The negative control of SV and FV tonoplast channel activity in quinoa leaves reduces Na+ leak. This improves the efficiency of Na+ sequestration in leaf vacuoles, thus enabling optimal photosynthetic performance and conferring salinity tolerance in this halophyte species.
Abstract
Halophyte species implement a “salt-including” strategy, sequestering significant amounts of Na+ to cell vacuoles. This requires a reduction of passive Na+ leak from the vacuole. In this work, we used quinoa (Chenopodium quinoa) to investigate the ability of halophytes to regulate Na+-permeable slow-activating (SV) and fast-activating (FV) tonoplast channels, linking it with Na+ accumulation in mesophyll cells and salt bladders as well as leaf photosynthetic efficiency under salt stress. Our data indicate that young leaves rely on Na+ exclusion to salt bladders, whereas old ones, possessing far fewer salt bladders, depend almost exclusively on Na+ sequestration to mesophyll vacuoles. Moreover, although old leaves accumulate more Na+, this does not compromise their leaf photochemistry. FV and SV channels are slightly more permeable for K+ than for Na+, and vacuoles in young leaves express less FV current and with a density unchanged in plants subjected to high (400 mm NaCl) salinity. In old leaves, with an intrinsically lower density of the FV current, FV channel density decreases about 2-fold in plants grown under high salinity. In contrast, intrinsic activity of SV channels in vacuoles from young leaves is unchanged under salt stress. In vacuoles of old leaves, however, it is 2- and 7-fold lower in older compared with young leaves in control- and salt-grown plants, respectively. We conclude that the negative control of SV and FV tonoplast channel activity in old leaves reduces Na+ leak, thus enabling efficient sequestration of Na+ to their vacuoles. This enables optimal photosynthetic performance, conferring salinity tolerance in quinoa species.
The increasing problem of global land salinization (Flowers, 2004; Rengasamy, 2006) and its associated multibillion dollar losses in agricultural production require a better understanding of the key physiological mechanisms that confer salinity tolerance in crops. One effective way of gaining such knowledge comes from studying halophytes (Glenn et al., 1999; Flowers and Colmer, 2008; Shabala and Mackay, 2011).
One of the prominent features of halophytes is their ability to efficiently sequester cytosolically toxic Na+ to the cell vacuole. The classic view is that this sequestration is achieved by tonoplast Na+/H+ antiporters (Barkla et al., 1995; Flowers and Colmer, 2008), a process energized by both vacuolar H+ pumps: ATPase (Ayala et al., 1996; Vera-Estrella et al., 1999; Wang et al., 2001) and pyrophosphatase (Parks et al., 2002; Vera-Estrella et al., 2005; Guo et al., 2006; Krebs et al., 2010). However, recent studies have added more complexity to the relationship between Na+/H+ antiporters and vacuolar Na+ sequestration, assigning a role to the transporter in the regulation of K+ and H+ homeostasis (for review, see Rodríguez-Rosales et al., 2009; Jiang et al., 2010; Bassil et al., 2011). Vacuolar Na+/H+ antiporters encoded by NHX genes have been shown to also act as K+/H+ antiporters, with a relatively weak selectivity between Na+ and K+. The Na+/K+ selectivity ratio, in turn, is regulated by vacuolar calmodulin in a pH- and Ca2+-dependent manner (Yamaguchi et al., 2005). Consequently, other transporters, in addition to and different from NHX, are likely to be involved in vacuolar Na+ sequestration. In addition, salt-induced up-regulation of Na+/H+ antiporter expression levels has been observed in leaves but not in roots (Cosentino et al., 2010), suggesting the importance of Na+ exclusion and intracellular sequestration, primarily in photosynthesizing cells. Thus, tissue- and species-specific differences in the respective mechanisms should be considered as well.
Whatever the actual mechanisms are for intracellular Na+ sequestration, efficient Na+ pumping into vacuole is only one side of the coin. To confer salinity tolerance, toxic Na+ ions must be prevented from leaking back into the cytosol. Indeed, given the at least 4- to 5-fold concentration gradient between the vacuole and the cytosol (Shabala and Mackay, 2011) and a zero or slightly negative cytosol-to-vacuole voltage difference across the tonoplast, Na+ leakage from the vacuole is thermodynamically favorable. Thus, to avoid energy-consuming futile Na+ cycling between the cytosol and the vacuole, and to achieve efficient vacuolar sequestration of toxic Na+, passive tonoplast Na+ conductance has to be kept to an absolute minimum. This implies strict and efficient control over Na+-permeable tonoplast channels.
Two major types of Na+-permeable channels are present in the tonoplast, the slow-activating (SV) and fast-activating (FV) vacuolar channels. The SV channel is permeable to both monovalent and divalent cations and is activated by cytosolic Ca2+ and positive vacuolar voltage (Hedrich and Neher, 1987; Ward and Schroeder, 1994; Pottosin et al., 1997, 2001). The FV channel is permeable for monovalent cations only, is activated by large voltages of either sign, and is inhibited by divalent cations from either side of the membrane (Tikhonova et al., 1997; Brüggemann et al., 1999a, 1999b). In Arabidopsis (Arabidopsis thaliana), SV channels are shown to be encoded by a TPC1 (for two-pore channel1) protein (Peiter et al., 2005; Pottosin and Schönknecht, 2007; Hedrich and Marten, 2011). Importantly, recent studies on mammalian two-pore channels have suggested that endolysosomal TPCs are, in fact, Na+-selective channels (Wang et al., 2012). In contrast, the molecular identity of FV channels remains elusive. Both SV and FV channels are ubiquitous and abundant (up to several copies per μm2) in plant tissues, including mesophyll cell vacuoles (Pottosin and Muñiz, 2002; Pottosin and Schönknecht, 2007). SV and FV channel activity is strongly controlled at physiologically attainable conditions (physiological tonoplast voltages and vacuolar and cytosolic divalent and polyvalent cation concentrations). Importantly, even with 0.1% to 1% of the total population of channels open at any one time, impressive monovalent cation currents in the range of tens of pA per vacuole can be conducted. This is equivalent to a current mediated by the whole vacuole population of H+ pumps (Hedrich et al., 1988). Thus, under saline conditions, SV and FV channel activity probably needs to be further reduced.
Early attempts to unravel any dramatic differences between the properties of tonoplast cation channels in salt-tolerant and salt-sensitive plants did not yield a clear outcome. Ivashikina and Hedrich (2005) studied the voltage dependence of the SV channels in vacuoles from Arabidopsis cell culture and found that an increase in luminal Na+/K+ ratio, mimicking the accumulation of Na+ in vacuoles during salt stress, shifted the threshold for SV activation to positive potentials, reducing SV channel open probability under saline conditions. Maathuis and coworkers (1992) found significant SV channel activity in leaf vacuoles isolated from the extreme halophyte Suaeda maritima, even when plants were grown under high (200 mm) NaCl conditions. The estimated activity of the transporter at physiologically relevant cytosolic Ca2+ levels and relatively small transmembrane voltage differences was low. Thus, the authors suggested that, rather than possessing some specific salt-induced control over the SV channel, the transporter’s low activity would mean that even under highly saline conditions, it would consume only about 30% of the H+-ATPase-generated power. Further studies from this laboratory demonstrated that voltage gating, unitary conductance, and Na+/K+ selectivity (PK = PNa) of SV channels from roots of Plantago media (salt sensitive) and Plantago maritima (salt tolerant) were essentially the same (Maathuis and Prins, 1990). However, when both species were grown under saline conditions, the SV channel activity greatly diminished. Yet, based on the original data of this study, it is not possible to decipher whether the SV channel activity in the two species was the same or different under control conditions and whether it was a statistically significant difference between the salt-induced decrease in the open probability of SV channels between P. media and P. maritima. As for FV channels, we are not aware of a single study on their properties/expression in relation to the salt tolerance.
While the total number of halophytic species is relatively small compared with glycophytes, it still amounts to at least several thousand species (Glenn et al., 1999; Flowers et al., 2010). Moreover, halophytes are present in about one-half of higher plant families (Flowers and Colmer, 2008). These species possess a wide range of anatomical and morphological features that may potentially enable their superior performance under saline conditions (Shabala and Mackay, 2011). Nonetheless, the extent to which the above considerations could be extrapolated to all halophytes remains to be assessed. In this work, we used quinoa (Chenopodium quinoa) mesophyll leaf vacuoles to address some of these issues. Quinoa is a facultative halophyte species that originates from the Andean region of South America and was domesticated for human consumption some 3,000 to 4,000 years ago. It can grow under extreme saline conditions with a soil electrical conductivity exceeding 40 dS m−1, approximately 500 mm NaCl (Jacobsen et al., 2003; Razzaghi et al., 2011). Optimal plant growth is usually observed at NaCl concentrations of around 100 mm (Hariadi et al., 2011), but this may be genotype specific (Adolf et al., 2012). Quinoa possesses some degree of leaf succulence as well as epidermal bladder cells (EBC), so it has the potential to employ two different sequestration strategies for cytosolic Na+ exclusion: internal (e.g. vacuolar sequestration) and external (sequestration in EBC). This makes quinoa an excellent model species to investigate the role of vacuolar Na+ sequestration in the overall salinity tolerance in this crop plant as well as to determine the contribution of SV and FV channels in this process. Here, we report a highly significant difference in SV and FV channel activity between old and young leaves of quinoa plants, a difference that is further enhanced under saline conditions. We conclude that the ability of quinoa plants to control ion leak via SV and FV tonoplast channels is essential for conferring salinity tolerance in this species. The possible implications of these findings for crop breeding for salinity tolerance are discussed.
