Abstract
The α(1,3)-fucosyltransferases, types IV and VII (FUT4 and FUT7, respectively), are required for the synthesis of functional selectin-type leukocyte adhesion molecule ligands. The selectins and their ligands modulate leukocyte trafficking, and P-selectin and its ligand, P-selectin glycoprotein ligand-1, can modulate hemostasis and thrombosis. Regulation of thrombosis by FUT4 and/or FUT7 activity was examined in mouse models of carotid artery thrombosis and collagen/epinephrine-induced thromboembolism. Mice lacking both FUT4 and FUT7 (Fut−/− mice) had a shorter time to occlusive thrombus formation in the injured carotid artery and a higher mortality due to collagen/epinephrine-induced pulmonary thromboemboli. Mice lacking P-selectin or P-selectin glycoprotein ligand-1 did not have a prothrombotic phenotype. Whole blood platelet aggregation was enhanced, and plasma fibrinogen content, clot weight, and clot strength were increased in Fut−/− mice, and in vitro clot lysis was reduced compared with wild type. Fut4−/−, but not Fut7−/−, mice had increased pulmonary thromboembolism-induced mortality and decreased thromboemboli dissolution in vivo. These data show that FUT4 and FUT7 activity regulates thrombosis in a P-selectin– and P-selectin glycoprotein ligand-1–independent manner and suggest that FUT4 activity is important for thrombolysis.
Abnormal post-translational glycosylation can change protein structure, subcellular localization, stability, enzymatic activity, or immunogenic properties. Many proteins of the coagulation system are heavily glycosylated,1 and variations in the presence or composition of their glycans are known to modulate their function, thereby affecting hemostasis and thrombosis.2–5 The fucosyltransferases are a family of glycosyltransferases that catalyze the transfer of L-fucose to an acceptor glycan on glycoproteins or glycolipids. They are classified according to the location and configuration of the catalyzed bond between fucose and the acceptor molecule. In humans, α(1,3/4)-fucosyltransferase III (FUT3) catalyzes steps in the synthesis of carbohydrate blood antigens, including the Lewis and Lewis-related antigens (ie, Lea, Leb, Lex, LeY, sialyl-Lex, and sialyl-Lea).6 Individuals who are Lewis negative or have an FUT3 polymorphism that reduces fucosyltransferase availability (T59G) or enzymatic activity (T1067A and T202C) experience an increased incidence of atherothrombotic disease.7,8
The FUT3 homologue in mice (Fut3) is a pseudogene,9 and the functional α(1,3)-fucosyltransferases, FUT4 and FUT7, catalyze the synthesis of Lex and sialyl-Lex on leukocytes and high endothelial venules. FUT7 plays a predominant role, and FUT4 plays a subsidiary role, in the synthesis of the sialyl-Lex tetrasaccharide moiety on selectin ligands10 that is critical for ligand-binding activity.11,12 Mice with targeted mutations in both Fut4 and Fut7 (Fut−/−) have marked defects in selectin-dependent leukocyte rolling in vitro and leukocyte recruitment in response to acute inflammation and delayed-type hypersensitivity challenges.12,13 Mice lacking these FUTs are protected from inflammation-induced pathological conditions, such as atherosclerosis14,15 and renal ischemia-reperfusion injury.16 FUT7 expression is restricted primarily to leukocytes and high endothelial venules, and it has precursor specificity for sialyl-Lex synthesis. In contrast, FUT4 has a broader tissue expression pattern and catalyzes the synthesis of sialyl-Lex, Lex, and Ley moieties.6,17
Two functions of FUT4 and FUT7 suggest an interesting potential role for these enzymes in modulating thrombosis and hemostasis. First, FUT4 and FUT7 catalyze the synthesis of functional P-selectin glycoprotein ligand-1 (PSGL-1; gene Selplg). Binding interactions of PSGL-1 with its receptor, P-selectin (gene Selp), contribute to microvascular thrombosis by enhancing the generation of tissue factor–bearing microparticles and their recruitment into a growing thrombus. Both actions enhance the local generation of fibrin to stabilize the growing thrombus.18,19 Second, human genetic association studies demonstrate links between Lewis-related antigens and the risk of atherothrombotic disease.7,8 We examined thrombosis and hemostasis in Fut−/− mice and found an unexpected prothrombotic phenotype, enhanced platelet aggregation, and a high plasma fibrinogen concentration. Blood clot size and strength were increased, and whole blood clots derived from Fut−/− mice were resistant to tissue-type plasminogen activator-initiated thrombolysis. These results show that loss of α(1,3)-fucosylation, mediated by FUT4 and FUT7, results in enhanced thrombosis due to decreased thrombolysis. They suggest that altered fucosylation of Lewis and Lewis-related antigen structures modulates the thrombotic phenotype in mice, and that altered fucosylation of these structures may modulate the thrombotic phenotype in humans with generalized fucosylation deficiencies20 or specific α(1,3/4)-FUT polymorphisms.20,21
Materials and Methods
Materials
Endotoxin-free PBS, saline, and water were from Fisher Scientific (Pittsburgh, PA). Rose bengal, prostaglandin E1 (PGE1), ADP, bovine serum albumin, and thrombin were from Sigma-Aldrich (St. Louis, MO). The platelet aggregation reagents, collagen and arachidonic acid (AA), were from Chrono-Log (Havertown, PA). An enzyme-linked immunosorbent assay (ELISA) specific for mouse fibrinogen was purchased from Kamiya Biomedical Company (Seattle, WA) and used according to the manufacturer's protocol. Fibronectin-depleted and von Willebrand factor–depleted human fibrinogen was purchased from Enzyme Research Laboratories (South Bend, IN). Tissue plasminogen activator (t-PA) was from EMD Chemicals (La Jolla, CA). Thrombin fluorogenic substrate (Z-Gly-Gly-Arg-AMC) and calibrator (α2-macroglobulin/thrombin) and tissue factor were the generous gift of Dr. Alisa S. Wolberg (University of North Carolina at Chapel Hill). Mice deficient in P-selectin or PSGL-1 were obtained from The Jackson Laboratory (Bar Harbor, ME), and mice deficient in FUT4 and/or FUT7 were maintained in the laboratory of one of the authors (J.W.H.). Experimental procedures and animal husbandry conformed to the guidelines of the University of North Carolina Institutional Animal Care and Use Committee. Veterinary care was provided by the University of North Carolina Department for Laboratory Animal Medicine.
Rose Bengal Model of Carotid Thrombosis
Photochemical-induced carotid artery thrombosis was performed as previously described.22 Mice were anesthetized with 1.5% isoflurane in 2% oxygen. Body temperature was determined with an anal probe and maintained at 37°C using a heating pad. A midline incision approximately 1.75 cm in length was made on the anterior neck to expose the area of the right proximal common carotid artery. The common carotid artery was dissected free of surrounding tissues and immersed in saline throughout the experiment. A Doppler transonic flow probe (Transonic Systems, Ithaca, NY) was placed under the vessel to measure arterial blood flow. A 540-nm green laser light beam of 1.5 mV (Melles Griot, Carlsbad, CA) was continuously applied to the artery from a distance of 6 cm. The photochemical, rose bengal (20 mg/mL in PBS, 50 mg/kg final concentration) was administered via tail vein as a bolus over 5 seconds. The blood flow was monitored continuously until a stable occlusion occurred (lasting a minimum of 60 seconds) or for 90 minutes if no stable occlusion occurred.
Collagen/Epinephrine Model of Thromboembolism
Collagen/epinephrine (Col/Epi)–induced pulmonary thromboembolism was performed as previously described.23 Mice were anesthetized i.p. with 25 mg/kg xylazine and 75 mg/kg ketamine. Platelet agonist solution containing 75 μg/mL collagen and 10 μg/mL epinephrine in PBS was freshly prepared and administered as a bolus into the tail vein at 80 or 100 μL per 20 g body weight, depending on the experiment. In some experiments, mice were made hyperfibrinogenemic by infusion of human fibrinogen (in 50 μL PBS without calcium) 2 minutes before infusion of the Col/Epi mixture. Mice were observed for up to 10 minutes. After euthanasia, the lungs were insufflated with formalin via the trachea to the end-expiration rib cage volume, removed, fixed insufflated in formalin for >48 hours, and paraffin embedded. Coronal sections (5 μm thick) of the lungs were cut just posterior to the lung hilum and stained with H&E. The entire lung section was imaged in multiple, high-power, nonoverlapping photomicrographs. Intra-arteriolar thrombi (dimensions >50 μm and 25 μm in the perpendicular direction) were counted using ImageJ image analysis software version 1.35n (NIH, Bethesda, MD), and the number of thrombi per cm2 was calculated.
