eCA enhances CO2 supply for photosynthesis in two marine diatoms.
Abstract
Many microalgae induce an extracellular carbonic anhydrase (eCA), associated with the cell surface, at low carbon dioxide (CO2) concentrations. This enzyme is thought to aid inorganic carbon uptake by generating CO2 at the cell surface, but alternative roles have been proposed. We developed a new approach to quantify eCA activity in which a reaction-diffusion model is fit to data on 18O removal from inorganic carbon. In contrast to previous methods, eCA activity is treated as a surface process, allowing the effects of eCA on cell boundary-layer chemistry to be assessed. Using this approach, we measured eCA activity in two marine diatoms (Thalassiosira pseudonana and Thalassiosira weissflogii), characterized the kinetics of this enzyme, and studied its regulation as a function of culture pH and CO2 concentration. In support of a role for eCA in CO2 supply, eCA activity specifically responded to low CO2 rather than to changes in pH or HCO3−, and the rates of eCA activity are nearly optimal for maintaining cell surface CO2 concentrations near those in the bulk solution. Although the CO2 gradients abolished by eCA are small (less than 0.5 μm concentration difference between bulk and cell surface), CO2 uptake in these diatoms is a passive process driven by small concentration gradients. Analysis of the effects of short-term and long-term eCA inhibition on photosynthesis and growth indicates that eCA provides a small energetic benefit by reducing the surface-to-bulk CO2 gradient. Alternative roles for eCA in CO2 recovery as HCO3− and surface pH regulation were investigated, but eCA was found to have minimal effects on these processes.
To overcome the inefficiencies of Rubisco, many phytoplankton operate a CO2-concentrating mechanism (CCM) that increases Rubisco’s rate of carbon fixation and reduces oxygen fixation by increasing the concentration of CO2 around the enzyme. CCMs typically consist of inorganic carbon (Ci) pumps, carbonic anhydrases (CAs) to equilibrate HCO3− and CO2, and a compartment to confine Rubisco, such as the pyrenoid or carboxysome, minimizing the volume in which CO2 is elevated (Badger et al., 1998; Kaplan and Reinhold, 1999; Giordano et al., 2005). Intracellular carbonic anhydrases (iCAs) play multiple roles in CCMs, including the conversion of accumulated HCO3− to CO2 around Rubisco and the prevention of CO2 leakage (Badger, 2003). Some organisms also have an extracellular carbonic anhydrase (eCA) associated with the cell wall, plasma membrane, or periplasmic space. The role of eCA has been enigmatic, although it is clearly related to the CCM. In Chlamydomonas reinhardtii, where eCA has been most thoroughly studied, the major eCA (Cah1) is up-regulated at low CO2, and its regulatory network includes a transcription factor that induces the expression of other CCM genes as well (Yoshioka et al., 2004; Ohnishi et al., 2010). In other organisms, eCA activity generally increases, in some cases dramatically, at low CO2, supporting its association with the CCM, but the genetic details of regulation are not known (Nimer et al., 1997; Rost et al., 2003).
When first discovered in microalgae, eCA was thought to facilitate CO2 influx by keeping surface CO2 at bulk solution concentrations (Moroney et al., 1985). Many microalgae take up CO2 to support photosynthesis, but because of the low concentration of CO2 in most natural waters and the slow rate of HCO3− dehydration, this uptake can lead to some depletion of CO2 in the diffusive boundary layer surrounding the cell. eCA accelerates the dehydration of HCO3− to CO2 within the boundary layer, increasing the surface CO2 concentration. Support for this role has come from experiments showing that inhibition of eCA reduces photosynthetic rates and Ci accumulation in disparate microalgae, including the green alga C. reinhardtii, the dinoflagellate Prorocentrum micans, the prymnesiophyte Phaeocystis globosa, and the diatom Thalassiosira weissflogii (Moroney et al., 1985; Nimer et al., 1999; Elzenga et al., 2000; Burkhardt et al., 2001).
Although there is strong support for the role of eCA in CO2 supply, some observations suggest that it may have additional or alternative roles. In some organisms, blocking eCA does not inhibit photosynthesis, and in C. reinhardtii, knocking out the major eCA had only minor effects on photosynthesis (Van and Spalding, 1999; Moroney et al., 2011). In C. reinhardtii, these results may be explained by the fact that only a small fraction of the total eCA activity is apparently required to support photosynthetic CO2 uptake, so that if inhibition is not fully effective, CO2 could still be kept high at the cell surface (Moroney et al., 1985; Palmqvist et al., 1990; Moroney et al., 2011). Such excess may point to other roles for eCA. On the basis of a correlation between HCO3− uptake and eCA activity, Trimborn et al. (2009) suggested that eCA in diatoms may be used to recover leaked CO2, converting it to HCO3− to enhance uptake. eCA may also have a role in pH homeostasis, which is a common role for CA in heterotrophic organisms (Boron, 2004; Swietach et al., 2010).
We sought to better understand the role of eCA in two diatoms by making quantitative measurements of eCA rates and modeling the effect of eCA on boundary-layer chemistry. Our approach to quantify eCA activity is an adaptation of a common method to measure CA activity based on the enzyme’s acceleration of 18O removal from labeled Ci. This technique has been used to measure the kinetics of isolated CA enzymes (Silverman, 1982), iCA activity (Tu et al., 1978), and eCA activity (Palmqvist et al., 1994; Delacruz et al., 2010). The advance presented here is to extract a quantitative, intrinsic measure of surface eCA activity by applying a simple box model of 18O-exchange kinetics to the data that accounts for the localization of the enzyme. In contrast, previous methods to measure eCA activity have effectively treated the activity as dispersed throughout the solution and, in some cases, are semiquantitative (Palmqvist et al., 1994; Elzenga et al., 2000; Delacruz et al., 2010). Previous approaches based on 18O exchange use the long-term rate of 18O removal as a quantitative but empirical measure of eCA activity (Palmqvist et al., 1994; Delacruz et al., 2010). Another technique uses the rate of equilibration of 14C between CO2 and HCO3−, quantifying eCA activity as an increase in the rate of CO2 hydration in the bulk solution (Elzenga et al., 2000; Martin and Tortell, 2006). A key advantage of our approach is that eCA catalysis is treated as a surface phenomenon, allowing the measured activities to be used in modeling the effects of eCA on boundary-layer chemistry. Using the eCA rates measured in the diatoms, we assessed the potential role of eCA in CO2 supply, CO2 recovery, and pH homeostasis.
RESULTS
To determine eCA activity on the cell surface, paired measurements of 18O-labeled Ci exchange were made in the presence and absence of an eCA inhibitor. When an eCA inhibitor is present, the 18O-CO2 data were used to determine iCA activity and CO2 and HCO3− fluxes into the cell (Tu et al., 1978). Subsequently, surface eCA activity was quantified by comparing simulations using a model that describes the temporal evolution of the isotopologs of Ci in and around a cell (Fig. 1; see “Materials and Methods”), with 18O removal rates measured in the absence of an eCA inhibitor.
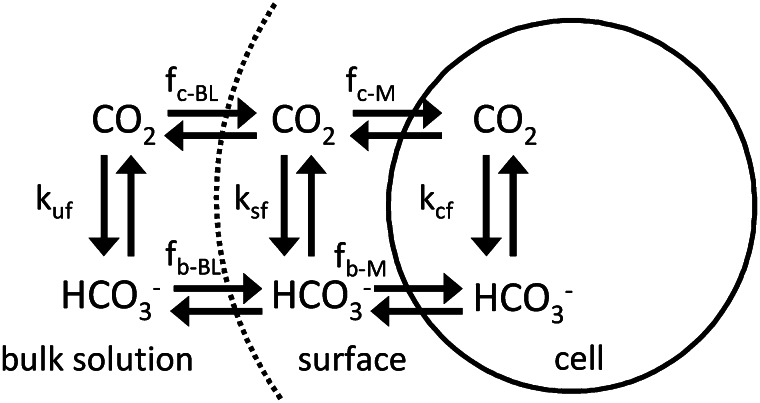
Figure 1.
Diagram of the compartments and fluxes in the box model used to determine eCA activity (Eqs. 1–6; Table III). Fluxes between the compartments are described using mass transfer coefficients, and the reactions are treated using first-order rate constants.
iCA Kinetics and Mass Transfer Coefficients
Our method for the determination of eCA activity requires that iCA activity and mass transfer coefficients for passive CO2 and HCO3− fluxes be known. These terms were determined from 18O-removal kinetics when eCA activity was inhibited using the CA inhibitor acetazolamide (AZ) or dextran-bound acetazolamide (DBAZ; Tu et al., 1978; Hopkinson et al., 2011). iCA activities depended on culture conditions, ranging between 80 and 200 s−1 for Thalassiosira pseudonana and between 20 and 150 s−1 for T. weissflogii. Mass transfer coefficients for CO2 (fc) were 1.3 ± 0.4 × 10−8 cm3 s−1 for T. pseudonana and 2.9 ± 0.9 × 10−8 cm3 s−1 for T. weissflogii, while HCO3− mass transfer coefficients were less than 1 × 10−12 cm3 s−1 for T. pseudonana and 1.9 ± 1.6 × 10−10 cm3 s−1 for T. weissflogii, similar to previously reported values (Hopkinson et al., 2011).
