Abstract
Diabetic macular edema (DME) remains one of the leading causes of moderate to severe vision loss. Although laser photocoagulation was the standard of care for several years, few patients achieved significant improvements in visual acuity. As a result, several pharmacotherapies and surgeries have been investigated. The fluocinolone acetonide devices are one of the latest therapies considered for the treatment of DME. Despite bringing significant improvements in visual acuity, fluocinolone devices are associated with cataract formation, increased intraocular pressure (IOP), and surgery to lower IOP. Due to the risk of complications, fluocinolone acetonide devices should be considered only in cases refractive to first-line therapies. In this review, we evaluate current and emerging therapies for DME, with special emphasis on fluocinolone acetonide intravitreal devices.
Keywords: diabetic macular edema, fluocinolone, anti-VEGF, triamcinolone, dexamethasone
Introduction
Diabetic retinopathy is the most common cause of moderate to severe visual impairment among working class adults.1 Within 15 years of diagnosis, 84% of type 1 diabetics and 57% of type 2 diabetics will have signs of diabetic retinopathy2. Among the sequelae of diabetic retinopathy, diabetic macular edema (DME) is the most common cause of moderate vision loss.3,4 Within 25 years of diagnosis, 29% of type 1 diabetics will have macular edema, and 17% will have clinically significant macular edema.5 After 2 years, over half of patients with DME will lose two or more lines of visual acuity (VA).6 Worldwide, it has been estimated that 21 million people have DME.7
The pathophysiology of DME is complex and multifactorial. Chronic hyperglycemia results in the formation of advanced glycation endproducts,8 oxidative stress,9 and the activation of protein kinase C.9 These changes lead to leukostasis and upregulation of vascular endothelial growth factor (VEGF), which cause endothelial damage,10 vascular leakage,10 hypoxia,11 the inactivation of tight junction proteins,12 and increased vessel permeability.12 As a result, the integrity of the blood–retinal barrier becomes permeable to fluid, leading to an accumulation of fluid in the extravascular space of the retina, and leading in turn, to DME.
Treatments for DME are evolving rapidly, and while DME was once a disease treated solely with laser therapy, today there are numerous alternative therapies that have demonstrated promise. Intravitreal anti-VEGF agents have revolutionized the treatment of DME by demonstrating visual improvement in multiple randomized clinical trials. Intravitreal steroids, most commonly the glucocorticoids dexamethasone and fluocinolone, have also shown efficacy in controlling DME and potentially in improving vision. While steroids are broad-spectrum anti-inflammatory medications that affect both gene transcription and protein upregulation, the anti-VEGF agents specifically target a molecule implicated in the pathogenesis of DME.
In this review, we evaluate the efficacy of intravitreal fluocinolone acetonide, via injection or implantation, in the treatment of DME and review the emerging treatment options for DME.
Current and emerging management strategies
Treatment for DME begins with treatment of the systemic disease by lifestyle modifications, including maintaining tight blood sugar and blood pressure control, increased exercise, weight loss, and the lowering of circulating triglycerides and cholesterol. The United Kingdom Prospective Diabetes Study (UKPDS) trial showed that an 11% reduction in hemoglobin A1c, from 7.9% to 7.0%, in type 2 diabetics reduced the risk of microvascular complications of diabetes, including the need for photocoagulation, by 25%.13 Similarly, tight blood pressure control reduced the risk of microvascular complications by 37%.14 However, once there is progression to DME, therapy is indicated to slow the rate of vision loss and to attempt to improve the long-term prognosis. Over the past 30 years, the first-line therapy has been laser photocoagulation; in the past few years, several other treatments have emerged, including corticosteroids, anti-VEGF agents, and vitrectomy.
Laser photocoagulation
The Early Treatment Diabetic Retinopathy Study (ETDRS) established focal laser therapy (FLT) as the standard treatment for DME. The ETDRS randomized 1490 eyes with mild to moderate clinically significant macular edema (CSME), a type of DME, to either focal laser treatment or observation. After 3 years, the results showed that focal laser treatment reduced vision loss of 15 letters or more by 50% compared with the control treatment. The most dramatic results were in groups with more extensive disease and worse baseline VA. The ETDRS established the criteria for CSME as well as defined the indications for FLT still in use today.15 Although laser therapy slows the progression of DME for many patients, nearly half do not respond to treatment, and few patients improve by more than three lines. A later study utilizing FLT as a control group had similar results, with minimal to no visual improvement in center-involving DME. FLT did show anatomic improvement however, by significantly reducing the foveal thickness at 12 months, on optical coherence tomography (OCT).16
Triamcinolone
Triamcinolone is a corticosteroid suspension that has been used in ophthalmology to treat conditions such as pseudophakic cystoid macular edema and posterior uveitis. It has demonstrated long-term depot stability within experimental animal vitreous for up to 41 days and has been shown to act to reduce VEGF production, decrease prostaglandin production, and to stabilize the blood–retina barrier.17 It is this action of decreasing vascular permeability that led initial investigators to try triamcinolone in the treatment diffuse DME.18
A few small, randomized trials have reported improvement in VA in eyes with recalcitrant diffuse DME treated with intravitreal triamcinolone (IVTA).19–21 In a study of 69 eyes randomized to receive either 4 mg IVTA injections or placebo, the improvement of five or more letters was found in 19 of 36 (56%) eyes treated with triamcinolone compared with nine of 35 (26%) eyes in the sham group (P = 0.006), after 2 years.19 OCT analysis demonstrated a significant decrease in central macular thickness in eyes treated with IVTA compared to controls.