Preparation of Washed Platelets
Platelets were isolated and washed according to published methods24 at room temperature. Citrate-treated venous blood containing 5 μg PGE1 (1 mL) was layered onto a 0.8-mL Accu-Prep lymphocyte gradient medium (Accurate Chemical, Westbury, NY) and spun at 620 × g for 20 minutes. The peripheral blood mononuclear cell and platelet band were transferred to a clean polypropylene tube and diluted with an equal volume of citrate-glucose buffer (13 mmol/L sodium citrate, 30 mmol/L glucose, and 120 mmol/L NaCl, pH 7.0) containing 5 μg/mL PGE1. The suspension was centrifuged at 122 × g for 10 minutes, and the platelets were purified from the supernatant using a Sepharose (Sigma CL-2B-300, Sigma, St. Louis, MO) column that was equilibrated with Tyrode's buffer (12 mmol/L NaHCO3, 138 mmol/L NaCl, 2.9 mmol/L KCl, 10 mmol/L HEPES, 5.5 mmol/L glucose, 1 mmol/L MgCl, 1 mmol/L CaCl2, and 1 mg/mL bovine serum albumin, pH 7.4). The effluent fractions with high platelet counts were collected.
Washed Platelet and Whole Blood Aggregation
Washed platelets were adjusted to 250 × 106/mL with Tyrode's buffer. Platelet aggregation was determined using an optical aggregometer (Chrono-Log Optical Aggregometer 470; Chrono-Log). Platelet aggregation was induced with 2 μg/mL final concentration collagen or 0.5 mmol/L final concentration AA. Human fibrinogen to a final concentration of 15 μg/mL was added to the washed platelet suspension to a final concentration of 10 μmol/L before ADP-induced aggregation.
Citrate-treated whole blood (500 μL) was diluted 1:1 with prewarmed 500 μL HBSS (Gibco, Grand Island, NY) with 2 mmol/L CaCl2. Whole blood aggregation was determined by impedance measurements using a Chronolog Lumi-Aggregometer (model 700; Chrono-Log). Baseline was set at 0%, and the impedance gain was set at 50%. Whole blood aggregation was induced by addition of 3 μg/mL collagen, 10 μmol/L ADP, or 0.5 mmol/L AA (all final concentrations).
Turbidometric Assay for Fibrin Polymerization
Wild-type (WT) platelet-poor plasma (PPP; 20 μL) was spiked with 0, 13.5, 27, or 45 μg of human fibrinogen in PBS. Clot formation was triggered by the addition of 80 μL HEPES buffered saline (20 mmol/L HEPES and 150 mmol/L NaCl, pH 7.4) containing a final concentration of 20 mmol/L CaCl2 and 0.1 U/mL thrombin. Clotting was detected by turbidity at 405 nm in a SpectraMax Plus340 plate reader (Molecular Devices, Sunnyvale, CA).
Whole Blood TEG
The elasticity of clotting blood was evaluated using the TEG Thromboelastograph Hemostasis analyzer (TEG 5000; Haemonetics, Braintree, MA), according to the manufacturer's protocol. Citrate-treated whole blood (360 μL) was quickly mixed with a final concentration of 20 mmol/L CaCl2 and immediately placed into untreated disposable cups for measurement. Clot strength is reflected by two parameters, maximum amplitude (measured in millimeters) and shear elastic modulus strength (measured in dyne/cm2). These parameters are a direct function of the maximum dynamic properties of fibrin and platelet bonding and represent the ultimate strength of the fibrin clot.25
Blood Clotting and Clot Weight Determination
Citrate-treated venous whole blood was centrifuged at 100 × g for 15 minutes. The platelet-rich supernatant and peripheral blood mononuclear cell/platelet layer were collected and centrifuged at 100 × g for 10 minutes to pellet peripheral blood mononuclear cells. Platelet-rich plasma (PRP) was collected and adjusted with PPP to a platelet concentration of 300 × 106/mL. PPP was obtained by centrifugation of the remaining red blood cell layer (4000 × g for 2 minutes). Clotting of 300 μL PRP was initiated with addition of CaCl2 to a final concentration of 20 mmol/L, and clot retraction was allowed to proceed overnight at room temperature. Clots were then rinsed with PBS, blotted to remove excess fluid, transferred to a preweighed tube, dried overnight, and weighed.
To determine clot lysis, a final concentration of 16 nmol/L t-PA or saline was spiked into 300 μL citrate-treated whole blood samples. Blood clotting was initiated with a final concentration of 20 mmol/L CaCl2. Clotting and clot retraction were allowed to proceed for 3 hours without disturbance. The clots were then gently removed from the tube wall if needed, and allowed to retract for an additional 2 hours, washed three times with PBS, dried, and weighed as described.
Clot Lysis Assay
Citrate-treated venous whole blood (400 μL) in a polypropylene tube was spiked with 10 μg fluorophore AF647-labeled human fibrinogen (hFG-AF647; Invitrogen, Grand Island, NY) to approximately 1% of total plasma fibrinogen, and then with either 16 nmol/L t-PA (final concentration) or saline as control. Clotting was triggered by the addition of a final concentration of CaCl2 to 20 mmol/L and thrombin to 0.1 U/mL. Clots formed immediately, and clot retraction was present after 20 minutes. At 40 minutes after clot initiation, 900 μL HEPES buffered saline with 2 mmol/L CaCl2 was added to the tube, and clots were gently peeled away from tube wall to freely float in the diluted serum. At the indicated times, the tube was gently inverted several times to equilibrate the concentration of the fibrin degradation products (FDPs) within the diluted serum; 110-μL aliquots of the solution were centrifuged at 4000 × g for 2 minutes to pellet cells. The fluorescence intensity (675 nm) of a 95-μL aliquot of the cell-free supernatant was determined. The concentration of hFDP-AF647 was determined from an hFG-AF647 standard curve. The total amount of hFDP-AF647 released from the clot at each time point was calculated using the hFDP-AF647 concentrations and diluted serum volumes. The percentage of clot lysis was calculated as total hFDP-AF647/initially spiked hFG-AF647 (10 μg).
Statistical Analysis
Kaplan-Meier survival curve and most other statistical analyses were performed with GraphPad Prism 3 software (La Jolla, CA). Survival curves were analyzed using the log-rank test. Thrombolysis data were analyzed by continued-measures analysis of variance using StatView software version 5.0.1 (Cary, NC). All other differences between means of two genotypes were analyzed with the unpaired, two-tailed Student's t-test. Differences between groups were considered significant if P ≤ 0.05.
Results
Time to Artery Occlusion after Photochemical Injury Is Shortened in Fut−/− Mice
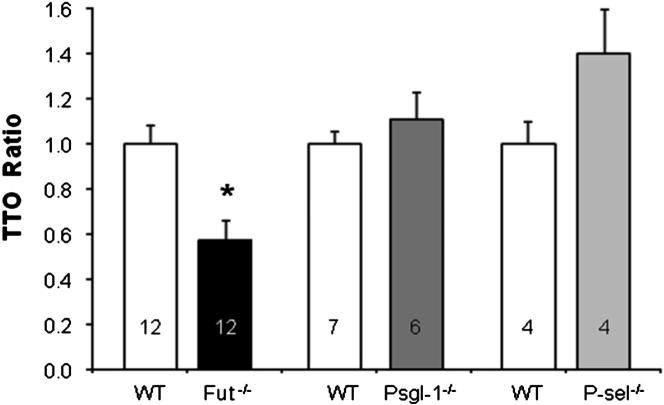
The thrombotic potential of Fut−/− mice was determined using photochemical-induced carotid artery thrombosis. Thrombus formation in this model is dependent on both platelet function and the coagulation cascade.26 Fut−/− mice had a 40% reduction in the time to occlusive thrombus formation (Figure 1) [time to occlusion (TTO) = 19.5 ± 9.6 minutes], compared with WT mice (TTO = 34 ± 9.7 minutes). The shortened TTO was not due to a functional defect in FUT-catalyzed PSGL-1 activity because Selplg−/− mice had a TTO similar to WT mice. In addition, Selp−/− mice had a prolonged TTO compared with WT mice. These results in mice lacking P-selectin or PSGL-1 are consistent with previously documented prothrombotic roles for these molecules.18,19 The data demonstrate that loss of fucosylation, mediated by FUT4 and FUT7, results in a prothrombotic phenotype, and that the phenotype is dominant to P-selectin– and PSGL-1–mediated mechanisms that promote thrombosis.
Figure 1.
Time to occlusion after carotid artery injury is decreased in Fut−/− mice. The TTO was determined in Fut−/−, Selplg−/−, Selp−/−, and corresponding WT (control) mice, all on a C57Bl/6 background, and normalized to the average (31 minutes) WT value. Fut−/− mice (n = 12) had a shortened TTO compared with WT (n = 12). ∗P = 0.002.