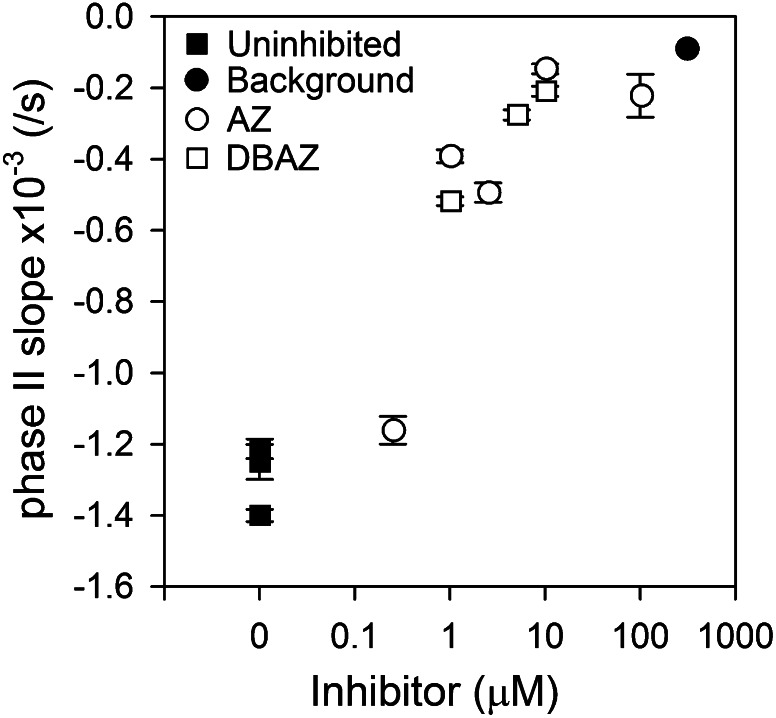
Accurate determination of iCA activity and CO2 and HCO3− mass transfer coefficients requires that eCA be fully inhibited. 18O removal follows a biphasic pattern in which there is an initial, rapid removal of 18O as CO2 enters CA-containing cells followed by a slower, long-term loss of 18O due to the depletion of 18O from HCO3− (Silverman et al., 1976; Fig. 2). We used the long-term rate of 18O removal or “phase II slope” (calculated as the slope of a linear fit through natural log-transformed 18O atom fraction data) as an empirical measure of eCA activity to establish that eCA effectively inhibited the CA inhibitors (Fig. 3; Delacruz et al., 2010). Application of increasing concentrations of AZ or DBAZ to cells expressing eCA reduces the phase II slope to near background, uncatalyzed exchange rates, verifying that eCA activity was effectively eliminated. The presence of iCA accelerates long-term 18O removal slightly above the background rate. We verified that neither AZ nor DBAZ had a detectable effect on iCA activity of low-pH-grown cells (where eCA is absent; see below) and so did not pass through the plasma membrane to any significant extent (data not shown).
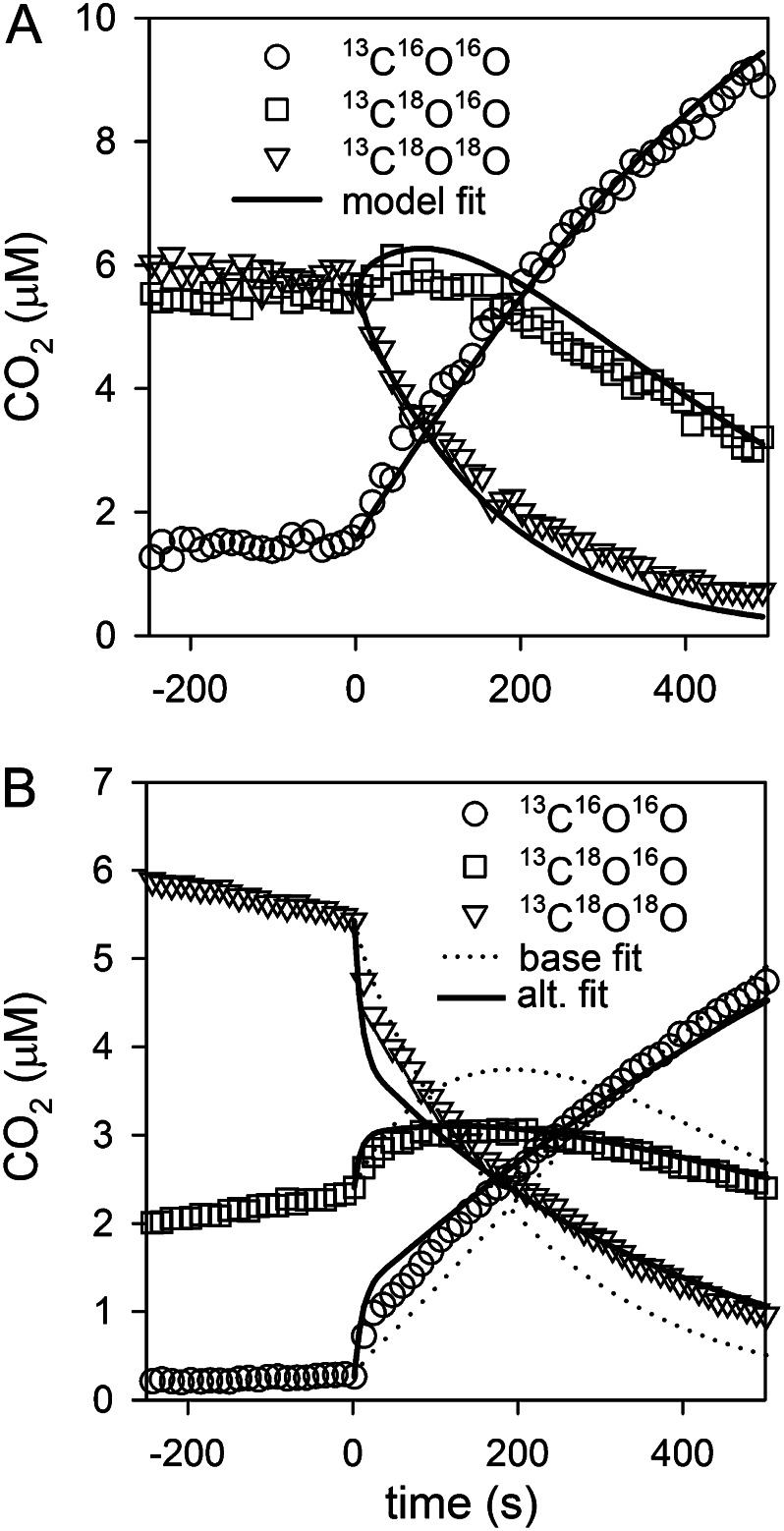
Figure 2.
Sample model fits to 18O-CO2 data for the determination of eCA activity. Cells expressing eCA were added to the assay solution at time 0, accelerating 18O removal. A shows a good fit to the data. B shows an example where a better fit can be achieved by reducing fb-BL 10-fold, but the eCA activities are similar in both fits (base, ksf = 1.7 × 10−6; reduced, fb-BL ksf = 1.5 × 10−6). For clarity, only every other 18O-CO2 data point is shown.
Figure 3.
Long-term rate of 18O removal (phase II slope) as a function of eCA inhibitor concentration. The background rate, prior to cell addition, is also plotted.
Determination of eCA Rate Constants
After determining the rate constants for iCA and CO2 and HCO3− mass transfer coefficients, eCA activity in T. pseudonana and T. weissflogii was quantified by fitting the box model of isotope exchange to the observations (see Eqs. 1–6 in “Materials and Methods”). ksf, the first-order rate constant for eCA-catalyzed CO2 hydration, is used as a measure for eCA activity, since it can be directly compared with the boundary-layer mass transfer coefficient for CO2 (fc-BL) to assess the effectiveness of eCA (see “Materials and Methods”). The model gave good fits to the 18O-CO2 data in most cases, and the eCA activities were consistent in replicate runs (Fig. 2A). eCA activities (ksf) varied with culture and assay conditions, ranging between 0 and 3.5 × 10−7 cm3 s−1 for T. pseudonana and between 0 and 40 × 10−7 cm3 s−1 for T. weissflogii.
In some runs, the model fits could not account for a depletion of the 13C18O16O intermediate species later in the assay (Fig. 2B). This signature suggests reduced exchange between the surface layer and the bulk solution (Silverman et al., 1981), and the fit can be improved by reducing the diffusive HCO3− flux to the cell surface (e.g. due to the presence of an extracellular matrix that reduces the diffusivity of charged ions; Stewart, 2003). However, this improvement of the fit alters the estimated eCA activity by less than 20% (Fig. 2B). Similarly, eCA activity is not sensitive to the choice of the size of the surface boundary-layer volume (Table I). The effect of potential residual eCA activity when determining iCA rate constants and cross-membrane fluxes was also assessed using the eCA box model. eCA activity and HCO3− permeability have similar effects on 18O-CO2 behavior, because both expose the extracellular HCO3− pool to CA and so accelerate the long-term rate of 18O removal. If eCA was not fully inhibited during the determination of iCA activity and mass transfer coefficients, the activity would be treated as increased HCO3− transfer through the cell membrane (greater fb-M). To determine the maximal residual eCA activity, we fit 18O-CO2 data from runs in which eCA should have been completely inhibited (50 μm or greater AZ or DBAZ) to the eCA box model (Eqs. 1–6), setting HCO3− membrane permeability to zero (fb-M = 0) and treating iCA activity (kcf), CO2 permeability (fc-M), and eCA activity (ksf) as unknowns. The potential residual eCA activities (T. pseudonana, 5.2 ± 7.1 × 10−9 cm3 s−1; T. weissflogii, 5.4 ± 4.6 × 10−8 cm3 s−1) were low relative to measured eCA activities. Thus, both the empirical analysis (Fig. 3) and the modeling approach show that the eCA inhibitors were highly effective.
