Coenzyme A made in the cytosol is imported into plant mitochondria by twin transporters from the mitochondrial carrier family that are cognates of coenzyme A transporters of animals and yeast and can functionally replace the yeast transporter.
Abstract
Plants make coenzyme A (CoA) in the cytoplasm but use it for reactions in mitochondria, chloroplasts, and peroxisomes, implying that these organelles have CoA transporters. A plant peroxisomal CoA transporter is already known, but plant mitochondrial or chloroplastic CoA transporters are not. Mitochondrial CoA transporters belonging to the mitochondrial carrier family, however, have been identified in yeast (Saccharomyces cerevisiae; Leu-5p) and mammals (SLC25A42). Comparative genomic analysis indicated that angiosperms have two distinct homologs of these mitochondrial CoA transporters, whereas nonflowering plants have only one. The homologs from maize (Zea mays; GRMZM2G161299 and GRMZM2G420119) and Arabidopsis (Arabidopsis thaliana; At1g14560 and At4g26180) all complemented the growth defect of the yeast leu5Δ mitochondrial CoA carrier mutant and substantially restored its mitochondrial CoA level, confirming that these proteins have CoA transport activity. Dual-import assays with purified pea (Pisum sativum) mitochondria and chloroplasts, and subcellular localization of green fluorescent protein fusions in transiently transformed tobacco (Nicotiana tabacum) Bright Yellow-2 cells, showed that the maize and Arabidopsis proteins are targeted to mitochondria. Consistent with the ubiquitous importance of CoA, the maize and Arabidopsis mitochondrial CoA transporter genes are expressed at similar levels throughout the plant. These data show that representatives of both monocotyledons and eudicotyledons have twin, mitochondrially located mitochondrial carrier family carriers for CoA. The highly conserved nature of these carriers makes possible their reliable annotation in other angiosperm genomes.
CoA acts as an acyl carrier in many reactions of primary and secondary metabolism, and some 8% of the nearly 4,900 enzymes described in the Enzyme Commission database are CoA dependent (Bairoch, 2000). CoA occupies a central position in lipid metabolism, respiration, gluconeogenesis, and other pathways (Leonardi et al., 2005). It is present in all forms of life, but while all organisms can synthesize it from pantothenate (vitamin B5), only prokaryotes, plants, and fungi are able to synthesize pantothenate; animals obtain pantothenate from the diet (Daugherty et al., 2002; Leonardi et al., 2005; Webb and Smith, 2011).
In plants, the steps that convert pantothenate to CoA are almost certainly cytosolic (Webb and Smith, 2011; Gerdes et al., 2012). CoA, however, is required in mitochondria for the citric acid cycle, in chloroplasts for fatty acid synthesis, and in peroxisomes for β-oxidation. CoA, therefore, must be imported into these organelles from the cytosol, and indeed, early work demonstrated a CoA transport system in potato (Solanum tuberosum) mitochondria (Neuburger et al., 1984). Yeast (Saccharomyces cerevisiae) and mammalian mitochondria and peroxisomes likewise import CoA because they cannot make it (Fiermonte et al., 2009; Agrimi et al., 2012b). The compartmentation of CoA in all eukaryotes appears to be closely regulated, with cytosol and organelles maintaining separate CoA pools whose levels can modulate fluxes through CoA-dependent reactions (Hunt and Alexson, 2002; Leonardi et al., 2005; De Marcos Lousa et al., 2013).
Mitochondrial CoA transporters belonging to the mitochondrial carrier family (MCF) have been identified in yeast (Leu-5p; Prohl et al., 2001) and human (SLC25A42; Fiermonte et al., 2009). Furthermore, peroxisomal CoA carriers from human (SLC25A17; Agrimi et al., 2012b) and Arabidopsis (Arabidopsis thaliana; peroxisomal CoA and NAD carrier [PXN]; Agrimi et al., 2012a) have also been identified. However, no transporters for CoA are known for plant mitochondria or chloroplasts (Palmieri et al., 2011; Gerdes et al., 2012).
In this study, a comparative genomic analysis first identified close Arabidopsis and maize (Zea mays) homologs of the yeast and mammalian mitochondrial CoA carriers as candidates for the missing plant mitochondrial or chloroplast transporters. Experimental evidence then demonstrated that the candidate proteins transport CoA when expressed in yeast, that they are targeted to mitochondria in vitro and in planta, and that they are expressed throughout the plant.
RESULTS
Identification of Candidate Plant CoA Carriers
An earlier analysis of Arabidopsis, human, and yeast MCF proteins identified At1g14560 and At4g26180 as potential CoA carriers, based on the presence of sequence motifs (symmetry-related amino acid triplets) similar to those in known, mitochondrially localized CoA carriers (Palmieri et al., 2011). At1g14560 and At4g26180 share only 59% amino acid identity and so are substantially diverged.