Pulmonary Thromboembolism Is Increased in Fut−/− Mice
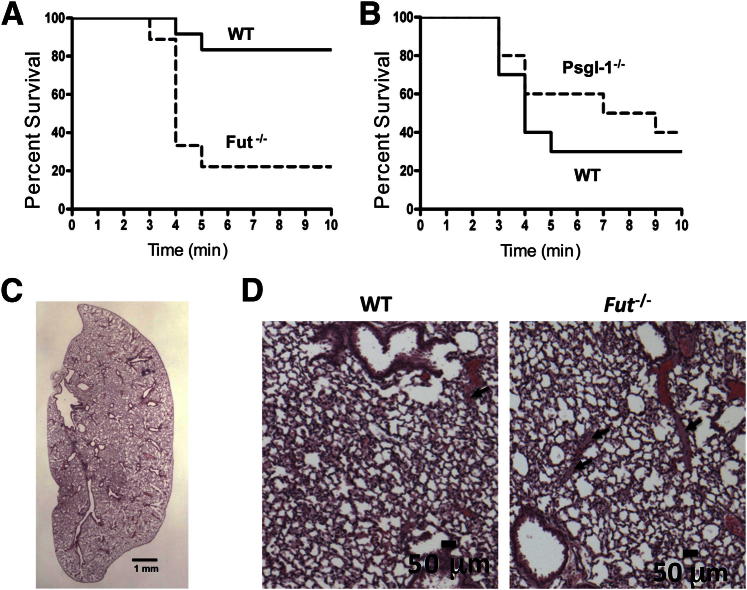
The prothrombotic phenotype was confirmed in Fut−/− mice using a model of pulmonary thromboembolism induced by infusion of a Col and Epi mixture. Mouse survival in this model is dependent on the number, size, and stability of thromboemboli formed as the result of the Col/Epi infusion. Fut−/− mice had a higher rate of mortality (P = 0.003) compared with WT mice in this model. Of WT mice, 83% (10 of 12) survived the challenge (300 μg Col/48 μg Epi per kg i.v.) compared with only 22% (2 of 9) of Fut−/− mice (Figure 2A). The thrombotic potential of PSGL-1–deficient mice was also examined using this model. However, at this dose, WT mice had a high rate of survival. Therefore, we used a higher Col/Epi dose (375 μg Col/60 μg Epi per kg i.v.) that resulted in a higher WT mortality rate to detect possible protection afforded by loss of PSGL-1. At this higher dose, 3 of 10 WT mice survived, and 4 of 10 PSGL-1–deficient mice survived (P = 0.27) (Figure 2B). Histological sections of lung tissue were analyzed for the presence of thromboemboli (Table 1 and Figures 2C and 2D). Fut−/− mice had a higher count of thromboemboli in lung tissue compared with WT mice, consistent with the higher mortality of these mice. No difference was found in thromboemboli count between WT and PSGL-1–deficient mice.
Figure 2.
Pulmonary thromboembolism-induced mortality is higher in Fut−/− mice. A: Kaplan-Meier survival curves for thromboembolus-induced mortality after treatment with 300 μg/kg Col and 48 μg/kg Epi. Mortality was significantly higher in Fut−/− mice (7 of 9) compared with WT mice (2 of 12). P = 0.003. B: Mortality of Selplg−/− mice (6 of 10) was similar to mortality for WT mice (7 of 10) after treatment with 375 μg/kg Col and 60 μg/kg Epi. P = 0.5. C: Representative low-power image of H&E-stained Fut−/− whole lung section used to determine the thrombus count. D: Representative high-power images of WT (left panel) or Fut−/− (right panel) lung sections showing the formed thrombi (arrows).
Table 1.
Genotype-Dependent Quantification of Pulmonary Thromboemboli
| Dose (μg/kg BW) | Genotype | Thrombi/cm2 | P value |
|---|---|---|---|
| Col 300/Epi 48 | WT | 110 ± 35 (n = 9) | 0.007 |
| Fut−/− | 251 ± 30 (n = 9) | ||
| Col 375/Epi 60 | WT | 341 ± 45 (n = 9) | 0.27 |
| Psgl-1−/− | 279 ± 33 (n = 10) |
Data are given as means ± SD.
BW, body weight.
Whole Blood Platelet Aggregation Is Enhanced in Fut−/− Mice
Platelets are a central contributor to both arterial and collagen/epinephrine-induced thrombosis. Circulating platelet counts were not different between WT (776 ± 118 × 106/mL, n = 13) and Fut−/− (852 ± 153 × 106/mL, n = 13) (P = 0.18) mice. Platelet aggregation was assessed using impedance aggregometry in whole blood (Table 2). These studies showed a consistently shortened lag time and increased rate of aggregation in Fut−/− whole blood triggered by 10 μmol/L of the platelet agonist, ADP, or 3 μg/mL collagen, compared with WT whole blood. The aggregation amplitude for these agonists was not significantly altered. Enhanced aggregation in Fut−/− mice was most pronounced when triggered by 0.5 mmol/L AA. The lag time was shortened by 52%, the rate was increased 2.1-fold, and the amplitude was increased 3.3-fold. Washed platelet aggregation was performed with the same agonists (Table 3), in an absorbance-based aggregometry assay, to determine whether the enhanced aggregation is the result of factors intrinsic or extrinsic to the platelet. No genotype-dependent differences in washed platelet aggregation were observed.
Table 2.
Whole Blood Platelet Aggregation
| Variable | Whole blood |
|||||
|---|---|---|---|---|---|---|
| ADP (10 μmol/L; n = 8) |
Col (3 μg/mL; n = 8) |
AA (0.5 mmol/L; n = 12) |
||||
| WT | Fut−/− | WT | Fut−/− | WT | Fut−/− | |
| LT (seconds) | 27.0 ± 0.6 | 22.2 ± 0.5∗ | 88.1 ± 3.2 | 78.0 ± 2.1∗ | 39.8 ± 6.2 | 20.8 ± 1.3∗ |
| Slope | 16.4 ± 1.0 | 21.4 ± 0.9∗ | 12.0 ± 0.8 | 14.8 ± 0.7∗ | 9.1 ± 2.2 | 19.3 ± 0.8∗ |
| MA (%) | 12.1 ± 0.6 | 13.0 ± 0.7 | 14.1 ± 0.6 | 13.5 ± 0.6 | 3.0 ± 0.9 | 9.8 ± 0.7∗ |
All values represent means ± SD.
AA, arachidonic acid; Col, collagen; LT, lag time; MA, maximum amplitude.
P < 0.05 vs the corresponding WT.
Table 3.
Washed Platelet Aggregation
| Variable | Washed platelets |
|||||
|---|---|---|---|---|---|---|
| ADP (10 μmol/L; n = 3)∗ |
Col (2 μg/mL; n = 5) |
AA (0.5 mmol/L; n = 5) |
||||
| WT | Fut−/− | WT | Fut−/− | WT | Fut−/− | |
| LT (seconds) | 14.0 ± 2.1 | 14.0 ± 2.5 | 95.2 ± 7.7 | 110 ± 11.3 | 35.0 ± 0.8 | 36.6 ± 1.1 |
| Slope | 67.7 ± 3.4 | 70.0 ± 2.5 | 59.0 ± 1.8 | 58.7 ± 1.5 | 71.1 ± 5.6 | 67.4 ± 1.5 |
| MA (%) | 77.7 ± 0.7 | 82.3 ± 3.3 | 81.8 ± 1.2 | 84.4 ± 0.5 | 73.5 ± 6.2 | 74.8 ± 1.3 |
All values represent means ± SD.
AA, arachidonic acid; Col, collagen; LT, lag time; MA, maximum amplitude.
Human fibrinogen (15 μg/mL final concentration) was added for ADP-stimulated washed platelet aggregation.
Prothrombin Time, Partial Thromboplastin Time, and Thrombin Generation Are Not Altered in Fut−/− Mice
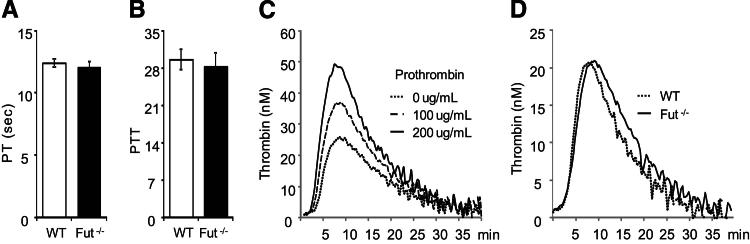
We examined whether fucosyltransferase deficiency altered traditional measures of coagulation pathway activation, including prothrombin time (PT) and activated partial thromboplastin time. There was no detectable difference in the plasma PT or activated partial thromboplastin time between Fut−/− and WT mice (Figure 3, A and B). Calibrated automated thrombography detected increased thrombin generation in a prothrombotic state mimicked by the addition of exogenous prothrombin (Figure 3C) and was, therefore, used to assess the prothombotic state of mouse plasma ex vivo. No difference in thrombin generation was detected between Fut−/− and WT plasma (Figure 3D), as determined by the lag time to thrombin generation, time to peak thrombin generation, peak height, or endogenous thrombin potential (area under the thrombin generation curve).
