The evolutionary origin of a novel fruit morphology in legumes and the importance of changes in coding regions of master genes to generate diversity.
Abstract
Angiosperms are the most diverse and numerous group of plants, and it is generally accepted that this evolutionary success owes in part to the diversity found in fruits, key for protecting the developing seeds and ensuring seed dispersal. Although studies on the molecular basis of morphological innovations are few, they all illustrate the central role played by transcription factors acting as developmental regulators. Here, we show that a small change in the protein sequence of a MADS-box transcription factor correlates with the origin of a highly modified fruit morphology and the change in seed dispersal strategies that occurred in Medicago, a genus belonging to the large legume family. This protein sequence modification alters the functional properties of the protein, affecting the affinities for other protein partners involved in high-order complexes. Our work illustrates that variation in coding regions can generate evolutionary novelties not based on gene duplication/subfunctionalization but by interactions in complex networks, contributing also to the current debate on the relative importance of changes in regulatory or coding regions of master regulators in generating morphological novelties.
Fruits are a major evolutionary innovation of flowering plants, essential for reproductive success and adaptation. Morphological and functional diversity in fruits found in nature is impressive and often allows for offspring dispersal, in the form of adaptations of fruits as vectors for seed dissemination. Thus, fruit morphology and associated dispersal strategies are evolutionarily labile characters of ecological importance under strong selective pressure (Bremer and Eriksson, 1992; Mummenhoff et al., 1997; Clausing et al., 2000), suggesting that modest genetic changes in key genes could be responsible for large phenotypic changes affecting fruit function observed within closely related taxa.
Most of the knowledge on the genetic networks that control fruit patterning has been obtained in Arabidopsis (Arabidopsis thaliana), a species with a dry dehiscent fruit formed from a bicarpellate pistil that opens through dehiscence zones formed at the valve margins and releases seeds upon maturity (Scutt et al., 2006; Ferrándiz et al., 2010). In Arabidopsis, the MADS-box genes SHATTERPROOF1 (SHP1) and SHP2 and the basic helix-loop-helix gene INDEHISCENT (IND) are the major regulators directing dehiscence zone formation, which includes the lignification of a precise subset of cells along the dehiscence zone, the lignified layer, and also the differentiation of an adjacent domain of cells with restricted growth, the separation layer. In shp1 shp2 or ind mutants, the dehiscence zone does not form, and therefore neither the lignified nor the separation layers develop at the valve margins (Liljegren et al., 2000, 2004). FRUITFULL (FUL), another MADS-box gene, is required for ovary growth and expansion by restricting the expression domain of SHP and IND to the valve margins, and thus, in ful mutants, misregulation of SHP/IND causes extensive ectopic lignification in the valves (Gu et al., 1998; Ferrándiz et al., 2000b; Liljegren et al., 2004).
In spite of our increasing knowledge of genetic networks directing fruit patterning in Arabidopsis, little is known about the conservation of these networks in distantly related species and virtually nothing on how variations in these networks account for fruit morphological evolution in other higher plant taxa. Legumes are the third largest family of angiosperms, comprising more than 19,000 species and second only to grasses in agricultural importance (Lewis et al., 2005). The fruit (a legume, or more generally, a pod) is the defining characteristic of this group and typically consists of a dry dehiscent pod similar to the Arabidopsis silique, but is only derived from a monocarpellate pistil (Polhill, 1994). While the dry dehiscent pod is the most common fruit morphology in the legume family, fruit morphological innovations are also found (Lewis et al., 2005). Especially interesting are the fruits found in the Medicago genus. The Medicago genus comprises about 87 species and is phylogenetically very close to economically important crop legumes such as pea (Pisum sativum), fava beans (Vicia faba), and lentils (Lens culinaris; Small and Jomphe, 1989; Small, 2011). Many of the species in the genus show a striking example of a morphological novelty, forming spiral pods, frequently spiny, that likely have an adaptive value. Medicago spp. coiled pods represent a new mechanism of collective seed dispersal that upon development of spines can be more adapted to epizoochory, which allows long-range dissemination of the spiny ball-shaped fruits that attach to passing animals. In addition, some authors have also associated Medicago spp. coiled pod morphology to increased pest resistance by reducing accessibility to insects (Small and Brookes, 1984).
In this work, we have addressed the molecular basis underlying the acquisition of coiled fruit morphology in Medicago spp. by studying morphological traits associated with gene functions previously characterized for their role in fruit patterning in other angiosperm model systems. Our study correlates coiled pod morphology in Medicago spp. with strong lignification at valve margins. Moreover, we show that the likely cause of this altered lignification pattern is a change in the protein coding sequence of orthologs of the SHP MADS-box gene, whose conserved function in fruit lignin deposition has already been demonstrated in distantly related angiosperm species (Liljegren et al., 2000; Tani et al., 2007; Fourquin and Ferrándiz, 2012).
RESULTS
Monophyletic Origin of Coiled Pod Morphology in the Medicago Genus
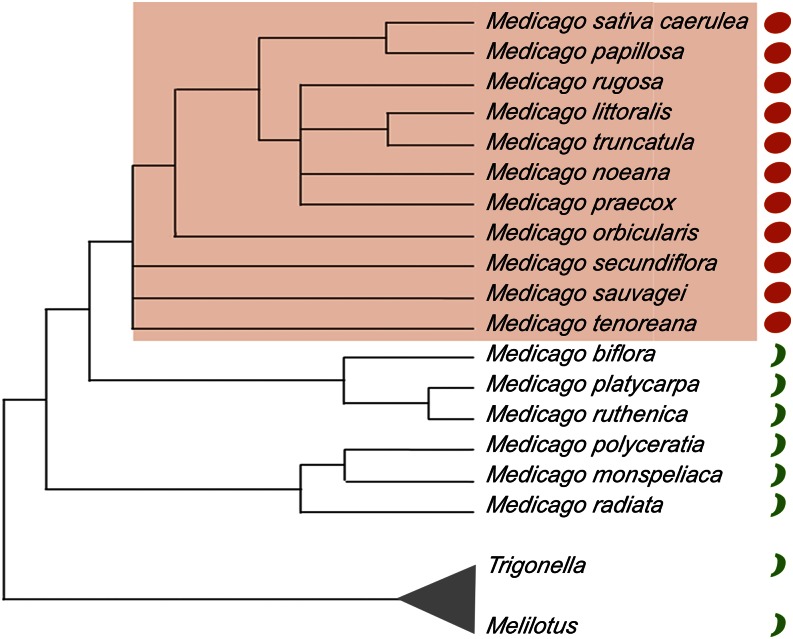
We chose for our study 17 annual species of the Medicago genus that have a diploid genome and that flowered and formed fertile fruits under our greenhouse conditions. Figure 1 shows the phylogenetic relationships among these 17 species on a tree based on the plastid maturaseK (matK) gene sequence distances (Steele et al., 2010), which is in good agreement with other phylogenetic analyses of the genus (Bena, 2001; Maureira-Butler et al., 2008; Yoder et al., 2013). In the tree, species with coiled fruits, defined by pods that complete at least a 360° turn, and uncoiled fruits, defined by straight or slightly curved pods never completing a full 360° turn, are indicated (for pictures of fruits, see Supplemental Fig. S1 and Fig. 2). Species with coiled fruits grouped together, suggesting a common evolutionary origin within the genus for this novel trait, which would be derived from the ancestral uncoiled condition.
Figure 1.
Simplified species tree of the Medicago genus. The phylogenetic relationships among the species included in this study are shown. The tree has been redrawn based on the phylogeny based on the gene sequence distances of the plastid matk genes published in Steele et al. (2010), eliminating the species not used in our study. Species with coiled pod morphology are shaded in orange and identified with an orange circle. Species with uncoiled pod morphology are indicated with green bars. The related genera Trigonella and Melilotus are used to root the tree.