Table I. Effect of model surface volume on derived eCA activity.
| Surface Volume | eCA Activity |
|---|---|
| cm3 | cm3 s−1 |
| 2.0 × 10−12 | 7.29 × 10−8 |
| 8.2 × 10−12 | 7.34 × 10−8 |
| 4.8 × 10−11 | 7.31 × 10−8 |
| 1.1 × 10−10 | 7.32 × 10−8 |
eCA Kinetics: Effect of pH and Ci
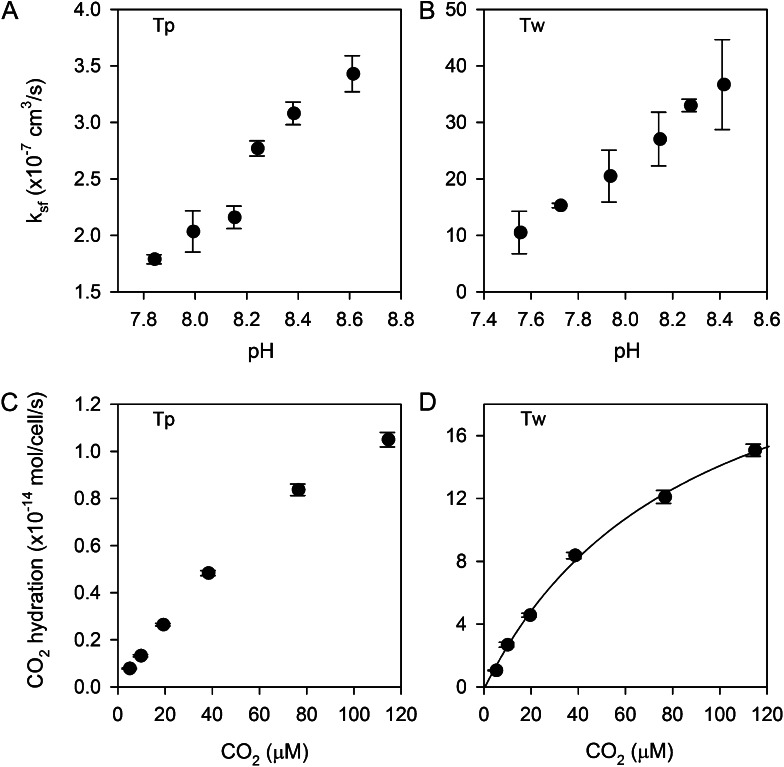
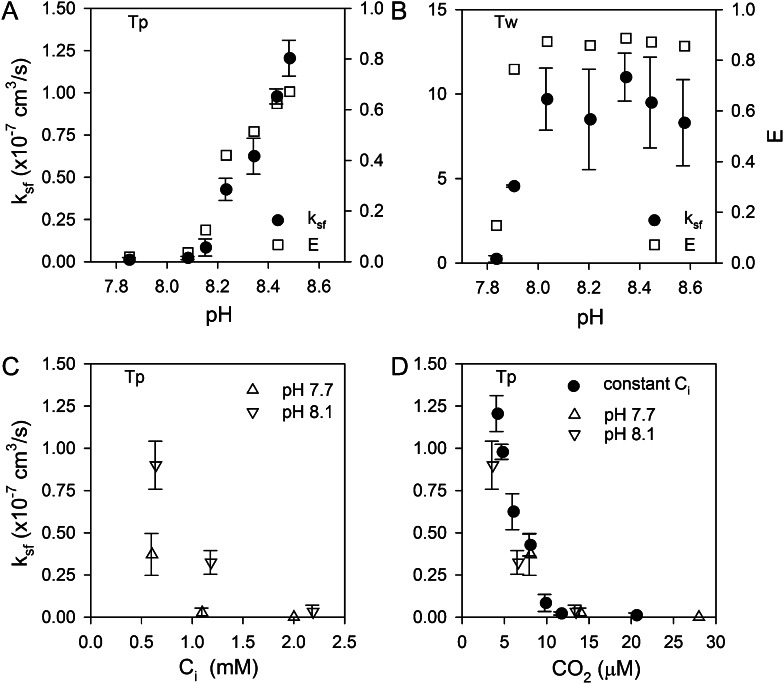
To characterize eCA enzyme kinetics, strong eCA activity was induced by growing cultures to high density without pH or CO2 control (final culture pH of 8.6–8.8). The cells were then concentrated and immediately assayed for eCA activity at a range of pHs and Ci concentrations. Enzyme activity (ksf) increased linearly with pH in T. pseudonana (7.8–8.6) and in T. weissflogii (7.5–8.4; Fig. 4, A and B) over a pH range typical of marine environments. CA activity typically shows a logarithmic increase with pH as water at the active site is deprotonated (Silverman and Lindskog, 1988). Our observations indicate a linear response, which may reflect the small pH range tested or result from differences between solution and cell surface pH.
Figure 4.
eCA kinetics. A and B, Effects of assay pH on eCA activity in T. pseudonana (Tp; A) and T. weissflogii (Tw; B). C and D, Reaction rates (CO2 hydration rate) as a function of substrate concentration (CO2) in T. pseudonana (C) and T. weissflogii (D). A Michaelis-Menten fit to the T. weissflogii data, which shows some saturation, gives a CO2 half-saturation constant of 87 ± 10 μm and a maximal reaction rate of 26 ± 2 × 10−14 mol cell−1 s−1.
We assessed the effect of Ci concentration on eCA reaction rates. eCA activity was measured at pH 7.9 and varying Ci from 0.5 to 12 mm, with corresponding CO2 concentrations ranging from 5 to 115 μm (Fig. 4, C and D). In T. pseudonana, nonlinearity in the reaction rate (CO2 hydration rate) versus substrate concentration (CO2) was observed only at the very highest CO2 concentration. In T. weissflogii, there is more significant nonlinearity, allowing a Michaelis-Menten function to be fit to the data. From this fit, the half-saturation constant of eCA for CO2 is 87 ± 10 μm. These results show that CO2 hydration and HCO3− dehydration are effectively first order with respect to substrate concentration in the environmentally relevant range (approximately 2 mm Ci, CO2 of 5–20 μm), validating our use of a first-order rate constant as a measure of eCA activity.
eCA Expression: Effect of CO2 and pH
T. pseudonana and T. weissflogii were grown for several generations at different pHs (7.7–8.6) maintained by a pH buffer 4-(2-Hydroxyethyl)piperazine-1-propanesulfonic acid (EPPS) at constant Ci (2 mm), concentrated, and assayed for eCA activity at constant pH (8.0) and Ci (2 mm) to determine the effect of growth conditions on eCA expression. Under these culture conditions, pH and CO2 covary with CO2, ranging between 24 μm at pH 7.8 and 3 μm at pH 8.6. In T. pseudonana, eCA activity was undetectable from pH 7.8 to 8.1 but then increased dramatically above this pH (Fig. 5A). eCA activity in T. weissflogii was undetectable only in cells grown at the lowest pH (7.8) and then increased quickly to a high, constant value from pH 8.0 to 8.6 (Fig. 5B). iCA activity (kcf) also increased substantially from low to high culture pH in both species (T. pseudonana, 80–200 s−1; T. weissflogii, 20–150 s−1). Complementary experiments in which the culture pH was held constant but Ci was varied also showed that low CO2 induced eCA expression in T. pseudonana (Fig. 5C). The T. pseudonana eCA activity data from the constant Ci and constant pH experiments converge, following a single trend, when plotted as a function of CO2 concentration in the growth medium, showing that eCA expression was primarily controlled by CO2 (Fig. 5D).
Figure 5.
Expression of eCA as a function of culture pH and Ci. A and B, When grown at a constant total Ci (2 mm) but varying pH, both diatoms induce eCA at high pH/low CO2 (A, T. pseudonana [Tp]; B, T. weissflogii [Tw]). The level of activity is sufficient to reduce surface-to-bulk CO2 gradients, as shown by the effectiveness index (E; Eq. 13). C, When T. pseudonana was grown at constant pH (7.7 or 8.1) but varying Ci, eCA was induced at low Ci. D, All the T. pseudonana data converge when plotted as a function of CO2, showing that it is the major control on eCA expression.
