS4 and ADAMTS5 mRNA and protein during disk degeneration.17,19,37 Patel et al37 and Seki et al38 showed that, in both human and rabbit, although ADAMTS-4 protein levels increase with severity of the disease, ADAMTS-5 levels are similar at early and late stages. Moreover, Seki et al38 showed that silencing of ADAMTS-5 alone is sufficient to block aggrecan degradation in rabbit disks. Lending support to these earlier reports, in the present study expression of ADAMTS-4 was more responsive to TNF-α in NP cells from intervertebral disks of more degenerate grades; nevertheless, silencing of either ADAMTS-4 or ADAMTS-5 in grade 2 human NP cells resulted in inhibition of TNF-α–induced aggrecan neoepitope generation. Based on these findings, it is not unreasonable to conclude that ADAMTS-4 and -5 are nonredundant and therefore that therapeutic blocking of the activity of even one of these proteases could be expected to limit breakdown of the aggrecan-rich matrix and possibly mitigate degenerative disk disease.
Footnotes
Supported by NIH grants AR050087 (to I.M.S. and M.V.R.) and AR055655 (to M.V.R.).
Y.T. and W.Y. contributed equally to this work.
Supplemental Data
A: Western blot analysis of ADAMTS4 and 5 in human nucleus pulposus (NP) cells isolated from severely degenerated disks (grade G4 and G5). ADAMTS-4 showed more pronounced increase, compared with ADAMTS-5, after TNF-α treatment. B: Role of MAPK signaling in controlling ADAMTS-4 promoter activity in NP cells. Rat NP cells were cotransfected with DN-p38δ, and ADAMTS-4 promoter activity was measured after IL-1β treatment. Unlike other p38 isoforms, p38δ plays no role in controlling ADAMTS-4 promoter activity. C and D: Cotransfection of cells with DN-ERK2 had no effect on TNF-α–dependent (C) and IL-1β–dependent (D) induction in ADAMTS-4 promoter activity. Data are expressed as means ± SEM from three independent experiments. *P < 0.05.
A: TNF-α–mediated induction in NRE reporter activity in rat NP cells was completely blocked by both DN-NF-κB and the NF-κB inhibitor SM7368. B and C: RelA/p65 wild-type (B) and null (C) MEFs were transfected with ADAMTS-4 reporter constructs and treated with TNF-α and IL-1β. Only wild-type cells showed increase in ADAMTS-4 reporter activity. Data are expressed as means ± SEM from three independent experiments. *P < 0.05.
References
- 1.Stemple D.L. Structure and function of the notochord: an essential organ for chordate development. Development. 2005;132:2503–2512. doi: 10.1242/dev.01812. [DOI] [PubMed] [Google Scholar]
- 2.Feng H., Danfelter M., Strömqvist B., Heinegård D. Extracellular matrix in disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):25–29. doi: 10.2106/JBJS.E.01341. [DOI] [PubMed] [Google Scholar]
- 3.Setton L.A., Chen J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):52–57. doi: 10.2106/JBJS.F.00001. [DOI] [PubMed] [Google Scholar]
- 4.Ng L., Grodzinsky A.J., Patwari P., Sandy J., Plaas A., Ortiz C. Individual cartilage aggrecan macromolecules and their constituent glycosaminoglycans visualized via atomic force microscopy. J Struct Biol. 2003;143:242–257. doi: 10.1016/j.jsb.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Urban J.P., Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts S., Evans H., Trivedi J., Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88(Suppl 2):10–14. doi: 10.2106/JBJS.F.00019. [DOI] [PubMed] [Google Scholar]