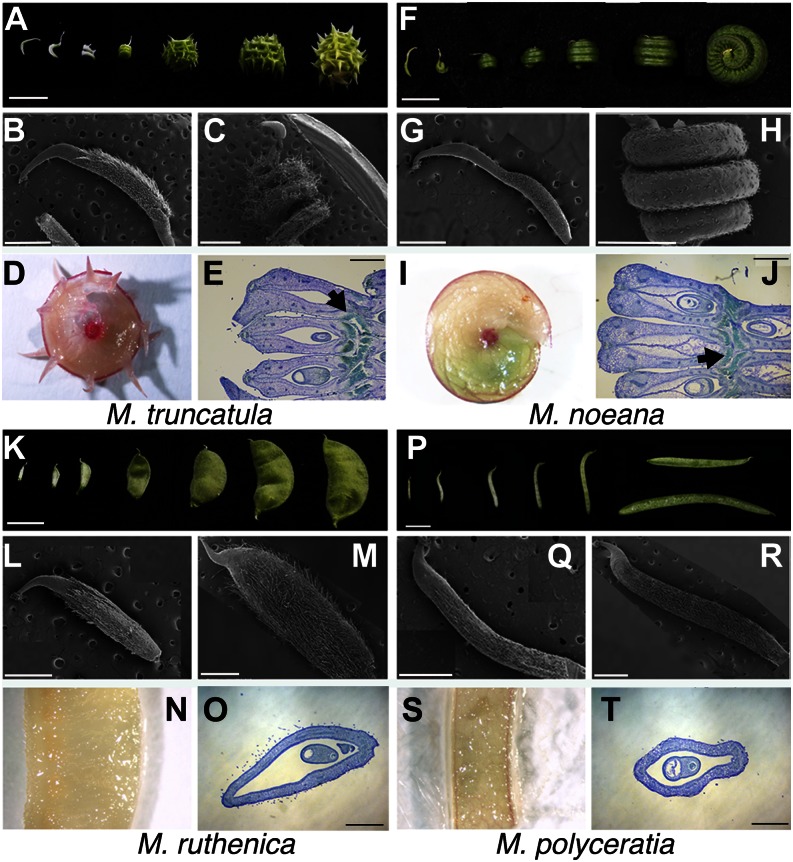
Figure 2.
Comparative morphological study of fruit development in Medicago spp. with coiled and uncoiled pod morphology. A to E, M. truncatula fruits. F to J, M. noeana fruits. K to O, M. ruthenica fruits. P to T, M. polyceratia fruits. A, F, K, and P, Seven developmental stages of fruit development from anthesis to mature fruit. Bars = 0.5 cm. B, C, G, H, L, M, Q, and R, Scanning electron micrographs of fruits at anthesis (B, G, L, and Q) and at stage 3 (C, H, M, and R) according to the developmental series in A, F, K, and P. Bars = 1 mm. D, I, N, and S, Whole-mount phloroglucinol staining of Medicago spp. fruits at stage 5. Lignin appears in pink/red. E, J, O, and T, Lignification patterns observed in histological sections of Medicago spp. fruits at stage 5. The sections have been stained with toluidine blue, and the lignin, only evident in coiled pods, appears in turquoise blue (arrow). The orientation of the sections of coiled fruits corresponds to a plane parallel to the background in C or H and perpendicular to the background in D or I. Bars = 0.5 mm.
Coiled Pod Morphology in Medicago Genus Correlates with Strong Lignification of the Carpel Margins
Medicago spp. fruits, like the vast majority of legumes, are derived from a monocarpellate pistil. By anthesis, the carpel is fused along the margin, forming a tubular structure with marginal placentation, which becomes the pod after fertilization (Benlloch et al., 2002; Wang and Grusak, 2005). To identify possible mechanisms underlying fruit coiling, we compared pod growth patterns and morphology in coiled and uncoiled fruits from four different species, Medicago truncatula, Medicago noeana, Medicago ruthenica, and Medicago polyceratia (Fig. 2, A–T). At anthesis, when fertilization takes place and fruit development starts, ovary morphology looked strikingly similar in all four species (Fig. 2, B, G, L, and Q). However, differences in ovary growth patterns were evident from early postanthesis stages. In uncoiled-pod species, the ovary grew mainly through symmetrical elongation in the longitudinal axis and in width, followed by expansion in pod diameter, similarly to what has been described for other legume species such as pea, soybean (Glycine max), or Lotus japonicus (Ozga et al., 2002; Nautrup-Pedersen et al., 2010). Mature pods in these species had a similar shape to the anthesis ovaries, only larger (Fig. 2, K, M, P, and R). By contrast, for species with coiled pods, ovary elongation proceeded asymmetrically. The fused valve margins showed restricted elongation from early stages of fruit development while the rest of the ovary grew at a much higher rate, resulting in the formation of a spiral structure where the fused valve margins remained at interior positions (Fig. 2, A, C, F, and H).
Whole-mount phloroglucinol staining of fruits from 12 of the species included in this study suggested differences in lignification patterns (Fig. 2, D, I, N, and S; Supplemental Fig. S1). Transverse sections of elongated fruits from four species stained with toluidine blue to reveal ovary tissue organization confirmed that differences were observed in lignin deposition patterns between coiled and uncoiled pods. In coiled pods, the valve margins showed extensive lignification with large patches of lignified tissue on the interior of the spiral structure, while a small domain of lignification associated with the medial vascular bundle was observed on the exterior side of the spiral (Fig. 2 E and J). In uncoiled pods, the lignified tissues were much less evident both at the medial bundle and the valve margins (Fig. 2, O and T). In all cases, a positive correlation was found between strong lignification at valve margins and pod coiling. Likewise, uncoiled pods showed weak symmetrical lignification at medial and marginal positions.
Are Different Pod Morphologies Defined by Changes in the Regulatory Networks Directing Dehiscence Zone Formation?
In Arabidopsis, the FUL/SHP/IND pathway controls critical aspects of dehiscence zone formation and lignification of the fruit (Ferrándiz, 2002; Balanzá et al., 2006). In shp1 shp2 or ind mutants, valve margins are not lignified, and the typical small cells of the separation layer adjacent to the lignified patches are missing (Liljegren et al., 2000, 2004). Conversely, in ful mutants, SHP and IND expression expands to the valves, causing ectopic lignification and restricted cell elongation in the valves (Ferrándiz et al., 2000b; Fig. 3, A–C). Additional evidence obtained through functional studies in other distantly related species indicates that the role of the FUL/SHP network in fruit dehiscence and lignification is widely conserved, at least in dicot plants (Smykal et al., 2007; Fourquin and Ferrándiz, 2012; Pabón-Mora et al., 2012).
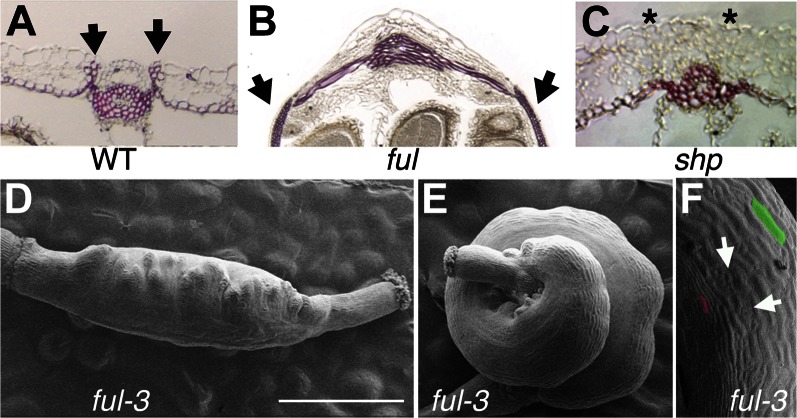
Figure 3.