Effect of eCA Inhibition on Photosynthesis
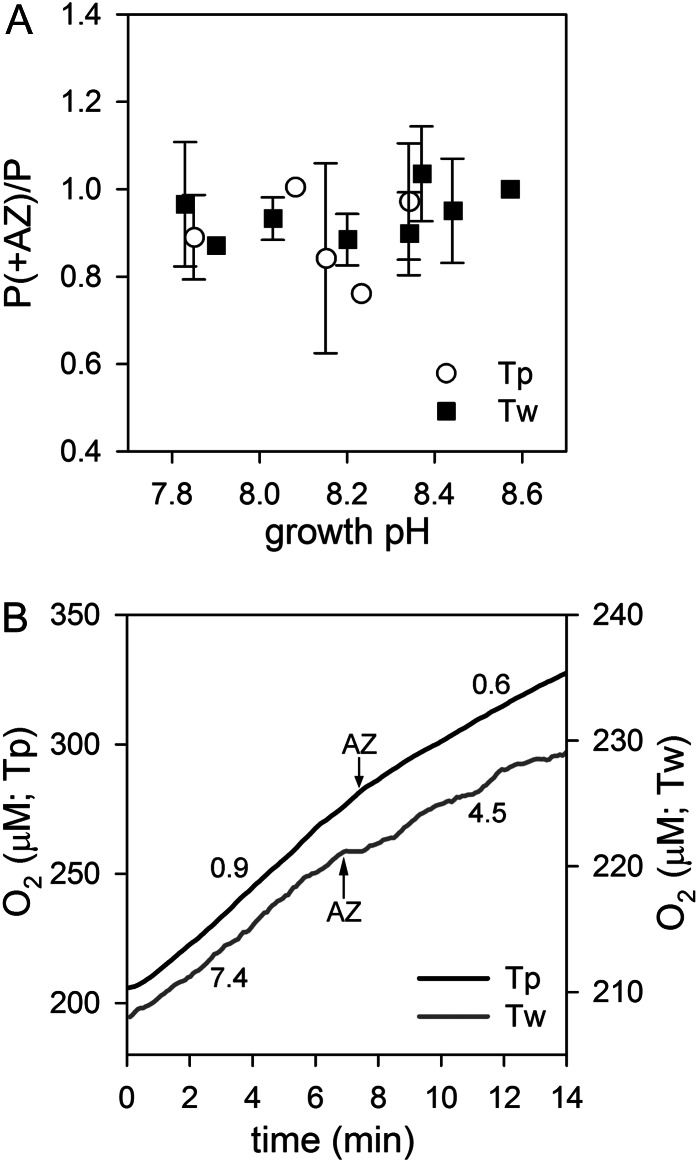
We tested the effects of short-term eCA inhibition on photosynthesis. At a concentration of AZ sufficient to inhibit all detectable eCA activity (50 μm), there was no consistent effect of short-term eCA inhibition on photosynthesis under environmentally relevant conditions of Ci availability (Ci, 2 mm; pH 7.8–8.6; Fig. 6A). We assessed the effects of AZ ranging from 1 to 500 μm on photosynthesis in T. pseudonana and T. weissflogii grown without pH control to pH 8.7 and assayed at the same pH, but we found no significant inhibition of photosynthesis even at the highest AZ concentrations (data not shown). Only at very low CO2 concentrations (1 μm; pH 8.7; Ci, 1 mm) did short-term eCA inhibition consistently reduce photosynthesis, inhibiting oxygen production by 42% ± 5% in T. weissflogii and by 25% ± 8% in T. pseudonana (Fig. 6B).
Figure 6.
Effect of the eCA inhibitor AZ on photosynthesis. A, Effect of short-term inhibition of eCA on photosynthesis shown as the ratio of the photosynthetic rate in the presence of the inhibitor (P+AZ) to the rate without the inhibitor (P). The diatoms (T. pseudonana [Tp] and T. weissflogii [Tw]) were grown and assayed at a range of pHs with constant Ci (2 mm). B, Oxygen traces showing inhibition of photosynthesis at 1 μm CO2 after the addition of AZ. The numbers next to the traces indicate the rate of oxygen production (×10−17 mol cell−1 s−1) before and after AZ addition.
Effect of eCA Inhibition on Growth
To test the long-term effects of eCA inhibition, growth rates of the two diatoms were measured in the presence and absence of DBAZ (Table II). The cultures were grown for 4 to 6 d under the same environmental conditions as other cultures (20°C, 16/8-h photoperiod at 125–150 μmol photons m−2 s−1) with 20 μm DBAZ added initially and 5 μm additional DBAZ added in the morning of day 3. In T. weissflogii, DBAZ reduced growth by approximately 10% at pH 8.4 (5 μm CO2) but had no effect on growth at pH 7.8 (24 μm CO2), demonstrating that the inhibitor did not have any nonspecific effects on metabolism. DBAZ had no significant effect on growth of T. pseudonana at pH 8.4.
Table II. Effect of DBAZ on growth rate.
The asterisk indicates that the DBAZ treatment was significantly different (P < 0.05; n = 4) from the control based on Student’s t test.
| Diatom | Culture pH | Growth Rate |
|
|---|---|---|---|
| Control | DBAZ | ||
| d−1 ± sd | |||
| T. pseudonana | 8.4 | 1.15 ± 0.01 | 1.14 ± 0.02 |
| T. weissflogii | 8.4 | 0.55 ± 0.01 | 0.51 ± 0.03* |
| T. weissflogii | 7.8 | 0.94 ± 0.02 | 0.94 ± 0.02 |
Model Assessment of eCA on Surface Boundary-Layer Chemistry
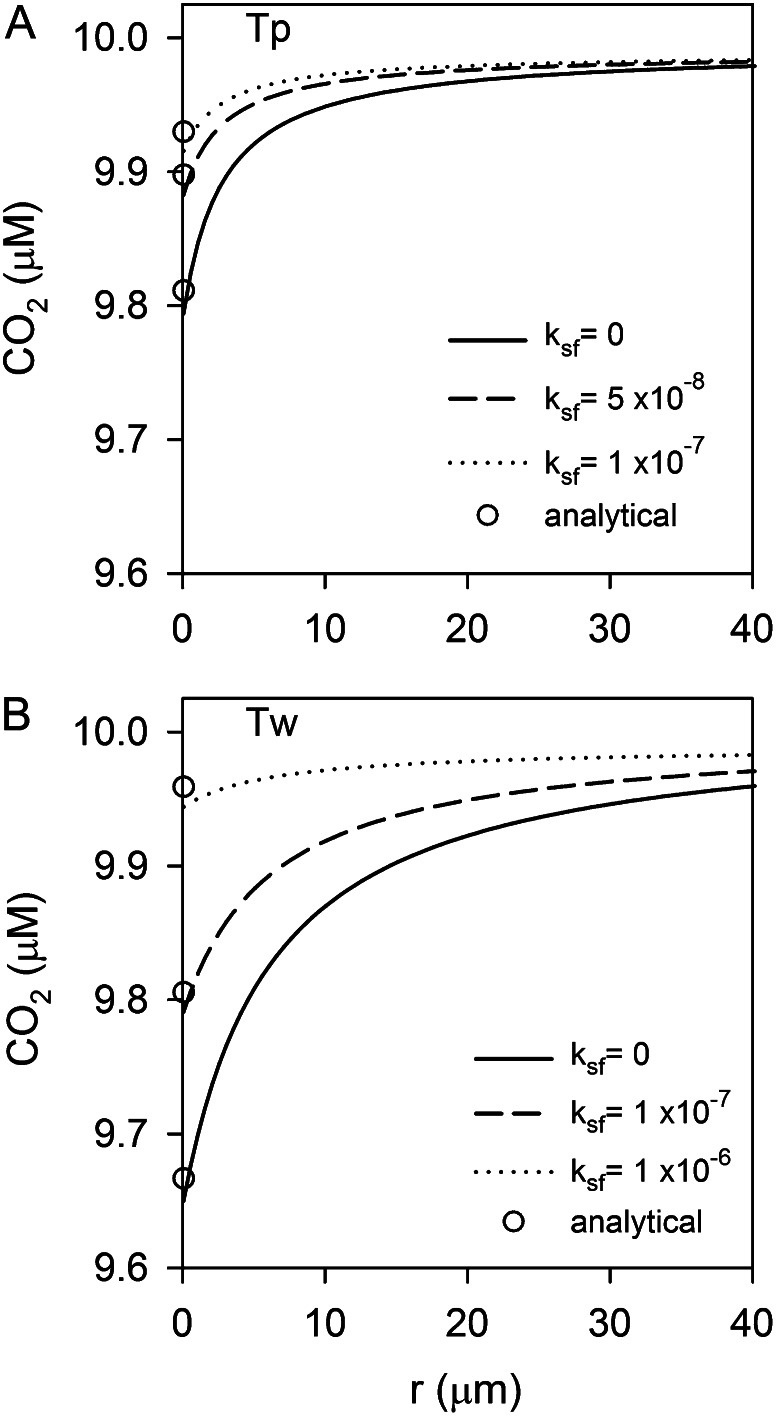
We used a spatially resolved reaction-diffusion model of the diffusive boundary layer to quantify the effect of eCA on boundary-layer chemistry (for details, see “Materials and Methods”). The primary role of eCA is thought to be the maintenance of bulk solution CO2 concentrations at the cell surface during photosynthesis. To assess this role, a model simulation was run in which a diatom takes up CO2 at two-thirds its photosynthetic rate, which is typical of cultured marine diatoms (Burkhardt et al., 2001; Rost et al., 2003; Hopkinson et al., 2011). Average photosynthetic rates measured in this study were used in the simulation (T. pseudonana, 1.7 × 10−17 mol cell−1 s−1; T. weissflogii, 7 × 10−17 mol cell−1 s−1). The analysis shows that the measured eCA activities are sufficient to maintain cell surface CO2 near bulk concentrations despite photosynthetic carbon uptake in both species (Fig. 7). In all cases, however, the absolute size of the CO2 gradient is relatively small. In the absence of eCA, the concentration difference between the bulk solution and the cell surface is only 0.2 μm for T. pseudonana and 0.35 μm for T. weissflogii.
Figure 7.