Figure 3.
Prothrombin time (PT), partial thromboplastin time (PTT), and thrombin generation in Fut−/− mice. A and B: The time to initial fibrin formation via the extrinsic (A) or intrinsic (B) pathway was not altered in Fut−/− mice. C: Calibrated automated thrombography detects increased thrombin generation in WT mouse PPP spiked with prothrombin. D: Thrombin generation and activity were not different between WT and Fut−/− mice, respectively, as determined by lag time (4.1 ± 1.4 versus 3.8 ± 0.8 seconds; n = 5 per group, P = 0.73), maximum thrombin generation peak (21.8 ± 3.1 versus 21.5 ± 1 nmol/L; P = 0.93), time to maximum thrombin generation (9.4 ± 1.7 versus 8.7 ± 1.4 seconds; P = 0.44), or endogenous thrombin potential (326 ± 84 versus 269 ± 20; P = 0.18).
Plasma Fibrinogen Concentration and Clot Strength Are Increased in Fut−/− Mice
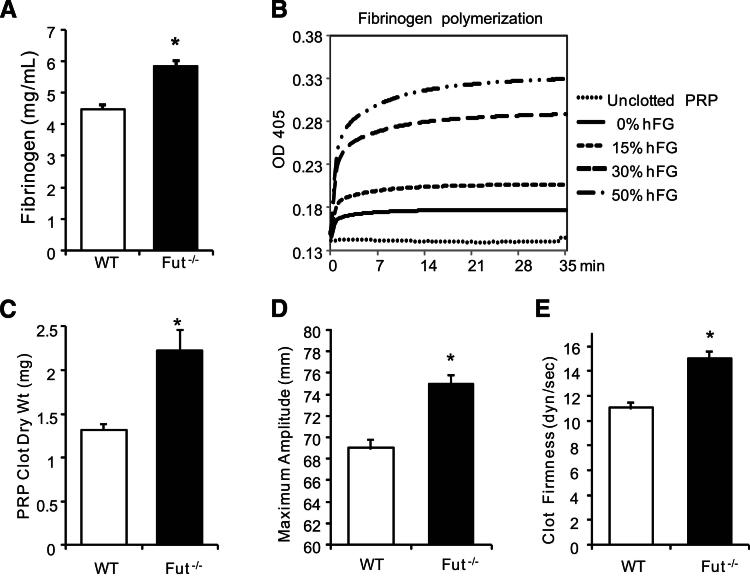
Plasma fibrinogen concentration (Figure 4A) was determined using a mouse fibrinogen-specific ELISA. Fut−/− plasma had a significantly higher concentration of fibrinogen (5.8 ± 0.4 mg/mL; 30% increase) compared with WT (4.5 ± 0.3 mg/mL). The fibrinogen level in Selplg−/− plasma (4.7 ± 0.6 mg/mL) was not different from WT, indicating that the increase is independent of PSGL-1 activity. A turbidometric measure of plasma fibrin polymerization (Figure 4B) showed that a 30% increase in fibrinogen concentration, achieved in vitro by addition of purified human fibrinogen, results in significantly more fibrin polymerization that could contribute to enhanced thrombus stabilization. Consistent with these data, clots that formed in Fut−/− PRP resulted in higher clot dry weight compared with WT (2.2 ± 0.6 versus 1.3 ± 0.2 mg; from 300-μL PRP with platelet counts at 300 × 106/mL) (Figure 4C).
Figure 4.
Plasma fibrinogen concentration, clot size, and clot strength are increased in Fut−/− mice. A: The fibrinogen concentration in Fut−/− plasma (5.83 ± 0.4 mg/mL, n = 6) is 30% higher than that in WT plasma (4.47 ± 0.6 mg/mL, n = 6). B: Fibrin polymerization was enhanced in WT mouse PPP spiked with human fibrinogen (hFG) to a final fibrinogen concentration of 4.5 mg/mL (0% hFG), 5.18 mg/mL (15% hFG), 5.85 mg/mL (30% hFG), and 6.75 mg/mL (50% hFG). Polymerization curves are representative of three independent experiments. C: Platelet-rich plasma (PRP) clot size is increased in Fut−/− plasma compared with WT plasma (2.2 ± 0.2 versus 1.3 ± 0.1 mg dry weight; n = 7 per genotype). D and E: Thromboelastogram parameters showed that an Fut−/− clot had an increased maximum amplitude (75 ± 0.8 versus 69 ± 0.9 mm; n = 7 per genotype; D) and increased clot firmness (shear elastic modulus strength, 15 ± 0.6 versus 11 ± 0.5 dyne/second; n = 7 per genotype; E) compared with WT control. ∗P < 0.001.
High fibrinogen concentrations facilitate formation of a densely packed fibrin network, and fibrin network structure can determine clot mechanical properties.27 Therefore, we tested the mechanical properties of whole blood clots from Fut−/− or WT mice using thromboelastography (TEG). Compared with WT mice, clots from Fut−/− whole blood were stronger and more elastic (Figure 4, C, D, and E), as measured by the TEG parameters of maximum amplitude and shear elastic modulus strength. These results are consistent with previous reports that elevated fibrinogen,27 in combination with enhanced platelet activation (Table 2), results in increased mechanical clot strength.
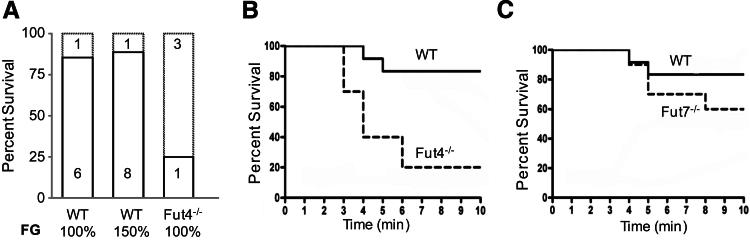
FUT4 Mediates Enhanced Pulmonary Thromboembolism Independent of Hyperfibrinogenemia
We tested whether the hyperfibrinogenemia observed in Fut−/− mice enhances Col/Epi-induced mortality. Pulmonary thromboembolism was induced in WT mice after infusion with PBS or human fibrinogen to obtain a plasma fibrinogen concentration equivalent to 150% of the WT concentration. The mortality rate was not higher in the hyperfibrinogenemic mice compared with the PBS-treated mice (Figure 5A). Mice deficient in FUT4 alone have slightly reduced plasma fibrinogen compared with WT (4.02 ± 0.4 versus 4.5 ± 0.3 mg/mL; n = 6 per group; P = 0.07), yet have a high rate of mortality (Figure 5B) comparable to the Fut−/− mice. In addition, mice deficient in FUT7 alone have a high plasma fibrinogen concentration compared with WT (5.6 ± 0.7 versus 4.5 ± 0.3 mg/mL; n = 6 per group; P = 0.01), but have a low mortality rate, similar to WT mice (Figure 5C). These data suggest that the 30% higher fibrinogen concentration in Fut−/− mice is not the primary cause of the high mortality in these mice and that increased thromboembolism-induced mortality is associated with loss of FUT4 activity.
Figure 5.
FUT4 mediates enhanced pulmonary thromboembolism independent of hyperfibrinogenemia. A: Col/Epi treatment caused high mortality of Fut4−/− mice (3 of 4 mice), but most WT mice survived (6 of 7 mice). Mortality of WT mice (1 of 9 mice) was not increased by hyperfibrinogenemia. B and C: Kaplan-Meyer survival curves show that thromboembolism-induced mortality was significantly higher in Fut4−/− mice (9 of 11, P = 0.002), but not in Fut7−/− mice (4 of 10, P = 0.25), compared with WT mice (2 of 12).