Arabidopsis phenotypes related to altered lignification patterns. A to C, Lignification patterns in transverse sections of mature fruits at valve (carpel) margins as revealed by phloroglucinol staining in pink/red. A, The wild type. Arrows mark the lignified patches at valve margins. The inner cell layer of the valve also lignifies. B, ful loss-of-function mutant. Arrow marks ectopic lignification in the valve (carpel wall). C, shp1 shp2 double mutant. Note the absence of lignin at the valve margin (asterisks). D to F, Scanning electron micrographs of two fruits from the same ful-3 mutant plant, which bears an active transposon element inserted in the coding sequence of the FUL gene. D, Fruit with typical ful phenotype. E, Fruit where the excision of the transposon element has created a FUL+ revertant sector, therefore restoring FUL activity and the concomitant repression of SHP. The FUL+ sector grows normally, while growth in the ful sector is restricted by the ectopic expression of genes promoting dehiscence zone formation and lignification (SHP and IND). F, Close up of the revertant sector of the ful-3 fruit shown in E. Arrows indicate developing stomata typical of wild-type valve tissue. For comparison of differences in cell expansion, one cell of the revertant sector has been shaded in green, while one cell of the mutant sector is colored in pink. WT, Wild type. Bar = 1 mm.
Because the FUL/SHP/IND genes are at the top of the genetic hierarchy controlling both lignification and cell differentiation and expansion in the fruit, we hypothesized that changes in this pathway could lead to the morphological variation of pod shape and lignification patterns occurring in the Medicago genus. This idea was supported by the phenotypes of the ful-3 mutant line. This line bears an insertion of a Suppresor-mutator (Spm)-transposon element in the FUL gene region that causes strong loss-of-function ful phenotypes (Ferrándiz et al., 2000a). Spontaneous somatic excision of the Spm-element restores FUL activity, and in the ful-3 line, we occasionally observed fruits where FUL wild-type sectors underwent cell growth and expansion, while cells in ful mutant sectors remained small and lignified, resulting in differential growth rates of FUL+ and ful– sectors in the ovary. These different patterns of cell elongation and lignification within the ovary resulted in coiling of the silique (Fig. 3, D–F).
Given the ful-3 phenotype, we hypothesized that changes in expression patterns or protein activities in the Medicago spp. orthologs of FUL, SHP, or IND could cause changes in ovary growth and lignification patterns in coiled-pod species. Thus, for example, changes in the expression patterns could lead to different patterns of lignin deposition and cell expansion. Alternatively, a change in protein activity, for example, a higher SHP or IND activity leading to a stronger activation of the lignin biosynthetic pathway, could also result in increased lignification of valve margins if these genes were only expressed at carpel/valve margins.
The Expression Patterns of SHP Orthologs Are Similar in Medicago ssp. with Coiled and Uncoiled Pod Morphology
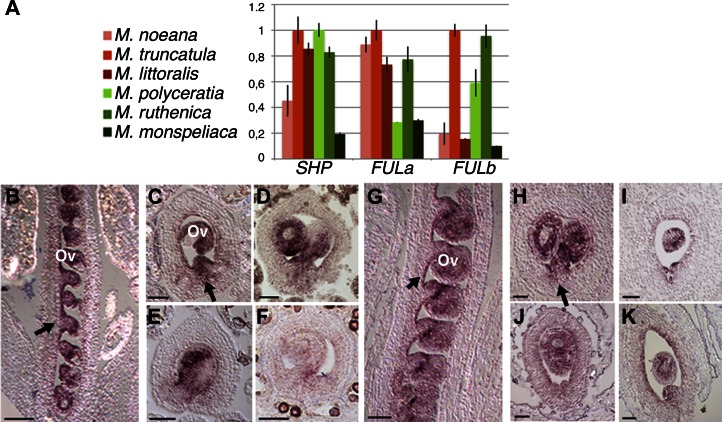
To test these hypotheses, we searched for orthologs of FUL, SHP, and IND in the fully sequenced M. truncatula genome. We found two FUL genes, MtFULa and MtFULb (Berbel et al., 2012), while only one SHP gene, which we named MtruSHP. No clear ortholog was found for IND, in agreement with the fact that IND is a paralog of HECATE3, which is only present in the Brassicaceae (Kay et al., 2013). Therefore, we focused our study on Medicago spp. orthologs of FUL and SHP. First, we cloned these genes in most of the species included in our study (for accession nos., see Supplemental Table S1). To check whether there was a correlation between the expression pattern of these genes and pod morphology, we performed a quantitative reverse transcription (RT)-PCR experiment for the three genes in six species, three having uncoiled pods and three with coiled pods, on young fruits at the time of anthesis, when morphological differences are just beginning to originate. No correlation was found between levels of expression and pod shape in these two groups (Fig. 4A), suggesting that it was unlikely that differences in pod coiling were based on differences in expression level of these genes. However, because in Arabidopsis the SHP expression domain at anthesis marks the subsequent differentiation of the dehiscence zone during fruit development, it still could be possible that more subtle changes in the spatial pattern of SHP genes (either caused by different elements in SHP regulatory sequences or by gain/loss of activity of FUL, a SHP negative regulator) had a significant effect in the extension of the lignified patches at carpel margins. Hence, we studied the expression pattern of SHP orthologs in eight species, four with uncoiled pods and four with coiled pods. In anthesis fruits of M. truncatula, a coiled-pod species, SHP was strongly expressed in ovules, at a lower level in the inner epidermal layer of the ovary, and in the carpel margins (Fig. 4, B and C), an expression pattern resembling that of the Arabidopsis SHP genes (Ferrándiz et al., 2000b). Highly similar patterns of SHP expression were observed for Medicago orbicularis, Medicago littoralis, and Medicago tenoreana, coiled-pod species, and M. polyceratia, Medicago platycarpa, Medicago monspeliaca, and M. ruthenica, uncoiled-pod species (Fig. 4, D–K). These results suggested that differences in cell growth and lignification patterns of coiled and uncoiled species were not likely caused by differences in regulatory elements of SHP genes or in the function of SHP upstream regulators such as FUL. A second important conclusion from these experiments was that SHP expression in the ovary of both coiled and uncoiled pods was not symmetrical but higher in the valve margins, the domain that is extensively lignified in coiled pods.
Figure 4.
Expression analyses of FUL and SHP genes in Medicago spp. with different pod morphology. A, Expression levels of FULa, FULb, and SHP in anthesis fruits of three Medicago spp. with coiled pod morphology (M. noeana, M. truncatula, and M. littoralis) and of three Medicago spp. with uncoiled pod morphology (M. polyceratia, M. ruthenica, and M. monspeliaca). No correlation of pod shape and overall levels of expression are observed for any of the genes. B to K, Expression patterns of MedicagoSHP in anthesis flowers of Medicago spp. with coiled (B–F) or uncoiled (G–K) pod morphologies. B, Longitudinal section of a M. truncatula flower. Expression is mainly detected in ovules (ov) and the inner epidermal layer of the carpel (arrow). C, Transversal section of a M. truncatula fruit. Expression is observed in ovules (ov) and the inner epidermal layer and at the carpel margin (arrow). D to F, Transversal sections equivalent to that shown in C for M. orbicularis (D), M. littoralis (E), and M. tenoreana (F), all showing very similar expression patterns. G, Longitudinal section of a M. polyceratia flower. Expression is detected in ovules (ov) and the inner epidermal layer of the carpel (arrow). H, Transversal section of a M. polyceratia fruit. Again, expression is observed in ovules and inner epidermal layer and at the carpel margin (arrow). I to K, Transversal sections equivalent to that shown in C or H for M. platycarpa (I), M. monspeliaca (J), and M. ruthenica (K), all showing very similar expression patterns. Bars = 50 μm.