RESULTS
Both Young and Old Leaves Are Capable of Maintaining Optimal Leaf Photochemistry
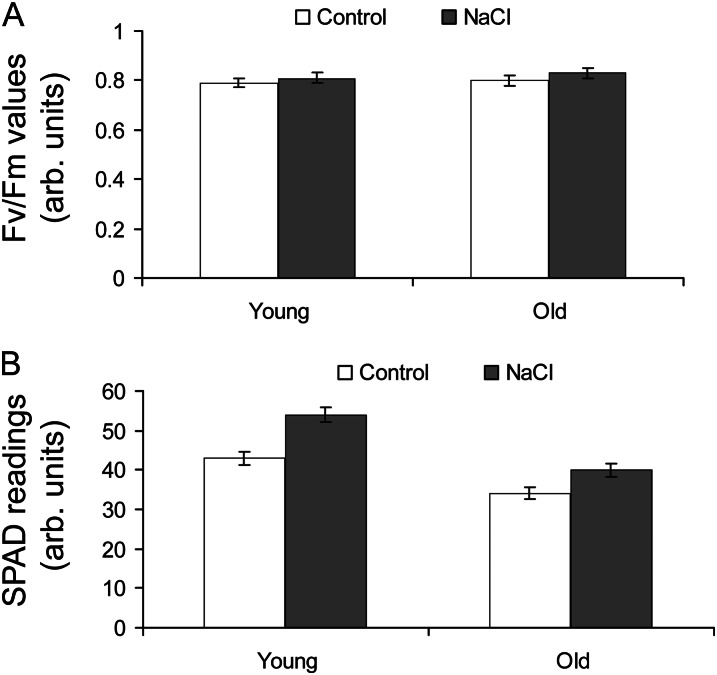
Quinoa can grow in highly saline environments and actually shows optimal grain yield when irrigated with saline water of approximately 100 mm NaCl (Fig. 1, A and B). To a large extent, this ability can be attributed to the presence of EBC on the leaf surface (Fig. 1C). Indeed, being a very large structure (100–200 µm diameter; Fig. 1C) and containing a sizeable central fluid-field vacuole, such cells serve as peripheral Na+ storage organs (Shabala and Mackay, 2011). In some species, over 50% of the accumulated Na+ may be excreted at the leaf surface via bladders, and mutants lacking EBC show much poorer growth (Agarie et al., 2007). However, EBC density is strikingly different between young (Fig. 1C) and old (Fig. 1D) leaves. While in young leaves EBC are numerous and “inflated,” EBC density in old leaves is at least 1 order of magnitude lower, and most of the bladders are ruptured (Fig. 1D; for quantitative data, see Shabala et al., 2012). At the same time, photosynthetic characteristics such as the Fv/Fm (for maximum photochemical efficiency of PSII) chlorophyll fluorescence value (Fig. 2A) and chlorophyll content (Fig. 2B) are not significantly (P < 0.05) different between control and salt-treated plants, regardless of whether measurements are made in old or young leaves. Indeed, Fv/Fm values are all around 0.8, indicating no negative impact of salinity on PSII, regardless of the EBC density. As for chlorophyll content (measured by the SPAD meter), the latter was slightly higher in salt-treated samples (Fig. 2B). The decreased density of EBC in old leaves and the fact that most are ruptured indicate that additional mechanisms to cope with the excessive sodium accumulated must exist in mesophyll cells from older leaves.
Figure 1.
A and B, Quinoa, a facultative C3 halophytic plant capable of producing grain even when irrigated with high (seawater) levels of salinity. C and D, To a large extent, the remarkable salinity tolerance of quinoa plants is attributed to salt bladders (EBC) located at both the adaxial and abaxial leaf surfaces. Salt bladder density, however, is very different between young (C) and old (D) leaves. [See online article for color version of this figure.]
Figure 2.
Photosynthetic characteristics of quinoa leaves grown under optimal (50 mm; control) and saline (400 mm NaCl for 3 weeks) growth conditions. Values are means ± se (n = 8–10). A, Chlorophyll fluorescence Fv/Fm value. B, Chlorophyll content (SPAD readings). arb., Arbitrary.
Young Leaves Rely More on Using EBC for Na+ Storage
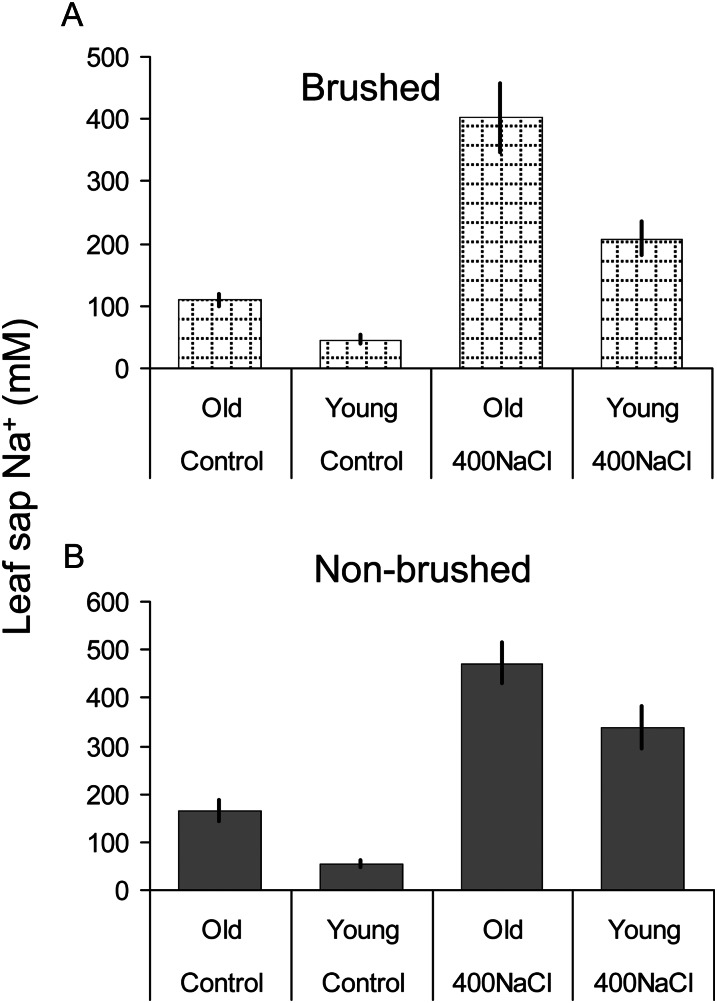
Additional supporting evidence for an essential role of EBC as peripheral Na+ storage structures comes from comparing brushed and nonbrushed leaves (Fig. 3). In old leaves (where bladder density is low and most are ruptured), salinity treatment causes an almost identical (3- to 3.5-fold) increase in Na+ accumulation (from 164 ± 21 to 471 ± 42 mm in nonbrushed leaves, and from 109 ± 10 to 402 ± 56 mm in brushed leaves; the difference between brushed and nonbrushed leaves is not significant at P < 0.05). In contrast, salinity treatment in young leaves results in a 6.2-fold increase in Na+ content in nonbrushed leaves (from 56 ± 7 to 339 ± 43 mm) but only in a 4.4-fold increase (from 47 ± 6 to 208 ± 27 mm) in brushed leaves (Fig. 3). This difference between brushed and nonbrushed leaves is significant at P < 0.05. Thus, it appears that young leaves rely more on EBC for Na+ storage.
Figure 3.
Leaf sap sodium content in brushed (A) and nonbrushed (B) quinoa leaves of a different physiological age (young and old) from control and salt-grown (400 mm NaCl for 3 weeks) plants. Values are means ± se (n = 6).
Old Leaves Accumulate Larger Amounts of Na+ and Are More Efficient in Sequestering It in Vacuoles
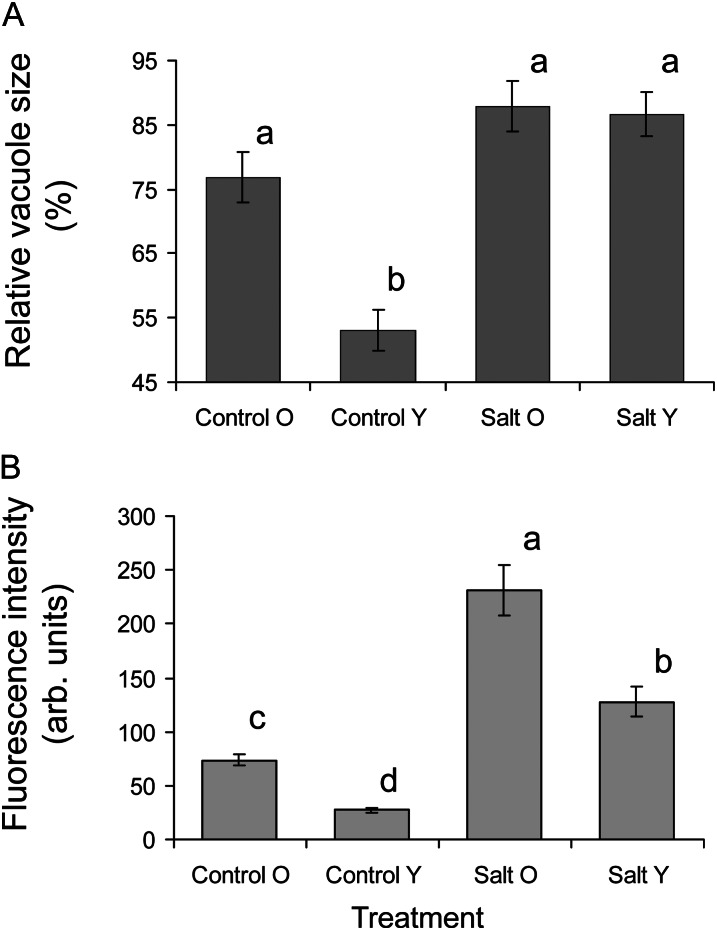
The only reasonable alternative to sequestering Na+ in external structures (e.g. salt bladders) is vacuolar Na+ sequestration. Although no significant (at P < 0.05) difference in cell size or volume was observed between young and old leaves (data not shown), a substantial difference in the extent of cell vacuolation was observed for plants grown under control conditions (Fig. 4A). While in old leaf mesophyll cells, vacuoles contributed 77% ± 4% of the total cell volume, in young leaves, this percentage was much lower (53% ± 3%; significant at P < 0.01). Salt-grown plants increased the extent of their vacuolation to nearly 90% (Fig. 4A), and no significant (P < 0.05) difference between old and young leaves was observed in this case. At the same time, 3 weeks of 400 mm salinity treatment resulted in about a 10-fold increase in the amount of vacuolar Na+ (Fig. 4B), and old leaves accumulated higher amounts of Na+ compared with young leaves (Figs. 4B and 5; all differences significant at P < 0.05).
Figure 4.
A, Relative vacuole volume (percentage of the total cell volume) in old (O) and young (Y) quinoa leaf cells grown under control (50 mm NaCl) and saline (400 mm NaCl) conditions. Values are means ± se (n = 40 cells from five plants). Data with different lowercase letters are significantly different at P < 0.05. B, Vacuolar Na+ content in quinoa mesophyll vacuoles, quantified using corrected total vacuole fluorescence of CoroNa Green, expressed in arbitrary (arb.) units. Values are means ± se (n = 55–97).