- 7.Le Maitre C.L., Freemont A.J., Hoyland J.A. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732–R745. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee S., Moon C.S., Sul D., Lee J., Bae M., Hong Y., Lee M., Choi S., Derby R., Kim B.J., Kim J., Yoon J.S., Wolfer L., Kim J., Wang J., Hwang S.W., Lee S.H. Comparison of growth factor and cytokine expression in patients with degenerated disc disease and herniated nucleus pulposus. Clin Biochem. 2009;42:1504–1511. doi: 10.1016/j.clinbiochem.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Lee J.M., Song J.Y., Baek M., Jung H.Y., Kang H., Han I.B., Kwon Y.D., Shin D.E. Interleukin-1β induces angiogenesis and innervation in human intervertebral disc degeneration. J Orthop Res. 2011;29:265–269. doi: 10.1002/jor.21210. [DOI] [PubMed] [Google Scholar]
- 10.Séguin C.A., Pilliar R.M., Roughley P.J., Kandel R.A. Tumor necrosis factor-alpha modulates matrix production and catabolism in nucleus pulposus tissue. Spine (Phila Pa 1976) 2005;30:1940–1948. doi: 10.1097/01.brs.0000176188.40263.f9. [DOI] [PubMed] [Google Scholar]
- 11.Séguin C.A., Bojarski M., Pilliar R.M., Roughley P.J., Kandel R.A. Differential regulation of matrix degrading enzymes in a TNFalpha-induced model of nucleus pulposus tissue degeneration. Matrix Biol. 2006;25:409–418. doi: 10.1016/j.matbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Millward-Sadler S.J., Costello P.W., Freemont A.J., Hoyland J.A. Regulation of catabolic gene expression in normal and degenerate human intervertebral disc cells: implications for the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2009;11:R65. doi: 10.1186/ar2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tortorella M.D., Liu R.Q., Burn T., Newton R.C., Arner E. Characterization of human aggrecanase 2 (ADAM-TS5) substrate specificity studies and comparison with aggrecanase 1 (ADAM-TS4) Matrix Biol. 2002;21:499–511. doi: 10.1016/s0945-053x(02)00069-0. [DOI] [PubMed] [Google Scholar]
- 14.Malfait A.M., Liu R.Q., Ijiri K., Komiya S., Tortorella M.D. Inhibition of ADAM-TS4 and ADAM-TS5 prevents aggrecan degradation in osteoarthritic cartilage. J Biol Chem. 2002;277:22201–22208. doi: 10.1074/jbc.M200431200. [DOI] [PubMed] [Google Scholar]
- 15.Tortorella M.D., Pratta M., Liu R.Q., Austin J., Ross O.H., Abbaszade I., Burn T., Arner E. Sites of aggrecan cleavage by recombinant human aggrecanase1 (ADAMTS-4) J Biol Chem. 2000;275:18566–18573. doi: 10.1074/jbc.M909383199. [DOI] [PubMed] [Google Scholar]
- 16.Tortorella M.D., Burn T.C., Pratta M.A., Abbaszade I., Hollis J.M., Liu R., Rosenfeld S.A., Copeland R.A., Decicco C.P., Wynn R., Rockwell A., Yang F., Duke J.L., Solomon K., George H., Bruckner R., Nagase H., Itoh Y., Ellis D.M., Ross H., Wiswall B.H., Murphy K., Hillman M.C., Jr., Hollis G.F., Newton R.C., Magolda R.L., Trzaskos J.M., Arner E.C. Purification and cloning of aggrecanase-1: a member of the ADAMTS family of proteins. Science. 1999;284:1664–1666. doi: 10.1126/science.284.5420.1664. [DOI] [PubMed] [Google Scholar]
- 17.Pockert A.J., Richardson S.M., Le Maitre C.L., Lyon M., Deakin J.A., Buttle D.J., Freemont A.J., Hoyland J.A. Modified expression of the ADAMTS enzymes and tissue inhibitor of metalloproteinases 3 during human intervertebral disc degeneration. Arthritis Rheum. 2009;60:482–491. doi: 10.1002/art.24291. [DOI] [PubMed] [Google Scholar]