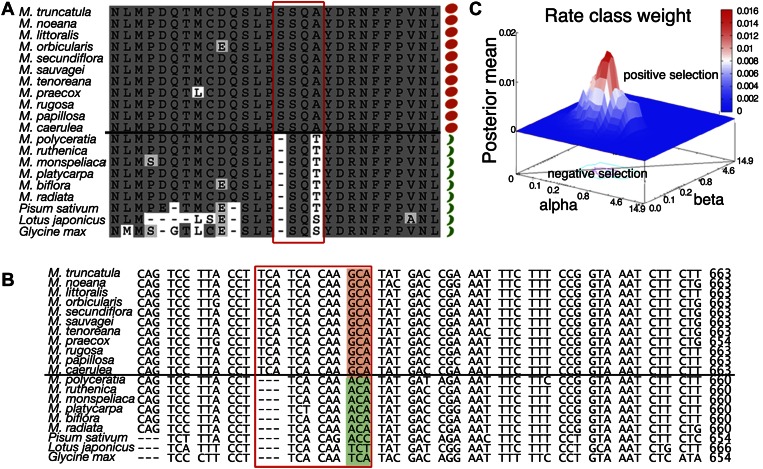
A Sequence Polymorphism in SHP Orthologs Correlates with Coiled/Uncoiled Pod Morphology
Our results led us to investigate the alternative hypothesis, that differences in protein activity resulted in differences in the extent of lignified tissues associated with valve margins. For this reason, we compared the deduced protein sequences of FUL and SHP from different Medicago spp. For FULa and FULb orthologs, no correlation was found between any sequence polymorphism and fruit shape (Supplemental Fig. S2). However, for SHP orthologs, a sequence signature was found that perfectly correlated with coiled and uncoiled pod morphology in 17 species (Fig. 5A; Supplemental Fig. S3). Moreover, we found the uncoiled signature in other legume SHP orthologs found in public databases (pea, soybean, and L. japonicus), all of which are from species with similar uncoiled pods (Fig. 5A).
Figure 5.
Sequence analyses on SHP genes from Medicago spp. with coiled or uncoiled pod morphology. A, Alignment of a fragment of SHP protein sequences from Medicago spp. coiled- (orange circles) and uncoiled-pod species (green bars). Sequences from SHP proteins from other legumes species with uncoiled pods (pea, accession no. AY884292; L. japonicus, BT135371; and soybean, XM_003551863) have also been added. The polymorphism is framed in red, and the amino acid corresponding to the transition codon used for the evolutionary fingerprint analyses is marked by a red asterisk. B, Alignment of the corresponding nucleotide sequences of SHP genes around the polymorphism analyzed. The transition codon is shaded in orange for species with coiled fruits and in green for species with uncoiled fruits. C, Selection pressure evidences in the transition ACA-GCA site in Medicago spp. SHATTERPROOF orthologs suggested by the Bayesian analysis. Posterior means shown on the vertical axis indicate mean values of sites. Rate class weights (individual sites) around the transition site are shown as a red peak rising above the neutral selection level (blue plane).
If the correlation between the sequence variation and the coiled/uncoiled morphology had functional relevance, we would expect that the corresponding sequence polymorphism would be under selective pressure. To analyze the selective pressure on a given site, the ratio of replacement and silent mutations in this site is normalized to the number of replacement and silent sites in the gene (dN/dS), where the number of replacement mutations per replacement site is called dN and the number of silent mutations per silent site is called dS (Lemey et al., 2009). Through a sequence alignment, we compared SHP coding regions from species possessing coiled fruits against those with uncoiled fruits from Medicago spp. and other legume plants in various codon positions. Each codon position was tested for the selective pressure acting on that site, using all homologs from coiled and uncoiled-pod species. The evolutionary fingerprint mean observed for all codons in the ortholog SHP genes indicates a negative selection (Supplemental Fig. S4). However, using the single likelihood ancestor counting (SLAC) method available in the HyPhy package (Pond et al., 2005), we found evidence for diversifying positive selection, where the nonsynonymous changes outweigh the synonymous changes (dN/dS > 1), in the coiled/uncoiled signature sequence found in the SHP gene (ACA to GCA or Thr to Ala in coiled-pod species; Fig. 5, A–C; Table I). These results suggested that the codon transition was under positive Darwinian selection. The selection rates for the ACA-GCA transition, measured for the three possible conditions, i.e. coiled, uncoiled, and all species, are shown in Table II. The results indicate that the evolutionary rates at the ACA codon are higher than at the GCA, or that natural/domestication forces are higher toward the formation of coiled phenotypes, being more conservative on the genotypes presenting the GCA codon.
Table I. SLAC results.
Positively and negatively selected sites (nucleotide changes), as well as mean dN/dS (difference between synonymous and nonsynonymous sites) and sites with evidence of directional selection, i.e. those having a concerted substitution toward a particular residue. For each codon, estimates of the numbers of inferred synonymous and nonsynonymous substitutions were calculated along with the numbers of sites that are estimated to be synonymous and nonsynonymous. These estimates were produced using the joint maximum likelihood reconstructions of ancestral states under a Muse-Gaut model of codon substitution and Tamura-Nei model of nucleotide substitution for coiled-like and uncoiled-like homologs and general time reversible model for all genes. For estimating maximum likelihood values, a tree topology was automatically computed. The statistical test dN/dS was used for detecting codons that have undergone positive selection.
| Genes | Positive Sites (SLAC)a | Negative Sites (SLAC)a | Mean dN/dS | Sites with Evidence of Directional Selectionb |
|---|---|---|---|---|
| Coiled | 1 | 52 | −0.050 | 0 |
| Uncoiled | 0 | 0 | −0.010 | 0 |
| All homologs | 0 | 50 | −0.095 | 2 |
P = 0.05. bMixed-effects model of evolution.
Table II. Selection rate in the ACA-AGA transition site (codon identified as associated with the Coiled condition) using the coiled/uncoiled homologs and all genes clustered.
These are normalized values such that sequence evolution rates across all sites is 1. Relative rates were estimated under the Tamura model (+gamma) for coiled-like and uncoiled-like homologs and under the general time-reversible model (+gamma+invar) for all genes. There were a total of 609 positions in the final data set.
| Triplet | Selectiona Coiled | Sequence Evolution Rateb | Selection Uncoiled | Sequence Evolution Rateb | Selectiona All Genes | Sequence Evolution Rateb |
|---|---|---|---|---|---|---|
| ACA | n/a | n/a | 0 | 0.496 | 0.644 | 0.496 |
| GCA | 0 | 0.226 | n/a | n/a | 0.500 | 0.461 |
Selection acting on the site. bMean (relative) sequence evolution rates are shown for each site next to the aimed site.