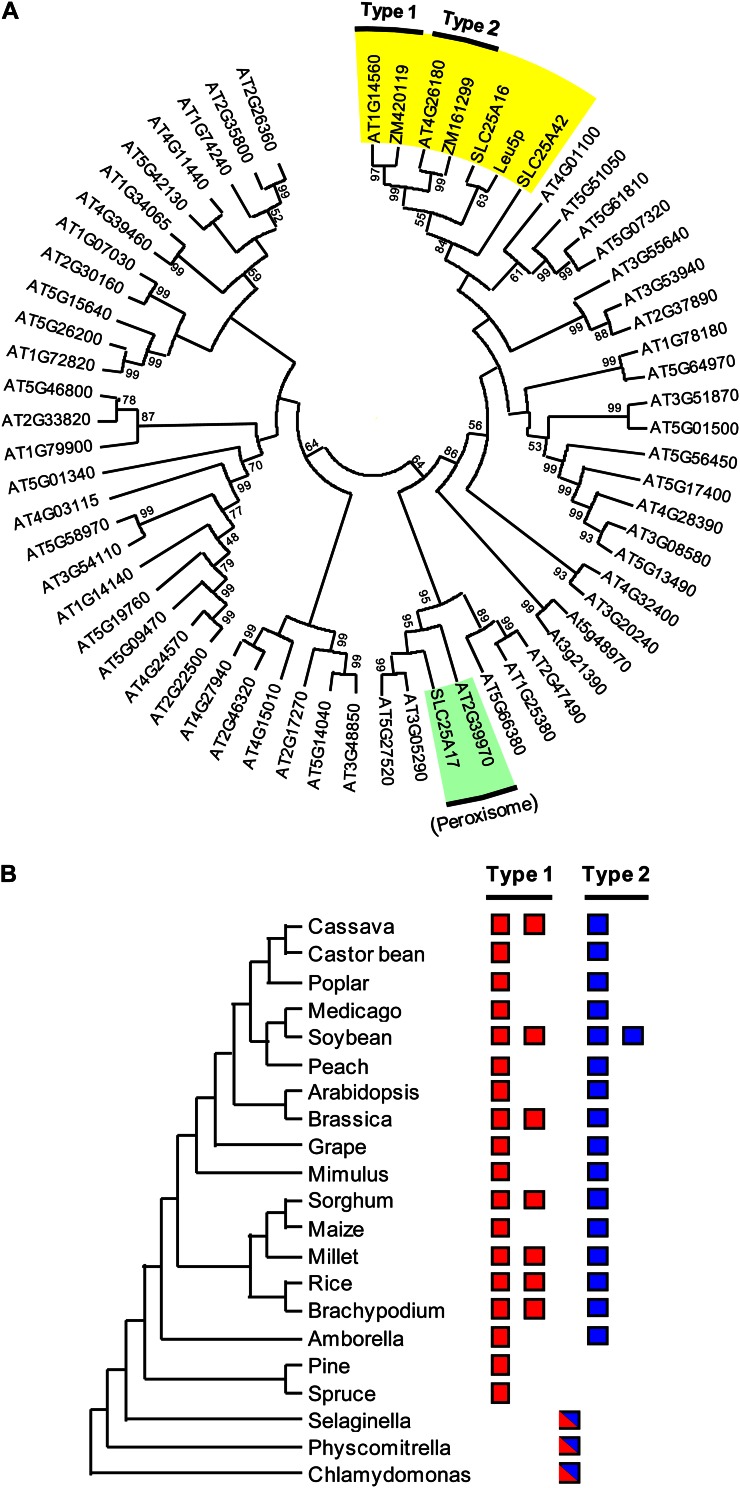
BLAST searches of the maize genome using each Arabidopsis sequence as query detected GRMZM2G420119 and GRMZM2G161299 (henceforth ZM420119 and ZM161299) as the closest homologs (66%–67% identity) of At1g14560 and At4g26180, respectively. All four proteins have the tripartite structure that characterizes the MCF (Palmieri et al., 2011) and are predicted to contain the usual six transmembrane domains (Supplemental Fig. S1). Phylogenetic analyses of these proteins, the human and yeast mitochondrial CoA carriers, and all Arabidopsis, yeast, and human MCF proteins consistently placed all four candidates and the yeast and human CoA carriers in the same clade. Thus, among all 58 Arabidopsis MCF proteins, those closest to the known mitochondrially localized CoA carriers are the candidates (Fig. 1A), and among all yeast or human MCF proteins, the candidates are closest to the known mitochondrial CoA carriers (Supplemental Fig. S2). The recently identified peroxisomal CoA carriers from human (Agrimi et al., 2012b) and Arabidopsis (Agrimi et al., 2012a) occupy a very different clade (Fig. 1A).
Figure 1.
Phylogenetic analysis of known and predicted CoA transporters. A, Phylogenetic tree of the experimentally validated CoA transporters from yeast, human, and Arabidopsis, the candidate CoA transporters from Arabidopsis (At1g14560 and At4g26180) and maize (GRMZM2G420119 and GRMZM2G161299; abbreviated to ZM420119 and ZM161299), and all other Arabidopsis MCF proteins. Sequences were aligned with ClustalW; the tree was constructed by the neighbor-joining method with MEGA5. Bootstrap values (%) for 1,000 replicates are shown next to nodes; those of less than 50% are omitted. Only the tree topology is shown. The yellow highlighted sector contains the validated human and yeast mitochondrial CoA transporters and their Arabidopsis and maize homologs; the type 1 and type 2 plant carriers are indicated. The green highlighted sector contains the validated human and Arabidopsis peroxisomal CoA transporters. B, Phylogenetic distribution of type 1 and type 2 predicted CoA transporters among angiosperms, gymnosperms, and lower plants. Red boxes and blue boxes indicate the number of type 1 and type 2 sequences, respectively, represented in each genome or transcriptome. Piebald red/blue boxes indicate homologs in lower plants that could not be assigned to either type 1 or type 2.
The phylogenetic trees all separate the four candidate genes into two types: type 1 composed of At1g14560 and ZM420119, and type 2 composed of At4g26180 and ZM161299 (Fig. 1A; Supplemental Fig. S2). One or two genes of each type were found in all other eudicotyledon and monocotyledon genomes and transcriptomes examined as well as in that of the basal angiosperm Amborella trichopoda (Fig. 1B; Supplemental Fig. S3). In contrast, gymnosperms had type 1 but not type 2 sequences, whereas lower plants each had a single sequence that could not be confidently assigned to either type (Fig. 1B; Supplemental Fig. S3).