When compared with laser therapy however, the results have been inconclusive. In a small study of 26 eyes randomized to receive a 25 mg triamcinolone injection compared with 16 eyes undergoing macular grid laser photocoagulation, treatment with 25 mg triamcinolone was associated with improved VA at 6 weeks (P = 0.003), 10 weeks (P = 0.01), and 6 months (P = 0.02) compared with laser photocoagulation.20 Patients in the triamcinolone group had a baseline VA of 0.12 ± 0.08 and improved to a maximum of 0.19 ± 0.14 compared with a small, nonsignificant decrease in VA in the laser photocoagulation group, during the mean 6-month follow-up. The Diabetic Retinopathy Clinical Research Network (DRCR.net) compared 840 eyes randomized to receive either FLT (N = 330), 1 mg IVTA (N = 256), or 4 mg triamcinolone (N = 254), with the option to retreat for persistent edema every 4 months. After 4 months, the 4 mg triamcinolone group had better VA than both the laser group (P < 0.001) and the 1 mg triamcinolone group (P < 0.001). However, at 1 year there was no statistical difference between the three groups. At the primary endpoint of 2 years, the FLT group had a small but significant improvement in best-corrected VA (BCVA) (+1 letter vs −2 and −3 letter loss) over both of the IVTA groups.22
It has been suggested that the acute anti-inflammatory action of the steroid is effective in the short term, but that the gradual decline in concentration is not beneficial as a long-term therapy. As was seen in multiple other studies,19,20 the eyes treated with 4 mg IVTA had significantly higher rates of increased intraocular pressure (IOP) (33%), need for antiglaucoma medication (30%), and need for cataract surgery (51%) compared with the FLT group in the DRCR.net study.22 There was a doubling of cataract development and IOP anomalies in the 4 mg group compared with the 1 mg group. When compared to FLT, the intravitreal steroids demonstrated no benefit and increased side effects that could increase ocular morbidity.
With the high rate of complications and without evidence for its superiority over FLT, IVTA is generally reserved for patients refractory to FLT and anti-VEGF agents.18,23 Despite the adverse events associated with steroids, a recent Cochrane review supported the use of intravitreal steroids in the treatment of DME refractory to FLT.24 IVTA has an advantage over FLT in that it can be repeated multiple times, as long as the IOP rise and cataract risk is assessed at every visit. FLT can lead to an increase in foveal nonperfusion after repeated treatments and to macular scarring. A literature review from 2010 found that the addition of triamcinolone to FLT had no significant effect on VA.25
Dexamethasone
The dexamethasone intravitreous drug delivery system (DDS) was recently designed and US Food and Drug Administration (FDA) approved for the treatment of macular edema due to retinal vein occlusion and noninfectious posterior segment uveitis. Dexamethasone differs from triamcinolone in that it is more potent and has a much shorter half-life (3.5 hours vs 1.6 days).26,27 Thus, intravitreal injections of suspended dexamethasone would have a very short window of efficacy and would not be very useful in the management of chronic retinal disease. To overcome this, a delivery system was developed that utilizes a slowly dissolving copolymer of lactic and glycolic acid, similar to the material of absorbable sutures, with impregnated dexamethasone. This allows for the slow release of a constant amount of drug over the lifespan of the implant. At the end of the implant life, the polymer dissolves completely into its breakdown products of water and carbon dioxide.
Currently, one clinical trial has studied the effects of the DDS compared with observation, in eyes with DME previously treated with FLT. Kuppermann et al28 randomized 315 eyes to receive either 350 μg DDS, 700 μg DDS, or observation. After 90 days, an improvement of ten letters or more was observed in significantly more of the eyes treated with 700 μg DDS (35%) and 350 μg DDS (24%) compared with observation (13%). In a follow-up publication, significant improvements in VA, retinal thickness, and fluorescein leakage were maintained for at least 6 months.29 Of both treatment groups, 15% developed IOP elevations of greater than 10 mmHg at some point during follow-up, though the authors pointed out that the elevations were generally singular, and only 2% of patients had sustained IOP elevations at 90 days. No subjects required IOP-reduction surgery. The follow-up time was too short to comment on cataractogenesis.
Similar to IVTA, DDS has the advantage of repeatability, as long as the IOP and cataract effects are mitigated. Significantly different from IVTA, the level of drug within the vitreous is stable during the life of the implant, whereas with IVTA, the level initially peaks, then slowly trends downward.
Anti-VEGF
Vascular endothelial growth factor (VEGF) has been implicated in the development of DME, decreasing tight junction proteins and in turn, increasing vascular permeability.30 Following the success of several trials that showed their superiority to sham treatment, anti-VEGF agents, specifically the monoclonal antibodies ranibizumab and bevacizumab, have been widely used to treat DME.31–37 To date, only ranibizumab has received FDA approval for use in DME; however, bevacizumab is widely used as an off-label treatment by retina specialists.
Over the past few years, several prospective, randomized, controlled trials have been published that compared anti-VEGF agents with laser therapy.16,38–42 These results are summarized in Table 1. In the Ranibizumab for Edema of the Macula in Diabetes (READ-2) study, patients were randomized to receive either ranibizumab intravitreal injections, the combination of ranibizumab with laser therapy, or laser therapy alone. After 6 months, ranibizumab-alone group showed significantly better VA than both the laser group and the ranibizumab-plus-laser groups (ranibizumab group, 7.24 letters; laser group, −0.43 letters; ranibizumab plus laser, 3.80 letters). At 2 years, the mean differences in VA between the three groups were not significant, but since patients in the laser-only group had the option to use ranibizumab after 6 months, these results may reflect the use of ranibizumab in the control group.38 Also, the READ-2 study showed that ranibizumab exerts the greatest improvement in VA within 6 months of initiating therapy. At 6 months, the eyes injected with ranibizumab had improved 7.24 letters. From 6 months until 24 months, the further improvement was minimal, with a total BCVA gain of 7.70 letters. In the Phase III DRCR.net trial evaluating DME treatment, participants randomized to both ranibizumab plus prompt laser (+9 ± 11 letters) (P < 0.001) and ranibizumab plus deferred laser (+9 ± 12 letters) (P < 0.001) demonstrated superior visual gains compared with participants in the sham-injection-plus-prompt-laser group (+3 ± 13 letters), after 1 year.39 In the RESTORE study,16 both ranibizumab alone or in combination with laser improved BCVA significantly more compared with laser therapy alone, after 12 months. The BOLT study40 also showed the superiority of bevacizumab over FLT at 24 months, with the bevacizumab cohort gaining a mean of 8.6 letters compared with a mean loss of 0.5 letters for FLT. The Pan-American Collaborative Retina Study Group (PACORES) study41 is the largest and most recent study further supporting the use of intravitreal bevacizumab over bevacizumab plus photocoagulation and photocoagulation alone. After 2 years, 53% of eyes treated with intravitreal bevacizumab alone achieved two or more lines improvement in VA compared with 37% and 30% of eyes treated with bevacizumab plus photocoagulation and photocoagulation, respectively.