The Two Sequence Variants of SHP Have Different Protein Activities
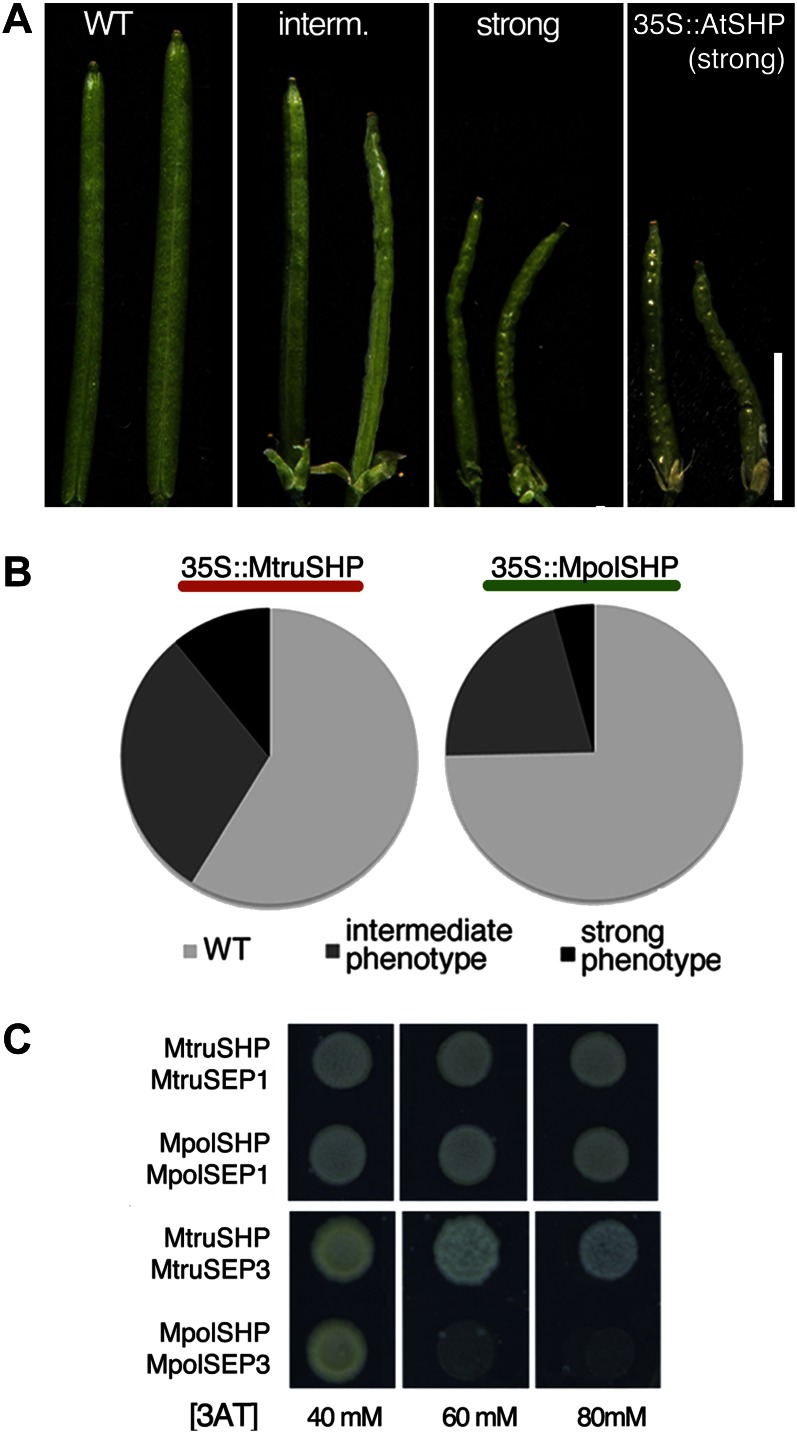
To test whether this change in sequence could have an effect on protein activity, we generated two constructs expressing the coding sequence of the SHP ortholog from a coiled (M. truncatula) and an uncoiled-pod species (M. polyceratia) under the control of the constitutive Cauliflower mosaic virus 35S promoter (Benfey et al., 1989). We transformed Arabidopsis wild-type plants with these constructs, recovered more than 150 independent primary transgenic lines (T1) for each, and compared the phenotypes of the individual plants. Both transgenes caused a range of phenotypes in T1 lines related to those observed in Arabidopsis lines that overexpressed the endogenous AtSHP genes, with different degrees of leaves curled upwards, early flowering, conversion of sepals into carpelloid structures, and shorter wrinkled fruits (Fig. 6A; Liljegren et al., 2000). However, we were able to observe significant differences in the relative effects of both constructs: overexpression of MpolSHP, the SHP gene from M. polyceratia (uncoiled pods), only produced short wrinkled fruit phenotypes in 25% of the T1 lines, while the overexpression of MtruSHP, the SHP gene from M. truncatula (coiled pods) affected a significantly higher proportion of lines (41%; Fig. 6B). Moreover, the strongest phenotypes appeared in 11% of the transgenic lines overexpressing the gene from the coiled-pod species (MtruSHP), while only in 4% of the lines overexpressing the gene from the uncoiled-pod species (MpolSHP), therefore indicating that the MtruSHP protein was more active than MpolSHP (Fig. 6B).
Figure 6.
Different activities of SHP proteins from Medicago spp. with coiled or uncoiled pod morphology. A and B, Effect of the overexpression of Medicago spp. SHP genes in Arabidopsis. A, Wild-type Arabidopsis fruit (left) and examples of intermediate and strong fruit phenotypes observed in 35S::MpolSHP or 35S::MtruSHP T1 lines (center). The right section also shows the strongest fruit phenotypes observed when the Arabidopsis SHP1 gene is overexpressed. B, Relative proportions of phenotypes observed in the different T1 plants transformed with 35S::MtruSHP (from M. truncatula, coiled pods) and 35S::MpolSHP (from M. polyceratia, uncoiled pods). More that 150 T1 plants were generated for each construct. C, Yeast two-hybrid experiment to reveal dimer formation between SEP and SHP proteins from M. truncatula (coiled pods) and M. polyceratia (uncoiled pods). 3-AT concentration (mm) for each experiment is indicated. MpolSEP1 and MpolSEP3 were cloned for these analyses (see accession nos. in Supplemental Table S1). Accession numbers are MtSEP1, AC146650a and MtSEP3, AC144644. WT, Wild type. [See online article for color version of this figure.]
Because protein-protein interactions among MADS-box proteins are crucial for their function (Honma and Goto, 2001), we speculated that the sequence differences between the two SHP variants from coiled- and uncoiled-pod species could affect the range of these interactions. MADS-box proteins from the AGAMOUS (AG)/SHP subfamily have been shown to interact with SEPALLATA (SEP) MADS-box proteins to direct carpel development (de Folter et al., 2005; Immink et al., 2009). We used yeast (Saccharomyces cerevisiae) two-hybrid experiments to test the ability of MtruSHP and MpolSHP to interact with SEP orthologs from the corresponding species. We found both MtruSHP and MpolSHP interacted with their corresponding SEP1 and SEP3 orthologs, but not with SEP4, in agreement with previously reported interactions for AG/SHP factors from other species (de Folter et al., 2005; Immink et al., 2009; Airoldi et al., 2010). However, we also found that the SHP-SEP3 dimers interacted with different affinities: the dimer from the coiled-pod species M. truncatula interacted more strongly than the dimer from the uncoiled-pod species M. polyceratia. The interaction of the SHP-SEP3 dimer from M. truncatula was maintained even when 80 mm 3-aminotriazole (3-AT) was added to the growth medium, while the SHP-SEP3 dimer from M. polyceratia was not able to form at 3-AT concentrations higher than 40 mm (Fig. 6C).
In summary, these results suggested that differential SHP activity could be at the origin of coiled fruit morphology and that this differential activity did not seem to be based on differential expression, but on different protein functional properties.
DISCUSSION
Our work shows a tight correlation between coiled pod morphology and increased valve margin lignification in Medicago spp. fruits. The lignified areas in coiled pods fail to elongate during fruit development, similar to what is observed in partially revertant fruits of ful-3 mutants that also adopt a coiled morphology. We also show that SHP genes in Medicago spp. fruits are expressed in the valve margins, the domains that lignify extensively in coiled-pod species, and we have found a correlation of coiled pod morphologies with a sequence polymorphism under positive Darwinian selection in SHP sequences, strongly suggesting that the modification of SHP protein underlies the evolutionary origin of pod coiling in Medicago spp.