Reduced Fibrinolysis in Fut−/− Mice
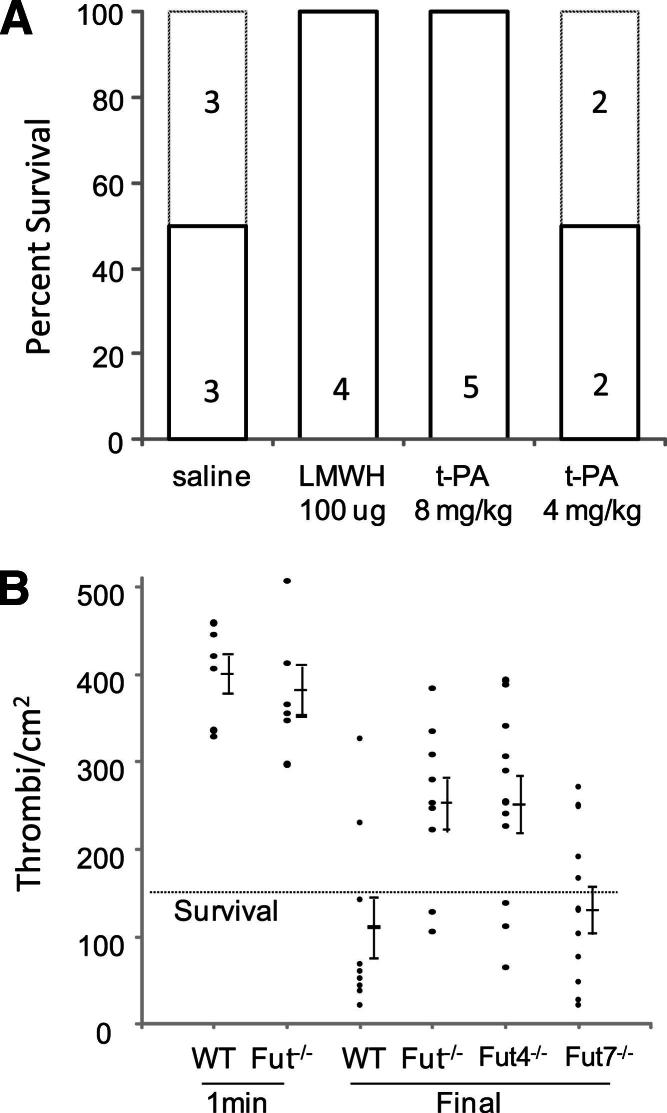
Experiments tested whether fibrinolysis is altered in Fut−/− mice. Endothelial cell–derived tissue-type plasminogen activator (t-PA) and urokinase-type plasminogen activator incorporate into the forming clot and convert plasminogen to plasmin, which lyses fibrin into soluble FDPs. We first demonstrated the role of the coagulation system in the pathogenesis of pulmonary thromboembolism induced by the platelet-activating agents, collagen and epinephrine. Pulmonary thromboembolism was induced in control WT mice or WT mice i.v. treated with either 100 μg low-molecular-weight heparin (Lovenox; Sanofi US, Bridgewater, NJ) or 4 or 8 mg/kg t-PA (human t-PA; Activase; EMD Chemicals, La Jolla, CA). Treatment with low-molecular-weight heparin or the higher dose of t-PA protected the mice from pulmonary thromboembolism–induced mortality (Figure 6A). These data confirm that the coagulation cascade plays a critical role in collagen/epinephrine-induced thromboembolism, likely by stabilizing formed platelet-rich microthrombi, and suggest that thrombolysis may be an important protective mechanism in this model.
Figure 6.
Thrombolysis protects mice from collagen/epinephrine-induced pulmonary thromboembolism. A: Thromboembolism was triggered in WT mice treated as indicated. All mice that received PBS or low dose t-PA (4 mg/kg) had respiratory distress, and 50% succumbed to the treatment. In contrast, mice treated with low-molecular-weight heparin or 8 mg/kg t-PA showed no signs of pulmonary distress, and 100% survived. B: At 1 minute after treatment with Col/Epi, all mice were alive and the number of thrombi/cm2 lung was similar between WT and Fut−/− mice. At the final time point (death or 10 minutes), most WT and Fut7−/− mice survived, and had reduced thrombi counts. In contrast, most Fut−/− and Fut4−/− mice died and had high thrombi counts. All mice below the dotted line survived, except one Fut4−/− mouse, and all mice above the line died.
The temporal appearance and resolution of pulmonary thromboemboli were determined to assess genotype-dependent alterations in thrombolysis in this model. The number of thromboemboli present in the lungs was not different between WT and Fut−/− mice (WT, 399 ± 22; Fut−/−, 381 ± 29; n = 6 per group; P = 0.6) (Figure 6B) when counted early (1 minute) after Col/Epi infusion. In contrast, when thromboemboli were counted late (at death or 10 minutes) after treatment, there were far fewer thromboemboli in lungs of WT mice (110 ± 35/cm2, n = 9) or Fut7−/− mice (129 ± 28/cm2, n = 10; P = 0.67), most of which survived, compared with the Fut−/− (251 ± 30/cm2, n = 9; P = 0.007) or Fut4−/− (250 ± 33/cm2, n = 11; P = 0.009) mice, most of which died. These temporal changes in thromboemboli counts indicate that fucosyltransferase deficiency does not significantly alter initial thrombus formation, but that subsequent thrombus dissolution (thrombolysis) is reduced in Fut−/− or Fut4−/− mice that have high mortality rates. For standardization, lungs from mice that died prematurely were harvested at the 10-minute time point, theoretically allowing for changes in thrombus size or number in the interim. However, without hemodynamic forces or circulation to deliver or remove plasma or thrombus components, such changes should be limited.
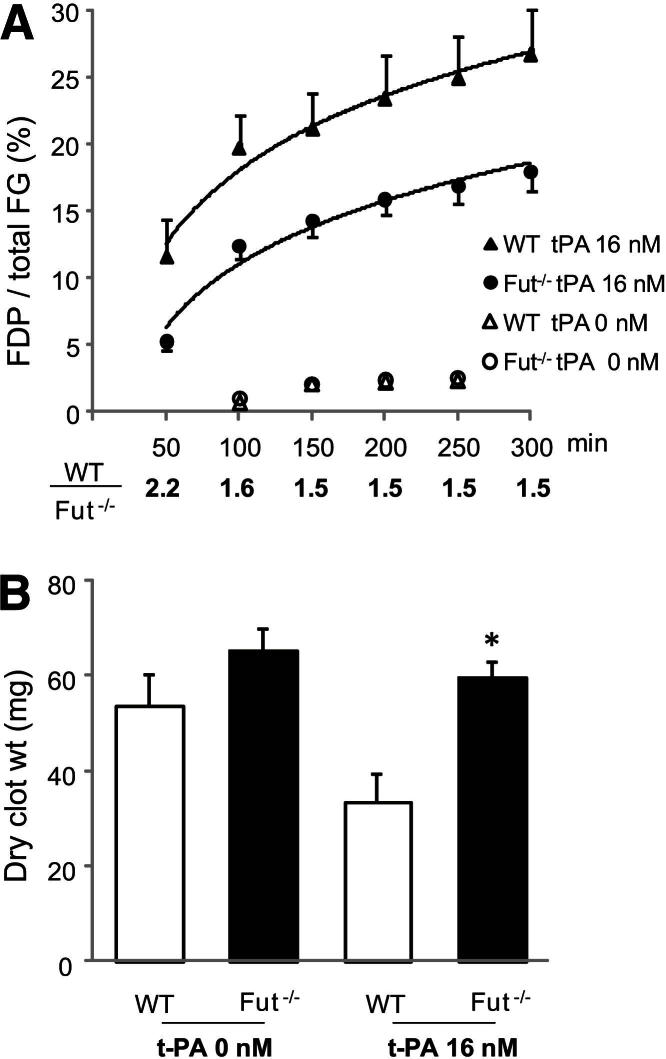
Fibrinolysis of whole blood clot was directly evaluated by mixing 16 nmol/L exogenous t-PA into whole blood before clot formation was initiated with CaCl2 and excess thrombin (final, 1 U/mL). This procedure was used because a 400-fold greater concentration of t-PA is required to achieve the same degree of lysis if the t-PA is added subsequent to clot formation.28 Fluorescence-labeled human fibrinogen (fibrinogen-AF647; 10 μg), which is approximately 1% of total fibrinogen, was added to whole blood before coagulation initiation, and the extent of clot lysis was determined by measuring the percentage of total fibrinogen-AF647 released from the clot as FDP-AF647. Minimal clot lysis occurred in the absence of exogenous t-PA (Figure 7A) (t-PA, 0 nmol/L), and there was no difference in lysis between genotypes. In contrast, significant lysis occurred with 16 nmol/L t-PA, but the lysis of Fut−/− whole blood clot was significantly reduced at each time point by an average of 46% compared with WT clot (Figure 7A). In a separate experiment, the dry weight of whole blood clot was measured after clotting in the presence or absence of 16 nmol/L t-PA. Consistent with the clot lysis data, Fut−/− whole blood clot retained its clot mass better than WT whole blood clot. Clot lysis with 16 nmol/L t-PA reduced WT clot dry weight by 38% from the weight without t-PA (Figure 7B). In contrast, t-PA only reduced Fut−/− dry clot weight by 9%. Together, these data demonstrate that clots from Fut−/− mice are resistant to lysis, and provide additional evidence that fucosyltransferase-dependent modulation of thrombolysis contributes to the observed prothrombotic phenotype.