Table 1.
Comparison of changes in BCVA among laser and anti-VEGF studies
| Photocoagulation | Ranibizumab | Bevacizumab | Aflibercept | |||
|---|---|---|---|---|---|---|
|
|
|
|
|
|||
| DRCR.net22 n = 272 2 years | READ238 n = 37 2 years | RESTORE16 n = 115 1 year | BOLT40 n = 37 2 years | PACORES41 n = 141 2 years | DA VINCI42 n = 45 1 year | |
| Visual acuity | ||||||
| ≥3 line improvement | 20% | 24.0% | 23.0% | 32.0% | – | 42.2% |
| ≥2 line improvement | 34% | – | 37.0% | 49.0% | 53% | 62.0% |
| ≥2 line loss | 19% | – | 4% | – | 4% | – |
| ≥3 line loss | 13% | – | <1% | 0.0% | – | 0% |
| Mean letter improvement | 2 | 7.7 | 6.1 | 8.6 | 11.8 | 12.0 |
Abbreviations: BCVA, best-corrected visual acuity; VEGF, vascular endothelial growth factor.
The newest anti-VEGF agent, aflibercept, a recombinant fusion protein with binding sequences from VEGF receptors 1 and 2, has been investigated as a possible treatment option for DME. The DA VINCI study42 randomized patients to one of four doses of aflibercept or laser photocoagulation. Participants in the aflibercept group had significantly improved VA and retinal thickness versus laser, at 24 weeks and at 52 weeks. The mean improvements in BCVA at 52 weeks ranged from 9.7 to 13.1 in the four aflibercept groups compared with a loss of 1.3 letters in the laser group (P < 0.0001). Similarly, aflibercept was associated with a significant reduction in central retinal thickness compared with laser (P < 0.0001).42
Reported ocular and nonocular adverse events in the anti-VEGF trials were low. In a meta-analysis of 1567 eyes injected with ranibizumab and 4882 eyes injected with bevacizumab, van der Reis et al43 found the incidence of endophthalmitis, retinal pigment epithelium tear, retinal detachment, and increased IOP to be <1% in the studied eyes. The authors noted that increased IOP was usually transient and in all but one case, controlled with medications. Cataract progression and anterior chamber inflammation were variable, with a cumulative incidence of 8% and 2% in the eyes that were injected with ranibizumab, respectively but less than <1% in the eyes that were injected with bevacizumab. There have been reports of increased stroke and myocardial infarction with the use of intravitreal anti-VEGF therapy, although the frequencies of these events have not proven to be significantly different from those seen in controls, in large meta-analyses.44 Since patients with a recent heart attack and stroke were excluded from anti-VEGF clinical trials due to the theoretical increased risk of causing a subsequent event, anti-VEGF therapies should be used cautiously in patients with a cardiovascular event in the preceding 3 months. To that end, some authors advocate the use of a reduced dose in poorly controlled diabetics, to mitigate the cardiovascular risk that may be present at higher doses.36
Anti-VEGF therapy has emerged as the most efficacious treatment for DME, with minimal ocular and systemic side effects. Although the results of these trials suggest that anti-VEGF therapy may be superior, laser therapy, when paired with anti-VEGF therapy, may still be beneficial in some populations.
Vitrectomy
After Nasrallah et al45 reported a higher prevalence of DME in eyes without a posterior vitreous detachment compared with eyes with a posterior vitreous detachment, it was hypothesized that the vitreous may play a role in the pathophysiology of DME. Pars plana vitrectomy with posterior hyaloid removal was then successfully used for patients with DME and thickened, taut, posterior hyaloidal traction.46–48 A few years later, various studies supported the expanded use of vitrectomy for patients without macular traction, by finding that vitrectomy improved VA compared with observation in eyes unresponsive to laser.49,50 The procedure was refined when other studies showed successful results from vitrectomy performed with peeling of the internal limiting membrane.51,52
With the majority of evidence coming from small retrospective studies with large interstudy variability, the interpretation of the vitrectomy data has been complicated. As a result, the DRCR.net study was designed to evaluate factors associated with favorable outcome after vitrectomy for DME. The researchers found that 6 months after vitrectomy, median central subfield thickness decreased from 412 μm to 278 μm, but the median VA was unchanged. In a subset analysis, a greater improvement in VA was associated with poor preoperative VA (P < 0.001) and with removal of the internal limiting membrane (P = 0.003). The use of vitrectomy in the treatment of DME remains controversial, but these data suggest that vitrectomy may be therapeutic in patients with poor baseline VA that is refractive to other therapy.53
Efficacy, safety, tolerability of fluocinolone
Fluocinolone acetonide (FAc) is an attractive drug in the treatment of DME due to its high lipophilicity and potency.54 Two FAc intravitreal devices have been studied for the treatment of DME: a FAc implant (Retisert®; Bausch and Lomb Inc, Rochester, NY, USA) and a FAc insert (Illuvien®; Alimera Sciences, Inc, Alpharetta, GA, USA). Currently, neither device has received FDA approval for the treatment of DME. The results of the studies examining the use of FAc are summarized in Table 2.