Model simulations of the effect of eCA on surface boundary-layer chemistry during CO2 uptake. (A, T. pseudonana [Tp]; B, T. weissflogii [Tw]). The effect of eCA on the CO2 profiles is shown at two eCA activities, the lower at a magnitude similar to fc-BL and the higher approximating maximal expression levels. ksf values used in the simulations are indicated. Analytical approximations for surface CO2 concentrations obtained using Equation 12 are indicated with white circles, agreeing well with the numerical solution.
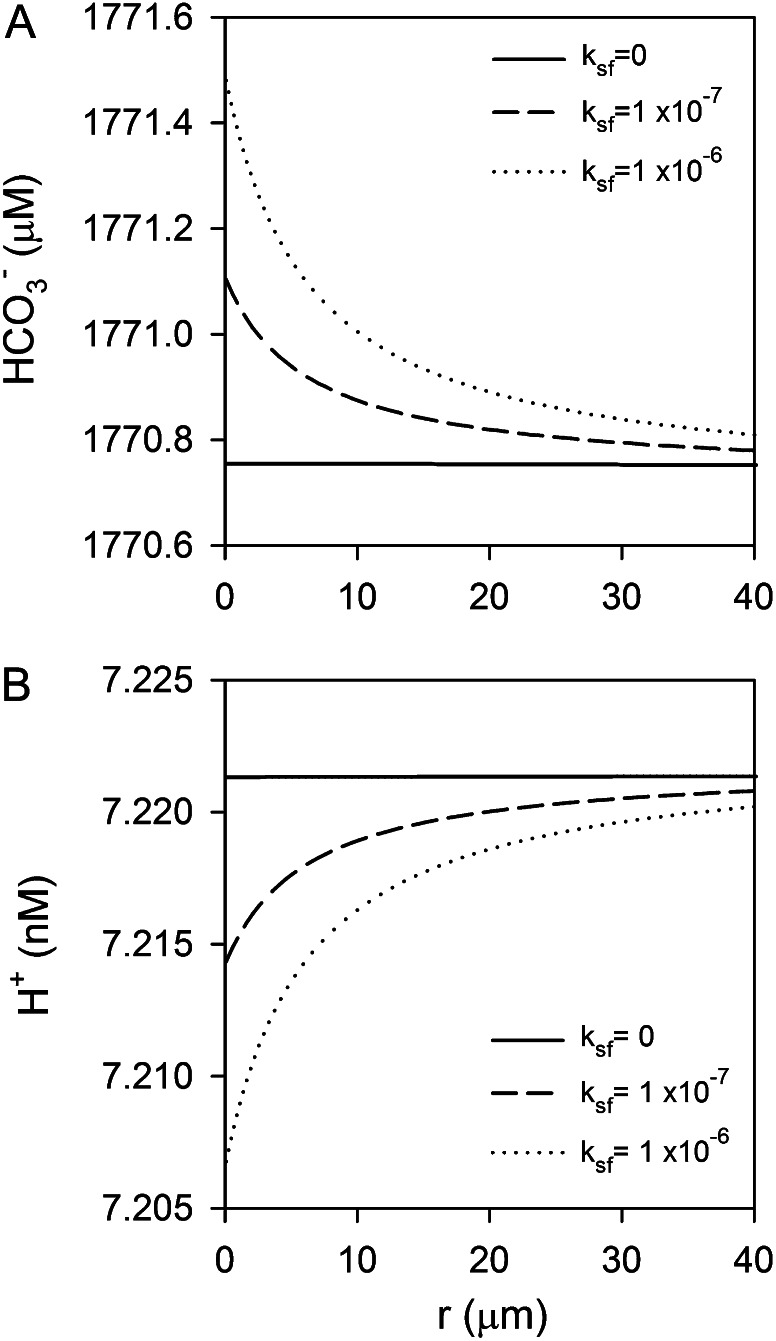
An alternative role for eCA in the recovery of leaked CO2 as HCO3− for subsequent uptake has also been suggested (Trimborn et al., 2008). As CO2 leakage is just the inverse of CO2 uptake, the modeled effects of eCA on CO2 gradients are very similar. In both diatoms, fully induced eCA activity is able to convert nearly all the leaked CO2 to HCO3−. However, even when eCA is effective, the changes in surface HCO3− concentrations are minuscule. The largest increase in HCO3− concentration is 0.7 μm, which is only a 0.04% increase (Fig. 8A).
Figure 8.
Model simulations showing that the effects of eCA on surface boundary-layer HCO3− and H+ concentrations are small. A, If CO2 leaks from the cell, eCA can convert this leaked CO2 to HCO3−, but the surface HCO3− concentration increases by less than 1 μm. B, eCA has only a minor effect on the H+ concentration during CO2 uptake. Both panels show results from simulations with T. weissflogii, since the effects of eCA, although still small, are more significant for this species.
Finally, eCA could also be involved in the control of surface pH, since the Ci system dominates pH buffering in seawater. One scenario, discussed below, involves H+ uptake equimolar to HCO3− uptake to compensate for intracellular H+ consumption required to convert HCO3− to CO2 for photosynthesis. This scenario was simulated with rates of H+ and HCO3− uptake at the cell surface equal to photosynthetic rates, but we found that eCA activity had no effect on the surface H+ perturbation induced by this uptake, because of rapid acid/base equilibration among buffer species (data not shown). CO2 uptake creates a pH disequilibrium in the boundary layer that can be reestablished by eCA (Fig. 8B), but the effect on the H+ concentration is very small.
DISCUSSION
Although there is strong evidence that eCA is linked to the CCM, its exact role has been controversial (Van and Spalding, 1999; Trimborn et al., 2008; Moroney et al., 2011). Enhancement of CO2 availability for photosynthesis is the most logical role for eCA, since CO2 concentrations are low in most natural waters and can become depleted in cell boundary layers. But alternative roles in the recovery of leaked CO2 and pH homeostasis have also been suggested, based in part on observations that the elimination of eCA does not always reduce photosynthesis. The lack of a quantitative, intrinsic measure of eCA activity has hindered the evaluation of its role. Here, we have developed an approach to quantify eCA activity, applied it to two marine diatoms, and evaluated the potential role of eCA in these organisms through an analysis of the effect of eCA activity on surface boundary-layer chemistry.
Enhancement of CO2 Supply
eCA is commonly thought to facilitate CO2 influx by dehydrating HCO3− at the cell surface. Many microalgae take up CO2 for photosynthesis (Badger et al., 1994; Burkhardt et al., 2001), which is most likely driven by a diffusive gradient (Kaplan and Reinhold, 1999; Hopkinson et al., 2011), despite the fact that HCO3− is much more abundant than CO2 in the ocean. Net CO2 uptake into the cell can be supported either by diffusion of CO2 from the bulk solution or generation of CO2 from HCO3− within the boundary layer, which for microalgae would need to be catalyzed by eCA because uncatalyzed HCO3− dehydration is slow (Wolf-Gladrow and Riebesell, 1997).
Consistent with a role for eCA in CO2 supply, we find that eCA is up-regulated at low CO2 concentrations and that induction occurs at a higher CO2 concentration in the larger diatom T. weissflogii, which is more prone to diffusive limitation (Fig. 5; Pasciak and Gavis, 1974; Riebesell et al., 1993). Typically, the effect of CO2 on eCA expression is assessed by varying pH at a constant Ci concentration or by bubbling cultures with air containing varying levels of CO2 (Nimer et al., 1997; Rost et al., 2003). In both approaches, CO2 and pH are closely coupled, with CO2 decreasing as pH increases, but it is generally assumed that CO2 is the key variable regulating eCA activity. Both T. pseudonana and T. weissflogii expressed eCA at low CO2/high pH (Fig. 5, A and B) in such an experiment, as is commonly observed. But by additionally culturing T. pseudonana at constant pH but variable Ci (Fig. 5C), we were able to clearly show that eCA activity responds to low CO2 concentrations, rather than changes pH or HCO3−, consistent with a role for eCA in CO2 uptake (Fig. 5D).
A key finding, made possible by the quantitative nature of the eCA measurements, is that the amount of eCA expressed by both diatoms is appropriate to support CO2 uptake, but not excessive. As shown in Equations 11 to 13 (see “Materials and Methods”), the relative magnitude of the boundary-layer mass transfer coefficient (fc-BL) and eCA activity (ksf) controls the sources of CO2 (diffusion or dehydration) for net uptake and describes the extent to which eCA is able to mitigate CO2 drawdown in the boundary layer. eCA activity at low CO2 in T. pseudonana and T. weissflogii is two to 10 times greater than the boundary-layer mass transfer coefficient (fc-BL; T. pseudonana, 5.9 × 10−8 cm3 s−1; T. weissflogii, 1.4 × 10−7 cm3 s−1), such that eCA activity is nearly optimal for the abolishment of bulk solution to surface CO2 gradients (Fig. 5). A spatially resolved model confirms that the eCA activities are able to maintain surface CO2 concentrations at near bulk concentrations (Fig. 7; for model description, see “Materials and Methods”). The lack of excess eCA for CO2 supply in these diatoms contrasts with C. reinhardtii, where there is apparently excess eCA for CO2 supply (Moroney et al., 1985), which could be taken to imply that it has other roles.
