CYCLIN H;1 regulates reactive oxygen species-dependent but abscisic acid-independent inhibition of blue light-induced stomatal aperture required for drought stress responses.
Abstract
Arabidopsis (Arabidopsis thaliana) CYCLIN-DEPENDENT KINASE Ds (CDKDs) phosphorylate the C-terminal domain of the largest subunit of RNA polymerase II. Arabidopsis CYCLIN H;1 (CYCH;1) interacts with and activates CDKDs; however, the physiological function of CYCH;1 has not been determined. Here, we report that CYCH;1, which is localized to the nucleus, positively regulates blue light-induced stomatal opening. Reduced-function cych;1 RNA interference (cych;1 RNAi) plants exhibited a drought tolerance phenotype. CYCH;1 is predominantly expressed in guard cells, and its expression was substantially down-regulated by dehydration. Transpiration of intact leaves was reduced in cych;1 RNAi plants compared with the wild-type control in light but not in darkness. CYCH;1 down-regulation impaired blue light-induced stomatal opening but did not affect guard cell development or abscisic acid-mediated stomatal closure. Microarray and real-time polymerase chain reaction analyses indicated that CYCH;1 did not regulate the expression of abscisic acid-responsive genes or light-induced stomatal opening signaling determinants, such as MYB60, MYB61, Hypersensitive to red and blue1, and Protein phosphatase7. CYCH;1 down-regulation induced the expression of redox homeostasis genes, such as LIPOXYGENASE3 (LOX3), LOX4, ARABIDOPSIS GLUTATHIONE PEROXIDASE 7 (ATGPX7), EARLY LIGHT-INDUCIBLE PROTEIN1 (ELIP1), and ELIP2, and increased hydrogen peroxide production in guard cells. Furthermore, loss-of-function mutations in CDKD;2 or CDKD;3 did not affect responsiveness to drought stress, suggesting that CYCH;1 regulates the drought stress response in a CDKD-independent manner. We propose that CYCH;1 regulates blue light-mediated stomatal opening by controlling reactive oxygen species homeostasis.
The fine control of the stomatal aperture is essential for minimizing transpiration and optimizing CO2 fixation. The stomatal aperture is regulated by several environmental and internal signals, such as light, the phytohormone abscisic acid (ABA), CO2, calcium, reactive oxygen species (ROS), humidity, temperature, and pathogens (Assmann and Shimazaki, 1999; Blatt, 2000; Schroeder et al., 2001; Shimazaki et al., 2007).
Blue and red light are the major light sources that mediate stomatal opening, with blue light playing the major role. Two families of blue-light receptors, the phototropins (PHOT1 and PHOT2) and the cryptochromes (CRY1 and CRY2), participate in blue light-induced stomatal opening. PHOT1-mediated stomatal opening depends on the interaction between Root Phototropism2 (RPT2) and PHOT1 (Inada et al., 2004). On the other hand, Root Curling in N-naphthylphthalamic acid1 (RCN1), the A1 subunit of Arabidopsis (Arabidopsis thaliana) protein phosphatase2A (PP2A), interacts with and dephosphorylates PHOT2 (deactivation) to inhibit PHOT2-mediated stomatal opening (Tseng and Briggs, 2010). H+-ATPase functions downstream of PHOT-mediated stomatal opening. H+-ATPase is activated by blue light through phosphorylation of its C terminus, which facilitates the interaction with its regulator, 14-3-3 protein (Kinoshita and Shimazaki, 1999; Emi et al., 2001; Shimazaki et al., 2007). Plasma membrane H+-ATPases generate the H+ electrochemical potential that activates voltage-gated inward-rectifying K+ channels, which decreases the osmotic potential, leading to increased guard cell turgor, water influx, and stomatal opening. Hydrogen peroxide (H2O2) inhibits blue light-induced phosphorylation of H+-ATPase (activation), and consequently attenuates blue light-dependent H+ pumping (Zhang et al., 2004). MYB60 and MYB61, two members of the R2R3-MYB family of transcription factors, are specifically expressed in guard cells and regulate the stomatal aperture (Cominelli et al., 2005; Liang et al., 2005). MYB60 is a positive regulator of stomatal opening, and its expression is induced by blue light and rapidly down-regulated by dehydration (Cominelli et al., 2005). By contrast, MYB61 is a negative regulator of light-induced stomatal opening (Liang et al., 2005; Chen et al., 2012). Recently, two interacting proteins, PP7 and Hypersensitive to red and blue1 (HRB1), were identified as regulating stomatal movement under blue light (Sun et al., 2012).
In mammals, cyclin H binds to and activates CYCLIN-DEPENDENT KINASE 7 (CDK7) (Fisher and Morgan, 1994; Labbé et al., 1994; Mäkelä et al., 1994). The CDK7/cyclin H complex phosphorylates the C-terminal domain of RNA polymerase II (Serizawa et al., 1995; Shiekhattar et al., 1995). The Arabidopsis genome encodes three CDK7 homologs, CDKD;1, CDKD;2, and CDKD;3. Arabidopsis CYCLIN H;1 (CYCH;1) strongly interacts with CDKD;2 and CDKD;3 but weakly interacts with CDKD;1 in yeast (Saccharomyces cerevisiae) cells (Yamaguchi et al., 2000; Shimotohno et al., 2004). Recently, it has been shown that the Arabidopsis CDKDs function redundantly to phosphorylate the C-terminal domain of RNA polymerase II and are required for plant viability (Hajheidari et al., 2012). Here, we report that CYCH;1 is involved in the drought stress response. Reduced-function cych;1 RNA interference (RNAi) plants exhibit enhanced drought tolerance, which is associated with reduced transpiration. Further physiological and molecular analyses indicate that CYCH;1 positively regulates blue light-induced stomatal opening by negatively regulating ROS production. Moreover, expression of CYCH;1 is down-regulated by dehydration. These results reveal that water deficit represses CYCH;1 expression, which leads to elevated levels of ROS in guard cells. The increased levels of ROS inhibit blue light-induced stomatal opening, which reduces transpiration and enhances drought tolerance.
RESULTS
CYCH;1 Gene Expression and Subcellular Protein Localization
To investigate the role of CYCH;1 in abiotic stress responses, we evaluated the expression of CYCH;1 in response to dehydration, ABA, mannitol, and NaCl. Quantitative real-time (qRT)-PCR analysis established that CYCH;1 transcript abundance is reduced by all of these treatments (Fig. 1A). These results demonstrate that CYCH;1 may be involved in the abiotic stress response.
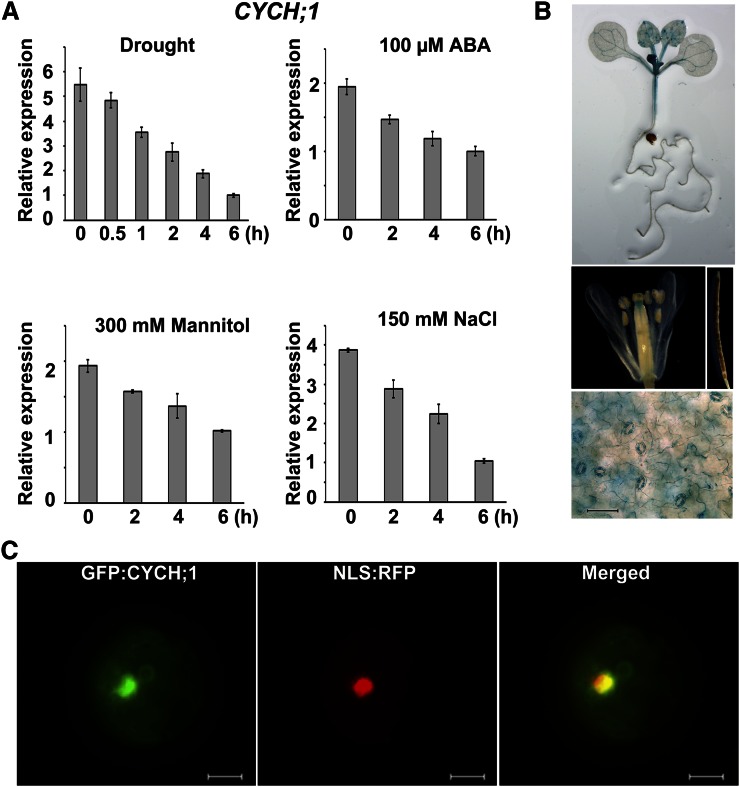
Figure 1.