Table 2.
Endpoints and results among fluocinolone device studies for the treatment of diabetic macular edema
| Author | Eyes | Endpoints | Results |
|---|---|---|---|
| FAc implant | |||
| Bausch and Lomb55 | 80 eyes (1:1 to 0.5 mg FAc or SOC) |
Retinal thickening after 6 mo | FAc significantly better (P = 0.03) |
| Improvement in severity of DR after 6 mo | FAc significantly better (P = 0.01) | ||
| Improvement or stable VA after 6 mo | 80% in treatment group vs 50% in SOC (P < 0.01) | ||
| Pearson et al57 | 197 eyes (2:1 to 0.59 mg FAc or SOC) |
Visual acuity gain of ≥three lines | 28% in FAc vs 15% in SOC at 3 yrs (P < 0.05) |
| Visual acuity loss of ≥three lines | 19% in FAc vs 16% in SOC at 3 yrs (NS) | ||
| Resolution of edema at center of macula | 58% in FAc vs 30% SOC at 3 yrs (P < 0.001) | ||
| Improvement in diabetic retinopathy scores | 13% in FAc vs 4% SOC at 3 yrs (P < 0.001) | ||
| Pearson et al56 | 196 eyes (2:1 0.59 mg FAc or SOC) |
Visual acuity gain of ≥15 letters | FAc significantly better until 1 yr |
| Improvement in macular edema | FAc significantly better until 2 yrs | ||
| ETDRS diabetic retinopathy severity scale | FAc improved faster, declined slower | ||
| Leakage by FA | FAc significantly better until 2 yrs | ||
| Maximum cystoid score | FAc significantly better until 1 yr | ||
| FAc insert | |||
| Campochiaro et al58 | 37 eyes (2:1 0.2 μg/day, 0.59 μg/day) | Change in BCVA at month 12 | 1.3 letters (low dose), 5.7 letters (high dose) |
| Campochiaro et al60 | 956 eyes (2:2:1 0.2 μg/day, 0.5 μg/day, or sham) | ≥15-letter increase in VA | FAc group better through 3 years |
| Mean improvement VA from baseline | FAc groups better through 3 years | ||
| Decreased retinal thickness | FAc groups better through 2 years | ||
Abbreviations: BCVA, best-corrected visual acuity; DR, diabetic retinopathy; ETDRS, Early Treatment Diabetic Retinopathy Study; FA, fluorescein angiography; FAc, fluocinolone acetonide; SOC, standard of care; VA, visual acuity; NS, not significant.
The FAc implant is a surgically implanted, nonbiodegradable intravitreal insert that releases 0.59 μg/day of fluocinolone over the approximately 3 year lifespan of the implant via the controlled breakdown of a central polymer-drug matrix. It received initial FDA approval for the treatment of chronic, posterior uveitis; however, more recently investigators have examined its utility in the treatment of DME.55–57 In 2002, Bausch and Lomb published the first results of 80 patients randomized to receive a 0.5 mg FAc implant or the standard of care (either laser or observation). After 6 months, the eyes treated with FAc had significantly less retinal thickening and a higher proportion of eyes with improved or stable VA than did the standard of care group.55 In 2006 and 2011, Pearson et al published the results of two trials with 197 and 196 eyes randomized in a 2:1 ratio, to receive either 0.59 mg FAc or the standard of care.56,57 After 3 years, 28% (in 2006) and 31% (in 2011) of the FAc groups had ≥3 line improvement in VA compared with 15% (P < 0.05) and 20% (P = 0.16), respectively, in the standard of care groups. Moreover, more eyes implanted with FAc had resolution of their macular edema in both studies; however, after 3 years, this was only statistically significant in the 2006 study.
A surgically implanted steroid eluting device is not without other ocular effects, and the implant has the highest rates of steroid-induced ocular comorbidities of any delivery form currently available. Compared with the standard of care, patients treated with the FAc implant had significantly higher rates of cataract extraction (91% vs 20%), IOP above 30 mmHg (61% vs 6%), and surgery to relieve elevated IOP (34% vs not reported). While potentially effective for recalcitrant DME, the implant demonstrates a substantial risk of glaucoma and cataract progression.
The FAc insert is a smaller, nonbiodegradable cylindrical tube with a central drug-polymer matrix similar to the FAc implant that is inserted intravitreally via a 25-gauge needle. There are two clinical trials that studied the effects of the FAc insert on DME.58–60 In a study of 37 eyes randomized to receive either a 0.2 μg/day or 0.50 μg/day FAc insert, treatment for 12 months with the low dose and high dose inserts was associated with a 1.3- and 5.7-letter increase in VA, respectively.58 The Fluocinolone Acetonide for Macular Edema (FAME) studies were two large prospective, randomized, controlled studies that followed 956 eyes randomized to receive 0.2 μg/day (low dose) or 0.50 μg/day (high dose) inserts or sham. After 3 years, a BCVA letter score ≥ 15 letters was achieved in 29% of the low-dose group (P = 0.018) and in 28% of the high-dose group compared with 19% of the sham group. Within 1 month, 10% of patients with inserts improved by three lines. Interestingly, in an analysis of only patients with DME for ≥3 years prior to enrollment, patients in both the low-dose (P < 0.001) and high-dose groups (P = 0.002) were more likely to achieve a ≥15 letter gain compared with patients in the sham group. Retinal thickness was significantly reduced in both the low-dose and high-dose groups compared with the sham group until month 30.59,60
Adverse effects were common in both the low-dose and high-dose insert groups and included cataract formation (43% and 53%, respectively), cataract surgery (80% and 87%, respectively), increased IOP (37% and 46%, respectively), the need for IOP-lowering medication (38% and 47%, respectively), incisional glaucoma surgery (5% and 8%, respectively), and trabeculoplasty (1% and 3%, respectively). Although the incidence of adverse events was high, the low-dose group had a consistently lower rate of adverse events yet achieved equally efficacious results compared with the high-dose group. These data suggest that an even lower dose may be possible and that further reductions in the delivered dose may maintain efficacy and reduce side effects.