Several pieces of evidence support this hypothesis. First, there is a strong correlation between pod coiling, extent of valve margin lignification, and a functionally relevant polymorphism in the SHP sequence. The role of SHP genes in the control of lignin deposition during late fruit development has been described for other distantly related dicot species. Thus, in Arabidopsis or Nicotiana benthamiana, SHP directs lignification of dehiscence zones (Liljegren et al., 2000; Fourquin and Ferrándiz, 2012), and in peach (Prunus persica), increased levels of PpSHP are found in varieties prone to split-pit formation and overlignification of the fruit (Tani et al., 2007). These conserved roles across dicot species support the idea that modifications in SHP sequence, causing a change in protein activity, would be likely to induce changes in lignification patterns in valve margins such as those observed in the coiled pods of Medicago spp. Second, we have proven that the variation in SHP sequence observed in coiled-pod species causes a change in SHP functional properties, as seen in the relative effects of SHP overexpression in Arabidopsis and in protein interaction studies. A naturally occurring variation in a single amino acid of the related PLENA (PLE) and FARINELLI (FAR) MADS-box proteins from Antirrhinum majus has been shown to have profound effects on their ability to specify male and female reproductive organs by altering its range of protein-protein interactions with SEP homologs (Airoldi et al., 2010). Thus, we have uncovered a similar scenario where a small sequence modification in SHP amino acid sequence increases the SHP affinity for SEP3 protein partners that could lead to hyperactivation of the downstream targets. Finally, positive selective pressure in the ACA-GCA transition indicates a selective sweep fixing the new allele. Interestingly, the remaining codons are under a negative selection, indicating a strong conserving force that suggests an essential role of SHP for plant survival. Coiled pod morphology in the Medicago genus appears to be evolutionarily fixed, suggesting an adaptive value of this novel trait. In fact, pod coiling in Medicago spp. has been associated with an increase in reproductive success due to enhanced resistance to certain phytophagous insects, which encounter increased physical constraints to feed on the seeds of coiled pods (Small and Brookes, 1984). Moreover, the coiled pod morphology represents a change in seed dispersal strategies with a likely impact on population structures and fitness (Heyn, 1963; Lesins and Lesins, 1979; Polhill, 1994; Levin et al., 2003; Yan et al., 2009). The possible evolutionary advantages of the novel pod morphology in Medicago spp. could explain the low variability in the coiled-SHP sequence signature and supports the hypothesis of this signature as the origin of the trait.
We are aware that additional functional data would be desirable for the validation of our hypothesis. For this purpose, we identified an insertion in the MtruSHP gene by reverse genetics in the collection of lines tagged with the transposable element of Nicotiana tabacum cell type1 at the Noble Foundation (Tadege et al., 2008), but the original primary line from which genomic DNA was isolated did not produce seeds, and therefore, the corresponding mutants were lost. Likewise, when we generated transgenic lines harboring a hairpin RNA interference construct designed to silence MtruSHP, we observed unusually reduced transformation efficiency, and in the small number of lines recovered, almost no reduction in MtruSHP levels was obtained. Thus, these experiments did not help to characterize the role of SHP in pod development but might suggest an essential role of SHP for reproductive success in Medicago spp., as already indicated by the analyses on sequence evolution that we have performed.
Our work on Medicago spp. SHP orthologs provides an example of a modest change in the protein sequence of a key regulatory factor as the likely origin of a developmental innovation, in this case a dramatic change in fruit morphology likely affecting fitness and seed dispersal strategies. A widely accepted postulate of evolutionary developmental biology science (evo-devo) is that many developmental innovations affecting morphology are more likely linked to changes in the cis-regulatory regions than in the protein-coding regions of key developmental genes. This idea is mainly based on the modular nature of cis-regulatory elements, which allows local modifications of the activity of key regulators of developmental pathways without other deleterious pleiotropic effects (Carroll, 2000, 2008). However, there is a lively debate on the relative importance of changes in protein activity to create new developmental functions, which some authors consider also to be highly significant (Hoekstra and Coyne, 2007; Haag and Lenski, 2011). In addition to our study and the PLE/FAR case already mentioned, we can find other examples of amino acid substitutions underlying the evolution of novel traits, for instance, an Asn-to-Lys substitution in the coding sequence of the SH4 (for grain shattering quantitative trait locus on chromosome4) gene in rice (Oryza sativa) causing a reduction in seed shattering that was selected by humans in the process of rice domestication (Li et al., 2006), a Lys-to-Glu substitution in the ENHANCER OF TRY AND CPC2 factor, which determines trichome patterning in natural Arabidopsis populations (Hilscher et al., 2009), or a Lys-to-Asn substitution in the teosinte glume architecture1 gene of maize (Zea mays) that appears to be responsible for the origin of the naked grains selected in domesticated maize (Wang et al., 2005). Taken together, these studies highlight the importance of modified protein activity acting as a driving force in evolution. This expands the general postulates of evo-devo, which state that form evolves mainly through cis-regulatory changes that alter expression patterns of functionally conserved proteins with highly pleiotropic functions.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Medicago spp. plants were grown in the greenhouse at 22°C (day) and 18°C (night) with a 16-h-light/8-h-dark photoperiod in soil irrigated with Hoagland No. 1 solution supplemented with oligoelements (Hewitt, 1966). Arabidopsis (Arabidopsis thaliana) plants were grown in cabinets at 21°C under long-day conditions (16-h-light) illuminated by cool-white fluorescent lamps (150 μE m–2 s–1) in a 1:1:1 mixture of sphagnum:perlite:vermiculite.
For Arabidopsis transformation, MtruSHP and MpolSHP coding sequences were amplified with primers Sal1MSHPFor and BamH1MSHPRev and cloned in the pBIN-JIT vector (Ferrándiz et al., 2000b). Each vector was introduced into Agrobacterium tumefaciens PMP90 for Arabidopsis transformation using the floral dip protocol (Clough and Bent, 1998). T1 plants were selected based on kanamycin selection.
Phenotypic categories for 35S::MtruSHP/MpolSHP fruits were scored as follows. Silique length was measured for 10 fully developed fruits in the main inflorescence of five wild-type plants and each of the T1 plants obtained for each construct showing morphological alterations such as modified sepals or curled leaves. Average silique length of each transgenic plant was compared to the wild-type value and categorized as “strong” if it was less than 75% of the wild type and the ovary showed an evident bumpy or wrinkled surface, while “intermediate” phenotypes corresponded to plants where average silique length was between 75% and 95% of the wild type and ovary surface was not smooth.
Cloning and Sequence Analysis
All sequences were isolated by RT-PCR on complementary DNA (cDNA) of young flowers. The full-length coding sequence of MtruSHP gene was isolated using primers PeaSHPFor and PeaSHPRev designed from the sequence of the pea (Pisum sativum) SHP gene (PM8, AY884292). From the identified MtruSHP gene, the primers MSHPFor and MSHPRev were designed and used to isolate the SHP genes from the rest of the Medicago spp. The primers MFULaFor/MFULaRev and MFULbFor/MFULbRev were designed from the Medicago truncatula FUL available sequences (MtFULa, TC84496 and MtFULb, TC82227) and used to amplify the FULa and FULb genes from the different Medicago spp. The deduced amino acid sequences alignments were analyzed using the Macvector 12.5 software. See Supplemental Table S2 for primers sequences and Supplemental Table S1 for the accession numbers of the identified sequences.
For the analyses on patterns of sequence polymorphism, an evolutionary fingerprint analysis was made to infer about the main selection pressure in the overall codon sites within the SHP orthologs from Medicago spp. and other legume species. To verify the putative selection, the transitional ACA-GCA site was analyzed individually. The analyses were run using the Fast Unbiased AppRoximate Bayesian approach for selection pressure evidences. To verify the existence of mixed effects in the selected codon site, a Multiple Expectation Maximization for Motif Elucidation analysis was carried out. All analyses were made using the HyPhy package (Pond et al., 2005) in situ or the webserver available (http://www.datamonkey.org).
In Situ Hybridization
RNA in situ hybridization with digoxigenin-labeled probes was performed on 8-μm paraffin sections of Medicago spp. flowers as described (Ferrándiz et al., 2000a). The RNA antisense and sense probes were generated from a 478-bp fragment of the MtruSHP cDNA (positions 258–735 from start codon). The same probe was used for the eight Medicago spp. studied, as the sequences present more than 95% of identity.