CYCH;1 expression is down-regulated by drought stress and CYCH;1 is localized to the nucleus. A, qRT-PCR analysis of the time course of CYCH;1 expression in wild-type plants after treatment with drought, ABA, mannitol, and NaCl. Error bars represent the se of three independent experiments. B, Histochemical GUS staining of PCYCH;1:GUS transgenic plants. GUS staining of a 12-d-old seedling (top) of a flower (middle, left) and silique (middle, right) of a 5-week-old plant and of the epidermal layer of a mature rosette leaf of a 3-week-old plant (bottom). Bar = 60 μm. C, Subcellular localization of GFP:CYCH;1. A nuclear localization signal (NLS) fused with red fluorescent protein (RFP) was cotransfected into protoplasts with GFP:CYCH;1. Bars = 10 μm.
Next, we used the GUS reporter to determine the tissue-specific expression pattern of CYCH;1. The 2,056-bp promoter region of CYCH;1 was used to drive the expression of the GUS (uidA) gene in transgenic Arabidopsis plants. GUS activity was detected mainly in the vascular tissues of young leaves and stems (Fig. 1B, top) and in the stigma apex (Fig. 1B, middle). GUS activity was detected predominantly in guard cells in the epidermal layer of mature rosette leaves (Fig. 1B, bottom). However, GUS activity was not observed in roots, petals, or siliques (Fig. 1B, top and middle). These observations suggest that CYCH;1 functions in stomata.
It was reported previously that Arabidopsis CYCH;1 was localized in the cytoplasm and nucleus in heterologous systems, such as tobacco (Nicotiana tabacum) Bright Yellow-2 and onion (Allium cepa) epidermal cells (Shimotohno et al., 2006). In Arabidopsis protoplasts, GFP:CYCH;1 was targeted to the nucleus (Fig. 1C).
Morphological Phenotypes of the cych;1 RNAi Mutants
To determine whether CYCH;1 is involved in the abiotic stress response, we obtained three CYCH;1 transfer DNA insertion mutants, Salk_108040, GABI_590B10, and Salk_115564; however, the level of CYCH;1 expression was unfortunately not significantly altered in these mutants (Supplemental Fig. S1). Therefore, we generated a loss-of-function cych;1 mutant (referred to as cych;1 RNAi) through RNA interference-mediated gene silencing using a CYCH;1-specific region (Supplemental Fig. S2). CYCH;1 expression was substantially reduced in three independent cych;1 RNAi lines, which were subjected to further analysis (Supplemental Fig. S2C). Additional microarray and qRT-PCR analyses indicated that the expression levels of other CYCLIN family genes were not altered in cych;1 RNAi plants (Supplemental Fig. S2, D–F; Supplemental Table S1). Whereas the rosettes of the three cych;1 RNAi plants were slightly smaller than those of the wild type and the leaves were yellow green, root growth was normal (Supplemental Fig. S2B).
Reduced Transpiration Rate and Increased Drought Tolerance in cych;1 RNAi Plants
Because the level of CYCH;1 expression was reduced in response to abiotic stresses and ABA (Fig. 1A), CYCH;1 may be involved in the regulation of abiotic stress and ABA responses. Seed germination and seedling growth of the cych;1 RNAi mutants were similar to those of the wild type under various concentrations of ABA, mannitol, and NaCl (Supplemental Fig. S3). These results indicate that CYCH;1 does not regulate ABA, mannitol, and NaCl stress responses during seed germination and seedling development.
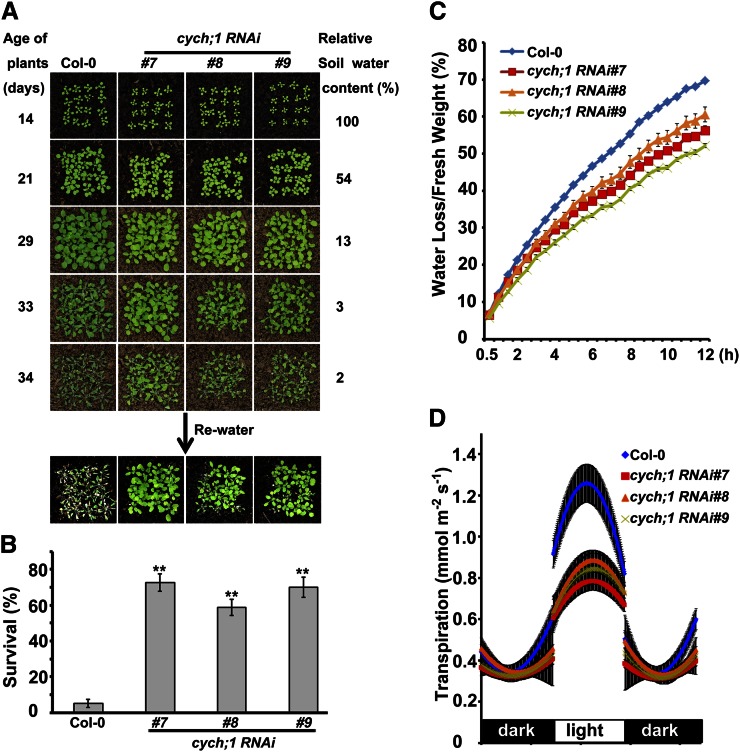
Next, we examined the drought stress responses of soil-grown cych;1 RNAi mutant plants (Fig. 2A). Although most wild-type plants wilted at a relative soil water content of 2%, the mutant plants exhibited less wilting. After rewatering, only 3% of wild-type plants survived, compared with approximately 70% of the mutant plants (Fig. 2B). These results indicate that CYCH;1 negatively regulates the drought stress response. The drought tolerance phenotype of cych;1 RNAi mutants was further evaluated by measuring water loss from detached leaves. We found that the detached leaves of the cych;1 RNAi mutants lost water more slowly compared with the wild type (Fig. 2C). Thus, the drought tolerance phenotype of the cych;1 RNAi mutant may be due to lower transpiration rates. To confirm this, we investigated the transpiration rate by means of gravimetric analyses over diurnal light/dark periods (Yoo et al., 2010). The cych;1 RNAi plants exhibited a lower transpiration rate than the wild type during the light period, but transpiration rates were similar in mutant and wild-type plants during the dark period (Fig. 2D). These results indicate that the reduced transpiration rate of cych;1 RNAi plants contributes to the drought tolerance phenotype.
Figure 2.
Drought tolerance phenotypes of the cych;1 RNAi mutants. A, Responses to water stress were determined for 2-week-old wild type and cych;1 RNAi mutants grown in soil. B, Survival rate was determined 4 d after rewatering. Data are presented as means ± se. **P < 0.01, Student’s t test. C, Comparison of water loss from the detached leaves of wild-type and cych;1 RNAi plants. Values are means ± se . D, Diurnal transpiration rates in 5-week-old wild-type and cych;1 RNAi plants grown under a 10-h-light/14-h-dark photoperiod were determined by gravimetric analysis (mean ± se, n = 4).
CYCH;1 Regulates Stomatal Aperture under Blue Light
It is possible that CYCH;1 knockdown affects stomatal density and/or aperture to reduce transpiration rate. We first evaluated stomatal density and index in the leaf abaxial epidermal layer of wild-type and cych;1 RNAi plants under water-sufficient conditions. Figure 3, A and B, shows that both cych;1 RNAi and wild-type plants had similar stomatal densities and indices, indicating that down-regulation of CYCH;1 does not affect stomatal development. As the cych;1 RNAi mutants and the wild type showed similar transpiration rates in the dark (Fig. 2D), CYCH;1 does not regulate dark-induced stomatal closure but may regulate ABA-induced stomatal closure and/or light-induced stomatal opening (Shimazaki et al., 2007). Treatment with different ABA concentrations caused equivalent degrees of stomatal closure in both the cych;1 RNAi mutants and the wild type, indicating that CYCH;1 knockdown does not affect ABA-mediated stomatal closure (Fig. 3C). To determine whether CYCH;1 is involved in light-induced stomatal opening, we measured the stomatal aperture widths of the cych;1 RNAi and wild-type plants in the dark and under both red and blue light (Mao et al., 2005). The stomatal apertures of the cry1 and cry2 mutants were measured as controls. Under blue light, the cych;1 RNAi mutants had a much smaller stomatal aperture width than the wild type, similar to the cry1 and cry2 mutants (Fig. 3D). There were no significant differences in stomatal width between the mutants and the wild type in the dark and under red light (Fig. 3D), indicating that CYCH;1 mediates blue light-induced stomatal opening, consistent with the predominant expression of CYCH;1 in guard cells (Fig. 1B).