Figure 7.
A whole blood clot from Fut−/− mice is resistant to t-PA–mediated lysis. A: Clot lysis was assessed by the release of AF647-labeled fibrin degradation products (FDP-AF647). The WT and Fut−/− curves are significantly different (WT, n = 4; Fut−/−, n = 5; P = 0.03). The percentage lysis ratio (WT to Fut−/−) at each time point is indicated below the graph. B: Clot size in the absence and presence of t-PA. The addition of 16 nmol/L t-PA reduced WT (n = 8) dry clot weight by 38%. In contrast, Fut−/− clot weight (n = 7) was only reduced by 9% by the addition of t-PA. The t-PA–treated Fut−/− whole blood clot size was significantly heavier than the t-PA–treated WT clots. ∗P = 0.005.
Discussion
Selectin-dependent processes can modulate thrombosis and hemostasis by several recently reviewed mechanisms.18,19 PSGL-1 or glycoprotein Ib expressed on platelets binds endothelial P-selectin to enable platelet rolling and recruitment to a forming thrombus. Binding of membrane-bound or soluble P-selectin to leukocyte PSGL-1 induces expression of tissue factor on monocytes and the formation of procoagulant leukocyte microparticles that can subsequently be recruited to the forming thrombus. Mice harboring targeted mutations of Fut4 and Fut7 lack functional selectin ligand activity11,12 and, therefore, lack P-selectin/PSGL-1 binding interactions. Together, these findings suggested a potential role for FUT4 and FUT7 in modulating selectin-dependent thrombotic processes. They predict that Fut−/− mice would have an anti-thrombotic phenotype because they lack the fucosylation of PSGL-1 that is necessary for P-selectin binding. In contrast to the prediction, our findings show that these mice have a shortened time to thrombotic occlusion after photochemical-induced carotid artery injury. This model was initially chosen to examine large-artery thrombosis instead of microvascular thrombosis, in which P-selectin interactions with PSGL-1 are known to enhance thrombosis, and because of the limited vascular injury compared with the transmural injury caused by ferric chloride treatment. The thrombus in this model of carotid artery injury is platelet rich, and this model has been used to study the role of platelets and platelet inhibitors in arterial thrombosis.29 In addition, coagulation-modifying factors, such as plasminogen activator inhibitor-1 and vitronectin, also modulate thrombosis in this model.22,30 The prothrombotic phenotype was confirmed in a second thrombosis model by showing that Fut−/− mice have increased mortality in a model of pulmonary thromboembolism. The role of platelets in this model is evident in the model's dependence on the platelet-activating agents, epinephrine and collagen, and modulation of platelet function in this model alters thromboembolus formation.31,32 However, coagulation factors are also critical in this model.33 In addition, P-selectin/PSGL-1–mediated processes, including microparticle formation, are known to be important in venous thrombus formation and resolution.34 Thus, thrombus formation in both models involves platelet activation and aggregation, as well as activation of the coagulation cascade and fibrin formation, to stabilize the thrombus.
The plasma fibrinogen concentration in Fut−/− mice was 30% higher than in WT mice. Fibrinogen plays important roles in hemostasis and thrombosis by bridging platelets during aggregation, forming the fibrin network, and enabling platelet-dependent clot retraction. Hyperfibrinogenemia decreases time to occlusion after arterial injury and increases platelet-dependent clot retraction.27,35 Machlus et al27 showed a decreased time to carotid artery thrombosis in mice with human fibrinogen-induced hyperfibrinogenemia (170% of WT) using the ferric chloride–induced injury model. Our data in the photochemical-induced injury model showed a shortened time to arterial thrombosis in Fut−/− mice with less severe hyperfibrinogenemia (130% of WT). However, the time to occlusion was not shortened in Fut7−/− mice with hyperfibrinogenemia of a similar magnitude (125% of WT), suggesting that hyperfibrinogenemia is not the primary mechanism for enhanced arterial thrombosis in the Fut−/− mice.
In our pulmonary thromboembolism studies, hyperfibrinogenemia did not correlate with thromboembolism-induced mortality. Mortality was enhanced in mice lacking both FUT4 and FUT7 that were hyperfibrinogenemic (130% of WT) and in mice lacking only FUT4 that had normal to slightly low plasma fibrinogen, but not in mice lacking FUT7 that were hyperfibrinogenemic (125% of WT). In addition, mortality was not increased in WT mice made hyperfibrinogenemic (150% of WT) by treatment with fibrinogen. These data show that mortality in this model is largely independent of the plasma fibrinogen concentration if it is near or higher than the concentration in WT mice. A possible alternative mechanism to account for a high mortality rate is decreased thrombolysis. Support for this mechanism is provided by the in vitro data showing that t-PA–mediated Fut−/− fibrin clot degradation is reduced by 45% compared with WT (Figure 7) and the in vivo data showing that thromboembolus dissolution during the experiment was reduced in Fut−/− and Fut4−/− mice, resulting in higher mortality compared with WT and Fut7−/− mice (Figure 6B).
Moderate enhancement of Fut−/− whole blood aggregation was measured in response to several agonists, but was most pronounced in response to AA. These data suggest that the mechanism of enhancement, not specifically determined by our experiments, is independent of pathways specific to a given agonist/receptor. Aggregation of washed Fut−/− platelets was not altered compared with WT. This finding may be the result of the wash protocol (ie, treatment with PGE1 or the removal of plasma components, including fibrinogen) or the experimental methods (impedance versus absorbance), or may suggest that the enhancement is not due to a mechanism intrinsic to the platelet. The enhanced whole blood platelet aggregation in Fut−/− mice was not associated with more thromboemboli in the lungs of Fut−/− mice early after collagen-epinephrine infusion. These data suggest that the moderate enhancement of platelet aggregation also does not lead to increased thromboemboli formation in Fut4−/− mice. However, activated platelets contribute to processes that stabilize a thrombus, once formed. They enhance thrombin generation through the release of coagulation factors and the assembly of thrombin-generating molecular complexes on their surface.36,37 Activated platelets also decrease fibrinolysis by releasing plasminogen activator inhibitor-138,39 from α granules, by enhancing clot retraction,40,41 and by increasing platelet release and activation of thrombin-activatable fibrinolysis inhibitor.41 Therefore, it is possible that the increased platelet function in Fut−/− mice contributed to the prothrombotic phenotype of these mice by helping to stabilize the formed thrombi.
Many coagulation factors are heavily glycosylated,1 and variation in the glycosylation pattern can significantly alter the function of a factor. Glycosylation affects the kinetics of fibrinogen polymerization,42 clearance of von Willebrand factor43 and antithrombin,3 isoform activity of factor V,2,44 post-translational folding of factor VII,45 activation of factor IX,46 activity of plasminogen activator inhibitor-1 and protein S4, and biochemical properties of thrombin-activatable fibrinolysis inhibitor.47 Our data show that glycan α(1,3)-fucosylation modulates the thrombotic process by mechanisms that are independent of P-selectin/PSGL-1–mediated events and dependent on FUT4 activity, suggesting that altered fucosylation of a coagulation factor may account for the phenotype.
Fut−/− mice have a marked deficiency in selectin ligand activity that severely limits selectin-dependent trafficking of neutrophils and monocytes, and homing of naive T lymphocytes.11,12 In addition, loss of P-selectin–dependent interactions has been shown to reduce microvascular thrombosis.18,19 These two phenotypes would be expected to limit atherogenesis in mice deficient in apolipoprotein E (ApoE) and FUT7 (ApoE−/−/Fut7−/−). However, Fut−/− mice have a marked neutrophilia and a moderate monocytosis,12 and the studies reported herein show a dominate prothrombotic phenotype in these mice. In contrast to the first two phenotypes, the latter two would be expected to exacerbate atherogenesis in ApoE−/−/Fut7−/− mice. Previous studies showed that atherosclerotic lesion size in ApoE−/− mice is limited by loss of FUT7 activity.14 Together, these findings suggest that the anti-inflammatory effects afforded by reduced leukocyte trafficking and lymphocyte homing are the dominant phenotype with respect to atherogenesis in these mice.