Quantitative RT-PCR
Total RNA was extracted from flowers in anthesis from the different Medicago spp. with the RNeasy Plant Mini Kit (Qiagen). Four micrograms of total RNA were used for cDNA synthesis with the First Strand cDNA Synthesis Kit (Invitrogen), and the quantitative PCR master mix was prepared using the iQTM SYBR Green Supermix (Bio-Rad). Results were normalized to the expression of the M. truncatula O-linked GlcNAc Transferase-like mRNA (XM_003590858) amplified with primers qMConstiFor/qMConstiRev. The primers used to amplify the SHP, FULa, and FULb genes of Medicago spp. generated products of 51 bp and did not show any cross amplification. The efficiency in the amplification of MedicagoSHP, MedicagoFULa, MedicagoFULb and the reference gene was similar. The PCR reactions were run and analyzed using the ABI PRISM 7700 Sequence Detection System (Applied Biosystems). See Supplemental Table S2 for primer sequences.
Scanning Electron Microscopy
Samples were vacuum infiltrated with FAE (3.7% formaldehyde, 5% acetic acid, 50% ethanol [v/v]) for 10 min and fixed with fresh solution for 16 h at 4°C. Samples were dehydrated in an ethanol series and critical point dried in liquid CO2 (Polaron E300 apparatus). Dried samples were mounted on stubs and coated with gold palladium (4:1) in a Sputter Coater SCD005 (Baltec). Scanning electron microscopy was performed with a JEOL JSM-5410 microscope (10 kV).
Lignin Staining
Fruits were fixed in formaldehyde-acetic acid-ethanol overnight and then embedded into paraffin. Twelve-micrometer sections were stained in a 0.2% (p/v) toluidine blue solution for 2 min and then washed in water. Alternatively, sections were stained in phloroglucinol 2.5% (p/v) for 30 min and then soaked 30 s in 50% HCl (v/v) before being photographed under the microscope. For whole-mount lignin observation, fruits were fixed in formaldehyde-acetic acid-ethanol overnight, stained for 30 min in 2.5% phloroglucinol, and soaked 30 s in 50% HCl.
Yeast Two-Hybrid Assays
The yeast (Saccharomyces cerevisiae) two-hybrid assays were performed in the yeast strain PJ69-4α using the vectors pGADT7 (pAD) and pGBKT7 (pBD; kindly provided by Monica Colombo and Barry Causier). The coding sequences were cloned using TOPO cloning and Gateway recombination (Invitrogen). MtruSHP and MpolSHP were cloned into the pBD vector using primers MSHPFor/MSHPRev. MtruSEP1, MtruSEP3, MpolSEP1, and MpolSEP3 were cloned into the pAD vector using primers MSEP1For/MSEP1Rev and MSEP3For/MSEPRev. Two-hybrid interactions were assayed on selective yeast synthetic dropout medium lacking Leu, Trp, and His and supplemented with different concentrations of 3-AT (40, 60, and 80 mm). Selections were performed at 28°C. The experiments were repeated four times, and growth of the colonies on control and selective plates was assessed.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers: AY884292, BT135371, XM_003551863, AC146650a; AC144644, TC84496, TC82227, XM_003590858, JX308825, JX297560, JX297556, JX297557, JX297558, JX297559, JX297561, JX297562, JX297563, JX297564, JX297565, JX297566, JX297567, JX297568, JX297569, JX297570, JX297571, JX308806, JX308802, JX308803, JX308804, JX308805, JX308807, JX308808, JX308809, JX308810, JX308815, JX308811, JX308812, JX308813, JX390719, JX308814, JX308816, JX308817, JX308818, JX308819, JX308820, JX308821, JX308822, JX308823, JX308824, JX390717, and JX390718.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Fruit lignification pattern of pods from Medicago spp. included in this study.
Supplemental Figure S2. Alignment of FULa and FULb proteins from different Medicago spp.
Supplemental Figure S3. Alignment of SHP proteins from different Medicago spp.
Supplemental Figure S4. Evolutionary fingerprint of the SHP homolog codons.
Supplemental Table S1. Accession numbers for sequences identified in this work.
Supplemental Table S2. Primers used in this work.
Acknowledgments
We thank Rafael Martínez-Pardo and Eugenio Grau (Instituto de Biologia Molecular y Celular de Plantas) for technical support and Barbara Ambrose (New York Botanical Garden), Mario Fares, and Francisco Madueño (Instituto de Biología Molecular y Celular de Plantas) for helpful discussions and critical reading of the manuscript. Germplasm used in this study was obtained from the National Genetic Resources Program (USA).
Glossary
- RT
reverse transcription
- dN
number of replacement mutations per replacement site
- dS
number of silent mutations per silent site
- SLAC
single likelihood ancestor counting
- cDNA
complementary DNA
- 3-AT
3-aminotriazole
References
- Airoldi CA, Bergonzi S, Davies B. (2010) Single amino acid change alters the ability to specify male or female organ identity. Proc Natl Acad Sci USA 107: 18898–18902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balanzá V, Navarrete M, Trigueros M, Ferrándiz C. (2006) Patterning the female side of Arabidopsis: the importance of hormones. J Exp Bot 57: 3457–3469 [DOI] [PubMed] [Google Scholar]
- Bena G. (2001) Molecular phylogeny supports the morphologically based taxonomic transfer of the “medicagoid” Trigonella species to the genus Medicago L. Plant Syst Evol 229: 217–236 [Google Scholar]
- Benfey PN, Ren L, Chua NH. (1989) The CaMV 35S enhancer contains at least two domains which can confer different developmental and tissue-specific expression patterns. EMBO J 8: 2195–2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benlloch R, Navarro C, Beltran JP, Canas LA. (2002) Floral development of the model legume Medicago truncatula: ontogeny studies as a tool to better characterize homeotic mutations. Sex Plant Reprod 15: 231–241 [Google Scholar]
- Berbel A, Ferrándiz C, Hecht V, Dalmais M, Lund OS, Sussmilch FC, Taylor SA, Bendahmane A, Ellis TH, Beltrán JP, et al. (2012) VEGETATIVE1 is essential for development of the compound inflorescence in pea. Nat Commun 3: 797. [DOI] [PubMed] [Google Scholar]
- Bremer B, Eriksson O. (1992) Evolution of fruit characters and dispersal modes in the tropical family Rubiaceae. Biol J Linn Soc Lond 47: 75–95 [Google Scholar]
- Carroll SB. (2000) Endless forms: the evolution of gene regulation and morphological diversity. Cell 101: 577–580 [DOI] [PubMed] [Google Scholar]
- Carroll SB. (2008) Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell 134: 25–36 [DOI] [PubMed] [Google Scholar]
- Clausing G, Meyer K, Renner S. (2000) Correlations among fruit traits and evolution of different fruits within Melastomataceae. Bot J Linn Soc 133: 303–326 [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- de Folter S, Immink RGH, Kieffer M, Parenicová L, Henz SR, Weigel D, Busscher M, Kooiker M, Colombo L, Kater MM, et al. (2005) Comprehensive interaction map of the Arabidopsis MADS Box transcription factors. Plant Cell 17: 1424–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrándiz C. (2002) Regulation of fruit dehiscence in Arabidopsis. J Exp Bot 53: 2031–2038 [DOI] [PubMed] [Google Scholar]
- Ferrándiz C, Fourquin C, Prunet N, Scutt C, Sundberg E, Trehin C, Vialette-Guiraud A. (2010) Carpel development. Adv Bot Res 55: 1–74 [Google Scholar]
- Ferrándiz C, Gu Q, Martienssen R, Yanofsky MF. (2000a) Redundant regulation of meristem identity and plant architecture by FRUITFULL, APETALA1 and CAULIFLOWER. Development 127: 725–734 [DOI] [PubMed] [Google Scholar]