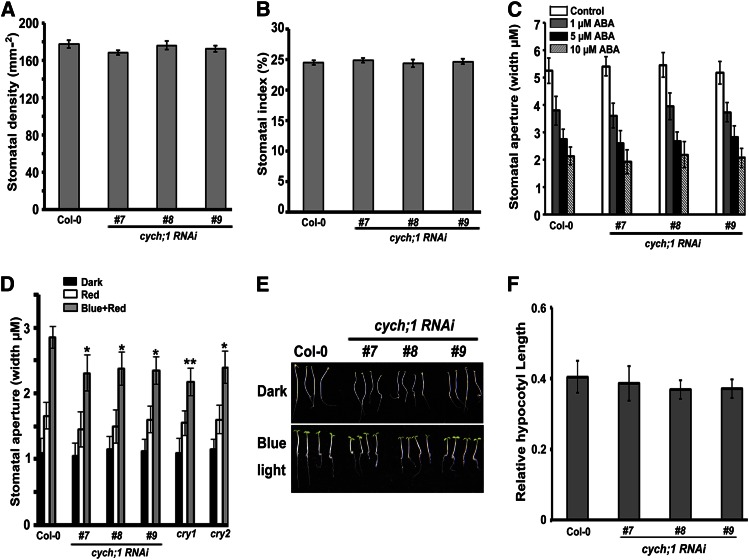
Figure 3.
CYCH;1 regulates the stomatal aperture under blue light. Stomatal density (A) and stomatal index (B) of wild-type and cych;1 RNAi plants. Data are means ± se. C, Stomatal closure in wild-type and cych;1 RNAi plants in response to various concentrations of ABA. D, Stomatal aperture under different light conditions in wild-type, cych;1 RNAi, cry1, and cry2 plants (*P < 0.05 and **P < 0.01, Student’s t test). E, Four-day-old seedlings of Col-0 and cych;1 RNAi mutants were grown under 10 μmol m–2 s–1 blue light or in darkness. F, Blue light response of Col-0 and cych;1 RNAi. The y axis represents the length of hypocotyls grown in blue light relative to that of hypocotyls grown in darkness. Data are means ± sd. [See online article for color version of this figure.]
Blue light can regulate hypocotyl elongation in addition to stomatal opening. Therefore, we examined whether CYCH;1 regulates the inhibition of hypocotyl elongation by blue light. As shown in Figure 3E, cych;1 RNAi seedlings had shorter hypocotyls than the wild-type controls in darkness and under 10 µmol m–2 s–1 blue light. However, relative hypocotyl length (i.e. blue light-grown hypocotyl length relative to dark-grown hypocotyl length) was not significantly affected in the mutant seedlings (Fig. 3F). This result indicates that CYCH;1 does not affect the blue light-mediated inhibition of hypocotyl elongation.
Mutation of CDKD;2 or CDKD;3 Does Not Impair Responsiveness to Dehydration
In Arabidopsis, CYCH;1 interacts with CDKD;2 and CDKD;3 and facilitates their kinase activity (Shimotohno et al., 2006). The individual cdkd;2 and cdkd;3 single mutants do not cause abnormal morphological phenotypes (Hajheidari et al., 2012); however, it is unknown if CDKD;2 or CDKD;3 is involved in drought stress responses.
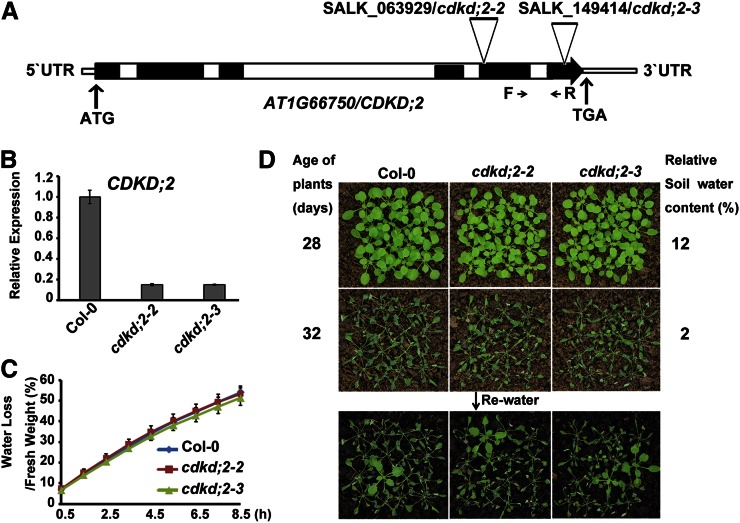
We acquired cdkd;2-2 (Salk_063929; Hajheidari et al., 2012) and a previously uncharacterized allele (Salk_149414; cdkd;2-3) from the Arabidopsis Biological Resource Center (Fig. 4A). qRT-PCR detected very low levels of CDKD;2 transcript in both mutants (Fig. 4B). We compared the water loss of detached leaves taken from wild-type, cdkd;2-2, and cdkd;2-3 plants. As shown in Figure 4C, the detached leaves of the cdkd;2-2 and cdkd;2-3 mutants showed similar amounts of water loss compared with the wild-type controls. Then, we measured the drought stress responsiveness of each of these plants by withholding water for the indicated periods of time. Wild-type, cdkd;2-2, and cdkd;2-3 plants wilted to the same extent at a relative soil water content of 2% (Fig. 4D), while cych;1 RNAi mutants showed less wilting compared with the wild type (Fig. 2A). After rewatering, the survival rates of cdkd;2-2 and cdkd;2-3 mutants were similar to those of the wild type (Fig. 4D). Moreover, cdkd;3-1 (Salk_120536) and cdkd;3-2 (Salk_007756) mutants (Hajheidari et al., 2012) also exhibited similar amounts of water loss and drought stress responsiveness as the wild-type controls (Supplemental Fig. S4). These results suggest that CDKD;2 and CDKD;3 do not function with CYCH;1 to regulate drought stress responses.
Figure 4.
The cdkd;2 mutants are not resistant to drought stress. A, Transfer DNA insertion sites in CDKD;2. Black bars indicate exons, and white bars indicate introns. F and R indicate the location of the primers used for gene expression analysis. B, CDKD;2 expression levels in 10-d-old wild-type, cdkd;2-2, and cdkd;2-3 seedlings were determined by qRT-PCR analysis. C, Water loss of detached leaves from wild-type and cdkd;2 plants. Values are means ± se. D, Comparison of drought stress responsiveness of wild-type and cdkd;2 plants.
Downstream Targets of CYCH;1
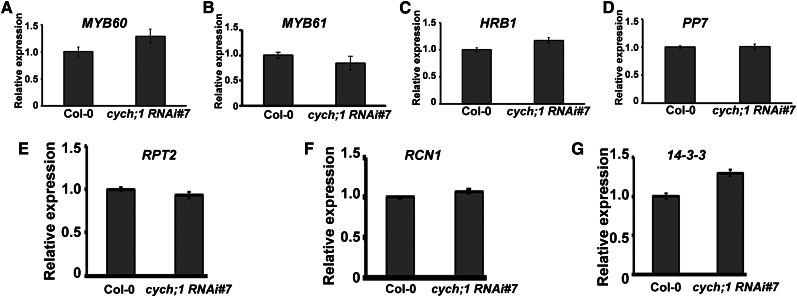
To further determine the role of CYCH;1 in blue light-induced stomatal opening, we assessed the expression of genes involved in this process, such as MYB60 (Cominelli et al., 2005), MYB61 (Liang et al., 2005), HRB1 (Sun et al., 2012), PP7 (Sun et al., 2012), RPT2 (Inada et al., 2004), RCN1 (Tseng and Briggs, 2010), and 14-3-3 (Kinoshita et al., 2003), in the cych;1 RNAi mutants and wild-type controls using qRT-PCR. No significant changes in the expression levels of these genes were found, indicating that CYCH;1 does not affect blue light-induced stomatal opening by regulating the transcription of these genes (Fig. 5).
Figure 5.
Expression of genes involved in blue light-mediated stomatal opening in wild-type and cych;1 RNAi#7 plants. qRT-PCR was performed using total RNA from 3-week-old wild-type and cych;1 RNAi#7 plants under normal conditions. Error bars represent the se of three independent experiments.