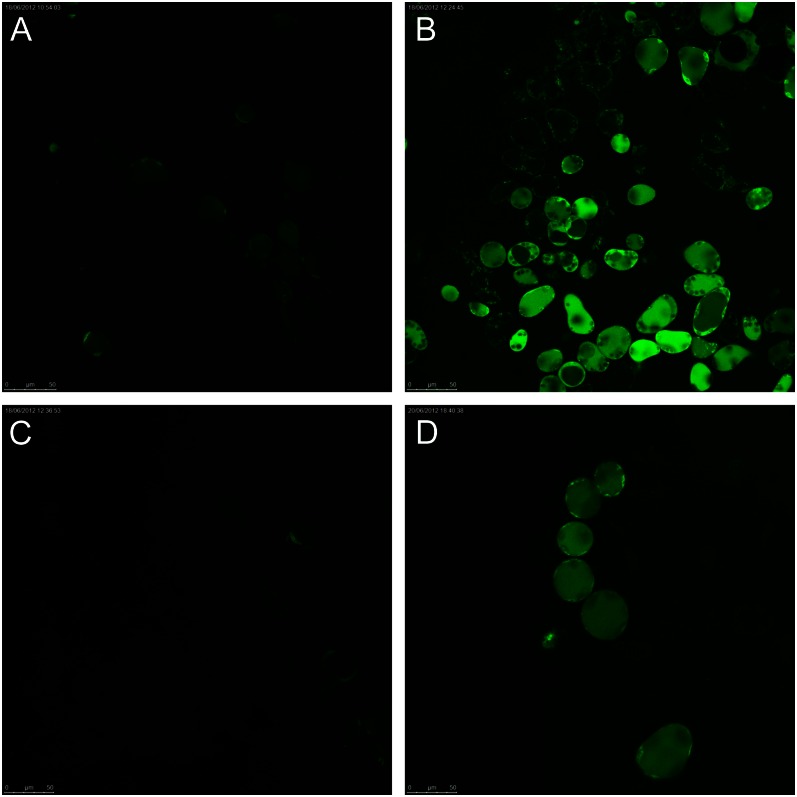
Figure 5.
Sodium sequestration in quinoa leaf vacuoles imaged by CoroNa Green dye. One (of six to 10) representative images is shown for each treatment. A, Old leaf in control plant. B, Old leaf grown at 400 mm NaCl salinity. C, Young leaf in control plant. D, Young leaf at 400 mm NaCl.
Properties of FV Channels in Quinoa Leaf Mesophyll
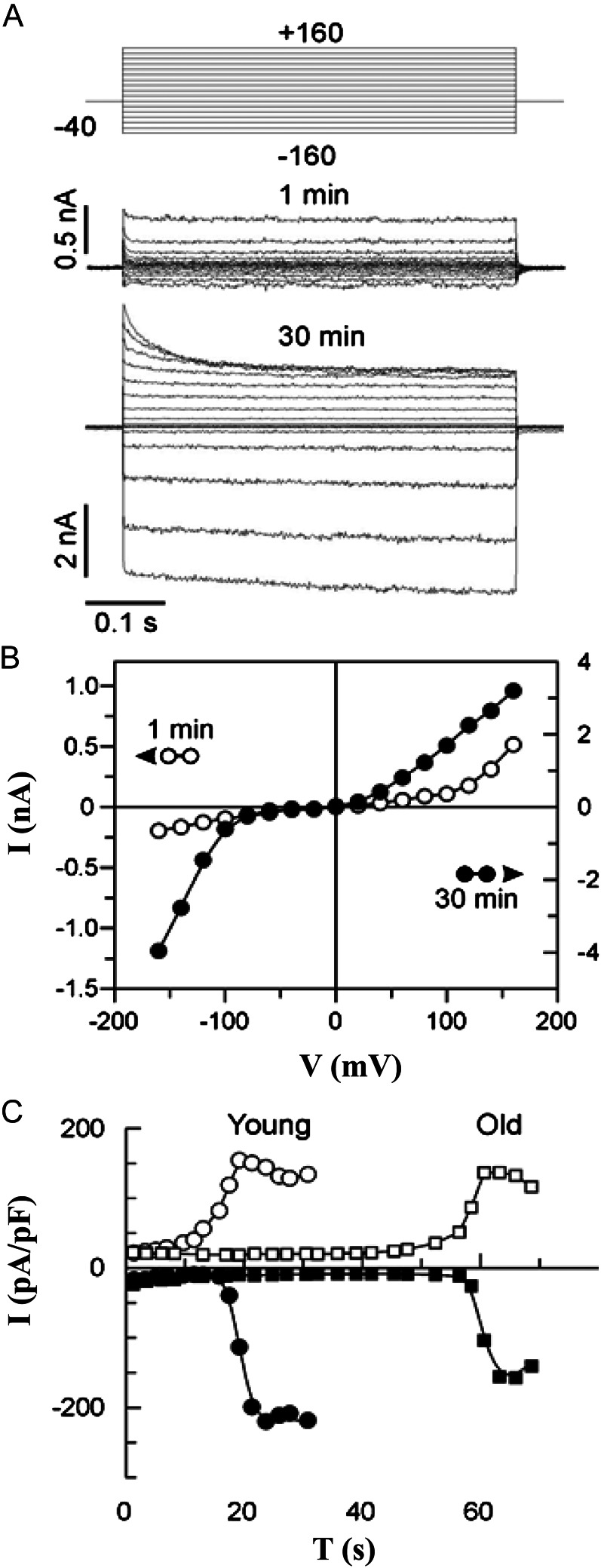
When the whole cell configuration was achieved, a series of voltage steps was applied. This evoked an instantaneous outwardly rectifying current (Fig. 6A, top trace). The current remained stable for some minutes, after which it rapidly grew, reaching a new stable state with a different (bipolar) rectification pattern (Fig. 6A, bottom trace). The respective current/voltage (I/V) relations are presented in Figure 6B. In vacuoles isolated from old leaves, the transition occurred with a larger delay (Fig. 6C). Run-up behavior is a characteristic of the FV current (Tikhonova et al., 1997; Allen et al., 1998). Obviously, some intravacuolar component(s), washed up by perfusion with a patch pipette solution when in the whole-vacuole mode, handicaps the FV conductance at cytosol negative potentials in the intact vacuole.
Figure 6.
The FV current undergoes run-up in the whole-vacuole configuration. A, Typical FV current recordings from a small (approximately 12 pF) vacuole, isolated from a young quinoa leaf, shortly (1 min) and 30 min after the whole-vacuole configuration was obtained. The instantaneous current amplitudes are plotted against voltage in both cases. B, I/V relations for FV current records presented in A. C, Typical time course of run-up transition from the low- to the high-conductance state for the tonoplast FV current in vacuoles from young (same sample as in A) and old quinoa leaves. Steady-state FV currents at +140 (white symbols) and −140 mV (black symbols) are plotted against the time spent in the whole-vacuole configuration. Symmetric 100 mm KCl, standard bath, and pipette solution were used for all FV current recordings (see “Materials and Methods”).
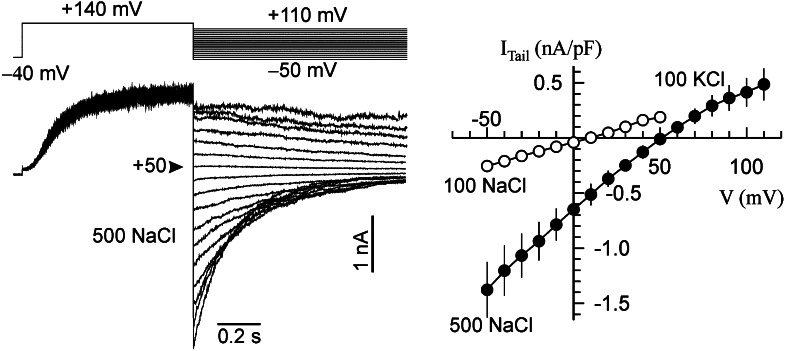
For evaluating the relative permeability of Na+/K+, the 100 mm KCl in the pipette solution was substituted with 500 mm NaCl (to account for the higher osmolality, leaves of salt-grown plants were used for this experiment). The reversal potential of the whole-vacuole FV current under these conditions was about +40 mV (+37 mV [Fig. 7A] and +43 mV [Fig. 7B]). This implies an almost equal permeability for K+ and Na+ (PNa/K = 0.96–1.21). The latter value is more reliable due to the higher specific FV current in young leaves, minimalizing the contribution of unspecific leak current. Consequently, for assays of the FV channel activity, either cation may be used. For simplicity, we assayed the FV voltage-gated conductance at symmetric 100 mm KCl.
Figure 7.
The FV current poorly discriminates between Na+ and K+. I/V relations in a high-conductance stable state, 100 mm KCl in the bath, and either 100 mm KCl or 500 mm NaCl in the pipette (the arrow indicates the reversal potential of the whole-vacuole current for the latter case). A and B, Young (A) and old (B) quinoa leaves. Values are means ± se (n = 5–6 mesophyll vacuoles). C, Plot of conductance versus voltage. Symbols are as in A and B. Measurements were taken 30 min after vacuole perfusion, when currents reached their maximum values.