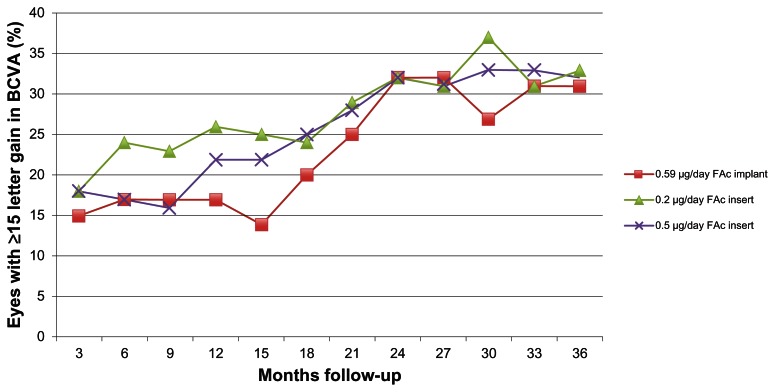
While comparing the studies of FAc devices head-to-head is complicated because of differences in their inclusion/exclusion criteria, retreatment protocols, and follow-up time, both FAc devices appear to have similar efficacy in the treatment of DME. In both studies, ≥15% of eyes achieved a ≥15 letter increase in BCVA. By 18 months, 20% eyes treated with the FAc implant or either doses of the FAc insert maintained a ≥15 letter increase in BCVA. By 3 years, 30% of eyes treated with a FAc device achieved a ≥15 letter increase in BCVA (Figure 1). The rate of cataract progression was similar between the two FAc devices, with more than 80% of phakic eyes requiring cataract surgery within 4 years of treatment. However, the risk of increased IOP and glaucoma progression was significantly higher in the FAc implant trial compared with the FAc insert. An increased IOP occurred in 69.7% of implanted eyes compared with only 45.5% of high-dose inserted eyes and 37.1% of low-dose inserted eyes; most significantly, 33.8% of FAc-implanted eyes required incisional surgery to relieve increased IOP compared with only 4.8% and 8% of low- and high-dose FAc-inserted eyes. Although the improvement in BCVA may be similar, the significantly lower rate of ocular hypertension in patients treated with FAc inserts make this the preferred FAc device in the treatment of DME.
Figure 1.
Percentage of eyes achieving ≥15 letter gain in BCVA during follow-up between intravitreal fluocinolone acetonide devices.
Abbreviations: BCVA, best-corrected visual acuity; FAc, fluocinolone acetonide.
Due to differences in follow-up time and patient populations, it is problematic to compare results from several trials; however, large variations in rates may elucidate important differences in the efficacy and rates of adverse events between fluocinolone, dexamethasone, and triamcinolone (Table 3). When comparing effects on VA, triamcinolone injections appear inferior to both fluocinolone and dexamethasone devices, with fewer patients achieving a BCVA improvement of ≥15 letters. Although it is still too early to reach definitive conclusions regarding the efficacy of the dexamethasone insert, the percentage of eyes achieving ≥15 letter improvement with this insert is comparable with that seen in the FAc device studies and is superior to that seen in the triamcinolone studies at similar follow-up times.22,28,29,56,60 Overall, these data show greater efficacy of the FAc and dexamethasone devices compared with triamcinolone injections that is unlikely attributable simply to variations in follow-up time or study design.
Table 3.
Visual acuity, macular thickness, and adverse events among different steroid treatment options for diabetic macular edema
| Triamcinolone (2-year follow-up) | Dexamethasone (6-month follow-up)† | FAc implant (3-year follow-up)‡ | FAc insert (3-year follow-up) | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||
| DRCR.net22 | Haller et28,29 | Pearson et al56 | Campochiaro et al60 | ||||
|
|
|
|
|
||||
| 1 mg (n = 256) | 4 mg (n = 254) | 350 μg (n = 103) | 700 μg (n = 105) | 0.59 mg (n = 127) | 0.2 μg (n = 375) | 0.5 μg (n = 393) | |
| Visual acuity | |||||||
| ≥15 letter improvement | 15% | 16% | 15% | 18% | 31% | 33% | 32% |
| ≥15 letter loss | 21% | 21% | NR | NR | 17%* | NR | NR |
| Macular thickness | |||||||
| Baseline mean foveal thickness (μm) | 405 | 396 | 446 | 428 | 419 | 451 | 461 |
| Follow-up mean foveal thickness (μm) | 319 | 319 | 403 | 296 | 309* | 280 | 300 |
| Mean change in foveal thickness (μm) | 86 | 77 | 43 | 132 | 110* | 171 | 161 |
| Ocular adverse events | |||||||
| Cataract surgery, phakic eyes | 23% | 51% | NR | NR | 91% | 80% | 87% |
| Increased IOP ≥ 10 mmHg from baseline | 16% | 33% | 15% | 15% | NR | NR | NR |
| IOP ≥ 30 mmHg | 9% | 21% | NR | NR | 61% | NR | NR |
| Initiation of IOP-lowering meds | 12% | 30% | NR | NR | NR | 38% | 47% |
| Glaucoma surgery | 0% | 1% | 0% | 0% | 33% | 6% | 11% |
| Hypotony (IOP ≤ 7 mmHg) | NR | NR | NR | NR | 22% | NR | NR |
| Endophthalmitis | 0% | 0% | 0% | 0% | NR | NR | NR |
Notes:
Macular thickness measured at 3 months;
adverse events reported after 4 years of follow-up;
estimated from published figures.