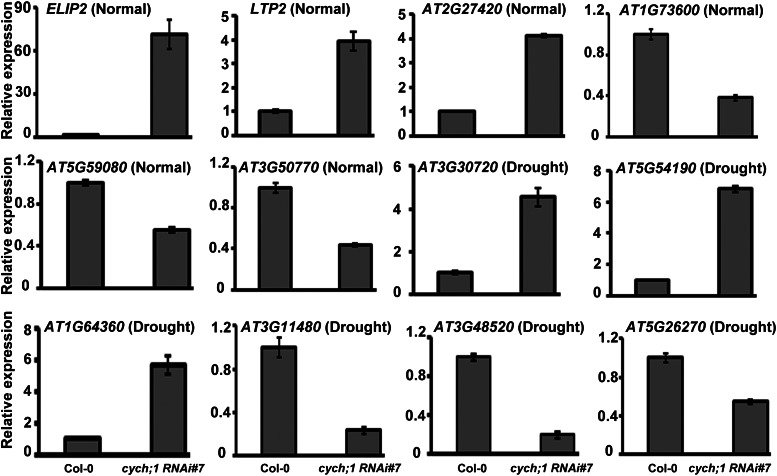
To identify CYCH;1-regulated genes, we performed a microarray analysis that investigated the effects of CYCH;1 disruption on global gene expression using Affymetrix GeneChips. Total mRNAs derived from 3-week-old cych;1 RNAi#7 mutants and wild-type plants under normal conditions or after 1 h of drought stress treatment were used. Two biological replicates were performed for wild-type and cych;1 RNAi#7 plants for each treatment. We confirmed the microarray expression data for 12 selected genes by qRT-PCR analysis (Fig. 6). Under water-sufficient conditions, only 49 genes showed more than a 2-fold change in expression, of which 35 were up-regulated and 14 were down-regulated in the cych;1 RNAi#7 mutant (Table I). The microarray results indicated increased transcript levels of ARABIDOPSIS RIBULOSE-BISPHOSPHATE CARBOXYLASE/OXYGENASE X1 (ATRBCX1) (Kolesiński et al., 2011), ECERIFERUM1 (CER1) (Bourdenx et al., 2011), JASMONATE-ZIM-DOMAIN PROTEIN5 (JAZ5; Pauwels and Goossens, 2011), LIPID TRANSFER PROTEIN2 (LTP2; Chae et al., 2010), and At1g06620 in the cych;1 RNAi#7 mutant, all of which can be induced by water deprivation. Interestingly, the 35 up-regulated genes included at least 12 genes involved in redox homeostasis (i.e. LIPOXYGENASE3 [LOX3], LOX4, ATGPX7, EARLY LIGHT-INDUCIBLE PROTEIN1 [ELIP1], ELIP2, PROTOCHLOROPHYLLIDE OXIDOREDUCTASE A [PORA], SQUALENE MONOXYGENASE6 [SQE6], CYTOCHROME P450 96 A1 [CYP96A15], CER1, At4g15690, At4g15680, and At1g06620). LOX proteins are involved in biotic and abiotic stress responses in plants (Feussner and Wasternack, 2002; Umate, 2011). ATGPX7 regulates cellular photooxidative tolerance and immune responses (Chang et al., 2009). ELIP1/ELIP2 were proposed to have a photoprotective function under conditions of light stress, which they brought about by transiently binding to released chlorophyll, preventing the formation of free radicals, and participating in energy dissipation (Adamska et al., 1999; Montané and Kloppstech, 2000; Adamska et al., 2001). Overexpression of light-dependent PORA protects against photooxidative damage (Sperling et al., 1997). SQE6 had squalene monooxygenase activity similar to SQE1. sqe1-5 is hypersensitive to drought stress and had an impaired stomatal response and defective root development, which involved the altered production of ROS (Posé et al., 2009). CYP96A15 has monooxygenase activity (Greer et al., 2007). The proteins encoded by At4g15690 and At4g15680 have glutaredoxin activity, indicating that they belong to the thioredoxin superfamily. Many of the 14 genes down-regulated in the cych;1 RNAi#7 mutant encode proteins of unknown function. Two of these genes encoded calcium ion-binding proteins (i.e. At3g50770 and At1g21550). Three genes encoded proteins involved in the regulation of transcription (i.e. REGULATOR OF THE ATPASE OF THE VACUOLAR MEMBRANE2 [RAV2], ARABIDOPSIS MYB-LIKE2 (MYBL2), and Arabidopsis NAC domain containing protein36 [anac036]; Table I).
Figure 6.
Expression of genes regulated by CYCH;1 under normal and drought stress conditions. The relative expression levels of 12 genes selected based on the microarray data (Tables I and II; Supplemental Table S3) were measured by qRT-PCR. Three-week-old wild-type and cych;1 RNAi#7 plants were used in this analysis. For drought stress, leaves were detached from 3-week-old plants and placed on the bench for 1 h. Error bars represent the se of three independent experiments.
Table I. Microarray gene expression analysis of wild-type and cych;1 RNAi#7 plants grown under normal conditions.
Gene identification and description correspond to gene designation and annotation obtained from The Arabidopsis Information Resource (http://www.arabidopsis.org).
| Gene ID | Fold Change | Description |
|---|---|---|
| At4g15690 | 19.70 | Glutaredoxin, cell redox homeostasis |
| At1g18830 | 17.15 | Transducin/WD40 repeat-like superfamily protein |
| At4g15680 | 6.96 | Glutaredoxin, cell redox homeostasis |
| At2g27420 | 6.50 | Cys proteinases superfamily protein, Cys-type peptidase activity |
| At2g43590 | 6.50 | Chitinase family protein, chitinase activity |
| At5g58310 | 6.06 | METHYL ESTERASE18, methyl IAA esterase activity in vitro |
| At4g14690 | 5.66 | ELIP2, response to UV |
| At1g57750 | 4.29 | CYP96A15, oxidation reduction process monooxygenase activity |
| At5g54190 | 4.00 | PORA, light-dependent NADPH:protochlorophyllide oxidoreductase A |
| At3g30720 | 3.73 | QUA-QUINE STARCH, starch biosynthetic process |
| At5g24160 | 3.73 | SQE6, oxidation reduction process squalene monooxygenase activity |
| At4g04330 | 3.48 | ARABIDOPSIS RIBULOSE-BISPHOSPHATE CARBOXYLASE/OXYGENASE X1 (ATRBCX1) response to water deprivation, cold, and salt stress |
| At3g63160 | 3.25 | OUTER ENVELOPE PROTEIN6, cell differentiation, positive regulation of transcription |
| At1g02205 | 3.25 | CER1, response to water deprivation, defense response to bacterium and fungus, oxidoreductase activity |
| At1g53885 | 3.03 | Unknown protein |
| At1g17420 | 3.03 | LOX3, response to high light intensity, oxidoreductase activity |
| At4g23680 | 2.83 | Polyketide cyclase/dehydrase and lipid transport superfamily protein, defense response, response to biotic stimulus |
| At5g57785 | 2.64 | Unknown protein, response to Suc stimulus, response to UV-B |
| At5g42830 | 2.64 | HXXXD-type acyltransferase family protein, transferase activity |
| At1g06620 | 2.64 | Similar to a 2-oxoglutarate-dependent dioxygenase, response to water deprivation and wounding, oxidoreductase activity |
| At4g34410 | 2.46 | REDOX RESPONSIVE TRANSCRIPTION FACTOR1, intracellular signal transduction, regulation of transcription |
| At1g17380 | 2.46 | JAZ5, response to water deprivation |
| At5g37300 | 2.46 | WAX ESTER SYNTHASE/ACYL-COA:DIACYLGLYCEROL ACYLTRANSFERASE1 (WSD1) (diacylglycerol O-acyltransferase/long-chain-alcohol O-fatty-acyltransferase), wax biosynthetic process |
| At3g53650 | 2.46 | Histone superfamily protein |
| At1g72520 | 2.46 | ATLOX4, defense response, oxidation reduction process |
| At3g51450 | 2.30 | Calcium-dependent phosphotriesterase superfamily protein, biosynthetic process |
| At2g38530 | 2.14 | LTP2, response to water deprivation |
| At4g27657 | 2.14 | Unknown protein |
| At2g36590 | 2.14 | PRO TRANSPORTER3, amino acid transport, Pro transport |
| At4g34590 | 2.14 | G-BOX BINDING FACTOR6, encodes a basic domain Leu zipper transcription factor bZIP11 |
| At4g27654 | 2.14 | Unknown protein |
| At3g28500 | 2.14 | Acidic ribosomal protein P2b, translational elongation |
| At3g22840 | 2.00 | ELIP1, response to UV |
| At4g31870 | 2.00 | ATGPX7, response to oxidative stress |
| At5g23660 | 2.00 | Arabidopsis homolog of Medicago truncatula MEDICAGO TRUNCATULA NODULIN3 (MTN3), Suc transport |
| At5g02760 | −4.29 | Protein phosphatase-like protein phosphatase 2C homolog, protein Ser/Thr phosphatase activity |
| At1g73600 | −3.48 | Conserved peptide upstream open reading frame32, methyltransferase activity |
| At5g12050 | −2.64 | Putative Ser-rich protein predicted proteins |
| At5g59080 | −2.46 | Unknown protein |
| At3g01290 | −2.46 | Unknown protein |
| At2g38310 | −2.46 | PYRABACTIN RESISTANCE1 (PYR1)-LIKE4, ABA mediated signaling pathway |
| At1g56220 | −2.30 | Unknown protein |
| At1g68840 | −2.30 | RAV2, transcription repressor activity |
| At1g71970 | −2.30 | Unknown protein |
| At3g50770 | −2.00 | Calmodulin-like41, calcium ion binding |
| At3g13980 | −2.00 | Unknown protein |
| At1g71030 | −2.00 | MYBL2, regulation of transcription |
| At1g21550 | −2.00 | Calcium-binding EF-hand family protein, calcium ion binding |
| At2g17040 | −2.00 | Anac036, sequence-specific DNA-binding transcription factor activity |
In the cych;1 RNAi#7 mutant, as many as 72 genes with expression ratio changes of above 2-fold were differentially expressed compared with the wild type under drought stress conditions (Table II; Supplemental Table S3). As shown in Table II, of the 23 genes up-regulated in cych;1 RNAi#7, nine were also up-regulated under water-sufficient conditions. Many of these encoded proteins are involved in oxidation reduction processes and defense responses. The 49 genes down-regulated in the mutants under conditions of drought stress are involved in the regulation of transcription, signal transduction, and the defense response (Supplemental Table S3).