Previous human studies have identified single-nucleotide polymorphisms that decrease FUT3 activity and lead to increased formation of the Le(a−b−) antigen, which is associated with atherothrombotic disease.7,8 Synthesis of Lea and Leb by FUT3 requires α(1,4)-fucosylation of a type I precursor, but FUT3 also has α(1,3) activity and can use type II precursors similar to FUT4.48 In contrast, FUT4 is not known to have α(1,4)-fucosyltransferase activity. Both FUT3 and FUT4 are expressed in a fairly broad and overlapping tissue distribution, including in the liver and epithelial tissues. However, FUT3 is a pseudogene in the mouse. Tissue expression of FUT7 is limited and does not overlap with FUT3. Given the prothrombotic phenotype associated with loss of FUT4 in mice, it is interesting to consider whether the atherothrombotic disease associated with decreased FUT3 activity in humans is due to loss of FUT3-mediated α(1,3)-fucosyltransferase activity that is redundant with FUT4 activity. Additional studies are warranted to better understand the mechanism(s) by which FUT4 and FUT7 contribute to homeostasis of the coagulation system.
Acknowledgments
We thank Dr. Robert Bagnell (Microscopy Services Laboratory, University of North Carolina) for expert assistance and Dr. Paul Monahan (University of North Carolina) for his assistance with the prothrombin time and partial thromboplastin time assays.
Footnotes
Supported by NIH grants HL090823 (J.W.H.) and HL073150 (D.T.E.).
References
- 1.Hansson K., Stenflo J. Post-translational modifications in proteins involved in blood coagulation. J Thromb Haemost. 2005;3:2633–2648. doi: 10.1111/j.1538-7836.2005.01478.x. [DOI] [PubMed] [Google Scholar]
- 2.Nicolaes G.A., Villoutreix B.O., Dahlback B. Partial glycosylation of Asn2181 in human factor V as a cause of molecular and functional heterogeneity: modulation of glycosylation efficiency by mutagenesis of the consensus sequence for N-linked glycosylation. Biochemistry. 1999;38:13584–13591. doi: 10.1021/bi991165r. [DOI] [PubMed] [Google Scholar]
- 3.Ni H., Blajchman M.A., Ananthanarayanan V.S., Smith I.J., Sheffield W.P. Mutation of any site of N-linked glycosylation accelerates the in vivo clearance of recombinant rabbit antithrombin. Thromb Res. 2000;99:407–415. doi: 10.1016/s0049-3848(00)00263-2. [DOI] [PubMed] [Google Scholar]
- 4.Gils A., Pedersen K.E., Skottrup P., Christensen A., Naessens D., Deinum J., Enghild J.J., Declerck P.J., Andreasen P.A. Biochemical importance of glycosylation of plasminogen activator inhibitor-1. Thromb Haemost. 2003;90:206–217. doi: 10.1160/TH03-01-0034. [DOI] [PubMed] [Google Scholar]
- 5.Lu D., Xie R.L., Rydzewski A., Long G.L. The effect of N-linked glycosylation on molecular weight, thrombin cleavage, and functional activity of human protein S. Thromb Haemost. 1997;77:1156–1163. [PubMed] [Google Scholar]
- 6.Ma B., Simala-Grant J.L., Taylor D.E. Fucosylation in prokaryotes and eukaryotes. Glycobiology. 2006;16:158R–184R. doi: 10.1093/glycob/cwl040. [DOI] [PubMed] [Google Scholar]
- 7.Djousse L., Karamohamed S., Herbert A.G., D'Agostino R.B., Cupples L.A., Ellison R.C. Fucosyltransferase 3 polymorphism and atherothrombotic disease in the Framingham Offspring Study. Am Heart J. 2007;153:636–639. doi: 10.1016/j.ahj.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellison R.C., Zhang Y., Myers R.H., Swanson J.L., Higgins M., Eckfeldt J. Lewis blood group phenotype as an independent risk factor for coronary heart disease (the NHLBI Family Heart Study) Am J Cardiol. 1999;83:345–348. doi: 10.1016/s0002-9149(98)00866-2. [DOI] [PubMed] [Google Scholar]
- 9.Gersten K.M., Natsuka S., Trinchera M., Petryniak B., Kelly R.J., Hiraiwa N., Jenkins N.A., Gilbert D.J., Copeland N.G., Lowe J.B. Molecular cloning, expression, chromosomal assignment, and tissue-specific expression of a murine alpha-(1,3)-fucosyltransferase locus corresponding to the human ELAM-1 ligand fucosyl transferase. J Biol Chem. 1995;270:25047–25056. doi: 10.1074/jbc.270.42.25047. [DOI] [PubMed] [Google Scholar]
- 10.Martinez M., Joffraud M., Giraud S., Baisse B., Bernimoulin M.P., Schapira M., Spertini O. Regulation of PSGL-1 interactions with L-selectin, P-selectin, and E-selectin: role of human fucosyltransferase-IV and -VII. J Biol Chem. 2005;280:5378–5390. doi: 10.1074/jbc.M410899200. [DOI] [PubMed] [Google Scholar]
- 11.Maly P., Thall A., Petryniak B., Rogers C.E., Smith P.L., Marks R.M., Kelly R.J., Gersten K.M., Cheng G., Saunders T.L., Camper S.A., Camphausen R.T., Sullivan F.X., Isogai Y., Hindsgaul O., von Andrian U.H., Lowe J.B. The alpha(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- 12.Homeister J.W., Thall A.D., Petryniak B., Maly P., Rogers C.E., Smith P.L., Kelly R.J., Gersten K.M., Askari S.W., Cheng G., Smithson G., Marks R.M., Misra A.K., Hindsgaul O., von Andrian U.H., Lowe J.B. The alpha(1,3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity. 2001;15:115–126. doi: 10.1016/s1074-7613(01)00166-2. [DOI] [PubMed] [Google Scholar]
- 13.Smithson G., Rogers C.E., Smith P.L., Scheidegger E.P., Petryniak B., Myers J.T., Kim D.S., Homeister J.W., Lowe J.B. Fuc-TVII is required for T helper 1 and T cytotoxic 1 lymphocyte selectin ligand expression and recruitment in inflammation, and together with Fuc-TIV regulates naive T cell trafficking to lymph nodes. J Exp Med. 2001;194:601–614. doi: 10.1084/jem.194.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Homeister J.W., Daugherty A., Lowe J.B. Alpha(1,3)fucosyltransferases FucT-IV and FucT-VII control susceptibility to atherosclerosis in apolipoprotein E-/- mice. Arterioscler Thromb Vasc Biol. 2004;24:1897–1903. doi: 10.1161/01.ATV.0000141844.28073.df. [DOI] [PubMed] [Google Scholar]
- 15.Gitlin J.M., Homeister J.W., Bulgrien J., Counselman J., Curtiss L.K., Lowe J.B., Boisvert W.A. Disruption of tissue-specific fucosyltransferase VII, an enzyme necessary for selectin ligand synthesis, suppresses atherosclerosis in mice. Am J Pathol. 2009;174:343–350. doi: 10.2353/ajpath.2009.080036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burne M.J., Rabb H. Pathophysiological contributions of fucosyltransferases in renal ischemia reperfusion injury. J Immunol. 2002;169:2648–2652. doi: 10.4049/jimmunol.169.5.2648. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto M., Yamamoto F., Luong T.T., Williams T., Kominato Y. Expression profiling of 68 glycosyltransferase genes in 27 different human tissues by the systematic multiplex reverse transcription-polymerase chain reaction method revealed clustering of sexually related tissues in hierarchical clustering algorithm analysis. Electrophoresis. 2003;24:2295–2307. doi: 10.1002/elps.200305459. [DOI] [PubMed] [Google Scholar]
- 18.Vandendries E.R., Furie B.C., Furie B. Role of P-selectin and PSGL-1 in coagulation and thrombosis. Thromb Haemost. 2004;92:459–466. doi: 10.1160/TH04-05-0306. [DOI] [PubMed] [Google Scholar]
- 19.Polgar J., Matuskova J., Wagner D.D. The P-selectin, tissue factor, coagulation triad. J Thromb Haemost. 2005;3:1590–1596. doi: 10.1111/j.1538-7836.2005.01373.x. [DOI] [PubMed] [Google Scholar]
- 20.Marquardt T., Brune T., Luhn K., Zimmer K.P., Korner C., Fabritz L., van der Werft N., Vormoor J., Freeze H.H., Louwen F., Biermann B., Harms E., von Figura K., Vestweber D., Koch H.G. Leukocyte adhesion deficiency II syndrome, a generalized defect in fucose metabolism. J Pediatr. 1999;134:681–688. doi: 10.1016/S0022-3476(99)70281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bengtson P., Larson C., Lundblad A., Larson G., Pahlsson P. Identification of a missense mutation (G329A;Arg(110)–> GLN) in the human FUT7 gene. J Biol Chem. 2001;276:31575–31582. doi: 10.1074/jbc.M104165200. [DOI] [PubMed] [Google Scholar]