Saline Conditions Reduce the Activity of the FV Channel More Efficiently in Old Leaves
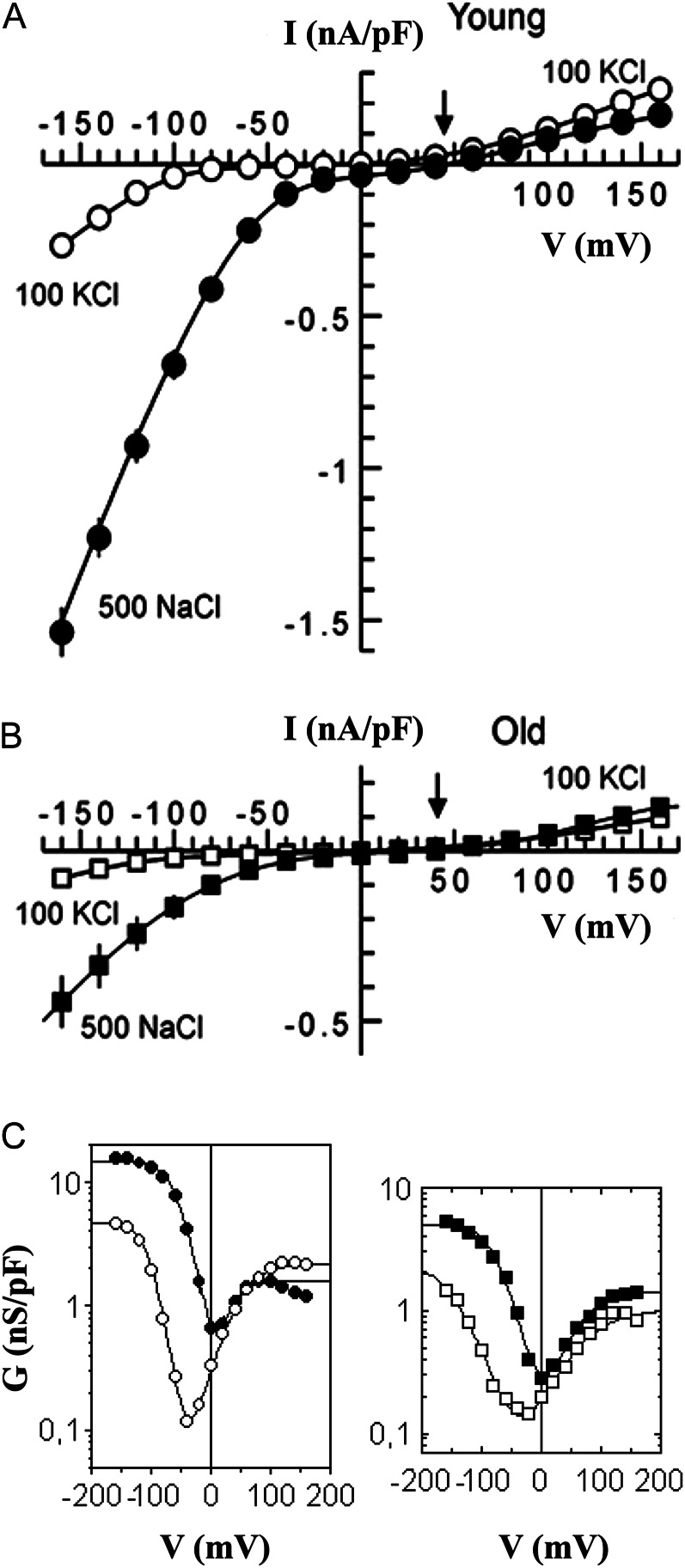
The I/V curve of the FV current is N shaped, representing a high-resistance (low-conductance) region around −40 mV in the final state under symmetrical 100 mm KCl conditions. At higher salt concentrations, the minimum is shifted to the right and the activity at negative potentials increases severalfold (Fig. 7C; for the respective mechanisms, see Pottosin and Martínez-Estévez, 2003). Such salt-induced stimulation of the vacuolar leak via FV requires an even tighter control of the activity.
For presentation of the FV voltage-dependent activity, a semilogarithmic plot of conductance versus voltage is the most suitable, allowing the visualization of conductance values that differ by several orders of magnitude (Pottosin and Martínez-Estévez, 2003). Measurements were taken at two time points: immediately after breaking into the whole-vacuole configuration, and after the FV current has reached its steady state (normally, 30 min later). In the former case, the vacuole luminal content is still “close to natural”; in the latter, all the soluble vacuolar components are washed out and currents reach their maximum values, allowing an accurate estimation of their density under fully controlled ionic and chemical conditions. FV conductance at physiologically relevant (±20-mV range) tonoplast potentials is similar in vacuoles isolated from old and young leaves of plants grown under control conditions (Figs. 8 and 9). In salt-grown plants, the conductance remains unchanged in young leaves at the initial state (Fig. 8A) and slightly decreased in the final state (Fig. 9A); a larger (more than 2-fold) decrease was observed in old leaves (Figs. 8B and 9B). This decrease is fairly constant over the whole voltage span. Thus, the changes in FV conductance in old leaves can be explained simply by a decrease in the number of active FV channels present in the vacuole under saline conditions.
Figure 8.
Saline conditions reduce FV currents in vacuoles from old quinoa leaves. Pipette and bath solutions contained 100 mm KCl. A and B, Whole vacuolar FV conductance was evaluated by taking the first derivative of the whole-vacuole I/V relation, measured immediately after achieving the whole-vacuole configuration. Vacuoles were isolated from either young or old quinoa leaves, grown in the presence of 50 mm NaCl (control) or saline (400 mm NaCl for 3 weeks; salt). Data are means ± se (n = 5–8 vacuoles). Solid lines are best fit to a three-state (open1-closed-open2) model (Pottosin and Martínez-Estévez, 2003). C, Same data as in A and B, but only the points within the physiological tonoplast potential range (±20 mV) are considered. O, Old leaves; Y, young leaves.
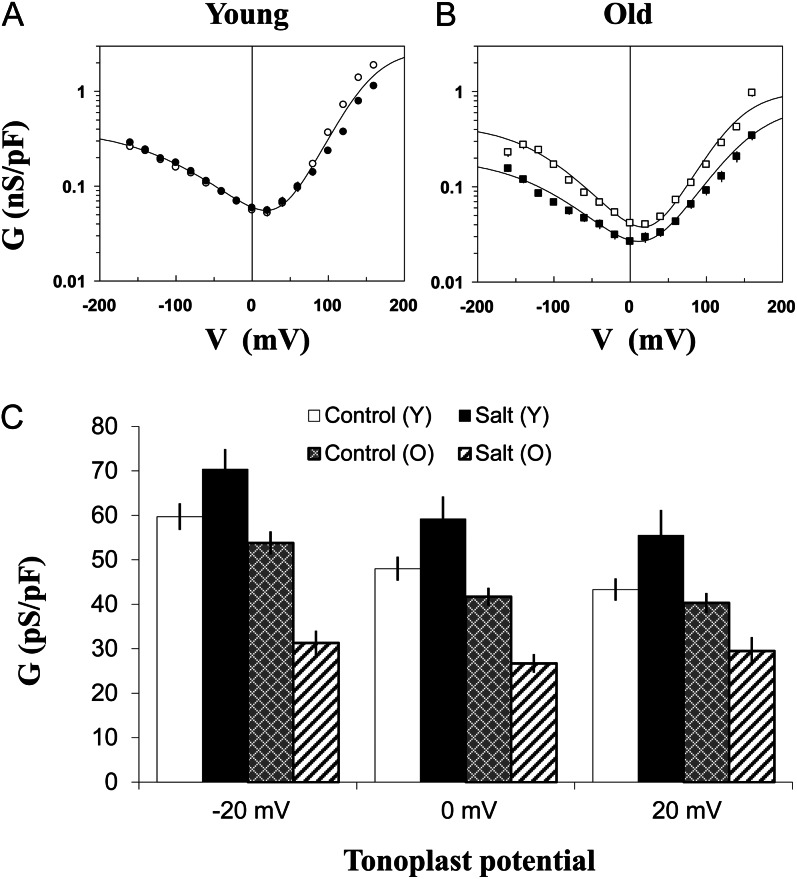
Figure 9.
Effects of salinity and leaf age on the FV channel activity in quinoa mesophyll vacuoles. A and B, Whole vacuolar FV conductance versus voltage, the first derivative of the whole-vacuole I/V relation, measured upon establishment of the high-conductance state. C, Same data as in A and B, but only the points within the physiological tonoplast potential range (±20 mV) are considered. Data with different lowercase letters are significantly different at P < 0.05. O, Old leaves; Y, young leaves. Data are means ± se (n = 3–7 vacuoles for each condition). Other conditions are as in Figure 8.
Most SV Channels Are Closed in Salt-Grown Old But Not Young Leaves
SV channels, which almost indiscriminately conduct monovalent and divalent cations (Pottosin et al., 2001), may be another source of Na+ leak from the vacuole. We tested the Na+/K+ selectivity of SV channels from quinoa mesophyll vacuoles at two biionic conditions: with 100 mm KCl in the cytosol and either 100 or 500 mm NaCl in the vacuole. Typical recordings of SV tail currents are given in Figure 10. The SV current was first activated by a prepulse to a large positive potential (+140 mV in this case), followed by a series of steps to lower potentials, where a partial or complete closure of SV channels (tail current) was observed. The reversal of the tail current was approximately +50 mV at a 100 KCl/500 NaCl gradient. Zero current voltage values can be estimated more accurately by plotting tail currents versus respective potentials, as presented in the summarizing I/V plot on the right side. The mean reversal potentials for 100 KCl/100 NaCl and 100 KCl/500 NaCl conditions were +8 ± 1 mV and +51 ± 2 mV, respectively. Because SV channels are permeable to Ca2+, the presence of relatively high (1 mm) Ca2+ at the cytosolic side could shift the reversal potentials toward more negative values (Ward and Schroeder, 1994; Pottosin et al., 1997). This shift was evaluated by measuring unitary I/V curves for SV channels at variable cytosolic Ca2+ in the background of a 100 KCl/100 NaCl gradient. Reversal potentials at virtually zero (few μm), 1 mm, and 5 mm free Ca2+ were +10, +8, and +1 mV, respectively (Supplemental Fig. S1). Thus, 1 mm Ca2+ in the bath solution introduced an approximately 2-mV shift for the reversal of SV-mediated current. Correction for this shift yielded PNa/K values between 1.6 and 1.8 (i.e. the SV channel from quinoa mesophyll vacuoles is slightly more permeable for Na+ than for K+). Keeping in mind that a high vacuolar Na+ decreases the threshold for SV channel voltage activation (Pérez et al., 2008), an even tighter control on SV channels is required under saline conditions to avoid Na+ leak via these channels.
Figure 10.
Selectivity of the SV current in quinoa mesophyll vacuoles to Na+ and K+. Typical whole-vacuole current recordings are shown. SV currents were activated by a prepulse to +140 mV, followed by a series of test steps to less positive voltages, resulting in a complete or partial closure of SV channels (tail currents). Vacuole (pipette solution) contains 500 mm NaCl, and bath contains 100 mm KCl. Reversal potential (approximately 50 mV) is indicated by the arrowhead. At right is a summary of tail current I/V relationships obtained with a standard 100 mm KCl bath and either 500 mm (n = 8) or 100 mm (n = 4) NaCl in the pipette (vacuolar side).