Abbreviations: FAc, fluocinolone acetonide; IOP, intraocular pressure; NR, not reported.
Serious adverse events were generally higher in the FAc studies compared with the triamcinolone and dexamethasone studies.22,28,29 First, the need for cataract surgery may be higher in the FAc-treated eyes, with more than 85% eyes needing cataract surgery after 3 years compared with the 37% of triamcinolone-injected eyes requiring this after 2 years; the follow-up period of the dexamethasone study is still not long enough to compare cataract progression rates. Second, rates of IOP increase were highest in the FAc-implanted group and lowest in the dexamethasone implant study. However, each study reported separate measures of increased IOP, making direct comparisons difficult. Of the patients with increased IOP, it appears that more patients treated with FAc device had progression leading to glaucoma surgery than did patients treated with triamcinolone and dexamethasone. Lastly, there appears to be no difference in endophthalmitis incidence, with this occurring in <1% of all eyes in all three types of steroid treatments, when reported. Although the FAc devices had higher rates of cataract progression and increased IOP, these differences may be artificially enhanced due to shorter follow-up times in the dexamethasone and triamcinolone studies.
Criteria for determining patient suitability
Both fluocinolone devices have distinct advantages in certain patients. Each offers a sustained release of medication over nearly 3 years, which obviates the need for repeat procedures during the treatment window. This can be particularly valuable in previously vitrectomized eyes, where drug clearance is higher than in eyes with an intact vitreous. Each device has shown clinical efficacy in both functional and anatomic measures. Lastly, unlike anti-VEGF therapies, there are no reported cases of systemic complications from local therapy in fluocinolone-treated eyes.
However, the use of fluocinolone devices is limited by their high rates of cataract progression, increased IOP, and need for glaucoma surgery, particularly with the FAc implant. In both devices, the rate of cataract progression and IOP elevation is so high that this should be expected with treatment with FAc devices. Campochiaro et al estimated that 3.7% of patients treated with the FAc insert develop the need for glaucoma surgery every year.
The ideal candidates for intravitreal FAc are pseudophakic patients with DME refractive to laser photocoagulation and anti-VEGF therapy for more than 3 years. Additionally, patients whose macular perfusion status limits further laser treatments or those who have developed central scotomas following therapy will be good candidates for intravitreal FAc. Other potential patients include those previously treated with anti-VEGF agents with a recent stroke or heart attack and patients who are unwilling or unable to receive monthly intravitreal injections. There are no other currently available options that can treat DME on a continuous basis without further intervention. Both the FAc devices can be valuable for eyes that are already pseudophakic and in eyes that have a pre-existing glaucoma drainage device.
Because of the high risk of developing ocular hypertension, FAc devices should be avoided in patients with pre-existing glaucoma, ocular hypertension, patients on IOP-lowering drugs, or patients who are likely to be lax in scheduling follow-ups, due to the need to monitor IOP.
Patient perspective, quality of life, tolerability
Besides vision-threatening adverse events, such as glaucoma and cataract progression, the FAc implant has also been associated with side effects that may affect patient quality of life. When compared with the standard of care group, patients implanted with the FAc implant reported increased pruritus (39% vs 22%), abnormal sensation of the eye (37% vs 12%), eye pain (27% vs 16%), eye irritation (22% vs 10%), and lacrimation (22% vs 9%).56 Moreover, since the FAc implant requires at least one trip to the operating room, treatment with the FAc implant may be anxiety-provoking for many patients with a fear of surgery. Conversely, since both FAc devices do not require regular injections and reduce the usage of anti-VEGF injections, it may be preferred in patients with excessive fear of needles.
Conclusion, place in therapy
Therapy for diabetic retinopathy begins with blood sugar control through lifestyle modifications, but there is an emerging role for pharmacologic and surgical therapy in the treatment of DME. These have been summarized (Table 4). Anti-VEGF therapies have emerged as the first line of treatment for center-involving DME, though FLT may be useful for noncenter-involving DME or to reduce the treatment burden of anti-VEGF agents. Their low incidence of complications, relative ease of administration, and proven efficacy make these agents a superior alternative to laser therapy alone. Injectable corticosteroids have also shown efficacy in recalcitrant cases, but the trials to date do not demonstrate that they are an improvement over FLT. Given the risk of endophthalmitis, cataract, and IOP elevations without demonstrated superiority to laser, no corticosteroid, be it fluocinolone, triamcinolone, or dexamethasone, should be a first-line therapy for DME. However, with the dexamethasone and fluocinolone devices, long-term control of DME is possible, if the potential effects on the pressure and lens status are monitored.
Table 4.
Advantages and disadvantages of different therapies for diabetic macular edema
| Advantages | Disadvantages | |
|---|---|---|
| Photocoagulation | Low risk of complications | Many patients do not respond |
| Well-studied | ||
| Inexpensive | ||
| Triamcinolone | Efficacy well-established | Many patients do not respond |
| Inexpensive | High rate of cataract progression | |
| High rate of increased IOP | ||
| Regular injections and follow-up | ||
| Dexamethasone | Decreased follow-up | High rate of cataract progression |
| High rate of increase IOP | ||
| Not well-studied in DME | ||
| Anti-VEGF therapy | Very strong efficacy | Regular injections and follow-up |
| Most patients responsive | ||
| Well-studied | ||
| Vitrectomy | Effective in patients with poor VA | Efficacy not well-established |
| Requires procedure in operating room | ||
| FAc implant | Strong efficacy | Very high rate of cataract progression |
| Decreased follow-up | High rate of increased IOP | |
| Increased risk for IOP-lowering surgery | ||
| Requires procedure in the operating room | ||
| FAc insert | Strong efficacy | Very high rate of cataract progression |
| Decreased follow-up | High rate of increased IOP | |
| Increased risk for IOP-lowering surgery |
Abbreviations: DME, diabetic macular edema; FAc, fluocinolone acetonide; IOP, intraocular pressure; VA, visual acuity; VEGF, vascular endothelial growth factor.