Functional Complementation of the Yeast leu5Δ Mutant
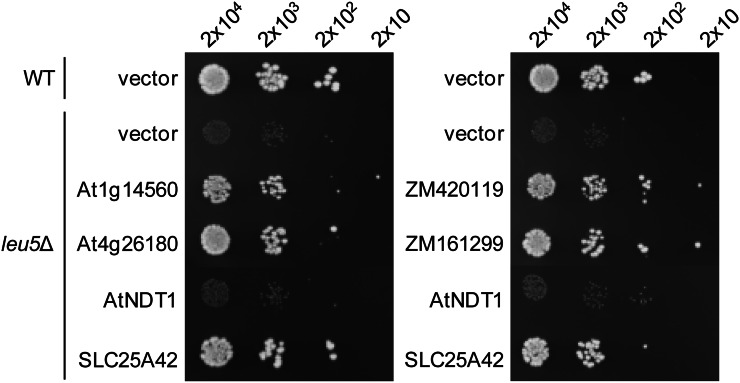
Deletion of the yeast LEU5 gene, encoding a mitochondrial CoA transporter, results in strongly retarded growth on rich medium containing a nonfermentable carbon source, and expression of a functional CoA carrier protein in the deletant (leu5Δ) strain restores normal growth (Prohl et al., 2001). The human Leu-5p homolog, SLC25A42, used as a positive control, restored normal growth on 1% yeast extract, 2% bacto-peptone (YP) medium plates containing 3% glycerol, as expected (Fiermonte et al., 2009). Similarly, all four candidate plant CoA transporters, At1g14560, At4g26180, ZM420119, and ZM161299, restored normal growth, as opposed to little or no growth for cells transformed with vector alone or a negative control (the Arabidopsis NAD+ carrier AtNDT1; Fig. 2). Similar results were obtained in liquid medium (Supplemental Fig. S4). These data indicate that the plant type 1 and type 2 proteins have CoA transport activity and that they are targeted to mitochondria in yeast. Furthermore, because the yeast Leu-5p protein is known to be in the mitochondrial inner membrane (Prohl et al., 2001), the observed complementation by the plant proteins indicates that they are likewise, at least in part, localized in the inner membrane when expressed in yeast.
Figure 2.
Complementation of the growth phenotype of the yeast CoA carrier deletant leu5Δ by the expression of candidate plant CoA carriers. Serial dilutions of wild-type (WT) cells harboring pYES2/CT (vector) or leu5Δ cells harboring pYES2/CT alone or encoding maize (ZM420119 and ZM161299) or Arabidopsis (At4g26180 and At1g14560) proteins were plated on YP medium containing 3% glycerol and incubated for 72 h at 30°C. pYES2/CT constructs encoding the human CoA transporter SLC25A42 or the Arabidopsis NAD+ transporter AtNDT1 were included as positive and negative controls, respectively.
Complementation Restores Mitochondrial CoA Levels in the leu5Δ Strain
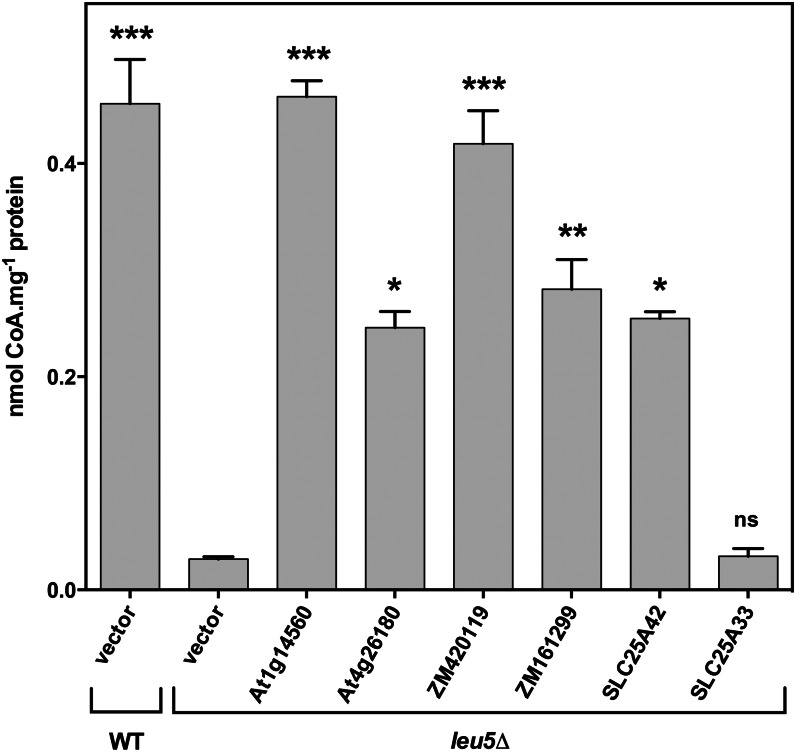
The CoA content in mitochondria of the leu5Δ strain is severely reduced compared with the wild type, reflecting the role of Leu-5p in mitochondrial CoA import (Prohl et al., 2001). The candidate proteins from maize and Arabidopsis complemented this biochemical phenotype of the leu5Δ strain: expression of the type 1 candidates At1g14560 and ZM420119 fully restored mitochondrial CoA levels to that of the wild type; expression of the type 2 candidates At4g26180 and ZM161299 restored levels to over one-half that of the wild type (Fig. 3). These data reinforce the conclusion that the plant proteins mediate CoA transport into yeast mitochondria.
Figure 3.
Effects of the expression of candidate plant CoA carriers on mitochondrial CoA contents of leu5Δ yeast cells. Cells were cultured in liquid YP medium containing 2% ethanol. Mitochondria were isolated from wild-type (WT) cells harboring pYES2/CT (vector) or from leu5Δ cells harboring pYES2/CT alone or encoding At1g14560, At4g26180, ZM420119, or ZM161299. pYES2/CT constructs encoding the human CoA transporter SLC25A42 or the human pyrimidine nucleotide transporter SLC25A33 were included as positive and negative controls, respectively. Data are means and se of at least three independent experiments and were subjected to one-way ANOVA followed by Bonferroni post hoc tests. Differences in CoA content between leu5Δ cells harboring pYES2/CT and other strains that are significant are indicated with asterisks: *P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant.