- 18.Mwale F., Masuda K., Pichika R., Epure L.M., Yoshikawa T., Hemmad A., Roughley P.J., Antoniou J. The efficacy of Link N as a mediator of repair in a rabbit model of intervertebral disc degeneration. Arthritis Res Ther. 2011;13:R120. doi: 10.1186/ar3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J., Markova D., Anderson D.G., Zheng Z., Shapiro I.M., Risbud M.V. TNF-α and IL-1β promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. J Biol Chem. 2011;286:39738–39749. doi: 10.1074/jbc.M111.264549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thirunavukkarasu K., Pei Y., Moore T.L., Wang H., Yu X.P., Geiser A.G., Chandrasekhar S. Regulation of the human ADAMTS-4 promoter by transcription factors and cytokines. Biochem Biophys Res Commun. 2006;345:197–204. doi: 10.1016/j.bbrc.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Risbud M.V., Guttapalli A., Stokes D.G., Hawkins D., Danielson K.G., Schaer T.P., Albert T.J., Shapiro I.M. Nucleus pulposus cells express HIF-1 alpha under normoxic culture conditions: a metabolic adaptation to the intervertebral disc microenvironment. J Cell Biochem. 2006;98:152–159. doi: 10.1002/jcb.20765. [DOI] [PubMed] [Google Scholar]
- 22.Pfirrmann C.W., Metzdorf A., Zanetti M., Hodler J., Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z., Bryan J.L., DeLassus E., Chang L.W., Liao W., Sandell L.J. CCAAT/Enhancer-binding protein beta and NF-kappaB mediate high level expression of chemokine genes CCL3 and CCL4 by human chondrocytes in response to IL-1beta. J Biol Chem. 2010;285:33092–33103. doi: 10.1074/jbc.M110.130377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wasserman W.W., Sandelin A. Applied bioinformatics for the identification of regulatory elements. Nat Rev Genet. 2004;5:276–287. doi: 10.1038/nrg1315. [DOI] [PubMed] [Google Scholar]
- 25.Tortorella M.D., Malfait A.M., Deccico C., Arner E. The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthritis Cartilage. 2001;9:539–552. doi: 10.1053/joca.2001.0427. [Erratum appeared in Osteoarthritis Cartilage 2002, 10:82] [DOI] [PubMed] [Google Scholar]
- 26.Hui W., Barksby E., Young D.A., Cawston T.E., McKie N., Rowan A.D. Oncostatin M in combination with tumour necrosis factor {alpha} induces a chondrocyte membrane-associated aggrecanase that is distinct from ADAMTS aggrecanase-1 or -2. Ann Rheum Dis. 2005;64:1624–1632. doi: 10.1136/ard.2004.028191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yatabe T., Mochizuki S., Takizawa M., Chijiiwa M., Okada A., Kimura T., Fujita Y., Matsumoto H., Toyama Y., Okada Y. Hyaluronan inhibits expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic chondrocytes. Ann Rheum Dis. 2009;68:1051–1058. doi: 10.1136/ard.2007.086884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koshy P.J., Lundy C.J., Rowan A.D., Porter S., Edwards D.R., Hogan A., Clark I.M., Cawston T.E. The modulation of matrix metalloproteinase and ADAM gene expression in human chondrocytes by interleukin-1 and oncostatin M: a time-course study using real-time quantitative reverse transcription-polymerase chain reaction. Arthritis Rheum. 2002;46:961–967. doi: 10.1002/art.10212. [DOI] [PubMed] [Google Scholar]
- 29.Yamanishi Y., Boyle D.L., Clark M., Maki R.A., Tortorella M.D., Arner E.C., Firestein G.S. Expression and regulation of aggrecanase in arthritis: the role of TGF-beta. J Immunol. 2002;168:1405–1412. doi: 10.4049/jimmunol.168.3.1405. [DOI] [PubMed] [Google Scholar]
- 30.Tsuji T., Chiba K., Imabayashi H., Fujita Y., Hosogane N., Okada Y., Toyama Y. Age-related changes in expression of tissue inhibitor of metalloproteinases-3 associated with transition from the notochordal nucleus pulposus to the fibrocartilaginous nucleus pulposus in rabbit intervertebral disc. Spine (Phila Pa 1976) 2007;32:849–856. doi: 10.1097/01.brs.0000259804.39881.62. [DOI] [PubMed] [Google Scholar]
- 31.Mizui Y., Yamazaki K., Kuboi Y., Sagane K., Tanaka I. Characterization of 5′-flanking region of human aggrecanase-1 (ADAMTS-4) gene. Mol Biol Rep. 2000;27:167–173. doi: 10.1023/a:1007253930568. [DOI] [PubMed] [Google Scholar]