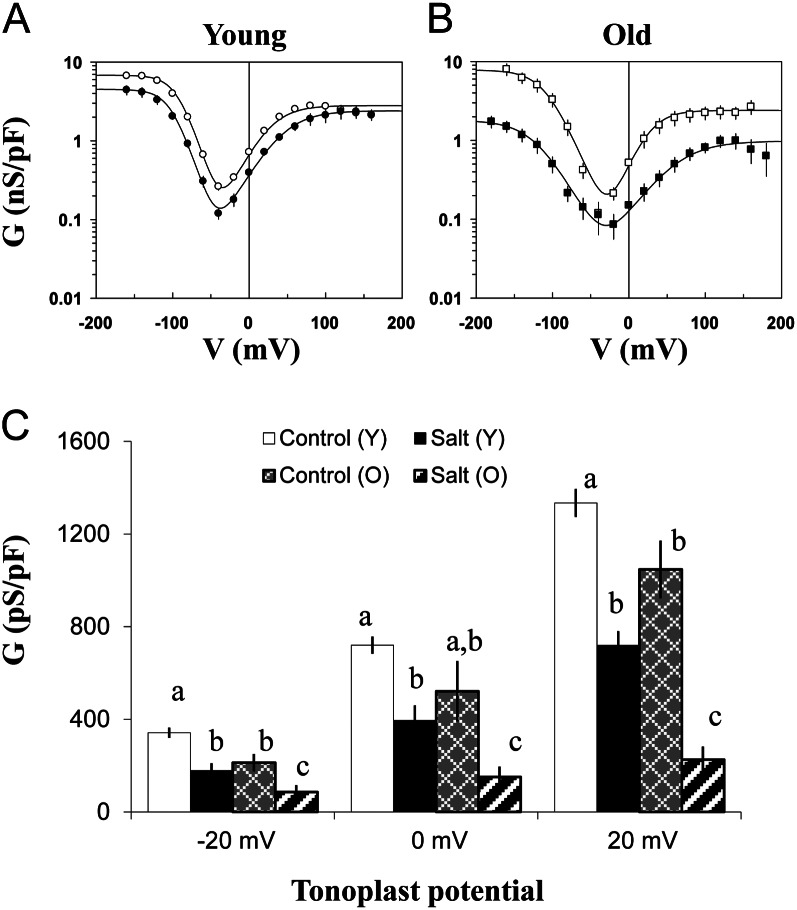
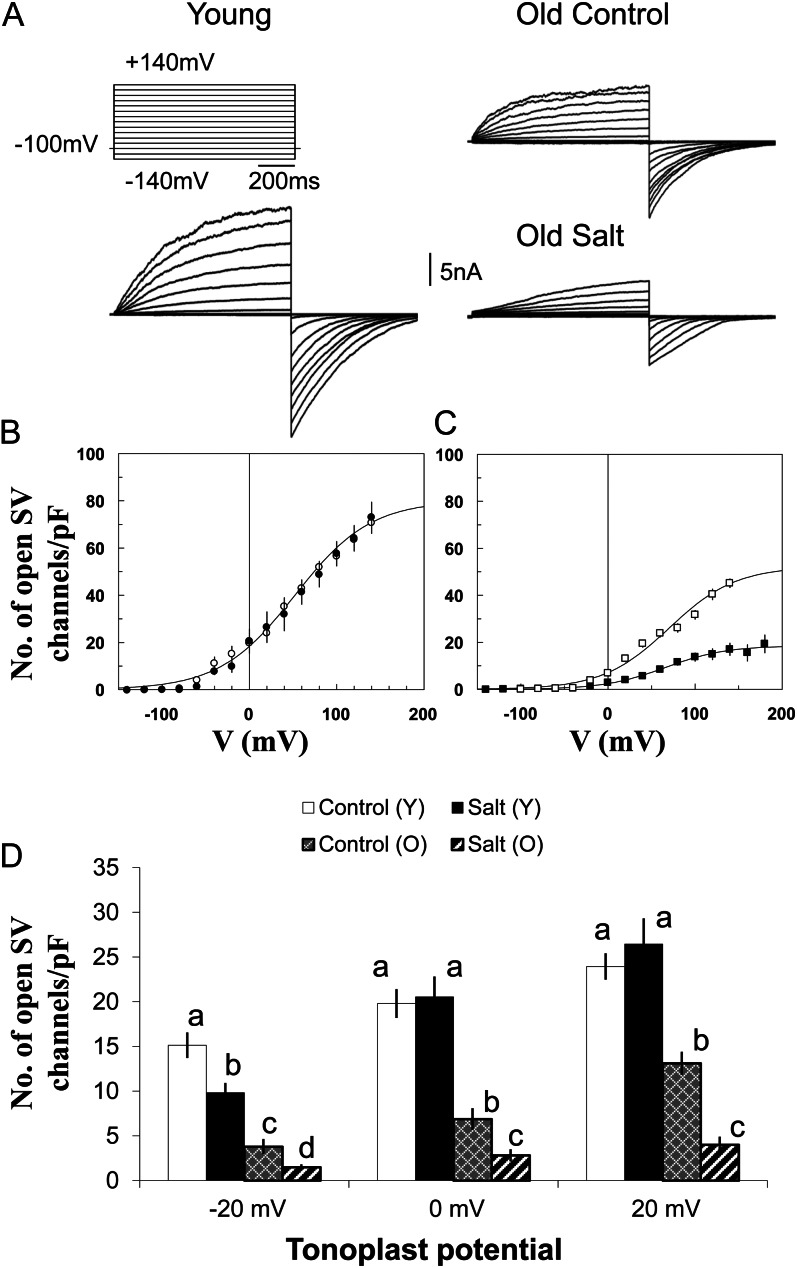
Typical records of whole-vacuole time- and voltage-dependent SV currents from old and young quinoa leaves under control conditions, and old leaves under saline conditions, are presented in Figure 11A. SV currents are substantially smaller in vacuoles from old leaves, especially from plants grown under saline conditions. In addition, the activation kinetics is slower in leaves of salt-grown plants (Fig. 11A). The relative activity of SV channels can be evaluated by taking the magnitude of tail currents at −100 mV as a function of the activation voltage preceding a step to −100 mV. We expressed the activity as the mean number of open SV channels per unit area (1 pF per 100 μm2 of membrane surface). To get the mean number of open SV channels, the whole-vacuole tail current was divided by a single channel current. The latter is illustrated in Supplemental Figure S2, showing a typical staircase closure of several SV channels after return to the holding potential of −100 mV in a small outside-out patch. The respective plots of the SV channel activity as a function of activation voltage are shown in Figure 11, B and C. No change of the SV channel activity was observed in young leaves of plants subjected to saline conditions (Fig. 11B). In old leaves, the maximal number of open SV channels at high positive potentials is approximately two times smaller than in young ones under control conditions, and it is 7-fold smaller in salt-grown plants (Fig. 11, B and C). The voltage dependence itself (midpoint potential, slope/voltage sensitivity) is not significantly different in old and young leaves under control or saline conditions. Rather, the number of active (open) SV channels is changed by the same factor at all tested potentials. Thus, SV channel activity is lower in vacuoles of old leaves compared with young ones, and this suppression is further enhanced under saline conditions, reducing cation (mainly Na+ under these conditions) leak from the vacuole.
Figure 11.
Effects of salinity and leaf age on the SV channel activity in quinoa mesophyll vacuoles. A, Typical whole-vacuole records of time- and voltage-dependent SV currents in vacuoles of young and old leaves from quinoa plants grown under control conditions (50 mm NaCl) and of old leaves from plants grown under saline conditions (400 mm NaCl). For symmetric 100 mm KCl, 1 mm free Ca2+ was added to the cytosolic side to activate SV and inhibit FV currents. B and C, Mean numbers of open SV channels in quinoa tonoplast as a function of membrane voltage. Solid lines are the best fits to the Boltzmann equation. D, Same data as in B and C, but only the points within the physiological tonoplast potential range (±20 mV) are considered. Data with different lowercase letters are significantly different at P < 0.05. O, Old leaves; Y, young leaves. Values are means ± se (n = 4–6 vacuoles).
DISCUSSION
Old and Young Leaves Accumulate Different Amounts of Na+ But Are Both Functionally Efficient under Saline Conditions
Despite halophytes being “salt-loving plants” (as per definition), the sensitivity of key cytosolic enzymes to Na+ appears to be similar for both glycophytes and halophytes (Flowers and Colmer, 2008). Therefore, efficient mechanisms must be in place preventing phytotoxic Na+ from interfering with key metabolic reactions in the cell cytosol and organelles.
Due to both physiological and anatomical reasons, nutrient delivery to young leaves is mediated mostly by phloem transport, while in old leaves the leaf nutrient loading occurs predominantly via the xylem (Shabala and Munns, 2012). In addition, old leaves are exposed to hostile (saline in this case) environments for a much longer period of time. Taken together, these two factors determine a substantial difference in Na+ accumulation between young and old leaves; much higher Na+ content occurs in the latter (Fig. 3). Despite this, both old and young leaves were equally photosynthetically competent, as can be judged by the amount of chlorophyll (SPAD readings; Fig. 2B) and the number of fully functional photosynthetic reaction centers (chlorophyll fluorescence characteristics; Fig. 2A). Thus, both types of leaves must have efficient mechanisms of dealing with the excessive Na+ arriving in the leaf lamina.
Young Leaves Rely on EBC for Na+ Sequestration, While Old Leaves Store Na+ in Mesophyll Cell Vacuoles
EBC are arguably the most remarkable feature of some halophytes. Being modified trichomes (Adams et al., 1998), they are thought to act as storage sites for excess Na+, Cl−, and K+ (Agarie et al., 2007). Other functions postulated for EBC include storage of water and various metabolic compounds (Adams et al., 1992; Agarie et al., 2007; Jou et al., 2007). However, at least 50% of halophytes do not utilize glands or external bladders to modulate their tissue ion concentrations (for review, see Shabala and Mackay, 2011). This poses the question, how do glandless halophytes control Na+ transport and sequestration? The answer to this question (at least in part) comes from this study.
Young quinoa leaves rely heavily on EBC for Na+ sequestration. The difference in Na+ content between old and young brushed leaves was about 2-fold (Fig. 3A), while in nonbrushed leaves, it was only 30% (Fig. 3B). This difference may be attributed to a much higher EBC density in young leaves (Fig. 1, C and D; Shabala et al., 2012). At the same time, old leaves possess only a few (if any) noncollapsed bladders (Fig. 1D), so they cannot rely on external Na+ sequestration. Hence, to survive and to be photosynthetically competent, old leaves must have an alternative: efficient mechanisms for intracellular (vacuolar) Na+ sequestration. This includes not only thermodynamically active Na+ loading into the vacuole but also efficient Na+ retention to avoid futile cycling that would drain the ATP pool required for tonoplast NHX Na+/H+ exchange (Zhang and Blumwald, 2001).