Subcellular Localization
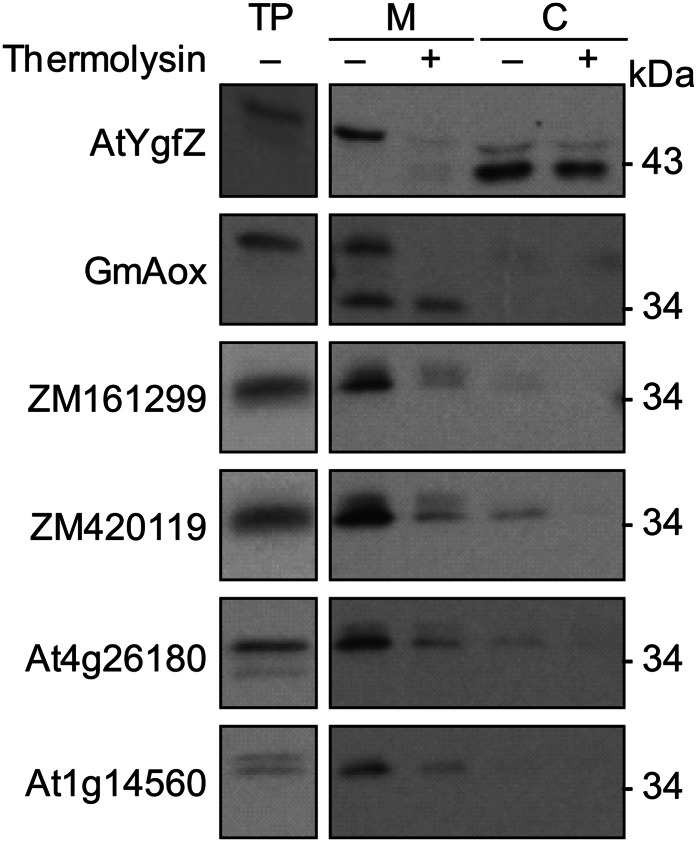
Organellar targeting of the four CoA carrier candidates was investigated using dual-import assays, in which labeled proteins are incubated with a mixture of purified pea (Pisum sativum) mitochondria and chloroplasts and then reisolated (Rudhe et al., 2002). Soybean (Glycine max) alternative oxidase (Rudhe et al., 2002) and Arabidopsis YgfZ protein (Waller et al., 2012) were used as positive controls for mitochondrial and chloroplast import, respectively. All four candidates behaved similarly to the mitochondria-targeted control protein (i.e. the reisolated mitochondria treated with thermolysin contained a labeled band corresponding to the imported protein; Fig. 4), which indicates an intramitochondrial location and is consistent with the complementation evidence above for an inner membrane location. The observed lack of difference between the molecular mass of the precursors and the imported proteins is expected, because MCF proteins typically lack cleavable targeting peptides (Palmisano et al., 1998; Zara et al., 2009). None of the candidates mirrored the behavior of the chloroplast-targeted control protein (Fig. 4).
Figure 4.
Protein uptake by purified pea mitochondria and chloroplasts in dual-import assays. The four CoA carrier cDNAs, plus the Arabidopsis YgfZ (AtYgfZ) and soybean alternative oxidase (GmAox) cDNAs as positive controls for chloroplast and mitochondrial import, respectively, were transcribed and translated in vitro in the presence of [3H]Leu. The translation products (TP) were incubated in the light with mixed pea chloroplasts (C) and mitochondria (M). The organelles were mock treated (−) or thermolysin treated (+) to remove adsorbed proteins and then reisolated on a Percoll gradient. Proteins were separated by SDS-PAGE and visualized by fluorography. Samples were loaded on the basis of equal chlorophyll or mitochondrial protein content next to an aliquot of the translation product. Exposure times were adjusted to give comparable band intensities in all tracks. Molecular masses are indicated on the right.
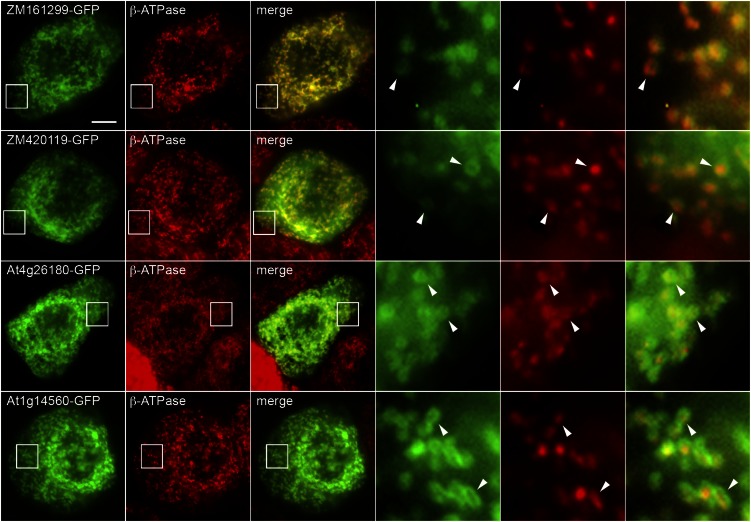
As a second approach for investigating subcellular localization, tobacco (Nicotiana tabacum) Bright Yellow-2 (BY-2) cells, serving as a model plant cell system for studying protein localization (Brandizzi et al., 2003; Miao and Jiang, 2007), were transiently transformed with each of the four candidates linked at their N or C termini to GFP and examined by epifluorescence microscopy. The expressed C-terminal fusions of both maize proteins and both Arabidopsis proteins showed a fluorescence pattern that colocalized with that attributable to the endogenous mitochondrial inner membrane marker, the β-ATPase subunit (Fig. 5). Notably, the fusion proteins often yielded a torus-like fluorescence pattern that circled the more punctate fluorescence attributable to the β-ATPase (Fig. 5, highly magnified images), suggesting that they may localize to two distinct subdomains of the inner mitochondrial membrane, the inner boundary membrane and the cristae membrane (for review, see Logan, 2006; Mannella, 2006). Thus, the toroid fluorescence pattern exhibited by the candidate fusion proteins in BY-2 cells resembles that of several yeast GFP-tagged proteins that preferentially localize to the inner boundary membrane, which lies parallel to the mitochondrial outer membrane (Wurm and Jakobs, 2006; Suppanz et al., 2009). The punctate fluorescence pattern exhibited by endogenous β-ATPase in BY-2 cells, on the other hand, is consistent with the localization of its yeast counterpart in the cristae membrane, which extends into the matrix (Wurm and Jakobs, 2006). There are, however, other possible explanations for the concentration of GFP-tagged inner membrane proteins at the mitochondrial rim (Donzeau et al., 2000), so this question needs to be studied in more detail.