While the magnitude of the CO2 drawdown at the cell surface is relatively small (0.2–0.35 μm; Fig. 7), it is similar in size to the CO2 gradient across the cytoplasmic membrane that drives CO2 influx. For example, given the permeability of the T. weissflogii membrane to CO2 (Hopkinson et al., 2011), a 0.4 μm CO2 gradient across the plasma membrane would be needed to support CO2 uptake at the rate estimated for our culture conditions. The high permeability of membranes to CO2 means that this influx occurs passively, which is potentially more energy efficient than active uptake of HCO3−, but requires the cell to generate a CO2 gradient across the cytoplasmic membrane. In the absence of eCA, intracellular CO2 would need to be drawn down further to maintain CO2 uptake, which diatoms are capable of doing, as shown by their ability to take up CO2 over a wide range of extracellular CO2 concentrations (Burkhardt et al., 2001), but at the cost of increased energetic expenditure.
Short-term inhibition of eCA at CO2 concentrations greater than 1 μm did not reduce photosynthesis, presumably because cytoplasmic CO2 concentrations could be lowered to maintain CO2 influx (Fig. 6A). Photosynthesis was only reduced when extracellular CO2 concentrations decreased to 1 μm (Fig. 6B), at which point the cytoplasmic CO2 concentration was 0.4 μm in T. pseudonana and 0.2 μm in T. weissflogii, as estimated using boundary-layer mass transfer coefficients and the cytoplasmic membrane permeability for each species. The cells apparently could not lower their intracellular CO2 concentrations further, and for T. weissflogii, the intracellular CO2 concentration was so low that greater reduction would not significantly increase CO2 influx. Long-term inhibition of eCA reduced the growth of T. weissflogii at low CO2 (Table II), consistent with an increased energetic cost of Ci acquisition without eCA, although inhibition of eCA had no detectable effect on the growth of T. pseudonana, perhaps because its smaller size minimizes the magnitude of the bulk-to-surface CO2 gradient and the energetic costs associated with compensating for this gradient. However, even a small energetic savings that allows minor increases in growth rate can have major consequences for the ecological success of plankton in the ocean (Tilman, 1977).
Alternative Roles for eCA
Alternative roles for eCA in the recovery of leaked CO2 and the regulation of cell surface pH have been suggested (Trimborn et al., 2008). The CCM is not perfectly efficient, in large part because of the high permeability of membranes to CO2, leading to CO2 leakage out of the chloroplast or plasma membranes. While eCA could effectively convert leaked CO2 to HCO3−, the increase in HCO3− concentration achieved by CO2 recovery would be very small compared with the approximately 1.8 mm HCO3− concentration in seawater (Fig. 8A). Assuming that diatom HCO3− transporters follow Michaelis-Menten kinetics, in which the greatest sensitivity of uptake rate to substrate is a linear increase at low substrate concentrations, the increased cell surface HCO3− concentration would allow at most a 0.04% increase in uptake rate. Additionally, diatoms exhibit a net CO2 influx rather than a net CO2 efflux under normal conditions (Burkhardt et al., 2001; Hopkinson et al., 2011), so there is no CO2 leaking from the cell to be recovered.
CAs are commonly involved in pH regulation (Boron, 2004), so it is conceivable that eCA may be involved in pH maintenance, since the Ci system is the main pH buffer in seawater. The best example of the role of eCA in pH maintenance comes from human cancer cells, where high rates of respiration lead to a high acid load (in the form of CO2). By converting CO2 to HCO3− and H+ outside the cell, eCA helps to maintain an alkaline internal environment at the expense of a more acidic extracellular environment (Swietach et al., 2010). Photosynthetic organisms such as diatoms, on the other hand, import CO2 and HCO3− for carbon fixation. Since CO2 is ultimately the species fixed by Rubisco, the import and subsequent consumption of CO2 has no net effect on intracellular acid/base balance. However, imported HCO3− must be converted to CO2 for fixation, consuming a proton in the process. Organisms that transport HCO3− then need to import a proton (or export OH−) for each molecule of HCO3−. eCA could help supply H+ to the cell surface to balance HCO3− uptake, potentially accounting for the observed correlation between eCA and HCO3− uptake in several marine phytoplankton (Trimborn et al., 2008). Simulation of the effect of eCA on boundary-layer pH for diatoms importing HCO3− and H+ revealed that the boundary-layer pH changes induced by uptake are very small because of rapid reactions among the buffer species HCO3−/CO32− and B(OH)4−/B(OH)3. Furthermore, eCA does not alter the small pH changes, because the slow CO2/HCO3− equilibrium is not significantly involved in boundary-layer pH buffering in this case. Alternatively, eCA could help establish pH equilibrium during CO2 uptake. We simulated the effect of eCA on boundary-layer pH, assuming that the diatoms obtain all their carbon for photosynthesis from CO2. eCA does have an effect on boundary-layer pH in this case, and the highest rates are effective at reestablishing pH equilibrium, but the absolute effect of eCA on H+ concentration is very small (Fig. 8B).
CONCLUSION
Using a newly developed approach to quantify eCA-catalyzed CO2 hydration rates, eCA activity was measured in two diatoms, T. pseudonana and T. weissflogii, and its potential roles in CO2 supply, CO2 recovery, and pH regulation were investigated. In support of the role of eCA in photosynthetic CO2 supply, its activity increased at low CO2 concentrations, was appropriate to abolish bulk-to-surface CO2 gradients, and was greater in the larger diatom (T. weissflogii), which is more prone to diffusive limitation. The differences in CO2 concentration between the bulk solution and the cell surface that are eliminated by eCA are small (less than 0.5 μm), but small gradients drive significant passive CO2 influxes in these diatoms (Hopkinson et al., 2011). The consequences of short-term inhibition of eCA can be overcome by decreasing the cytoplasmic CO2 concentration to maintain CO2 influx rates. However, this requires increased energetic expenditure, which may explain the decreased growth rate of T. weissflogii when eCA was inhibited. We investigated alternative roles for eCA in the recovery of leaked CO2 for HCO3− uptake and pH regulation but found that eCA activity would not have a significant effect on boundary-layer HCO3− and H+ concentrations. Taken together, these results support a role for eCA in CO2 supply.
MATERIALS AND METHODS
Culturing
The diatoms Thalassiosira pseudonana (CCMP1335) and Thalassiosira weissflogii (CCMP1336) were obtained from the National Center for Marine Algae and Microbiota and maintained in Aquil medium (Price et al., 1988). For most experimental work, the algae were grown in Aquil made from a natural seawater base with 5 mm 4-(2-Hydroxyethyl)piperazine-1-propanesulfonic acid (EPPS) buffer to maintain constant pH and Ci conditions. To assess the pH and Ci dependence of enzyme kinetics, cultures were grown in Aquil to high density without pH or CO2 control (final culture pH of 8.6–8.8) to induce strong eCA activity. For experiments in which Ci concentrations were varied, North East Pacific Culture Collection Enriched Seawater, Artificial Seawater (NEPCC ESAW) medium was used with 5 mm EPPS buffer added (Harrison et al., 1980). This medium uses an artificial seawater base, and Ci was left out in the initial medium preparation, being added later at the desired concentration. All cultures were grown in an incubator at 20°C under fluorescent lights (125–150 μmol photons m−2 s−1) on a light/dark cycle (16 h on, 8 h off). Cell numbers were counted daily with a Coulter Counter, and cells were harvested during exponential growth.
The initial pH of the culture medium was adjusted with HCl or NaOH (stored in sealed serum vials to avoid CO2 absorption) and measured on the total hydrogen ion scale using thymol blue (Zhang and Byrne, 1996). The Ci concentration was measured at the beginning and end of the experiments using membrane inlet mass spectrometry (MIMS; Beckmann et al., 2009; Hopkinson et al., 2011). Additional carbon system parameters (CO2, HCO3−, and CO32− concentrations) were calculated from pH and Ci (Dickson and Goyet, 1994; Lueker et al., 2000).
18O-Exchange Experiments
The rate of 18O removal from labeled Ci was used to determine iCA and eCA activities. 18O-labeled 13C-Ci (2 mm, unless otherwise noted) was added to assay buffer (Ci-free artificial seawater, 20 mm Tris at pH 8.0, unless otherwise noted) in a MIMS chamber. Temperature in the chamber was maintained at 20°C using a water jacket. 18O-CO2 species were monitored by MIMS for approximately 10 min to determine the background rate of CO2 hydration/HCO3− dehydration, after which cells were added to the chamber from a concentrated suspension. 18O removal catalyzed by cellular CA was then monitored in the dark for 15 to 20 min. To determine iCA activity, an inhibitor of eCA was added prior to the addition of cells. In most cases, 50 μm AZ was used, but 50 μm DBAZ (Ramidus) was used in select experiments to confirm that diatom membranes were not permeable to AZ.