- 22.Eitzman D.T., Westrick R.J., Nabel E.G., Ginsburg D. Plasminogen activator inhibitor-1 and vitronectin promote vascular thrombosis in mice. Blood. 2000;95:577–580. [PubMed] [Google Scholar]
- 23.Sather S., Kenyon K.D., Lefkowitz J.B., Liang X., Varnum B.C., Henson P.M., Graham D.K. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood. 2007;109:1026–1033. doi: 10.1182/blood-2006-05-021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolberg A.S., Monroe D.M., Roberts H.R., Hoffman M. Elevated prothrombin results in clots with an altered fiber structure: a possible mechanism of the increased thrombotic risk. Blood. 2003;101:3008–3013. doi: 10.1182/blood-2002-08-2527. [DOI] [PubMed] [Google Scholar]
- 25.Traverso C.I., Caprini J.A., Arcelus J.I. The normal thromboelastogram and its interpretation. Semin Thromb Hemost. 1995;21(Suppl 4):7–13. doi: 10.1055/s-0032-1313615. [DOI] [PubMed] [Google Scholar]
- 26.Rosen E.D., Raymond S., Zollman A., Noria F., Sandoval-Cooper M., Shulman A., Merz J.L., Castellino F.J. Laser-induced noninvasive vascular injury models in mice generate platelet- and coagulation-dependent thrombi. Am J Pathol. 2001;158:1613–1622. doi: 10.1016/S0002-9440(10)64117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Machlus K.R., Cardenas J.C., Church F.C., Wolberg A.S. Causal relationship between hyperfibrinogenemia, thrombosis, and resistance to thrombolysis in mice. Blood. 2011;117:4953–4963. doi: 10.1182/blood-2010-11-316885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishnamurti C., Vukelja S.J., Alving B.M. Inhibitory effects of lysine analogues on t-PA induced whole blood clot lysis. Thromb Res. 1994;73:419–430. doi: 10.1016/0049-3848(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 29.Saniabadi A.R., Umemura K., Matsumoto N., Sakuma S., Nakashima M. Vessel wall injury and arterial thrombosis induced by a photochemical reaction. Thromb Haemost. 1995;73:868–872. [PubMed] [Google Scholar]
- 30.Konstantinides S., Schafer K., Thinnes T., Loskutoff D.J. Plasminogen activator inhibitor-1 and its cofactor vitronectin stabilize arterial thrombi after vascular injury in mice. Circulation. 2001;103:576–583. doi: 10.1161/01.cir.103.4.576. [DOI] [PubMed] [Google Scholar]
- 31.Angelillo-Scherrer A., de Frutos P., Aparicio C., Melis E., Savi P., Lupu F., Arnout J., Dewerchin M., Hoylaerts M., Herbert J., Collen D., Dahlback B., Carmeliet P. Deficiency or inhibition of Gas6 causes platelet dysfunction and protects mice against thrombosis. Nat Med. 2001;7:215–221. doi: 10.1038/84667. [DOI] [PubMed] [Google Scholar]
- 32.Oury C., Kuijpers M.J., Toth-Zsamboki E., Bonnefoy A., Danloy S., Vreys I., Feijge M.A., De Vos R., Vermylen J., Heemskerk J.W., Hoylaerts M.F. Overexpression of the platelet P2X1 ion channel in transgenic mice generates a novel prothrombotic phenotype. Blood. 2003;101:3969–3976. doi: 10.1182/blood-2002-10-3215. [DOI] [PubMed] [Google Scholar]
- 33.Renne T., Pozgajova M., Gruner S., Schuh K., Pauer H.U., Burfeind P., Gailani D., Nieswandt B. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202:271–281. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramacciotti E., Hawley A.E., Farris D.M., Ballard N.E., Wrobleski S.K., Myers D.D., Jr., Henke P.K., Wakefield T.W. Leukocyte- and platelet-derived microparticles correlate with thrombus weight and tissue factor activity in an experimental mouse model of venous thrombosis. Thromb Haemost. 2009;101:748–754. [PMC free article] [PubMed] [Google Scholar]
- 35.Dempfle C.E., Kälsch T., Elmas E., Suvajac N., Lücke T., Münch E., Borggrefe M. Impact of fibrinogen concentration in severely ill patients on mechanical properties of whole blood clots. Blood Coagul Fibrinolysis. 2008;19:765–770. doi: 10.1097/MBC.0b013e32830f1b68. [DOI] [PubMed] [Google Scholar]
- 36.Walsh P.N. Platelet coagulation-protein interactions. Semin Thromb Hemost. 2004;30:461–471. doi: 10.1055/s-2004-833481. [DOI] [PubMed] [Google Scholar]
- 37.Nicolaes G.A., Dahlback B. Factor V and thrombotic disease: description of a janus-faced protein. Arterioscler Thromb Vasc Biol. 2002;22:530–538. doi: 10.1161/01.atv.0000012665.51263.b7. [DOI] [PubMed] [Google Scholar]
- 38.Fay W.P., Eitzman D.T., Shapiro A.D., Madison E.L., Ginsburg D. Platelets inhibit fibrinolysis in vitro by both plasminogen activator inhibitor-1-dependent and -independent mechanisms. Blood. 1994;83:351–356. [PubMed] [Google Scholar]
- 39.Levi M., Biemond B.J., van Zonneveld A.J., ten Cate J.W., Pannekoek H. Inhibition of plasminogen activator inhibitor-1 activity results in promotion of endogenous thrombolysis and inhibition of thrombus extension in models of experimental thrombosis. Circulation. 1992;85:305–312. doi: 10.1161/01.cir.85.1.305. [DOI] [PubMed] [Google Scholar]
- 40.Collet J.P., Montalescot G., Lesty C., Soria J., Mishal Z., Thomas D., Soria C. Disaggregation of in vitro preformed platelet-rich clots by abciximab increases fibrin exposure and promotes fibrinolysis. Arterioscler Thromb Vasc Biol. 2001;21:142–148. doi: 10.1161/01.atv.21.1.142. [DOI] [PubMed] [Google Scholar]
- 41.Carrieri C., Galasso R., Semeraro F., Ammollo C.T., Semeraro N., Colucci M. The role of thrombin activatable fibrinolysis inhibitor and factor XI in platelet-mediated fibrinolysis resistance: a thromboelastographic study in whole blood. J Thromb Haemost. 2011;9:154–162. doi: 10.1111/j.1538-7836.2010.04120.x. [DOI] [PubMed] [Google Scholar]
- 42.Langer B.G., Weisel J.W., Dinauer P.A., Nagaswami C., Bell W.R. Deglycosylation of fibrinogen accelerates polymerization and increases lateral aggregation of fibrin fibers. J Biol Chem. 1988;263:15056–15063. [PubMed] [Google Scholar]
- 43.Ellies L.G., Ditto D., Levy G.G., Wahrenbrock M., Ginsburg D., Varki A., Le D.T., Marth J.D. Sialyltransferase ST3Gal-IV operates as a dominant modifier of hemostasis by concealing asialoglycoprotein receptor ligands. Proc Natl Acad Sci U S A. 2002;99:10042–10047. doi: 10.1073/pnas.142005099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silveira J.R., Kalafatis M., Tracy P.B. Carbohydrate moieties on the procofactor factor V, but not the derived cofactor factor Va, regulate its inactivation by activated protein C. Biochemistry. 2002;41:1672–1680. doi: 10.1021/bi011304g. [DOI] [PubMed] [Google Scholar]
- 45.Bolt G., Kristensen C., Steenstrup T.D. Posttranslational N-glycosylation takes place during the normal processing of human coagulation factor VII. Glycobiology. 2005;15:541–547. doi: 10.1093/glycob/cwi032. [DOI] [PubMed] [Google Scholar]
- 46.Hertzberg M.S., Facey S.L., Hogg P.J. An Arg/Ser substitution in the second epidermal growth factor-like module of factor IX introduces an O-linked carbohydrate and markedly impairs activation by factor XIa and factor VIIa/tissue factor and catalytic efficiency of factor IXa. Blood. 1999;94:156–163. [PubMed] [Google Scholar]
- 47.Valnickova Z., Christensen T., Skottrup P., Thogersen I.B., Hojrup P., Enghild J.J. Post-translational modifications of human thrombin-activatable fibrinolysis inhibitor (TAFI): evidence for a large shift in the isoelectric point and reduced solubility upon activation. Biochemistry. 2006;45:1525–1535. doi: 10.1021/bi051956v. [DOI] [PubMed] [Google Scholar]
- 48.Kukowska-Latallo J.F., Larsen R.D., Nair R.P., Lowe J.B. A cloned human cDNA determines expression of a mouse stage-specific embryonic antigen and the Lewis blood group alpha(1,3/1,4)fucosyltransferase. Genes Dev. 1990;4:1288–1303. doi: 10.1101/gad.4.8.1288. [DOI] [PubMed] [Google Scholar]