Figure 5.
Representative epifluorescence images of tobacco BY-2 cells transiently expressing maize or Arabidopsis CoA carrier candidates C-terminally fused to GFP. Cells were biolistically bombarded with plasmid DNA encoding a GFP-tagged candidate. Approximately 4 h later, cells were formaldehyde fixed, permeabilized with pectolyase and Triton X-100, immunostained for endogenous mitochondrial β-ATPase, serving as a mitochondrial marker protein, and then examined by epifluorescence microscopy. As indicated by the labels, each row of images corresponds to the fluorescence attributable to the candidate fusion protein and endogenous β-ATPase immunostaining (green and red, respectively) and the corresponding merged image. Boxes represent the portion of the cell shown at higher magnification in the panels to the right. Arrowheads indicate examples of toroidal structures containing a candidate fusion protein enclosing a punctate structure containing β-ATPase. Bar = 10 μm.
Unlike the C-terminal fusions, the N-terminal GFP fusions for all four proteins localized only partially to mitochondria and mostly to the cytosol (data not shown), suggesting that the N-terminally appended GFP moiety interfered with the mitochondrial targeting information in the CoA carriers. Similar interference with mitochondrial targeting was observed for N-terminal fusions of GFP to thiamin diphosphate carriers (Frelin et al., 2012). Finally, there was no apparent targeting of either the C- or N-terminal GFP fusion protein to plastids or peroxisomes. Together, the results of dual-import assays and of cell imaging demonstrate the targeting of all four candidate proteins to plant mitochondria and not other organelles.
Expression Patterns of CoA Transporter Genes
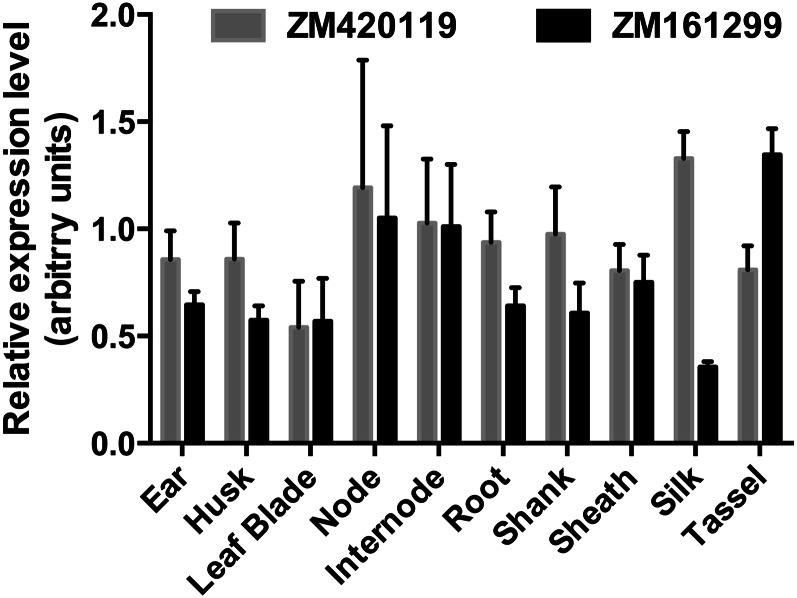
The relative levels of the ZM161299 and ZM420119 mRNAs were determined by quantitative reverse transcription-PCR in 10 tissues representing the whole adult maize plant (Fig. 6). Both genes are expressed in all tissues tested, with fairly little variation (maximum of 4.2-fold). Their expression patterns are similar, except that the type 1 gene ZM420119 predominates in silks and the type 2 gene ZM161299 predominates in tassels. Analysis of expression data for both maize genes from genome-wide RNA-seq data sets gave a similar picture (Supplemental Fig. S5A). Publicly available microarray data for the Arabidopsis genes likewise show expression throughout the plant, with relatively little variation except in stamens and pollen, and with the type 1 gene At1g14560 always predominant (Supplemental Fig. S5B). The ubiquitous expression of both types of CoA carrier gene in both species is consistent with the universal requirement of mitochondria for CoA, which is essential, among other things, for the oxidation of pyruvate via the tricarboxylic acid cycle.
Figure 6.
Expression patterns of maize genes GRMZM2G161299 and GRMZM2G420119 (abbreviated to ZM161299 and ZM420119). Relative mRNA levels were determined by quantitative reverse transcription-PCR using the comparative cycle threshold method against three reference genes (FPGS, CUL, and UBCP). Error bars represent the se for two technical replicates of two biological replicates.
DISCUSSION
The bioinformatic prediction that the Arabidopsis and maize homologs of the yeast and human mitochondrial CoA carriers themselves transport CoA is validated by the evidence that they functionally complement the growth defect of the yeast leu5Δ mutant and restore its mitochondrial CoA level. Therefore, we propose that these proteins be designated AtCoAc1 (At1g14560), AtCoAc2 (At4g26180), ZmCoAc1 (GRMZM2G420119), and ZmCoAc2 (GRMZM2G161299).
That these carriers belong to the MCF (Palmieri, 2013) does not necessarily mean that they are targeted to mitochondria, as several cases of plastid (Bedhomme et al., 2005; Kirchberger et al., 2008) or peroxisomal (Agrimi et al., 2012a, 2012b; Bernhardt et al., 2012) targeting are known. Nor does the fact that the plant proteins are targeted to mitochondria in yeast (i.e. they replace the missing mitochondrial CoA carrier in the leu5Δ mutant) prove that they are also targeted to mitochondria in planta. However, the results of in vitro dual-import assays and of in vivo localization of GFP fusion proteins provide strong evidence for mitochondrial (inner membrane) localization for both type 1 and type 2 transporters. As neither type showed any plastid targeting in vitro or in vivo, they are probably not responsible for CoA import into plastids. Thus, plastidial CoA transporters still remain to be identified.