Photosynthetic Rates
The effect of eCA inhibitors on photosynthesis was assessed from measurements of oxygen production made using MIMS. Assay buffer with 2 mm Ci, unless otherwise noted, was added to the MIMS chamber, and cells were added from a concentrated suspension. Light was provided from a tungsten lamp at 200 μmol photons m−2 s−1. Oxygen production was monitored for approximately 10 min, at which point AZ or DBAZ was added and oxygen production was monitored for a further approximately 10 min. Measurements were made at the same pH as the cultures were grown and at pH 8.0.
eCA Model Development
The eCA box model considers 18O-CO2 and 18O-HCO3− isotopologs in three compartments: the bulk solution, the boundary layer at the cell surface, and the intracellular space (Fig. 1; Table III). Fluxes between the compartments are described by mass transfer coefficients, and the uncatalyzed and catalyzed CO2 hydration/HCO3− dehydration reactions, responsible for 18O removal, are treated as first-order reactions, since the enzyme is undersaturated for Ci (Fig. 4). The model is described by the following system of differential equations:
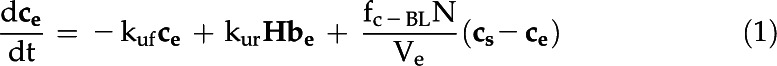 |
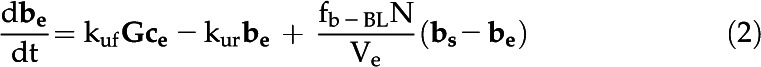 |
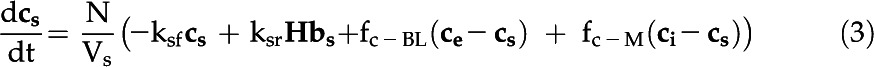 |
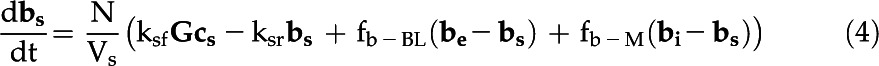 |
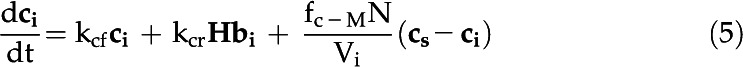 |
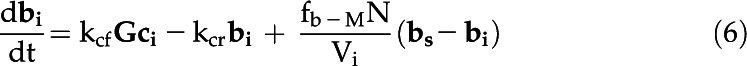 |
with variables and parameters as described in Table III.
Table III. Model notation.
Boldface symbols denote concentration vectors: c = [C16O2; C16O18O; C18O2]; b = [HC16O3−; HC16O218O−; HC16O18O2−; HC18O3−].
| Symbol | Definition | Units | Source/Method Reference |
|---|---|---|---|
| ce | Concentration of 18O-CO2 species in the bulk solution | mol cm−3 | MIMS measurement |
| cs | Concentration of 18O-CO2 species in the surface boundary layer | mol cm−3 | Modeled |
| ci | Concentration of 18O-CO2 species inside the cell | mol cm−3 | Modeled |
| be | Concentration of 18O-HCO3− species in the bulk solution | mol cm−3 | Modeled |
| bs | Concentration of 18O-HCO3− species in the surface boundary layer | mol cm−3 | Modeled |
| bi | Concentration of 18O-HCO3− species inside the cell | mol cm−3 | Modeled |
| kuf | Uncatalyzed CO2 hydration rate constant | s−1 | Background 18O removal rate |
| kur | Uncatalyzed HCO3− dehydration rate constant | s−1 | Background 18O removal rate |
| ksf | eCA-catalyzed CO2 hydration rate constant | cm3 s−1 | eCA assay (this study) |
| ksr | eCA-catalyzed HCO3− dehydration rate constant | cm3 s−1 | eCA assay (this study) |
| kcf | iCA-catalyzed CO2 hydration rate constant | s−1 | iCA assay (Tu et al., 1978) |
| kcr | iCA-catalyzed HCO3− dehydration rate constant | s−1 | iCA assay (Tu et al., 1978) |
| fc-BL | Boundary-layer mass transfer coefficient for CO2 | cm3 s−1 | iCA assay (Tu et al., 1978) |
| fb-BL | Boundary-layer mass transfer coefficient for HCO3− | cm3 s−1 | iCA assay (Tu et al., 1978) |
| fc-M | Membrane mass transfer coefficient for CO2 | cm3 s−1 | iCA assay (Hopkinson et al., 2011) |
| fb-M | Membrane mass transfer coefficient for HCO3− | cm3 s−1 | iCA assay (Hopkinson et al., 2011) |
| N | Number of cells in the assay chamber | – | Coulter Counter measurement |
| Ve | Bulk solution volume | cm3 | Measured |
| Vs | Surface layer volume | cm3 | Imposed, 8 × 10−12 cm3 |
| Vi | Intracellular volume | cm3 | Coulter Counter measurement |
| G | Stoichiometric matrix describing the hydration of CO2 species to HCO3− | – | Hopkinson et al. (2011) |
| H | Stoichiometric matrix describing the dehydration of HCO3− species to CO2 | – | Hopkinson et al. (2011) |
The solution volume (Ve) was measured directly, and the intracellular volume was determined using a Coulter Counter. The volume of the boundary layer compartment (Vs) was set to 8 × 10−12 cm3, reflecting a surface layer thickness on the order of 0.1 μm. The uncatalyzed CO2 hydration/HCO3− dehydration rates in the bulk solution (kuf, kur) were determined from 18O removal rates prior to the addition of cells, and the observed values agree well with published rates (Johnson, 1982). The mass transfer coefficients for diffusive flux between the bulk solution and the boundary layer (fc-BL, fb-BL) were calculated assuming the cells are spherical, with radii determined from Coulter Counter measurements (T. pseudonana, 2.5 μm; T. weissflogii, 6 μm):
where R is the cell radius and D is the diffusivity of CO2 (1.65 × 10−5 cm2 s−1 at 20°C) or HCO3− (1.05 × 10−5 cm2 s−1 at 20°C; Pasciak and Gavis, 1974). The parameters for iCA activity (kcf, kcr) and the mass transfer coefficients for membrane passage (fc-M, fb-M) were determined from analysis of 18O-exchange rates in the presence of an eCA inhibitor (Tu et al., 1978; Hopkinson et al., 2011). eCA-catalyzed hydration/dehydration rate constants (ksf, ksr) are related to each other via the CO2/HCO3− equilibrium constant, assuming microscopic reversibility. ksf, the first-order rate constant for eCA-catalyzed CO2 hydration, was determined by optimizing the model fit to the 18O-CO2 data (Supplemental Data S1).
Models of Surface Boundary-Layer Chemistry
To assess the effects of eCA on boundary-layer chemistry, we developed a simple analytical approximation and a one-dimensional numerical reaction diffusion model. The analytical model treats the case in which there is a net CO2 influx (photosynthetic uptake) to, or efflux (leakage) from, the cell. A net CO2 influx (an efflux is similar except for a change of sign) is supported by diffusion of CO2 from the bulk solution and, when eCA is present, the net generation of CO2 from eCA-catalyzed HCO3− dehydration. Diffusion through the boundary layer is dependent on the CO2 gradient between the bulk solution and the cell surface. For a spherical cell, the net diffusive CO2 flux (NCD) is:
The net generation of CO2 from HCO3− dehydration at the cell surface is:
In the marine environment, which our assay and culture conditions replicate, HCO3− concentrations are high, approximately 1.8 mm, and so are not significantly depleted at the cell surface. Taking the HCO3− concentration at the surface to be equal to the bulk solution, and using the fact that at equilibrium the forward and backward reaction rates will be equal (ksf[CO2] = ksr[HCO3−]), Equation 9 can be rewritten as:
In effect, the net CO2 supply from eCA depends on the same CO2 gradient as the diffusive supply, although the physicochemical processes underlying these net CO2 sources are very different. The diffusive flux is driven by the actual spatial CO2 gradient, whereas net CO2 production by eCA is the result of disequilibrium between CO2 and HCO3− at the cell surface. The total net CO2 influx is the sum of the diffusive and reactive supply:
This equation shows that the importance of diffusion or eCA activity for net CO2 supply depends on the relative sizes of fc-BL and ksf, justifying ksf as a measure of eCA activity.
Rearranging Equation 11, we can find an expression for the surface CO2 concentration:
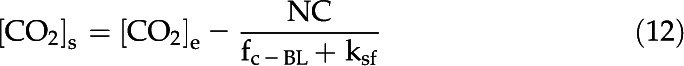 |
which shows that as eCA activity (ksf) increases, the surface CO2 concentration approaches that of the bulk solution. To measure the impact of eCA on CO2 exchange between the cell and the bulk solution, we define the effectiveness of eCA (E) as:
 |
which varies between 0 (no impact of eCA) and 1 (eCA dominates over diffusive exchange). E is both the fraction of net CO2 supply supported by eCA and the fraction of the maximum potential CO2 gradient abolished by eCA activity.
For a more detailed evaluation of the effect of eCA on boundary-layer carbon chemistry and pH, a spherical reaction-diffusion model was developed. The model domain extends from the cell surface 100 μm out to the bulk solution. Chemical concentrations are held constant in the bulk solution but are allowed to vary at the cell surface due to imposed uptake and export fluxes and reaction-diffusion within the boundary layer. Dissolved Ci species (CO2, HCO3−, and CO32−) and other important components determining seawater pH [H+, OH−, B(OH)3, and B(OH)4−] are included in the model. Bulk solution pH, Ci, and total boron were set at typical oceanic values (pH 8.14; Ci, 2 mm; total boron, 415 μm). All reactions are treated kinetically using rate constants from Zeebe and Wolf-Gladrow (2001) and diffusion coefficients from Boudreau (1997). The model was solved in Matlab.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Data S1. Description of scripts used to process isotope exchange data and implement the eCA box model.
Acknowledgments
We thank François Morel (Princeton University) for helpful advice and discussions.