Reduction in the Activity of Tonoplast FV and SV Channels Is More Pronounced in Old Leaves and Is Essential for Retention of Na+ in Cell Vacuoles
Two types of tonoplast channels, FV and SV vacuolar channels, mediate the leak of Na+ back to the cytosol (Brüggemann et al., 1999a; Pottosin et al., 2001, 2003; Pottosin and Muñiz, 2002; Figs. 7 and 10). These channels are oppositely regulated by cytosolic Ca2+: SV channels are activated and FV channels are efficiently inhibited by micromolar free Ca2+ (Hedrich and Neher, 1987; Tikhonova et al., 1997). Thus, at resting Ca2+ conditions, FV channels dominate the tonoplast passive conductance for monovalent cations such as K+, Na+, and NH4+. A peculiar characteristic of the FV current (bipolar gating, minimum of conductance close or slightly below the current reversal) means that this current is ideal for clamping the tonoplast potential. Under normal (nonsaline) conditions, K+ is the dominant monovalent cation species at both sides of the tonoplast, and the variation of the concentration of K+ on either membrane side results in a parallel shift of the reversal (equal to equilibrium potential for K+ in this case) and the position of the minimum (Pottosin and Martínez-Estévez, 2003). Single-channel conductance saturates at 100 mm monovalent cation concentration, so the stimulation effect of vacuolar salt increase on the inward (cytosol-directed) FV current is due to a positive shift of the voltage dependence for the inward FV current (Pottosin and Martínez-Estévez, 2003). The data presented in Figure 7C demonstrate a similar effect of high vacuolar Na+ (i.e. a shift of the FV voltage dependence to the right in both old and young leaves). This is approximately equivalent to the shift of reversal potential (approximately 40 mV) when changing from 100 to 500 mm vacuolar NaCl. Yet, the mean inward FV current in old leaves is smaller than in young leaves. For salt-grown plants, the difference in the FV current between young and old leaves is approximately 3-fold, and minimal activity (lower conductance) is observed as 0 mV under symmetrical ion conditions immediately after breaking into the whole-vacuole configuration (Fig. 8).
It should be commented that while both old and young leaves reach about the same maximum FV current at the end of run-up process (Fig. 6), this process is clearly delayed in vacuoles from old leaves. This suggests that the observed difference is most likely due to differences in regulation rather than channel expression. On the other hand, the initially recorded FV current, which also displays a different I/V pattern (Fig. 6B), has a conductance 1 order of magnitude lower within the physiological range of potentials (Figs. 8C and 9C). Consequently, vacuoles must naturally contain some factor that negatively controls the FV. Its effect on the I/V pattern is reminiscent of the effect of high vacuolar Ca2+ or Mg2+ (Tikhonova et al., 1997; Brüggemann et al., 1999b). In vacuoles, Ca2+ is largely buffered by organic bicarbonic and tricarbonic acids (Meyer and Popp, 1997). Thus, the run-up may reflect a relatively slow exchange of pipette EGTA with components of the intravacuolar Ca2+ buffer, so that an abrupt increase of the FV current possibly reflects a sudden decrease of the free vacuolar Ca2+ when EGTA becomes the dominant buffer. However, luminal Ca2+ levels in quinoa mesophyll cells are about 30 mm, and there are no significant differences between old and young leaves (Supplemental Fig. S3). Whatever the “vacuolar rectifying factor” is (e.g. free Ca2+, Ca2+ bound to some protein associated with the FV channel, or something else), its control on the FV conductance in old leaves appears to be stronger and less easy to remove than in young leaves (Fig. 6C). Interestingly, in salt-grown plants, the initial state is not altered in young leaves, whereas in old leaves, the FV current is reduced (Fig. 8B).
SV channels are controlled by a variety of cytosolic and vacuolar factors (Pottosin and Schönknecht, 2007). One key factor is luminal Ca2+, which shifts the SV voltage dependence, decreasing the SV activity at physiologically attainable transtonoplast potentials (Pottosin et al., 2004). The respective binding site in the TPC (SV) channel, sensitive to luminal Ca2+ changes between 0 and 10 mm, was recently characterized at the molecular level (Dadacz-Narloch et al., 2011). Under physiological conditions (relevant membrane voltage, cytosolic and vacuolar pH, and Ca2+, Mg2+, and other cations), the time-averaged SV channel activity yields from less than one to only a few open SV channels per vacuole. These conduct only a tiny (about 1 pA) Ca2+ current (Pérez et al., 2008). Nonetheless, due to their very high unitary conductance, even a few open SV channels can pass a monovalent cation current of several tens of pA, comparable to that generated by the whole vacuolar population of H+ pumps (Hedrich et al., 1988; see also below). SV channel density in young leaves is higher than in old leaves, especially under control conditions. This may be related to more intense vacuole-based Ca2+ signaling and/or a higher activity of H+ pumps, which require a substantially higher shunt conductance (Pottosin et al., 2003). On the other hand, under salt stress, a vacuolar Na+ increase (at physiologically relevant luminal Ca2+) causes a decrease of the threshold for SV channel activation (Pérez et al., 2008). Consequently, the overall pump activity may be short circuited, and building a high intravacuolar Na+ concentration would be virtually impossible. Therefore, an even tighter control of the SV channel activity is required under saline conditions. It appears that this problem is solved in old leaves simply by a decrease in the SV channel activity (mean number of simultaneously open channels at every tested potential) under saline conditions rather than by any alteration of their voltage dependence (Fig. 11C).
Relative Contributions of the SV- and FV-Mediated Na+ Influx under Physiological Conditions
An interesting question arising from this study is, what are the relative contributions of the SV- and FV-mediated Na+ influx under physiological conditions? Answering this question is not a trivial task; tonoplast channel activity can be controlled by myriad (only partly characterized) cytosolic components and other factors, most of which are not present under patch-clamp conditions. However, some plausible assumptions can be made. At physiological tonoplast potential differences (e.g. within a −20- to +20-mV range) and physiologically attainable free vacuolar and cytosolic Ca2+ and Mg2+ concentrations, the average monovalent cation current via FV and SV channels is about 1 pA per pF (Pottosin and Muñiz, 2002). This gives a Na+ current in the range of tens of pA per vacuole. In vacuole-attached patches at zero command voltage (i.e. at resting potential) and cytosolic Ca2+ of 0.1 to 20 μm, the SV current varies between 10 and 320 pA per 50-μm-diameter beet (Beta vulgaris) vacuole (Pérez et al., 2008). Thus, it appears that SV and FV currents make nearly equal contributions toward Na+ flux across the tonoplast, and the total channel-mediated current is fairly comparable to the current generated by the whole vacuolar population of H+ pumps (Hedrich et al., 1988). However, as commented above, tonoplast channel activity is under the control of numerous second messengers and, as such, may be significantly up- or down-regulated in planta (Brüggemann et al., 1998; Carpaneto et al., 1999; Dobrovinskaya et al., 1999; Hedrich and Marten, 2011; Gutla et al., 2012). This may change the balance between the relative contribution of SV and FV channels toward tonoplast Na+ fluxes under natural conditions. This issue warrants a separate investigation. Summarizing, old quinoa leaves rely mainly on Na+ sequestration in the vacuole; this requires a reduction of FV- and SV-mediated currents, sources of vacuolar Na+ leak. Consequently, the density of tonoplast FV and SV channels is decreased in old leaves, especially under saline conditions.
CONCLUSION
Salinity stress tolerance is a multifaceted trait, with numerous tissue- and age-specific components involved (Flowers, 2004; Shabala and Cuin, 2008). Because of this, a thorough and orchestrated pyramiding of complementary physiological traits is the only realistic way to create a “supertolerant” genotype. Indeed, despite numerous reports about a beneficial role of overexpressing NHX vacuolar antiporters for plant adaptive responses to salinity (Xia et al., 2002; Pardo et al., 2006; Hanana et al., 2009), crop breeding for salt tolerance has not met with much success (Yamaguchi and Blumwald, 2005). One possible explanation may be that a higher NHX Na+ sequestration ability is not matched by an efficient retention of Na+ in the cell vacuole. This work suggests that halophytes such as quinoa are very efficient in achieving the latter. Further electrophysiological and biochemical studies on halophytes could reveal the specific ways by which stress-inducible and constitutive suppression of the activity of vacuolar FV and SV channels is achieved. This knowledge can then be taken on board by molecular biologists and plant breeders to complement overexpressing NHX lines, thus achieving more efficient vacuolar sequestration and, ultimately, salinity tolerance in crop species.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Quinoa (Chenopodium quinoa variety 20) seeds were obtained from Prof. Sven-Eric Jacobsen (University of Copenhagen) and multiplied at the University of Tasmania. Plants were grown in 2-L plastic pots using a standard potting mix essentially as described in our previous publications (Hariadi et al., 2011). When plants were 3 weeks old, salinity stress was given by using 400 mm NaCl for irrigation. Control plants were irrigated with 50 mm NaCl (a concentration found to be stimulating for quinoa growth). Plants were grown under ambient light in a temperature-controlled glasshouse (between 19°C and 26°C and average humidity of approximately 65%) at the University of Tasmania between September 2011 and May 2012. Salinity treatment lasted 3 to 4 weeks. At that stage, leaves of two different positions (and, hence, of different physiological age) were collected: (1) young leaves (third or fourth youngest leaf; fully unfolded and actively growing) and (2) old leaves (fully expanded matured, but not senescing, leaf; positioned about two-thirds down the stem). Excised leaves were put into sealed plastic bags and brought to the laboratory for protoplast isolation.