The appropriate use of intravitreal FAc device still remains to be defined. However, this remains a viable second-line therapy, especially in pseudophakic patients who have been unresponsive to anti-VEGF and laser therapy for greater than 3 years. If intravitreal FAc levels can be optimized to the benefits of treatment with the adverse events, FAc devices may reach a higher clinical acceptance. Further research is needed to help define the role of FAc in clinical practice.
Footnotes
Disclosure
The authors report no conflicts of interest.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2005. US Department of Health and Human Services, Centers for Disease Control and Prevention; 2005. [Accessed May 12, 2013]. Available from: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2005.pdf. [Google Scholar]
- 2.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102(4):527–532. doi: 10.1001/archopht.1984.01040030405011. [DOI] [PubMed] [Google Scholar]
- 3.Tranos PG, Wickremasinghe SS, Stangos NT, Topouzis F, Tsinopoulos I, Pavesio CE. Macular edema. Surv Ophthalmol. 2004;49(5):470–490. doi: 10.1016/j.survophthal.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Gardner TW, Sander B, Larsen ML, et al. An extension of the Early Treatment Diabetic Retinopathy Study (ETDRS) system for grading of diabetic macular edema in the Astemizole Retinopathy Trial. Curr Eye Res. 2006;31(6):535–547. doi: 10.1080/02713680600746112. [DOI] [PubMed] [Google Scholar]
- 5.Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXIII: the twenty-five-year incidence of macular edema in persons with type 1 diabetes. Ophthalmology. 2009;116(3):497–503. doi: 10.1016/j.ophtha.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferris FL, 3rd, Patz A. Macular edema. A complication of diabetic retinopathy. Surv Ophthalmol. 1984;28(Suppl):S452–S461. doi: 10.1016/0039-6257(84)90227-3. [DOI] [PubMed] [Google Scholar]
- 7.Yau JW, Rogers SL, Kawasaki R, et al. Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stitt AW, Li YM, Gardiner TA, Bucala R, Archer DB, Vlassara H. Advanced glycation end products (AGEs) co-localize with AGE receptors in the retinal vasculature of diabetic and of AGE-infused rats. Am J Pathol. 1997;150(2):523–531. [PMC free article] [PubMed] [Google Scholar]
- 9.Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003;22(1):1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 10.Miyamoto K, Ogura Y. Pathogenetic potential of leukocytes in diabetic retinopathy. Semin Ophthalmol. 1999;14(4):233–239. doi: 10.3109/08820539909069542. [DOI] [PubMed] [Google Scholar]
- 11.Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol. 2001;158(1):147–152. doi: 10.1016/S0002-9440(10)63952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem. 1999;274(33):23463–23467. doi: 10.1074/jbc.274.33.23463. [DOI] [PubMed] [Google Scholar]
- 13.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. Erratum in: Lancet. 1999;354(9178):602. [PubMed] [Google Scholar]
- 14.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 15.Early Treatment Diabetic Retinopathy Study research group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103(12):1796–1806. [PubMed] [Google Scholar]
- 16.Mitchell P, Bandello F, Schmidt-Erfurth U, et al. RESTORE study group. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615–625. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 17.Wilson CA, Berkowitz BA, Sato Y, Ando N, Handa JT, de Juan E., Jr Treatment with intravitreal steroid reduces blood-retinal barrier breakdown due to retinal photocoagulation. Arch Ophthalmol. 1992;110(8):1155–1159. doi: 10.1001/archopht.1992.01080200135041. [DOI] [PubMed] [Google Scholar]
- 18.Martidis A, Duker JS, Greenberg PB, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002;109(5):920–927. doi: 10.1016/s0161-6420(02)00975-2. [DOI] [PubMed] [Google Scholar]
- 19.Gillies MC, Sutter FK, Simpson JM, Larsson J, Ali H, Zhu M. Intravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology. 2006;113(9):1533–1538. doi: 10.1016/j.ophtha.2006.02.065. [DOI] [PubMed] [Google Scholar]
- 20.Jonas JB, Kreissig I, Söfker A, Degenring RF. Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol. 2003;121(1):57–61. [PubMed] [Google Scholar]
- 21.Lam DS, Chan CK, Mohamed S, et al. A prospective randomised trial of different doses of intravitreal triamcinolone for diabetic macular oedema. Br J Ophthalmol. 2007;91(2):199–203. doi: 10.1136/bjo.2006.102848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115(9):1447–1449. doi: 10.1016/j.ophtha.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JE, Pollack JS, Miller DG, Mittra RA, Spaide RF Isis Study Group. ISIS-DME: a prospective, randomized, dose-escalation intravitreal steroid injection study for refractory diabetic macular edema. Retina. 2008;28(5):735–740. doi: 10.1097/IAE.0b013e318163194c. [DOI] [PubMed] [Google Scholar]
- 24.Grover D, Li TJ, Chong CC. Intravitreal steroids for macular edema in diabetes [review] Cochrane Database Syst Rev. 2008;1:CD005656. doi: 10.1002/14651858.CD005656.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steijns D, Duijvesz D, Breedijk MA, van der Heijden GJ. Steroid injection in addition to macular laser grid photocoagulation in diabetic macular oedema: a systematic review. Acta Ophthalmol. 2010;88(4):389–393. doi: 10.1111/j.1755-3768.2009.01657.x. [DOI] [PubMed] [Google Scholar]