Glossary
- CCM
CO2-concentrating mechanism
- Ci
inorganic carbon
- CA
carbonic anhydrase
- iCA
intracellular carbonic anhydrase
- eCA
extracellular carbonic anhydrase
- AZ
acetazolamide
- DBAZ
dextran-bound acetazolamide
- MIMS
membrane inlet mass spectrometry
References
- Badger MR. (2003) The roles of carbonic anhydrases in photosynthetic CO2 concentrating mechanisms. Photosynth Res 77: 83–94 [DOI] [PubMed] [Google Scholar]
- Badger MR, Andrews TJ, Whitney SM, Ludwig M, Yellowlees DC, Leggat W, Price GD. (1998) The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Can J Bot 76: 1052–1071 [Google Scholar]
- Badger MR, Palmqvist K, Yu J. (1994) Measurement of CO2 and HCO3− fluxes in cyanobacteria and microalgae during steady-state photosynthesis. Physiol Plant 90: 529–536 [Google Scholar]
- Beckmann K, Messinger J, Badger MR, Wydrzynski T, Hillier W. (2009) On-line mass spectrometry: membrane inlet sampling. Photosynth Res 102: 511–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron WF. (2004) Regulation of intracellular pH. Adv Physiol Educ 28: 160–179 [DOI] [PubMed] [Google Scholar]
- Boudreau BP (1997) Diagenetic Models and Their Implemenation: Modelling Transport and Reactions in Aquatic Sediments. Springer-Verlag, Berlin [Google Scholar]
- Burkhardt S, Amoroso G, Riebesell U, Sültemeyer D. (2001) CO2 and HCO3− uptake in marine diatoms acclimated to different CO2 concentrations. Limnol Oceanogr 46: 1378–1391 [Google Scholar]
- Delacruz J, Mikulski R, Tu C, Li Y, Wang H, Shiverick KT, Frost SC, Horenstein NA, Silverman DN. (2010) Detecting extracellular carbonic anhydrase activity using membrane inlet mass spectrometry. Anal Biochem 403: 74–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson AG, Goyet C, editors (1994) DOE Handbook of Methods for the Analysis of the Various Parameters of the Carbon Dioxide System in Sea Water, Version 2. US Department of Energy, Washington, DC, ORNL/CDIAC-74 [Google Scholar]
- Elzenga JTM, Prins HBA, Stefels J. (2000) The role of extracellular carbonic anhydrase activity in inorganic carbon utilization of Phaeocystis globosa (Prymnesiophyceae): a comparison with other marine algae using the isotopic disequilibrium technique. Limnol Oceanogr 45: 372–380 [Google Scholar]
- Giordano M, Beardall J, Raven JA. (2005) CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol 56: 99–131 [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Waters RE, Taylor FJR. (1980) A broad spectrum artificial seawater medium for coastal and open ocean phytoplankton. J Phycol 16: 28–35 [Google Scholar]
- Hopkinson BM, Dupont CL, Allen AE, Morel FMM. (2011) Efficiency of the CO2-concentrating mechanism of diatoms. Proc Natl Acad Sci USA 108: 3830–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KS. (1982) Carbon dioxide hydration and dehydration kinetics in seawater. Limnol Oceanogr 27: 849–855 [Google Scholar]
- Kaplan A, Reinhold L. (1999) CO2 concentrating mechanisms in photosynthetic microorganisms. Annu Rev Plant Physiol Plant Mol Biol 50: 539–570 [DOI] [PubMed] [Google Scholar]
- Lueker TJ, Dickson AG, Keeling CD. (2000) Ocean pCO2 calculated from dissolved inorganic carbon, alkalinity, and equations for K1 and K2: validation based on laboratory measurements of CO2 in gas and seawater at equilibrium. Mar Chem 70: 105–119 [Google Scholar]
- Martin CL, Tortell PD. (2006) Bicarbonate transport and extracellular carbonic anhydrase activity in Bering Sea phytoplankton assemblages: results from isotope disequilibrium experiments. Limnol Oceanogr 51: 2111–2121 [Google Scholar]
- Moroney JV, Husic HD, Tolbert NE. (1985) Effect of carbonic anhydrase inhibitors on inorganic carbon accumulation by Chlamydomonas reinhardtii. Plant Physiol 79: 177–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney JV, Ma YB, Frey WD, Fusilier KA, Pham TT, Simms TA, DiMario RJ, Yang J, Mukherjee B. (2011) The carbonic anhydrase isoforms of Chlamydomonas reinhardtii: intracellular location, expression, and physiological roles. Photosynth Res 109: 133–149 [DOI] [PubMed] [Google Scholar]
- Nimer NA, Brownlee C, Merrett MJ. (1999) Extracellular carbonic anhydrase facilitates carbon dioxide availability for photosynthesis in the marine dinoflagellate Prorocentrum micans. Plant Physiol 120: 105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimer NA, Iglesias-Rodriguez MD, Merrett MJ. (1997) Bicarbonate utilization by marine phytoplankton species. J Phycol 33: 625–631 [Google Scholar]
- Ohnishi N, Mukherjee B, Tsujikawa T, Yanase M, Nakano H, Moroney JV, Fukuzawa H. (2010) Expression of a low CO2-inducible protein, LCI1, increases inorganic carbon uptake in the green alga Chlamydomonas reinhardtii. Plant Cell 22: 3105–3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist K, Ramazanov Z, Samuelsson G. (1990) The role of extracellular carbonic anhydrase for accumulation of inorganic carbon in the green alga Chlamydomonas reinhardtii: a comparison between wild-type and cell wall-less mutant cells. Physiol Plant 80: 267–276 [Google Scholar]
- Palmqvist K, Yu JW, Badger MR. (1994) Carbonic anhydrase activity and inorganic carbon fluxes in low-Ci and high Ci cells of Chlamydomonas reinhardtii and Scenedesmus obliquus. Physiol Plant 90: 537–547 [Google Scholar]
- Pasciak WJ, Gavis J. (1974) Transport limitation of nutrient uptake in phytoplankton. Limnol Oceanogr 19: 881–898 [Google Scholar]
- Price NM, Harrison GI, Hering JG, Hudson RJ, Nirel PMV, Palenik B, Morel FMM. (1988) Preparation and chemistry of the artificial algal culture medium Aquil. Biol Oceanogr 6: 443–462 [Google Scholar]
- Riebesell U, Wolfgladrow DA, Smetacek V. (1993) Carbon dioxide limitation of marine phytoplankton growth rates. Nature 361: 249–251 [Google Scholar]
- Rost B, Riebesell U, Burkhardt S, Sültemeyer D. (2003) Carbon acquisition of bloom-forming marine phytoplankton. Limnol Oceanogr 48: 55–67 [Google Scholar]
- Silverman D, Lindskog S. (1988) The catalytic mechanism of carbonic anhydrase: implications of a rate-limiting protolysis of water. Acc Chem Res 21: 30–36 [Google Scholar]
- Silverman DN. (1982) Carbonic anhydrase: oxygen-18 exchange catalyzed by an enzyme with rate-contributing proton-transfer steps. Methods Enzymol 87: 732–752 [DOI] [PubMed] [Google Scholar]
- Silverman DN, Tu C, Wynns GC. (1976) Depletion of 18O from C18O2 in erythrocyte suspensions: the permeability of the erythrocyte membrane to CO2. J Biol Chem 251: 4428–4435 [PubMed] [Google Scholar]
- Silverman DN, Tu CK, Roessler N. (1981) Diffusion-limited exchange of 18O between CO2 and water in red cell suspensions. Respir Physiol 44: 285–298 [DOI] [PubMed] [Google Scholar]
- Stewart PS. (2003) Diffusion in biofilms. J Bacteriol 185: 1485–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swietach P, Hulikova A, Vaughan-Jones RD, Harris AL. (2010) New insights into the physiological role of carbonic anhydrase IX in tumour pH regulation. Oncogene 29: 6509–6521 [DOI] [PubMed] [Google Scholar]
- Tilman D. (1977) Resource competition between planktonic algae: an experimental and theoretical approach. Ecology 58: 338–348 [Google Scholar]
- Trimborn S, Lundholm N, Thoms S, Richter KU, Krock B, Hansen PJ, Rost B. (2008) Inorganic carbon acquisition in potentially toxic and non-toxic diatoms: the effect of pH-induced changes in seawater carbonate chemistry. Physiol Plant 133: 92–105 [DOI] [PubMed] [Google Scholar]
- Trimborn S, Wolf-Gladrow D, Richter KU, Rost B. (2009) The effect of pCO2 on carbon acquisition and intracellular assimilation in four marine diatoms. J Exp Mar Biol Ecol 376: 26–36 [Google Scholar]
- Tu C, Wynns GC, McMurray RE, Silverman DN. (1978) CO2 kinetics in red cell suspensions measured by 18O exchange. J Biol Chem 253: 8178–8184 [PubMed] [Google Scholar]
- Van K, Spalding MH. (1999) Periplasmic carbonic anhydrase structural gene (Cah1) mutant in Chlamydomonas reinhardtii. Plant Physiol 120: 757–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf-Gladrow D, Riebesell U. (1997) Diffusion and reactions in the vicinity of plankton: a refined model for inorganic carbon transport. Mar Chem 59: 17–34 [Google Scholar]
- Yoshioka S, Taniguchi F, Miura K, Inoue T, Yamano T, Fukuzawa H. (2004) The novel Myb transcription factor LCR1 regulates the CO2-responsive gene Cah1, encoding a periplasmic carbonic anhydrase in Chlamydomonas reinhardtii. Plant Cell 16: 1466–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeebe RE, Wolf-Gladrow D (2001) CO2 in Seawater: Equilibrium, Kinetics, Isotopes. Elsevier, Amsterdam [Google Scholar]
- Zhang HN, Byrne RH. (1996) Spectrophotometric pH measurements of surface seawater at in-situ conditions: absorbance and protonation behavior of thymol blue. Mar Chem 52: 17–25 [Google Scholar]