That homologs of both types of mitochondrial CoA transporter occur in all the sequenced angiosperm genomes that were analyzed, but not in those of gymnosperms or lower plants, implies that type 1 and type 2 genes are ancient paralogs that diverged not only before the split between eudicotyledons and monocotyledons but also before the Amborella lineage (Soltis et al., 2008) diverged from the lineage that gave rise to other angiosperms. Furthermore, the presence in gymnosperms of type 1 but not type 2 genes implies that type 1 is the ancestral gene and type 2 arose from it in the lineage that gave rise to angiosperms.
The existence of two similarly expressed genes in angiosperms but only one in other plants could imply either subfunctionalization or neofunctionalization (i.e. that gene duplication was followed either by [1] subdivision of an originally broad function or [2] acquisition of a novel function by the duplicated gene). The available data rule out neither possibility. However, assuming CoA transport to be an ancestral function (because it is shared with other eukaryotes) and that the type 2 gene is of quite recent origin (see above), it is reasonable to speculate that type 2 carriers have acquired a new function without losing the original one (Khersonsky and Tawfik, 2010). This new function is presumably a capacity to transport a substrate or substrates other than CoA, and in this regard, it is noteworthy that the Arabidopsis peroxisomal CoA carrier PXN accepts NAD+, NADH, AMP, ADP, and adenosine 3′,5′-phosphate as well as CoA (Agrimi et al., 2012a; Bernhardt et al., 2012). It is also noteworthy that type 1 carriers restored mitochondrial CoA levels in the yeast leu5Δ mutant more effectively than type 2 carriers, for this is consistent with some differentiation of function. Therefore, it will be valuable to assess the transport activities of type 1 and type 2 proteins in reconstituted liposomes; attempts to do this have so far been unsuccessful.
Lastly, from a practical standpoint, the conservation of the type 1 and type 2 proteins throughout the angiosperms, and the presence of close homologs in other plants, means that the functional annotation “mitochondrial CoA carrier” can be confidently propagated from maize and Arabidopsis to any plant genome.
MATERIALS AND METHODS
Bioinformatics
Sequences of Arabidopsis (Arabidopsis thaliana), human, and yeast (Saccharomyces cerevisiae) MCF carriers were taken from Palmieri et al. (2011). Maize (Zea mays) carrier sequences were from http://maizesequence.org/index.html; most other plant carrier sequences were from genomes at the National Center for Biotechnology Information, the Joint Genome Institute (http://www.jgi.doe.gov/), or the Amborella genome database (http://www.amborella.org/). Gymnosperm sequences were based on EST contigs at the Gene Index Project (http://compbio.dfci.harvard.edu/tgi/plant.html). Sequence alignments were made with ClustalW, and phylogenetic trees were constructed using MEGA5 (Tamura et al., 2011). Arabidopsis gene expression was analyzed at Botany Array Resource (http://bar.utoronto.ca/welcome.htm; Winter et al., 2007); maize gene expression data were from qTeller (http://qteller.com/).
Constructs
All PCRs were performed using high-fidelity polymerase Phusion TAQ (New England Biolabs). Maize open reading frames GRMZM2G420119 and GRMZM2G161299 were amplified from the plasmids ZM_BFb0285D11 and ZM_BFb0063E21 obtained from the Arizona Genomics Institute. Arabidopsis open reading frames At1g14560 and At4g26180 were amplified from a leaf complementary DNA (cDNA) library. For complementation of the yeast leu5Δ strain, coding sequences were cloned in pYES2/CT (Invitrogen). For dual-import assays, the coding sequences were cloned into pGEM-4Z (Promega) with the addition of the Kozak sequence ACC just upstream of the start codon. The plasmids (Frelin et al., 2012) used for in vivo localization studies were pRTL2ΔNS/mGFP-MCS for N-terminal GFP fusions of candidate proteins and pUC18/NcoI-mGFP for C-terminal GFP fusions. The Kozak sequence AACA was added just upstream of the transporter sequence start codon for C-terminal GFP fusions to match that in the pRTL2ΔNS/mGFP-MCS plasmid. The primers used to build these constructs are listed in Supplemental Table S1.
Complementation of the Yeast leu5Δ Mutant
The leu5Δ deletant and the corresponding wild-type W303-1B strain were grown in rich (YP) medium, containing 1% (w/v) yeast extract, 2% (w/v) Bacto-peptone, supplemented with 3% (w/v) glycerol. The final pH was adjusted to 4.8 with HCl. Strains were transformed with pYES2/CT alone or pYES2/CT constructs, including positive (human SLC25A42) and negative (Arabidopsis AtNDT1 [Palmieri et al., 2009] or human SLC25A33 [Floyd et al., 2007]) controls, as described previously (Fiermonte et al., 2009; Frelin et al., 2012).
Yeast Mitochondrial CoA Content Measurement
The leu5Δ strain transformed with recombinant pYES2/CT vectors was grown at 30°C to midlog phase in YP medium supplemented with 2% (v/v) ethanol. Mitochondria were isolated as described (Daum et al., 1982) and extracted with phenol:chloroform (1:1, v/v) for mass spectrometric analysis of CoA. A Quattro Premier mass spectrometer interfaced with an Acquity ultra-performance liquid chromatography system (Waters) was used for liquid chromatography-tandem mass spectrometry analysis. Chromatographic resolution was achieved using an HSS T3 column (2.1 × 100 mm, 1.8-µm particle size; Waters). The flow rate was 0.3 mL min−1. The reaction monitoring transition selected in the positive ion mode for CoA was mass-to-charge ratio 768 > 261. Calibration curves were established using standards, processed under the same conditions as the samples, at five concentrations. The best fit was determined using regression analysis of analyte peak area.
Dual-Import Assays
Dual-import assays with purified pea (Pisum sativum) mitochondria and chloroplasts were carried out as described (Rudhe et al., 2002; Frelin et al., 2012). The soybean (Glycine max) alternative oxidase protein GmAox (Rudhe et al., 2002) and the Arabidopsis YgfZ protein At1g60990 (Waller et al., 2012) served as positive controls for mitochondrial and chloroplast import, respectively. Import times used in the reactions were 20 min for CoA transporter candidates and GmAox and 5 min for Arabidopsis YgfZ.