- 32.Elsharkawy A.M., Oakley F., Lin F., Packham G., Mann D.A., Mann J. The NF-kappaB p50:p50:HDAC-1 repressor complex orchestrates transcriptional inhibition of multiple pro-inflammatory genes. J Hepatol. 2010;53:519–527. doi: 10.1016/j.jhep.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panzer U., Steinmetz O.M., Turner J.E., Meyer-Schwesinger C., von Ruffer C., Meyer T.N., Zahner G., Gómez-Guerrero C., Schmid R.M., Helmchen U., Moeckel G.W., Wolf G., Stahl R.A., Thaiss F. Resolution of renal inflammation: a new role for NF-kappaB1 (p50) in inflammatory kidney diseases. Am J Physiol Renal Physiol. 2009;297:F429–F439. doi: 10.1152/ajprenal.90435.2008. [DOI] [PubMed] [Google Scholar]
- 34.Oakley F., Mann J., Nailard S., Smart D.E., Mungalsingh N., Constandinou C., Ali S., Wilson S.J., Millward-Sadler H., Iredale J.P., Mann D.A. Nuclear factor-kappaB1 (p50) limits the inflammatory and fibrogenic responses to chronic injury. Am J Pathol. 2005;166:695–708. doi: 10.1016/s0002-9440(10)62291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J., Tian Y., Phillips K.L., Chiverton N., Haddock G., Bunning R.A., Cross A.K., Shapiro I.M., Le Maitre C.L., Risbud M.V. Tumor necrosis factor alpha- and interleukin-1beta-dependent induction of CCL3 expression by nucleus pulposus cells promotes macrophage migration through CCR1. Arthritis Rheum. 2013;65:832–842. doi: 10.1002/art.37819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Z., Xing X., Hensley G., Chang L.W., Liao W., Abu-Amer Y., Sandell L.J. Resistin induces expression of proinflammatory cytokines and chemokines in human articular chondrocytes via transcription and messenger RNA stabilization. Arthritis Rheum. 2010;62:1993–2003. doi: 10.1002/art.27473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel K.P., Sandy J.D., Akeda K., Miyamoto K., Chujo T., An H.S., Masuda K. Aggrecanases and aggrecanase-generated fragments in the human intervertebral disc at early and advanced stages of disc degeneration. Spine (Phila Pa 1976) 2007;32:2596–2603. doi: 10.1097/BRS.0b013e318158cb85. [DOI] [PubMed] [Google Scholar]
- 38.Seki S., Asanuma-Abe Y., Masuda K., Kawaguchi Y., Asanuma K., Muehleman C., Iwai A., Kimura T. Effect of small interference RNA (siRNA) for ADAM-TS5 on intervertebral disc degeneration in the rabbit anular needle-puncture model. Arthritis Res Ther. 2009;11:R166. doi: 10.1186/ar2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A: Western blot analysis of ADAMTS4 and 5 in human nucleus pulposus (NP) cells isolated from severely degenerated disks (grade G4 and G5). ADAMTS-4 showed more pronounced increase, compared with ADAMTS-5, after TNF-α treatment. B: Role of MAPK signaling in controlling ADAMTS-4 promoter activity in NP cells. Rat NP cells were cotransfected with DN-p38δ, and ADAMTS-4 promoter activity was measured after IL-1β treatment. Unlike other p38 isoforms, p38δ plays no role in controlling ADAMTS-4 promoter activity. C and D: Cotransfection of cells with DN-ERK2 had no effect on TNF-α–dependent (C) and IL-1β–dependent (D) induction in ADAMTS-4 promoter activity. Data are expressed as means ± SEM from three independent experiments. *P < 0.05.
A: TNF-α–mediated induction in NRE reporter activity in rat NP cells was completely blocked by both DN-NF-κB and the NF-κB inhibitor SM7368. B and C: RelA/p65 wild-type (B) and null (C) MEFs were transfected with ADAMTS-4 reporter constructs and treated with TNF-α and IL-1β. Only wild-type cells showed increase in ADAMTS-4 reporter activity. Data are expressed as means ± SEM from three independent experiments. *P < 0.05.