Table II. Genes up-regulated in cych;1 RNAi#7 plants under drought stress, as identified by microarray analysis.
Gene identification and description correspond to gene designation and annotation obtained from The Arabidopsis Information Resource (http://www.arabidopsis.org).
| Gene ID | Fold Change | Description |
|---|---|---|
| At4g23680a | 14.91 | Polyketide cyclase/dehydrase and lipid transport superfamily protein, defense response |
| At1g18830a | 6.13 | Transducin/WD40 repeat-like superfamily protein |
| At4g27530 | 5.81 | Unknown protein |
| At1g64360 | 4.63 | Unknown protein, response to oxidative stress |
| At3g30720a | 4.51 | QUA-QUINE STARCH, starch biosynthetic process |
| At5g54190a | 4.47 | PORA, light-dependent NADPH:protochlorophyllide oxidoreductase A |
| At2g38530a | 3.51 | LTP2, response to water deprivation |
| At2g20870 | 3.45 | Cell wall protein precursor, regulation of anthocyanin biosynthetic process |
| At4g14020 | 3.35 | Rapid alkalinization factor family protein |
| At2g43590a | 2.86 | Chitinase family protein, chitinase activity |
| At5g22430 | 2.68 | Pollen Ole e 1 allergen and extensin family protein |
| At1g65310 | 2.68 | XYLOGLUCAN ENDOTRANSGLUCOSYLASE/HYDROLASE17, carbohydrate metabolic process |
| At5g24160a | 2.48 | SQE6, oxidation reduction process squalene monooxygenase activity |
| At2g39330 | 2.37 | JACALIN-RELATED LECTIN23, molecular function unknown |
| At4g38840 | 2.24 | SMALL AUXIN UPREGULATED (SAUR)-like auxin-responsive protein family, response to cold and auxin stimulus |
| At5g57785a | 2.21 | Unknown protein, response to Suc stimulus, response to UV-B |
| At1g44970 | 2.20 | Peroxidase superfamily protein, oxidation reduction process, response to oxidative stress |
| At5g55620 | 2.17 | Unknown protein |
| At1g72260 | 2.17 | THIONIN2.1, defense response |
| At4g33420 | 2.09 | Peroxidase superfamily protein, response to oxidative stress |
| At4g27140 | 2.02 | Seed storage albumin1, lipid transport |
| At2g02130 | 2.01 | LOW-MOLECULAR-WEIGHT CYS-RICH68, defense response |
| At1g57750a | 2.01 | CYP96A15, oxidation reduction process monooxygenase activity |
Genes that were up-regulated under both normal and drought stress conditions.
Mutations in CYCH;1 Increased ROS Production
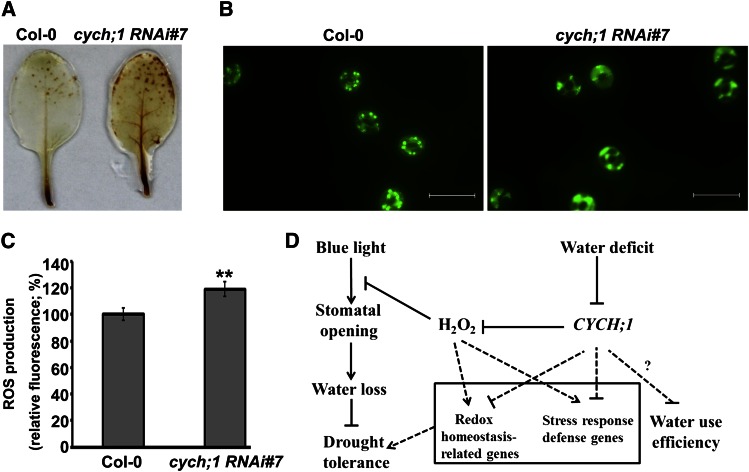
The microarray analysis indicated that the expression of many genes involved in cell redox homeostasis increased in the cych;1 RNAi mutant under normal conditions or conditions of drought stress. Therefore, we determined whether H2O2 accumulation was altered in the cych;1 RNAi mutant using 3,3′-diaminobenzidine (DAB) staining. As shown in Figure 7A, under normal conditions, the leaves of cych;1 RNAi accumulated higher levels of H2O2 than did those of wild-type plants. Next, H2O2 levels in guard cells were assessed using the 2′,7′-dichlorofluorescein diacetate (H2DCF-DA) staining assay (Hua et al., 2012). The cych;1 RNAi mutant showed higher levels of H2O2 production in guard cells than did wild-type plants (Fig. 7, B and C). These results reveal that the impaired stomatal function of cych;1 RNAi mutants under blue light may be due to increased ROS production in the mutant.
Figure 7.
The cych;1 RNAi#7 mutant accumulates more ROS than do wild-type plants. A, DAB staining of leaves from wild-type and cych;1 RNAi#7 plants. B, ROS production of guard cells was monitored using the ROS-sensitive fluorescent dye H2DCF-DA. Bar = 20 μm. C, Quantification of relative H2O2 production in guard cells of wild-type and cych;1 RNAi#7 plants grown under normal conditions. Values are means ± se of three independent experiments (n = 30 for each genotype per experiment). **P < 0.01. D, A model of CYCH;1 modulation of blue light-induced stomatal opening and drought stress through alterations in the accumulation of H2O2. Arrows indicate positive regulation, and T-bars indicate negative regulation. Dotted lines indicate hypothetical regulation.
DISCUSSION
Drought stress induces a range of physiological and biochemical responses in plants, including stomatal closure and repression of plant growth and photosynthesis, which consequently affect biomass and seed productivity. Here, we report that down-regulation of CYCH;1 reduces stomatal apertures and enhances drought tolerance. CYCH;1, by modulating ROS production in guard cells, positively regulates blue light-induced stomatal opening. In this study, cych;1 RNAi plants were found to be similar in size to wild-type plants (Supplemental Fig. S2B), but their transpiration rates and stomatal aperture widths (Figs. 2D and 3D) were significantly reduced under light conditions. It is possible that the reduced stomatal openings in cych;1 RNAi plants did not significantly affect CO2 assimilation and photosynthesis. Therefore, it would be interesting to determine if cych;1 RNAi plants exhibit increased water use efficiency in the future (Fig. 7D).