Expression and Visualization of GFP Fusion Proteins in BY-2 Cells
Expression and microscopic analyses of GFP fusion proteins in tobacco (Nicotiana tabacum) BY-2 suspension-cultured cells were performed as described previously (Frelin et al., 2012), except that endogenous mitochondrial β-ATPase protein was immunostained using mouse anti-maize mitochondrial β-ATPase E monoclonal antibodies (Luethy et al., 1993; provided by T. Elthon) and goat anti-mouse Rhodamine Red-X IgGs (Jackson ImmunoResearch Laboratories).
Analysis of Maize Gene Expression
RNA preparations were a gift from J.-C. Guan (University of Florida). Maize (inbred B73) plants were grown at the University of Florida Citra research farm in spring 2010; samples were harvested at tassel emergence. cDNAs were synthesized with the SuperScript III First-Strand Synthesis System (Life Technologies) using oligo(dT)20 primer. Real-time PCR was performed using the MyiQ2 Real Time PCR Detection System (Bio-Rad) using iQ SYBR Green Supermix (Bio-Rad). Amplification parameters were 95°C for 10 min followed by 40 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s with fluorescence measurement, followed by the establishment of a melting curve. Data were analyzed with the Bio-Rad Expression Macro using the comparative cycle threshold method. Expression was normalized against three reference genes: FPGS (GRMZM2G393334 T01), CUL (GRMZM2G166694 T04), and UBCP (GRMZM2G102471 T01; Vandesompele et al., 2002; Manoli et al., 2012). Primers used are given in Supplemental Table S2. Primer concentrations were optimized for the lowest threshold cycle values, and amplification efficiency was determined.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers At1g14560:NP_172908; At4g26180:NP_194348; GRMZM2G420119:AFW83095; GRMZM2G161299:AFW71266; and Leu5p:NP_011865.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Alignment of candidate CoA transporters and yeast known CoA transporter.
Supplemental Figure S2. Phylogenetic analysis of candidate CoA transporters from Arabidopsis (At1g14560, At4g26180) and maize (ZM420119, ZM161299), the known CoA transporters from yeast and human, and the complete set of MCF proteins from yeast or human.
Supplemental Figure S3. Phylogenetic analysis of candidate plant CoA transporters and the known yeast and human CoA transporters.
Supplemental Figure S4. Complementation of growth in liquid culture of the yeast CoA carrier deletant leu5Δ by expression of candidate plant CoA carriers.
Supplemental Figure S5. Expression data for maize ZM420119 and ZM161299 and Arabidopsis At1g14560 and At4g26180 in various tissues.
Supplemental Table S1. Primers used in this study.
Supplemental Table S2. Quantitative reverse transcription primers used in this study.
Acknowledgments
We thank M. Ziemak (University of Florida) for technical support and T. Elthon (University of Nebraska-Lincoln) for providing the anti-β-ATPase antibodies.
Glossary
- MCF
mitochondrial carrier family
- YP
yeast peptone
- BY-2
Bright Yellow-2
- cDNA
complementary DNA
- YP
1% yeast extract, 2% bacto-peptone
References
- Agrimi G, Russo A, Pierri CL, Palmieri F. (2012a) The peroxisomal NAD+ carrier of Arabidopsis thaliana transports coenzyme A and its derivatives. J Bioenerg Biomembr 44: 333–340 [DOI] [PubMed] [Google Scholar]
- Agrimi G, Russo A, Scarcia P, Palmieri F. (2012b) The human gene SLC25A17 encodes a peroxisomal transporter of coenzyme A, FAD and NAD+. Biochem J 443: 241–247 [DOI] [PubMed] [Google Scholar]
- Bairoch A. (2000) The ENZYME database in 2000. Nucleic Acids Res 28: 304–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedhomme M, Hoffmann M, McCarthy EA, Gambonnet B, Moran RG, Rébeillé F, Ravanel S. (2005) Folate metabolism in plants: an Arabidopsis homolog of the mammalian mitochondrial folate transporter mediates folate import into chloroplasts. J Biol Chem 280: 34823–34831 [DOI] [PubMed] [Google Scholar]
- Bernhardt K, Wilkinson S, Weber AP, Linka N. (2012) A peroxisomal carrier delivers NAD+ and contributes to optimal fatty acid degradation during storage oil mobilization. Plant J 69: 1–13 [DOI] [PubMed] [Google Scholar]
- Brandizzi F, Irons S, Kearns A, Hawes C. (2003) BY-2 cells: culture and transformation for live cell imaging. Curr Protoc Cell Biol 1.7.1–1.7.16 [DOI] [PubMed] [Google Scholar]
- Daugherty M, Polanuyer B, Farrell M, Scholle M, Lykidis A, de Crécy-Lagard V, Osterman A. (2002) Complete reconstitution of the human coenzyme A biosynthetic pathway via comparative genomics. J Biol Chem 277: 21431–21439 [DOI] [PubMed] [Google Scholar]
- Daum G, Böhni PC, Schatz G. (1982) Import of proteins into mitochondria: cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem 257: 13028–13033 [PubMed] [Google Scholar]
- De Marcos Lousa C, van Roermund CW, Postis VL, Dietrich D, Kerr ID, Wanders RJ, Baldwin SA, Baker A, Theodoulou FL. (2013) Intrinsic acyl-CoA thioesterase activity of a peroxisomal ATP binding cassette transporter is required for transport and metabolism of fatty acids. Proc Natl Acad Sci USA 110: 1279–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donzeau M, Káldi K, Adam A, Paschen S, Wanner G, Guiard B, Bauer MF, Neupert W, Brunner M. (2000) Tim23 links the inner and outer mitochondrial membranes. Cell 101: 401–412 [DOI] [PubMed] [Google Scholar]