Protoplast Isolation
The protocol for mesophyll protoplast preparation was modified from Zepeda-Jazo et al. (2011). Leaves were stripped from the abaxial epidermis and cut into sections, 3 mm wide and 15 mm long, avoiding major veins. Peel segments were placed in an Eppendorf tube containing 1 to 1.2 mL of enzyme solution: 2% (w/v) cellulose (Yakult Honsha), 1.2% (w/v) cellulysin (Biosciences), 0.1% (w/v) pectolyase, 0.1% (w/v) bovine serum albumin, 10 mm KCl, 10 mm CaCl2, and 2 mm MgCl2, pH 5.7, adjusted with 2 mm MES, and osmolality was adjusted with sorbitol to 750 to 800 and 1,700 to 1,800 mOsm for control and treatment, respectively. After 45 min of incubation in the enzyme solution (in the dark at 25°C; agitated on a 90-rpm rotary shaker), leaf segments were quickly but thoroughly rinsed in the wash solution (as above, minus enzymes) for several seconds. Mesophyll protoplasts were released by plasmolysis. Rinsed segments were placed into an Eppendorf tube containing 1 mL of release solution: 10 mm KCl, 2 mm CaCl2, 2 mm MgCl2, and 2 mm MES (pH 5.7, adjusted with KOH), and osmolality was adjusted with sorbitol to 350 to 380 mOsm for control leaves and 1,250 to 1,300 mOsm for saline-grown leaves. Tubes were centrifuged at 720g for 5 min. The supernatant was discarded, and the remaining pellet was redissolved in 1 mL of storage solution: 100 mm KCl, 0.5 mm EDTA, 10 mm HEPES (pH 7.4, adjusted with KOH), 10 mm Glc, and 10 mm Suc, adjusted with sorbitol to 500 to 570 mOsm for control leaves and 1,450 to 1,500 mOsm for saline-grown leaves. The latter step was repeated one more time, and isolated protoplasts were kept on ice during the day.
Patch-Clamp Electrophysiology
Single vacuoles were mechanically isolated from mesophyll protoplasts as described by Tikhonova et al. (1997). Current measurements were performed using an Axopatch 200B integrating patch-clamp amplifier (Axon Instruments). Records were low-pass filtered at 10 kHz, digitized using a DigiData 1320A interface (Axon Instruments), transferred to a personal computer using the pClamp 8.0 software package (Axon Instruments), and analyzed using GraFit version 6 (Erithacus Software). Currents were recorded in whole-vacuole and outside-out modes (Hamill et al., 1981). The current convention for vacuolar currents was according to Bertl et al. (1992). Pipette solution contained (in mm): 100 KCl, 5 EGTA, and 10 MES-KOH (pH 6). For selectivity measurements, 100 mm KCl was substituted with 100 or 500 mm NaCl. Bath solution for FV currents contained (in mm): 100 KCl, 0.5 EDTA, and 10 HEPES-KOH (pH 7.4). To inhibit FV and activate SV currents, 0.5 mm EDTA was substituted with 1 mm CaCl2. The osmolality of bath and pipette solutions was adjusted to 450 to 500 mOsm (control condition) or 1,450 to 1,500 mOsm (for saline-grown leaves) using sorbitol. Specific voltage protocols are given directly in the figures.
Tissue Nutrient Analysis
Leaves at the appropriate positions (from both control and salt-grown plants) were excised, placed in 2-mL Eppendorf tubes, and frozen immediately. From one-half of these leaves, salt bladders were mechanically removed by gentle but thorough brushing with a soft paintbrush. These samples are referred to as “brushed,” and intact leaf samples are referred to as “nonbrushed.”
Leaf Na+ content was determined essentially as described by Cuin et al. (2009). In brief, leaf samples were thawed and hand squeezed to extract all the sap, then measured for their Na+ concentration (in mm) using flame photometry (Corning 410C).
Leaf Photosynthetic Characteristics
The Fv/Fm was estimated by measuring the chlorophyll fluorescence-Fv/Fm ratio of old and young leaves using an OS-30p chlorophyll fluorometer (Opti-Sciences) as described elsewhere (Cuin et al., 2010). Leaf chlorophyll content was measured on the same leaf with a SPAD chlorophyll meter (SPAD-502; Minolta).
Vacuolar Na+ Measurements
The green fluorescent Na+ dye CoroNa Green acetoxymethyl ester (Invitrogen) was employed to measure vacuolar Na+ content in quinoa mesophyll cells. The dye has absorption and fluorescence emission maxima of approximately 492 and 516 nm, respectively. The dye was reconstituted as a stock with anhydrous dimethyl sulfoxide (Sigma) before use. The CoroNa Green indicator stock was added to 5 mL of measuring buffer (10 mm KCl, 5 mm Ca2+-MES, pH 6.1) and diluted to a final concentration of 15 μm. Quinoa plants were grown in a glasshouse as described above. Young and old leaves were collected. The abaxial epidermis was peeled off with a pair of fine forceps, and peeled leaf segments were incubated in the buffer solution containing CoroNa Green in the dark for 1 h before measurement.
Confocal imaging was conducted on a Leica inverted microscope fitted with a TCS SPII confocal head (Leica Microsystems) using 10× numerical aperture 0.35 dry and 40× Plan Apochromatic CS 0.75 oil objectives (Leica Microsystems). We used the 488-nm excitation line of an argon multiline laser and the triple dichroic TD 488/543/633-nm beam splitter. CoroNa Green fluorescence emission was detected in the photomultiplier at 505 to 525 nm. Chloroplast fluorescence was detected at 680 to 700 nm in order to separate the autofluorescence of chlorophyll in chloroplasts from the CoroNa Green fluorescence. Images were analyzed with LAS AF software (Leica Microsystems), and ImageJ software (National Institutes of Health) was used to calculate the corrected total vacuole fluorescence (Burgess et al., 2010). In the latter case, the vacuolar signal was first measured as the sum of the intensity of the pixels measured from the representative area of the standard size within the vacuole. The background signal was measured from the empty region of the similar size and subtracted from the vacuolar signal to obtain corrected total vacuole fluorescence values.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Ca2+ shifts the reversal potential for the unitary SV current.
Supplemental Figure S2. Resolving single SV channel currents in a small excised patch.
Supplemental Figure S3. Vacuolar Ca2+ content in old and young quinoa leaf cells grown under control and saline conditions.
Glossary
- SV
slow-activating
- FV
fast-activating
- EBC
epidermal bladder cells
- Fv/Fm
maximum photochemical efficiency of PSII
- I/V
current/voltage
References
- Adams P, Nelson D, Yamada S, Chmara W, Jensen RG, Bohnert HJ, Griffiths H. (1998) Growth and development of Mesembryanthemum crystallinum (Aizoaceae). New Phytol 138: 171–190 [DOI] [PubMed] [Google Scholar]
- Adams P, Thomas JC, Vernon DM, Bohnert HJ, Jensen RG. (1992) Distinct cellular and organismic responses to salt stress. Plant Cell Physiol 33: 1215–1223 [Google Scholar]
- Adolf VI, Shabala S, Andersen MN, Razzaghi F, Jacobsen S-E. (2012) Varietal differences of quinoa’s tolerance to saline conditions. Plant Soil 357: 117–129 [Google Scholar]
- Agarie S, Shimoda T, Shimizu Y, Baumann K, Sunagawa H, Kondo A, Ueno O, Nakahara T, Nose A, Cushman JC. (2007) Salt tolerance, salt accumulation, and ionic homeostasis in an epidermal bladder-cell-less mutant of the common ice plant Mesembryanthemum crystallinum. J Exp Bot 58: 1957–1967 [DOI] [PubMed] [Google Scholar]
- Allen GJ, Amtmann A, Sanders D. (1998) Calcium-dependent and calcium independent K+ mobilization channels in Vicia faba guard cell vacuoles. J Exp Bot 49: 305–318 [Google Scholar]
- Ayala F, O’Leary JW, Schumaker KS. (1996) Increased vacuolar and plasma membrane H+-ATPase activities in Salicornia bigelovii Torr in response to NaCl. J Exp Bot 47: 25–32 [DOI] [PubMed] [Google Scholar]
- Barkla BJ, Zingarelli L, Blumwald E, Smith JAC. (1995) Tonoplast Na+/H+ antiport activity and its energization by the vacuolar H+-ATPase in the halophytic plant Mesembryanthemum crystallinum L. Plant Physiol 109: 549–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil E, Tajima H, Liang YC, Ohto MA, Ushijima K, Nakano R, Esumi T, Coku A, Belmonte M, Blumwald E. (2011) The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell 23: 3482–3497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertl A, Blumwald E, Coronado R, Eisenberg R, Findlay G, Gradmann D, Hille B, Köhler K, Kolb HA, MacRobbie E, et al. (1992) Electrical measurements on endomembranes. Science 258: 873–874 [DOI] [PubMed] [Google Scholar]
- Brüggemann L, Pottosin I, Schönknecht G. (1998) Cytoplasmic polyamines block the fast activating vacuolar cation channel. Plant J 16: 101–105 [Google Scholar]
- Brüggemann LI, Pottosin II, Schönknecht G. (1999a) Selectivity of the fast activating vacuolar cation channel. J Exp Bot 50: 873–876 [Google Scholar]
- Brüggemann LI, Pottosin II, Schönknecht G. (1999b) Cytoplasmic magnesium regulates the fast activating vacuolar cation channel. J Exp Bot 50: 1547–1552 [Google Scholar]
- Burgess A, Vigneron S, Brioudes E, Labbé J-C, Lorca T, Castro A. (2010) Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc Natl Acad Sci USA 107: 12564–12569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpaneto A, Cantù AM, Gambale F. (1999) Redox agents regulate ion channel activity in vacuoles from higher plant cells. FEBS Lett 442: 129–132 [DOI] [PubMed] [Google Scholar]
- Cosentino C, Fischer-Schliebs E, Bertl A, Thiel G, Homann U. (2010) Na+/H+ antiporters are differentially regulated in response to NaCl stress in leaves and roots of Mesembryanthemum crystallinum. New Phytol 186: 669–680 [DOI] [PubMed] [Google Scholar]
- Cuin TA, Parsons D, Shabala S. (2010) Wheat cultivars can be screened for NaCl salinity tolerance by measuring leaf chlorophyll content and shoot sap potassium. Funct Plant Biol 37: 656–664 [Google Scholar]
- Cuin TA, Tian Y, Betts SA, Chalmandrier R, Shabala S. (2009) Ionic relations and osmotic adjustment in durum and bread wheat under saline conditions. Funct Plant Biol 36: 1110–1119 [DOI] [PubMed] [Google Scholar]