Drought triggers the accumulation of ABA, and increased ABA levels induce stomatal closure, reduce stomatal density (Bradford et al., 1983; Léon-Kloosterziel et al., 1996), and activate ABA-responsive gene expression (Marcotte et al., 1992). The cych;1 RNAi mutants showed no alterations in ABA-induced stomatal closure, stomata development, or ABA-responsive gene expression (Fig. 3; Table I). Moreover, inhibition of seed germination and seedling growth by ABA was not altered in cych;1 RNAi mutants (Supplemental Fig. S3A). These results suggest that CYCH;1 regulates drought stress responses through an ABA-independent pathway. In addition to the ABA-dependent pathway, the dehydration-responsive element-binding protein (DREB), NO APICAL MERISTEM (NAM), ATAF1/ATAF2, and CUP-SHAPED COTYLEDON2 (CUC2), and zinc finger homeodomain (ZFHD) transcription factors participate in drought stress responses by regulating the expression of downstream target genes (Nakashima et al., 2009). Microarray analysis under normal and dehydration conditions indicated that none of the CYCH;1-regulated genes were affected by DREBs, NAC, or ZFHD (Tables I and II; Supplemental Table S3), suggesting that regulation of the drought stress responses by CYCH;1 is independent of these transcription factors.
CYCH;1 is expressed predominantly in guard cells (Fig. 1B), and its expression level is significantly reduced by dehydration (Fig. 1A). Similar to CYCH;1, the expression level of MYB60 is also decreased under drought conditions (Cominelli et al., 2005). CYCH;1 and MYB60 both positively regulate blue light-induced stomatal opening, and the effects of ABA on stomatal aperture, seed germination, and vegetative growth were not impaired in cych;1 RNAi and atmyb60-1 mutants (Fig. 3C; Supplemental Fig. S3A). It is possible that down-regulation of CYCH;1 and MYB60 in response to drought conditions limits light-induced stomatal opening. Therefore, we assume that at least two regulatory mechanisms exist to minimize transpiration from leaves under drought conditions, one of which involves ABA-induced stomatal closure and the other of which involves a reduction in light-induced stomatal opening. qRT-PCR analysis showed that CYCH;1 did not regulate the expression of MYB60. However, we cannot exclude the possibility that genetic interactions exist between CYCH;1 and MYB60. CYCH;1 and MYB60 are nuclear proteins, and it will be interesting to determine whether these proteins function in the same genetic pathway.
Several signaling molecules involved in blue light-induced stomatal opening, such as CRYs, CONSTITUTIVE PHOTOMORPHOGENIC1 (COP1), and HRB1, are also involved in photomorphogenesis. The cry mutations impair blue light-mediated inhibition of hypocotyl elongation, the cop1 mutation causes short hypocotyls under both dark and light conditions, and hrb1 exhibits short hypocotyls under both red and blue light (Deng et al., 1992; Kang et al., 2005; Liu et al., 2011). However, CYCH;1 knockdown did not affect blue light-mediated inhibition of hypocotyl elongation (Fig. 3, E and F). Therefore, CYCH;1 is specifically involved in light-induced stomatal opening but not in photomorphogenesis.
Whereas CYCH;1 activates CDKD;2 and CDKD;3 kinase activity, the activation of CDKD;1 by CYCH;1 has not been established (Shimotohno et al., 2004). CDKD;2 or CDKD;3 single mutants showed a responsiveness to drought stress similar to that of the wild type (Fig. 4; Supplemental Fig. S4), suggesting that CYCH;1 may regulate drought stress responses independently of CDKD;2 or CDKD;3. However, recent findings indicate that the three CDKDs CDKD;1, CDKD;2, and CDKD;3 function redundantly to regulate plant viability (Hajheidari et al., 2012). Therefore, it is critical to determine if the three CDKDs also function redundantly to regulate drought stress responses. However, because the cdkd triple knockout mutant is lethal, it would be interesting to analyze the drought stress responsiveness of a conditional cdkd triple knockout mutant in the future. In addition to interacting with CDKDs, CYCH;1 interacts with 16 Arabidopsis core cell cycle proteins, including CDKs, CDK subunits, and Kip-related proteins (Boruc et al., 2010). Several lines of evidence indicate that cell cycle and non-cell cycle cyclins and CDKs regulate abiotic stress responses (Kitsios and Doonan, 2011). For example, rice (Oryza sativa) plants overexpressing OsCYCB1;1 showed enhanced resistance to cold stress (Ma et al., 2009). Some of these CYCH;1-interacting proteins may be involved in CYCH;1-mediated blue light-induced stomatal opening; however, this has yet to be confirmed.
H2O2 functions as a signal molecule in a wide range of abiotic stress responses, including the expression of antioxidant and defense genes (Karpinski et al., 1999), stomatal closure (Kwak et al., 2003), and the regulation of cell expansion and plant development (Kwak et al., 2003; He et al., 2012). Our results indicate that H2O2 accumulates (Fig. 7C), the expression of genes involved in redox homeostasis increases (Fig. 7, A–C; Table I), and blue light-induced stomatal opening is impaired in the cych;1 RNAi mutant (Fig. 3D). Therefore, we propose that CYCH;1 inhibits the generation of H2O2 to maintain the cell redox state under normal conditions. In this model (Fig. 7D), CYCH;1 expression will decrease under drought conditions, leading to increased H2O2 accumulation and consequently to an altered redox balance. The increased levels of H2O2 inhibit blue light-induced stomatal opening to reduce water loss from the stomata. Concurrently, the redox signal regulates the expression of stress response defense genes and redox homeostasis-related genes to increase drought tolerance. However, how CYCH;1 regulates ROS homeostasis remains to be investigated.
MATERIALS AND METHODS
Plant Materials
Seeds of Salk_108040, GABI_590B10, Salk_115564, Salk_063929, Salk_149414, Salk_120536, and Salk_007756 were obtained from the Arabidopsis Biological Resource Center. The cry1 and cry2 (Mao et al., 2005) mutants were kindly provided by Hong-Quan Yang (Shanghai Jiaotong University). The wild-type and mutant Arabidopsis (Arabidopsis thaliana) plants used in this work were of the Columbia (Col-0) ecotype.
RNA Interference
A 247-bp DNA fragment from the CYCH;1 complementary DNA (cDNA) sequence was PCR-amplified and cloned into the pFGC1008 vector. The forward primer was 5′-CAAAGGTCCAAGAAGTGTGC-3′ and the reverse primer was 5′-CTCTGAAGAACAGCCATCTC-3′. This construct was transformed into Col-0 by the Agrobacterium tumefaciens-mediated floral dip method (Clough and Bent, 1998).
RNA Isolation and Real-Time Reverse Transcription-PCR
Total RNA was isolated from seedlings grown under different conditions with TRIzol reagent (RNAiso Plus, Code D9108B; TaKaRa). The qRT-PCR was performed on an MX3000P QPCR system. Ubiquitin-conjugating enzyme (UBC) was used as an internal control. The primers used for qRT-PCR are listed in Supplemental Table S2.
GUS Staining Assay
The CYCH;1 promoter fragment, spanning the region from 2,056 bp upstream to 9 bp downstream of the translational start site, was subcloned into the XmaI and NcoI sites of the pCAMBIA1303 vector. The fragment was amplified by PCR with the primers PCYCH;1 (forward): 5′-CCCGGGAAACATTTGTGTCCTTTGTAAT-3′ and PCYCH;1 (reverse): 5′-CCATGGTATCCGCCATTAACTGAGTTTTC-3′. The construct was introduced into GV3101 and transformed into Col-0. Single-copy, homozygous transgenic lines were used for the GUS staining assay as described previously (Zhou et al., 2009).
Stomatal Aperture Assays
The apertures of mature stomata in the epidermal strips of 3-week-old plants grown under 16-h-light/8-h-dark conditions were measured under different light conditions. Plants were pretreated with darkness or red light (38 μmol m–2 s–1) or blue light (16 μmol m–2 s–1) plus red light (38 μmol m–2 s–1) for 48 h before stomatal apertures were measured. Leaves were then collected in the early morning, and epidermal strips were peeled off from the abaxial surface under dim red light (Mao et al., 2005). Stomata were imaged using an Olympus BX53 microscope with IpExp60C software. Measurements were performed using the free software ImageJ (National Institutes of Health). ABA-mediated stomatal closure was analyzed as described previously (Zhou et al., 2009). Stomatal density (i.e. the number of stomata per unit area) and the stomatal index (i.e. the ratio of the number of stomata to the total number of epidermal cells, including stomata, stomatal precursor cells, and pavement cells) were determined from the leaves of 5-week-old plants grown under 12-h-light/12-h-dark conditions.