- Ferrándiz C, Liljegren SJ, Yanofsky MF. (2000b) Negative regulation of the SHATTERPROOF genes by FRUITFULL during Arabidopsis fruit development. Science 289: 436–438 [DOI] [PubMed] [Google Scholar]
- Fourquin C, Ferrándiz C. (2012) Functional analyses of AGAMOUS family members in Nicotiana benthamiana clarify the evolution of early and late roles of C-function genes in eudicots. Plant J 71: 990–1001 [DOI] [PubMed] [Google Scholar]
- Gu Q, Ferrándiz C, Yanofsky MF, Martienssen R. (1998) The FRUITFULL MADS-box gene mediates cell differentiation during Arabidopsis fruit development. Development 125: 1509–1517 [DOI] [PubMed] [Google Scholar]
- Haag ES, Lenski RE. (2011) L’enfant terrible at 30: the maturation of evolutionary developmental biology. Development 138: 2633–2637 [DOI] [PubMed] [Google Scholar]
- Hewitt Y. (1966) Sand and Water Culture Methods Used in the Study of Plant Nutrition, Ed 2 Commonwealth Agricultural Bureau, Farnham, UK [Google Scholar]
- Heyn C. (1963) The Annual Species of Medicago. Magnes Press, Jerusalem [Google Scholar]
- Hilscher J, Schlötterer C, Hauser M-T. (2009) A single amino acid replacement in ETC2 shapes trichome patterning in natural Arabidopsis populations. Curr Biol 19: 1747–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra HE, Coyne JA. (2007) The locus of evolution: evo devo and the genetics of adaptation. Evolution 61: 995–1016 [DOI] [PubMed] [Google Scholar]
- Honma T, Goto K. (2001) Complexes of MADS-box proteins are sufficient to convert leaves into floral organs. Nature 409: 525–529 [DOI] [PubMed] [Google Scholar]
- Immink RG, Tonaco IA, de Folter S, Shchennikova A, van Dijk AD, Busscher-Lange J, Borst JW, Angenent GC. (2009) SEPALLATA3: the ‘glue’ for MADS box transcription factor complex formation. Genome Biol 10: R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay P, Groszmann M, Ross JJ, Parish RW, Swain SM. (2013) Modifications of a conserved regulatory network involving INDEHISCENT controls multiple aspects of reproductive tissue development in Arabidopsis. New Phytol 197: 73–87 [DOI] [PubMed] [Google Scholar]
- Lemey P, Salemi M, Vandamme AM. (2009) The Phylogenetic Handbook. A Practical Approach to Phylogenetic Analysis and Hypothesis Testing, Ed 2 Cambridge University Press, Cambridge, UK [Google Scholar]
- Lesins KA, Lesins I. (1979) Genus Medicago (Leguminosae): A Taxogenetic Study. W. Junk, The Hague, The Netherlands [Google Scholar]
- Levin SA, Muller-Landau HC, Nathan R, Chave J. (2003) The ecology and evolution of seed dispersal: a theoretical perspective. Annu Rev Ecol Evol Syst 34: 575–604 [Google Scholar]
- Lewis GP, Schrire B, Mackinder B, Lock M. (2005) Legumes of the World. Kew Publishing, Richmond, UK [Google Scholar]
- Li C, Zhou A, Sang T. (2006) Rice domestication by reducing shattering. Science 311: 1936–1939 [DOI] [PubMed] [Google Scholar]
- Liljegren SJ, Ditta GS, Eshed Y, Savidge B, Bowman JL, Yanofsky MF. (2000) SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature 404: 766–770 [DOI] [PubMed] [Google Scholar]
- Liljegren SJ, Roeder AHK, Kempin SA, Gremski K, Østergaard L, Guimil S, Reyes DK, Yanofsky MF. (2004) Control of fruit patterning in Arabidopsis by INDEHISCENT. Cell 116: 843–853 [DOI] [PubMed] [Google Scholar]
- Maureira-Butler IJ, Pfeil BE, Muangprom A, Osborn TC, Doyle JJ. (2008) The reticulate history of Medicago (Fabaceae). Syst Biol 57: 466–482 [DOI] [PubMed] [Google Scholar]
- Mummenhoff K, Franzke A, Koch M. (1997) Molecular data reveal convergence in fruit characters used in classification of Thlaspi sl (Brassicaceae). Bot J Linn Soc 125: 183–199 [Google Scholar]
- Nautrup-Pedersen G, Dam S, Laursen BS, Siegumfeldt AL, Nielsen K, Goffard N, Stærfeldt HH, Friis C, Sato S, Tabata S, et al. (2010) Proteome analysis of pod and seed development in the model legume Lotus japonicus. J Proteome Res 9: 5715–5726 [DOI] [PubMed] [Google Scholar]
- Ozga JA, van Huizen R, Reinecke DM. (2002) Hormone and seed-specific regulation of pea fruit growth. Plant Physiol 128: 1379–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabón-Mora N, Ambrose BA, Litt A. (2012) Poppy APETALA1/FRUITFULL orthologs control flowering time, branching, perianth identity, and fruit development. Plant Physiol 158: 1685–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polhill RM. (1994) Classification of the Leguminoseae and complete synopsis of legume genera. In Bisby FA, Buckingham J, Harborne JB, eds, Phytochemical Dictionary of the Leguminoseae, Vol 1 Chapman & Hall, London [Google Scholar]
- Pond SL, Frost SD, Muse SV. (2005) HyPhy: hypothesis testing using phylogenies. Bioinformatics 21: 676–679 [DOI] [PubMed] [Google Scholar]
- Scutt CP, Vinauger-Douard M, Fourquin C, Finet C, Dumas C. (2006) An evolutionary perspective on the regulation of carpel development. J Exp Bot 57: 2143–2152 [DOI] [PubMed] [Google Scholar]
- Small E. (2011) Alfalfa and Relatives: Evolution and Classification of Medicago. NRC Research Press, Ottawa [Google Scholar]
- Small E, Brookes B. (1984) Coiling of alfalfa pods in relation to resistance against seed chalcids: additional observations. Can J Plant Sci 64: 659–665 [Google Scholar]
- Small E, Jomphe M. (1989) A synopsis of the genus Medicago (Leguminosae). Can J Bot 67: 3260–3294 [Google Scholar]
- Smykal P, Gennen J, De Bodt S, Ranganath V, Melzer S. (2007) Flowering of strict photoperiodic Nicotiana varieties in non-inductive conditions by transgenic approaches. Plant Mol Biol 65: 233–242 [DOI] [PubMed] [Google Scholar]
- Steele KP, Ickert-Bond SM, Zarre S, Wojciechowski MF. (2010) Phylogeny and character evolution in Medicago (Leguminosae): evidence from analyses of plastid trnK/matK and nuclear GA3ox1 sequences. Am J Bot 97: 1142–1155 [DOI] [PubMed] [Google Scholar]
- Tadege M, Wen J, He J, Tu H, Kwak Y, Eschstruth A, Cayrel A, Endre G, Zhao PX, Chabaud M, et al. 2008) Large-scale insertional mutagenesis using the Tnt1 retrotransposon in the model legume Medicago truncatula. Plant J 54: 335–347 [DOI] [PubMed] [Google Scholar]
- Tani E, Polidoros AN, Tsaftaris AS. (2007) Characterization and expression analysis of FRUITFULL- and SHATTERPROOF-like genes from peach (Prunus persica) and their role in split-pit formation. Tree Physiol 27: 649–659 [DOI] [PubMed] [Google Scholar]
- Wang H, Nussbaum-Wagler T, Li B, Zhao Q, Vigouroux Y, Faller M, Bomblies K, Lukens L, Doebley JF. (2005) The origin of the naked grains of maize. Nature 436: 714–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Grusak MA. (2005) Structure and development of Medicago truncatula pod wall and seed coat. Ann Bot (Lond) 95: 737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Chu HJ, Wang HC, Li JQ, Sang T. (2009) Population genetic structure of two Medicago species shaped by distinct life form, mating system and seed dispersal. Ann Bot (Lond) 103: 825–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder JB, Briskine R, Mudge J, Farmer A, Paape T, Steele K, Weiblen GD, Bharti AK, Zhou P, May GD, et al. (2013) Phylogenetic signal variation in the genomes of Medicago (Fabaceae). Syst Biol 62: 424–438 [DOI] [PubMed] [Google Scholar]