- Fiermonte G, Paradies E, Todisco S, Marobbio CM, Palmieri F. (2009) A novel member of solute carrier family 25 (SLC25A42) is a transporter of coenzyme A and adenosine 3′,5′-diphosphate in human mitochondria. J Biol Chem 284: 18152–18159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd S, Favre C, Lasorsa FM, Leahy M, Trigiante G, Stroebel P, Marx A, Loughran G, O’Callaghan K, Marobbio CM, et al. (2007) The insulin-like growth factor-I-mTOR signaling pathway induces the mitochondrial pyrimidine nucleotide carrier to promote cell growth. Mol Biol Cell 18: 3545–3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelin O, Agrimi G, Laera VL, Castegna A, Richardson LG, Mullen RT, Lerma-Ortiz C, Palmieri F, Hanson AD. (2012) Identification of mitochondrial thiamin diphosphate carriers from Arabidopsis and maize. Funct Integr Genomics 12: 317–326 [DOI] [PubMed] [Google Scholar]
- Gerdes S, Lerma-Ortiz C, Frelin O, Seaver SM, Henry CS, de Crécy-Lagard V, Hanson AD. (2012) Plant B vitamin pathways and their compartmentation: a guide for the perplexed. J Exp Bot 63: 5379–5395 [DOI] [PubMed] [Google Scholar]
- Hunt MC, Alexson SE. (2002) The role acyl-CoA thioesterases play in mediating intracellular lipid metabolism. Prog Lipid Res 41: 99–130 [DOI] [PubMed] [Google Scholar]
- Khersonsky O, Tawfik DS. (2010) Enzyme promiscuity: a mechanistic and evolutionary perspective. Annu Rev Biochem 79: 471–505 [DOI] [PubMed] [Google Scholar]
- Kirchberger S, Tjaden J, Neuhaus HE. (2008) Characterization of the Arabidopsis Brittle1 transport protein and impact of reduced activity on plant metabolism. Plant J 56: 51–63 [DOI] [PubMed] [Google Scholar]
- Leonardi R, Zhang Y-M, Rock CO, Jackowski S. (2005) Coenzyme A: back in action. Prog Lipid Res 44: 125–153 [DOI] [PubMed] [Google Scholar]
- Logan DC. (2006) The mitochondrial compartment. J Exp Bot 57: 1225–1243 [DOI] [PubMed] [Google Scholar]
- Luethy MH, Horak A, Elthon TE. (1993) Monoclonal antibodies to the α- and β-subunits of the plant mitochondrial F1-ATPase. Plant Physiol 101: 931–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella CA. (2006) Structure and dynamics of the mitochondrial inner membrane cristae. Biochim Biophys Acta 1763: 542–548 [DOI] [PubMed] [Google Scholar]
- Manoli A, Sturaro A, Trevisan S, Quaggiotti S, Nonis A. (2012) Evaluation of candidate reference genes for qPCR in maize. J Plant Physiol 169: 807–815 [DOI] [PubMed] [Google Scholar]
- Miao Y, Jiang L. (2007) Transient expression of fluorescent fusion proteins in protoplasts of suspension cultured cells. Nat Protoc 2: 2348–2353 [DOI] [PubMed] [Google Scholar]
- Neuburger M, Day DA, Douce R. (1984) Transport of coenzyme A in plant mitochondria. Arch Biochem Biophys 229: 253–258 [DOI] [PubMed] [Google Scholar]
- Palmieri F. (2013) The mitochondrial transporter family SLC25: identification, properties and physiopathology. Mol Aspects Med 34: 465–484 [DOI] [PubMed] [Google Scholar]
- Palmieri F, Pierri CL, De Grassi A, Nunes-Nesi A, Fernie AR. (2011) Evolution, structure and function of mitochondrial carriers: a review with new insights. Plant J 66: 161–181 [DOI] [PubMed] [Google Scholar]
- Palmieri F, Rieder B, Ventrella A, Blanco E, Do PT, Nunes-Nesi A, Trauth AU, Fiermonte G, Tjaden J, Agrimi G, et al. (2009) Molecular identification and functional characterization of Arabidopsis thaliana mitochondrial and chloroplastic NAD+ carrier proteins. J Biol Chem 284: 31249–31259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmisano A, Zara V, Hönlinger A, Vozza A, Dekker PJ, Pfanner N, Palmieri F. (1998) Targeting and assembly of the oxoglutarate carrier: general principles for biogenesis of carrier proteins of the mitochondrial inner membrane. Biochem J 333: 151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohl C, Pelzer W, Diekert K, Kmita H, Bedekovics T, Kispal G, Lill R. (2001) The yeast mitochondrial carrier Leu5p and its human homologue Graves’ disease protein are required for accumulation of coenzyme A in the matrix. Mol Cell Biol 21: 1089–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudhe C, Chew O, Whelan J, Glaser E. (2002) A novel in vitro system for simultaneous import of precursor proteins into mitochondria and chloroplasts. Plant J 30: 213–220 [DOI] [PubMed] [Google Scholar]
- Soltis DE, Albert VA, Leebens-Mack J, Palmer JD, Wing RA, dePamphilis CW, Ma H, Carlson JE, Altman N, Kim S, et al. (2008) The Amborella genome: an evolutionary reference for plant biology. Genome Biol 9: 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suppanz IE, Wurm CA, Wenzel D, Jakobs S. (2009) The m-AAA protease processes cytochrome c peroxidase preferentially at the inner boundary membrane of mitochondria. Mol Biol Cell 20: 572–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller JC, Ellens KW, Alvarez S, Loizeau K, Ravanel S, Hanson AD. (2012) Mitochondrial and plastidial COG0354 proteins have folate-dependent functions in iron-sulphur cluster metabolism. J Exp Bot 63: 403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb ME, Smith AG. (2011) Pantothenate biosynthesis in higher plants. Adv Bot Res 58: 203–255 [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ. (2007) An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2: e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurm CA, Jakobs S. (2006) Differential protein distributions define two sub-compartments of the mitochondrial inner membrane in yeast. FEBS Lett 580: 5628–5634 [DOI] [PubMed] [Google Scholar]
- Zara V, Ferramosca A, Robitaille-Foucher P, Palmieri F, Young JC. (2009) Mitochondrial carrier protein biogenesis: role of the chaperones Hsc70 and Hsp90. Biochem J 419: 369–375 [DOI] [PMC free article] [PubMed] [Google Scholar]