Water Loss
Water deficit stress was induced by withholding water from 16 plants (2 weeks old) grown in containers of soil (Pindstrup Mosebrug, 16093421/LV/SEEDING; pH 5.0; 0–10 mm; 300 L; dry weight, 34 ± 0.1 g) in a growth room (12-h light/12-h dark, 22°C). Plants were irrigated with water to saturation and weighed at the start of the water deficit stress treatment (initial weight) and then periodically throughout the treatment period. Relative soil water content was calculated as (final weight–dry weight)/(initial weight–dry weight) × 100%. After 20 d, plants were rewatered, and the survival rate was assessed 4 d later. To measure water loss in detached leaves, the leaves were removed from 3-week-old Col-0 and mutant plants that had been grown under normal conditions (16-h-light/8-h-dark, 22°C). The detached leaves were placed on a laboratory bench and weighed periodically. Water loss rate was calculated as (initial fresh weight–final fresh weight)/initial fresh weight × 100%. Wild-type and mutant plants grown for 5 weeks in a growth room with 10-h light/14-h dark were subjected to a transpiration rate assay as described previously (Yoo et al., 2010).
Subcellular Localization
The full-length cDNA of CYCH;1 was fused downstream of GFP under the control of the Cauliflower mosaic virus 35S promoter in the 326 vector. The primers used for CYCH;1 cDNA were as follows: forward 5′-CCCGGGAAATGGCGGATTTTCAGACATC-3′ and reverse 5′-CTCGAGTCAACCTATGGGTGGCGG-3′. Plasmids were introduced into Arabidopsis protoplasts using polyethylene glycol-mediated DNA transfection (Jin et al., 2001). Images were obtained with an Olympus BX53 fluorescence microscope.
Measurement of H2O2 production
In situ detection of H2O2 was performed by DAB staining. The mature rosette leaves of 3-week old wild-type and mutant plants grown under normal conditions (16-h light/8-h dark; 22°C) were collected and vacuum infiltrated with DAB solution (1 mg mL–1, pH 3.8; Sigma-Aldrich). Infiltrated leaves were sampled and stained after 8 h and then fixed in a 3:1:1 solution of ethanol, lactic acid, and glycerol. When chlorophyll was completely depleted, the leaves were photographed under white light.
H2O2 production in guard cells was detected with the H2DCF-DA (Sigma-Aldrich) staining assay, as described previously (Hua et al., 2012). H2DCF-DA fluorescence in guard cells in the epidermal peels was detected using an Olympus BX53 fluorescence microscope, with excitation at 488 nm and emission at 525 nm. The fluorescence was then quantified using the free software ImageJ. The fluorescence intensities of the wild type were taken as 100%, and those of the other samples are presented as relative values. Approximately 10 images per sample were acquired, and three independent experiments were performed.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers CYCH;1 (At5g27620), CDKD;2 (At1g66750), CDKD;3 (At1g18040), UBC (At5g25760), MYB60 (At1g08810), MYB61 (At1g09540), HRB1 (At5g49230), PP7 (At5g63870), RPT2 (At2g30520), RCN1 (At1g25490), 14-3-3 (At1g22300), ELIP2 (At4g14690), CYCA1;1 (At1g44110), CYCB1;4 (At2g26760), CYCD3;3 (At3g50070), and LTP2 (At2g38530).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Analysis of CYCH;1 expression.
Supplemental Figure S2. The level of CYCH;1 transcript is greatly reduced in the cych;1 RNAi mutants.
Supplemental Figure S3. Seed germination and seedling growth of cych;1 RNAi mutants in the presence of ABA, mannitol, and NaCl were similar to those of wild-type plants.
Supplemental Figure S4. The cdkd;3 mutant and the wild type exhibit similar responsiveness to drought stress.
Supplemental Table S1. Expression of CYCLINs in cych;1 RNAi.
Supplemental Table S2. Primers used in the qRT-PCR experiments.
Supplemental Table S3. Genes down-regulated in cych;1 RNAi#7 plants under drought stress, as identified by microarray analysis.
Acknowledgments
We thank Michael V. Mickelbart (Purdue University) for technical support with the transpiration analysis and Hong-Quan Yang (Shanghai Jiaotong University) and the Arabidopsis Biological Resource Center for providing seeds.
Glossary
- ABA
abscisic acid
- ROS
reactive oxygen species
- H2O2
hydrogen peroxide
- qRT
quantitative real-time
- DAB
3,3′-diaminobenzidine
- H2DCF-DA
2′,7′-dichlorofluorescein diacetate
- cDNA
complementary DNA
- Col-0
Columbia
References
- Adamska I, Kruse E, Kloppstech K. (2001) Stable insertion of the early light-induced proteins into etioplast membranes requires chlorophyll a. J Biol Chem 276: 8582–8587 [DOI] [PubMed] [Google Scholar]
- Adamska I, Roobol-Bóza M, Lindahl M, Andersson B. (1999) Isolation of pigment-binding early light-inducible proteins from pea. Eur J Biochem 260: 453–460 [DOI] [PubMed] [Google Scholar]
- Assmann SM, Shimazaki Ki. (1999) The multisensory guard cell. Stomatal responses to blue light and abscisic acid. Plant Physiol 119: 809–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt MR. (2000) Cellular signaling and volume control in stomatal movements in plants. Annu Rev Cell Dev Biol 16: 221–241 [DOI] [PubMed] [Google Scholar]
- Boruc J, Van den Daele H, Hollunder J, Rombauts S, Mylle E, Hilson P, Inzé D, De Veylder L, Russinova E. (2010) Functional modules in the Arabidopsis core cell cycle binary protein-protein interaction network. Plant Cell 22: 1264–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdenx B, Bernard A, Domergue F, Pascal S, Léger A, Roby D, Pervent M, Vile D, Haslam RP, Napier JA, et al. (2011) Overexpression of Arabidopsis ECERIFERUM1 promotes wax very-long-chain alkane biosynthesis and influences plant response to biotic and abiotic stresses. Plant Physiol 156: 29–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford KJ, Sharkey TD, Farquhar GD. (1983) Gas exchange, stomatal behavior, and ΔC values of the flacca tomato mutant in relation to abscisic acid. Plant Physiol 72: 245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae K, Gonong BJ, Kim SC, Kieslich CA, Morikis D, Balasubramanian S, Lord EM. (2010) A multifaceted study of stigma/style cysteine-rich adhesin (SCA)-like Arabidopsis lipid transfer proteins (LTPs) suggests diversified roles for these LTPs in plant growth and reproduction. J Exp Bot 61: 4277–4290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Slesak I, Jordá L, Sotnikov A, Melzer M, Miszalski Z, Mullineaux PM, Parker JE, Karpinska B, Karpinski S. (2009) Arabidopsis chloroplastic glutathione peroxidases play a role in cross talk between photooxidative stress and immune responses. Plant Physiol 150: 670–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Xiao YG, Li X, Ni M. (2012) Light-regulated stomatal aperture in Arabidopsis. Mol Plant 5: 566–572 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cominelli E, Galbiati M, Vavasseur A, Conti L, Sala T, Vuylsteke M, Leonhardt N, Dellaporta SL, Tonelli C. (2005) A guard-cell-specific MYB transcription factor regulates stomatal movements and plant drought tolerance. Curr Biol 15: 1196–1200 [DOI] [PubMed] [Google Scholar]
- Deng XW, Matsui M, Wei N, Wagner D, Chu AM, Feldmann KA, Quail PH. (1992) COP1, an Arabidopsis regulatory gene, encodes a protein with both a zinc-binding motif and a G β homologous domain. Cell 71: 791–801 [DOI] [PubMed] [Google Scholar]
- Emi T, Kinoshita T, Shimazaki K. (2001) Specific binding of vf14-3-3a isoform to the plasma membrane H+-ATPase in response to blue light and fusicoccin in guard cells of broad bean. Plant Physiol 125: 1115–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feussner I, Wasternack C. (2002) The lipoxygenase pathway. Annu Rev Plant Biol 53: 275–297 [DOI] [PubMed] [Google Scholar]
- Fisher RP, Morgan DO. (1994) A novel cyclin associates with MO15/CDK7 to form the CDK-activating kinase. Cell 78: 713–724 [DOI] [PubMed] [Google Scholar]