- Dadacz-Narloch B, Beyhl D, Larisch C, López-Sanjurjo EJ, Reski R, Kuchitsu K, Müller TD, Becker D, Schönknecht G, Hedrich R. (2011) A novel calcium binding site in the slow vacuolar cation channel TPC1 senses luminal calcium levels. Plant Cell 23: 2696–2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrovinskaya OR, Muñiz J, Pottosin II. (1999) Inhibition of vacuolar ion channels by polyamines. J Membr Biol 167: 127–140 [DOI] [PubMed] [Google Scholar]
- Flowers TJ. (2004) Improving crop salt tolerance. J Exp Bot 55: 307–319 [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Colmer TD. (2008) Salinity tolerance in halophytes. New Phytol 179: 945–963 [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Galal HK, Bromham L. (2010) Evolution of halophytes: multiple origins of salt tolerance in land plants. Funct Plant Biol 37: 604–612 [Google Scholar]
- Glenn EP, Brown JJ, Blumwald E. (1999) Salt tolerance and crop potential of halophytes. Crit Rev Plant Sci 18: 227–255 [Google Scholar]
- Guo SL, Yin HB, Zhang X, Zhao FY, Li PH, Chen SH, Zhao YX, Zhang H. (2006) Molecular cloning and characterization of a vacuolar H+-pyrophosphatase gene, SsVP, from the halophyte Suaeda salsa and its overexpression increases salt and drought tolerance of Arabidopsis. Plant Mol Biol 60: 41–50 [DOI] [PubMed] [Google Scholar]
- Gutla PVK, Boccaccio A, De Angeli A, Gambale F, Carpaneto A. (2012) Modulation of plant TPC channels by polyunsaturated fatty acids. J Exp Bot 63: 6187–6197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391: 85–100 [DOI] [PubMed] [Google Scholar]
- Hanana M, Cagnac O, Zarrouk M, Blumwald E. (2009) Biological roles of NHX vacuolar antiport: achievements and prospects of plant breeding. Botany 87: 1023–1035 [Google Scholar]
- Hariadi Y, Marandon K, Tian Y, Jacobsen SE, Shabala S. (2011) Ionic and osmotic relations in quinoa (Chenopodium quinoa Willd.) plants grown at various salinity levels. J Exp Bot 62: 185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R, Barbier-Brygoo H, Felle H, Fluegge UI, Luettge U, Maathuis FJM, Marx S, Prins HBA, Raschke K, Schnabl H, et al. (1988) General mechanisms for solute transport across the tonoplast of plant vacuoles: a patch-clamp survey of ion channels and proton pumps. Bot Acta 101: 7–13 [Google Scholar]
- Hedrich R, Marten I. (2011) TPC1-SV channels gain shape. Mol Plant 4: 428–441 [DOI] [PubMed] [Google Scholar]
- Hedrich R, Neher E. (1987) Cytoplasmic calcium regulates voltage-dependent ion channels in plant vacuoles. Nature 329: 833–836 [Google Scholar]
- Ivashikina N, Hedrich R. (2005) K+ currents through SV-type vacuolar channels are sensitive to elevated luminal sodium levels. Plant J 41: 606–614 [DOI] [PubMed] [Google Scholar]
- Jacobsen SE, Mujica A, Jensen CR. (2003) The resistance of quinoa (Chenopodium quinoa Willd.) to adverse abiotic factors. Food Rev Int 19: 99–109 [Google Scholar]
- Jiang X, Leidi EO, Pardo JM. (2010) How do vacuolar NHX exchangers function in plant salt tolerance? Plant Signal Behav 5: 792–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou Y, Wang YL, Yen HCE. (2007) Vacuolar acidity, protein profile, and crystal composition of epidermal bladder cells of the halophyte Mesembryanthemum crystallinum. Funct Plant Biol 34: 353–359 [DOI] [PubMed] [Google Scholar]
- Krebs M, Beyhl D, Görlich E, Al-Raschied KA, Stierhof YD, Hedrich R, Schumacher K. (2010) Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation. Proc Natl Acad Sci USA 102: 3251–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis FJM, Flowers TJ, Yeo AR. (1992) Sodium chloride compartmentation in leaf vacuoles of the halophyte Suaeda maritima (L) Dum and its relation to tonoplast permeability. J Exp Bot 43: 1219–1223 [Google Scholar]
- Maathuis FJM, Prins HBA. (1990) Patch clamp studies on root cell vacuoles of a salt-tolerant and a salt-sensitive Plantago species: regulation of channel activity by salt stress. Plant Physiol 92: 23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AJ, Popp M. (1997) Free Ca2+ in tissue saps of calciotrophic CAM plants as determined with Ca2+-selective electrodes. J Exp Bot 48: 337–344 [Google Scholar]
- Pardo JM, Cubero B, Leidi EO, Quintero FJ. (2006) Alkali cation exchangers: roles in cellular homeostasis and stress tolerance. J Exp Bot 57: 1181–1199 [DOI] [PubMed] [Google Scholar]
- Parks GE, Dietrich MA, Schumaker KS. (2002) Increased vacuolar Na+/H+ exchange activity in Salicornia bigelovii Torr. in response to NaCl. J Exp Bot 53: 1055–1065 [DOI] [PubMed] [Google Scholar]
- Peiter E, Maathuis FJM, Mills LN, Knight H, Pelloux J, Hetherington AM, Sanders D. (2005) The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature 434: 404–408 [DOI] [PubMed] [Google Scholar]
- Pérez V, Wherrett T, Shabala S, Muñiz J, Dobrovinskaya O, Pottosin I. (2008) Homeostatic control of slow vacuolar channels by luminal cations and evaluation of the channel-mediated tonoplast Ca2+ fluxes in situ. J Exp Bot 59: 3845–3855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottosin II, Dobrovinskaya OR, Muñiz J. (2001) Conduction of monovalent and divalent cations in the slow vacuolar channel. J Membr Biol 181: 55–65 [DOI] [PubMed] [Google Scholar]
- Pottosin II, Martínez-Estévez M. (2003) Regulation of the fast vacuolar channel by cytosolic and vacuolar potassium. Biophys J 84: 977–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottosin II, Martínez-Estévez M, Dobrovinskaya OR, Muñiz J. (2003) Potassium-selective channel in the red beet vacuolar membrane. J Exp Bot 54: 663–667 [DOI] [PubMed] [Google Scholar]
- Pottosin II, Martínez-Estévez M, Dobrovinskaya OR, Muñiz J, Schönknecht G. (2004) Mechanism of luminal Ca2+ and Mg2+ action on the vacuolar slowly activating channels. Planta 219: 1057–1070 [DOI] [PubMed] [Google Scholar]
- Pottosin II, Muñiz J. (2002) Higher plant vacuolar ionic transport in the cellular context. Acta Bot Mex 60: 37–77 [Google Scholar]
- Pottosin II, Schönknecht G. (2007) Vacuolar calcium channels. J Exp Bot 58: 1559–1569 [DOI] [PubMed] [Google Scholar]
- Pottosin II, Tikhonova LI, Hedrich R, Schönknecht G. (1997) Slowly activating vacuolar channels can not mediate Ca2+-induced Ca2+ release. Plant J 12: 1387–1398 [Google Scholar]
- Razzaghi F, Ahmadi SH, Adolf VI, Jensen CR, Jacobsen S-E, Andersen MN. (2011) Water relations and transpiration of quinoa (Chenopodium quinoa Willd.) under salinity and soil drying. J Agron Crop Sci 197: 348–360 [Google Scholar]
- Rengasamy P. (2006) World salinization with emphasis on Australia. J Exp Bot 57: 1017–1023 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Rosales MP, Gálvez FJ, Huertas R, Aranda MN, Baghour M, Cagnac O, Venema K. (2009) Plant NHX cation/proton antiporters. Plant Signal Behav 4: 265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala L, Mackay A, Tian Y, Jacobsen S-E, Zhou D, Shabala S. (2012) Oxidative stress protection and stomatal patterning as components of salinity tolerance mechanism in quinoa (Chenopodium quinoa). Physiol Plant 146: 26–38 [DOI] [PubMed] [Google Scholar]
- Shabala S, Cuin TA. (2008) Potassium transport and plant salt tolerance. Physiol Plant 133: 651–669 [DOI] [PubMed] [Google Scholar]
- Shabala S, Mackay A. (2011) Ion transport in halophytes. Adv Bot Res 57: 151–187 [Google Scholar]
- Shabala S, Munns R (2012) Salinity stress: physiological constraints and adaptive mechanisms. In S Shabala, ed, Plant Stress Physiology. CAB International, Oxford, pp 59–93 [Google Scholar]
- Tikhonova LI, Pottosin II, Dietz KJ, Schönknecht G. (1997) Fast-activating cation channel in barley mesophyll vacuoles: inhibition by calcium. Plant J 11: 1059–1070 [Google Scholar]
- Vera-Estrella R, Barkla BJ, Bohnert HJ, Pantoja O. (1999) Salt stress in Mesembryanthemum crystallinum L. cell suspensions activates adaptive mechanisms similar to those observed in the whole plant. Planta 207: 426–435 [DOI] [PubMed] [Google Scholar]
- Vera-Estrella R, Barkla BJ, García-Ramírez L, Pantoja O. (2005) Salt stress in Thellungiella halophila activates Na+ transport mechanisms required for salinity tolerance. Plant Physiol 139: 1507–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BS, Lüttge U, Ratajczak R. (2001) Effects of salt treatment and osmotic stress on V-ATPase and V-PPase in leaves of the halophyte Suaeda salsa. J Exp Bot 52: 2355–2365 [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang XL, Dong XP, Samie M, Li XR, Cheng XP, Goschka A, Shen DB, Zhou YD, Harlow J, et al. (2012) TPC proteins are phosphoinositide-activated sodium-selective ion channels in endosomes and lysosomes. Cell 151: 372–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, Schroeder JI. (1994) Calcium-activated K+ channels and calcium-induced calcium-release by slow vacuolar ion channels in guard-cell vacuoles implicated in the control of stomatal closure. Plant Cell 6: 669–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T, Apse MP, Aharon GS, Blumwald E. (2002) Identification and characterization of a NaCl-inducible vacuolar Na+/H+ antiporter in Beta vulgaris. Physiol Plant 116: 206–212 [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Aharon GS, Sottosanto JB, Blumwald E. (2005) Vacuolar Na+/H+ antiporter cation selectivity is regulated by calmodulin from within the vacuole in a Ca2+- and pH-dependent manner. Proc Natl Acad Sci USA 102: 16107–16112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Blumwald E. (2005) Developing salt-tolerant crop plants: challenges and opportunities. Trends Plant Sci 10: 615–620 [DOI] [PubMed] [Google Scholar]
- Zepeda-Jazo I, Velarde-Buendía AM, Enríquez-Figueroa R, Bose J, Shabala S, Muñiz-Murguía J, Pottosin II. (2011) Polyamines interact with hydroxyl radicals in activating Ca2+ and K+ transport across the root epidermal plasma membranes. Plant Physiol 157: 2167–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HX, Blumwald E. (2001) Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotechnol 19: 765–768 [DOI] [PubMed] [Google Scholar]