- Greer S, Wen M, Bird D, Wu X, Samuels L, Kunst L, Jetter R. (2007) The cytochrome P450 enzyme CYP96A15 is the midchain alkane hydroxylase responsible for formation of secondary alcohols and ketones in stem cuticular wax of Arabidopsis. Plant Physiol 145: 653–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajheidari M, Farrona S, Huettel B, Koncz Z, Koncz C. (2012) CDKF;1 and CDKD protein kinases regulate phosphorylation of serine residues in the C-terminal domain of Arabidopsis RNA polymerase II. Plant Cell 24: 1626–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Duan Y, Hua D, Fan G, Wang L, Liu Y, Chen Z, Han L, Qu LJ, Gong Z. (2012) DEXH box RNA helicase-mediated mitochondrial reactive oxygen species production in Arabidopsis mediates crosstalk between abscisic acid and auxin signaling. Plant Cell 24: 1815–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua D, Wang C, He J, Liao H, Duan Y, Zhu Z, Guo Y, Chen Z, Gong Z. (2012) A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 24: 2546–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada S, Ohgishi M, Mayama T, Okada K, Sakai T. (2004) RPT2 is a signal transducer involved in phototropic response and stomatal opening by association with phototropin 1 in Arabidopsis thaliana. Plant Cell 16: 887–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JB, Kim YA, Kim SJ, Lee SH, Kim DH, Cheong GW, Hwang I. (2001) A new dynamin-like protein, ADL6, is involved in trafficking from the trans-Golgi network to the central vacuole in Arabidopsis. Plant Cell 13: 1511–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang X, Chong J, Ni M. (2005) HYPERSENSITIVE TO RED AND BLUE 1, a ZZ-type zinc finger protein, regulates phytochrome B-mediated red and cryptochrome-mediated blue light responses. Plant Cell 17: 822–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux P. (1999) Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 284: 654–657 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Emi T, Tominaga M, Sakamoto K, Shigenaga A, Doi M, Shimazaki K. (2003) Blue-light- and phosphorylation-dependent binding of a 14-3-3 protein to phototropins in stomatal guard cells of broad bean. Plant Physiol 133: 1453–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki Ki. (1999) Blue light activates the plasma membrane H+-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J 18: 5548–5558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitsios G, Doonan JH. (2011) Cyclin dependent protein kinases and stress responses in plants. Plant Signal Behav 6: 204–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesiński P, Piechota J, Szczepaniak A. (2011) Initial characteristics of RbcX proteins from Arabidopsis thaliana. Plant Mol Biol 77: 447–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI. (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé JC, Martinez AM, Fesquet D, Capony JP, Darbon JM, Derancourt J, Devault A, Morin N, Cavadore JC, Dorée M. (1994) p40MO15 associates with a p36 subunit and requires both nuclear translocation and Thr176 phosphorylation to generate cdk-activating kinase activity in Xenopus oocytes. EMBO J 13: 5155–5164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léon-Kloosterziel KM, Gil MA, Ruijs GJ, Jacobsen SE, Olszewski NE, Schwartz SH, Zeevaart JA, Koornneef M. (1996) Isolation and characterization of abscisic acid-deficient Arabidopsis mutants at two new loci. Plant J 10: 655–661 [DOI] [PubMed] [Google Scholar]
- Liang YK, Dubos C, Dodd IC, Holroyd GH, Hetherington AM, Campbell MM. (2005) AtMYB61, an R2R3-MYB transcription factor controlling stomatal aperture in Arabidopsis thaliana. Curr Biol 15: 1201–1206 [DOI] [PubMed] [Google Scholar]
- Liu H, Liu B, Zhao C, Pepper M, Lin C. (2011) The action mechanisms of plant cryptochromes. Trends Plant Sci 16: 684–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Dai X, Xu Y, Guo J, Liu Y, Chen N, Xiao J, Zhang D, Xu Z, Zhang X, et al. (2009) Enhanced tolerance to chilling stress in OsMYB3R-2 transgenic rice is mediated by alteration in cell cycle and ectopic expression of stress genes. Plant Physiol 150: 244–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä TP, Tassan JP, Nigg EA, Frutiger S, Hughes GJ, Weinberg RA. (1994) A cyclin associated with the CDK-activating kinase MO15. Nature 371: 254–257 [DOI] [PubMed] [Google Scholar]
- Mao J, Zhang YC, Sang Y, Li QH, Yang HQ. (2005) From the cover: a role for Arabidopsis cryptochromes and COP1 in the regulation of stomatal opening. Proc Natl Acad Sci USA 102: 12270–12275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotte WR, Jr, Guiltinan MJ, Quatrano RS. (1992) ABA-regulated gene expression: cis-acting sequences and trans-acting factors. Biochem Soc Trans 20: 93–97 [DOI] [PubMed] [Google Scholar]
- Montané MH, Kloppstech K. (2000) The family of light-harvesting-related proteins (LHCs, ELIPs, HLIPs): was the harvesting of light their primary function? Gene 258: 1–8 [DOI] [PubMed] [Google Scholar]
- Nakashima K, Ito Y, Yamaguchi-Shinozaki K. (2009) Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiol 149: 88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels L, Goossens A. (2011) The JAZ proteins: a crucial interface in the jasmonate signaling cascade. Plant Cell 23: 3089–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posé D, Castanedo I, Borsani O, Nieto B, Rosado A, Taconnat L, Ferrer A, Dolan L, Valpuesta V, Botella MA. (2009) Identification of the Arabidopsis dry2/sqe1-5 mutant reveals a central role for sterols in drought tolerance and regulation of reactive oxygen species. Plant J 59: 63–76 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D. (2001) Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 52: 627–658 [DOI] [PubMed] [Google Scholar]
- Serizawa H, Mäkelä TP, Conaway JW, Conaway RC, Weinberg RA, Young RA. (1995) Association of Cdk-activating kinase subunits with transcription factor TFIIH. Nature 374: 280–282 [DOI] [PubMed] [Google Scholar]
- Shiekhattar R, Mermelstein F, Fisher RP, Drapkin R, Dynlacht B, Wessling HC, Morgan DO, Reinberg D. (1995) Cdk-activating kinase complex is a component of human transcription factor TFIIH. Nature 374: 283–287 [DOI] [PubMed] [Google Scholar]
- Shimazaki K, Doi M, Assmann SM, Kinoshita T. (2007) Light regulation of stomatal movement. Annu Rev Plant Biol 58: 219–247 [DOI] [PubMed] [Google Scholar]
- Shimotohno A, Ohno R, Bisova K, Sakaguchi N, Huang J, Koncz C, Uchimiya H, Umeda M. (2006) Diverse phosphoregulatory mechanisms controlling cyclin-dependent kinase-activating kinases in Arabidopsis. Plant J 47: 701–710 [DOI] [PubMed] [Google Scholar]
- Shimotohno A, Umeda-Hara C, Bisova K, Uchimiya H, Umeda M. (2004) The plant-specific kinase CDKF;1 is involved in activating phosphorylation of cyclin-dependent kinase-activating kinases in Arabidopsis. Plant Cell 16: 2954–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling U, van Cleve B, Frick G, Apel K, Armstrong GA. (1997) Overexpression of light-dependent PORA or PORB in plants depleted of endogenous POR by far-red light enhances seedling survival in white light and protects against photooxidative damage. Plant J 12: 649–658 [DOI] [PubMed] [Google Scholar]
- Sun X, Kang X, Ni M. (2012) Hypersensitive to red and blue 1 and its modification by protein phosphatase 7 are implicated in the control of Arabidopsis stomatal aperture. PLoS Genet 8: e1002674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng TS, Briggs WR. (2010) The Arabidopsis rcn1-1 mutation impairs dephosphorylation of Phot2, resulting in enhanced blue light responses. Plant Cell 22: 392–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umate P. (2011) Genome-wide analysis of lipoxygenase gene family in Arabidopsis and rice. Plant Signal Behav 6: 335–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Fabian T, Sauter M, Bhalerao RP, Schrader J, Sandberg G, Umeda M, Uchimiya H. (2000) Activation of CDK-activating kinase is dependent on interaction with H-type cyclins in plants. Plant J 24: 11–20 [DOI] [PubMed] [Google Scholar]
- Yoo CY, Pence HE, Jin JB, Miura K, Gosney MJ, Hasegawa PM, Mickelbart MV. (2010) The Arabidopsis GTL1 transcription factor regulates water use efficiency and drought tolerance by modulating stomatal density via transrepression of SDD1. Plant Cell 22: 4128–4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang H, Takemiya A, Song CP, Kinoshita T, Shimazaki K. (2004) Inhibition of blue light-dependent H+ pumping by abscisic acid through hydrogen peroxide-induced dephosphorylation of the plasma membrane H+-ATPase in guard cell protoplasts. Plant Physiol 136: 4150–4158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Hua D, Chen Z, Zhou Z, Gong Z. (2009) Elongator mediates ABA responses, oxidative stress resistance and anthocyanin biosynthesis in Arabidopsis. Plant J 60: 79–90 [DOI] [PubMed] [Google Scholar]