- 26.Kwak HW, D’Amico DJ. Evaluation of the retinal toxicity and pharmacokinetics of dexamethasone after intravitreal injection. Arch Ophthalmol. 1992;110(2):259–266. doi: 10.1001/archopht.1992.01080140115038. [DOI] [PubMed] [Google Scholar]
- 27.Scholes GN, O’Brien WJ, Abrams GW, Kubicek MF. Clearance of triamcinolone from vitreous. Arch Ophthalmol. 1985;103(10):1567–1569. doi: 10.1001/archopht.1985.01050100143037. [DOI] [PubMed] [Google Scholar]
- 28.Kuppermann BD, Blumenkranz MS, Haller JA, et al. Dexamethasone DDS Phase II Study Group. Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Arch Ophthalmol. 2007;125(3):309–317. doi: 10.1001/archopht.125.3.309. [DOI] [PubMed] [Google Scholar]
- 29.Haller JA, Kuppermann BD, Blumenkranz MS, et al. Dexamethasone DDS Phase II Study Group. Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Arch Ophthalmol. 2010;128(3):289–296. doi: 10.1001/archophthalmol.2010.21. [DOI] [PubMed] [Google Scholar]
- 30.Aiello LP. Angiogenic pathways in diabetic retinopathy. N Engl J Med. 2000;353(8):839–841. doi: 10.1056/NEJMe058142. [DOI] [PubMed] [Google Scholar]
- 31.Cunningham ET, Jr, Adamis AP, Altaweel M, et al. Macugen Diabetic Retinopathy Study Group. A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 2005;112(10):1747–1757. doi: 10.1016/j.ophtha.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, et al. Pan-American Collaborative Retina Study Group. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology. 2007;114(4):743–750. doi: 10.1016/j.ophtha.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 33.Diabetic Retinopathy Clinical Research Network. Scott IU, Edwards AR, Beck RW, et al. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007;114(10):1860–1867. doi: 10.1016/j.ophtha.2007.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soheilian M, Ramezani A, Bijanzadeh B, et al. Intravitreal bevacizumab (avastin) injection alone or combined with triamcinolone versus macular photocoagulation as primary treatment of diabetic macular edema. Retina. 2007;27(9):1187–1195. doi: 10.1097/IAE.0b013e31815ec261. [DOI] [PubMed] [Google Scholar]
- 35.Faghihi H, Roohipoor R, Mohammadi SF, et al. Intravitreal bevacizumab versus combined bevacizumab-triamcinolone versus macular laser photocoagulation in diabetic macular edema. Eur J Ophthalmol. 2008;18(6):941–948. doi: 10.1177/112067210801800614. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen QD, Brown DM, Marcus DM, et al. RISE and RIDE Research Group. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 37.Massin P, Bandello F, Garweg JG, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33(11):2399–2405. doi: 10.2337/dc10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen QD, Shah SM, Khwaja AA, et al. READ-2 Study Group. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2010;117(11):2146–2151. doi: 10.1016/j.ophtha.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Diabetic Retinopathy Clinical Research Network. Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064–1077. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajendram R, Fraser-Bell S, Kaines A, et al. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol. 2012;130(8):972–979. doi: 10.1001/archophthalmol.2012.393. [DOI] [PubMed] [Google Scholar]
- 41.Arevalo JF, Lasave AF, Wu L, et al. Pan-American Collaborative Retina Study Group (PACORES) Intravitreal bevacizumab plus grid laser photocoagulation or intravitreal bevacizumab or grid laser photocoagulation for diffuse diabetic macular edema: results of the Pan-American Collaborative Retina Study Group at 24 Months. Retina. 2013;33(2):403–413. doi: 10.1097/IAE.0b013e3182695b83. [DOI] [PubMed] [Google Scholar]
- 42.Do DV, Nguyen QD, Boyer D, et al. DA VINCI Study Group. One-year outcomes of the DA VINCI Study of VEGF Trap-Eye in eyes with diabetic macular edema. Ophthalmology. 2012;119(8):1658–1665. doi: 10.1016/j.ophtha.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 43.van der Reis MI, La Heij EC, De Jong-Hesse Y, Ringens PJ, Hendrikse F, Schouten JS. A systematic review of the adverse events of intravitreal anti-vascular endothelial growth factor injections. Retina. 2011;31(8):1449–1469. doi: 10.1097/IAE.0b013e3182278ab4. [DOI] [PubMed] [Google Scholar]
- 44.Virgili G, Parravano M, Menchini F, Brunetti M. Antiangiogenic therapy with anti-vascular endothelial growth factor modalities for diabetic macular oedema [review] Cochrane Database Syst Rev. 2012;12:CD007419. doi: 10.1002/14651858.CD007419.pub3. [DOI] [PubMed] [Google Scholar]
- 45.Nasrallah FP, Jalkh AE, Van Coppenolle F, et al. The role of the vitreous in diabetic macular edema. Ophthalmology. 1988;95(10):1335–1339. doi: 10.1016/s0161-6420(88)33004-6. [DOI] [PubMed] [Google Scholar]
- 46.Lewis H, Abrams GW, Blumenkranz MS, Campo RV. Vitrectomy for diabetic macular traction and edema associated with posterior hyaloidal traction. Ophthalmology. 1992;99(5):753–759. doi: 10.1016/s0161-6420(92)31901-3. [DOI] [PubMed] [Google Scholar]
- 47.Pendergast SD, Hassan TS, Williams GA, et al. Vitrectomy for diffuse diabetic macular edema associated with a taut premacular posterior hyaloid. Am J Ophthalmol. 2000;130(2):178–186. doi: 10.1016/s0002-9394(00)00472-4. [DOI] [PubMed] [Google Scholar]
- 48.Harbour JW, Smiddy WE, Flynn HW, Jr, Rubsamen PE. Vitrectomy for diabetic macular edema associated with a thickened and taut posterior hyaloid membrane. Am J Ophthalmol. 1996;121(4):405–413. doi: 10.1016/s0002-9394(14)70437-4. [DOI] [PubMed] [Google Scholar]
- 49.Ikeda T, Sato K, Katano T, Hayashi Y. Vitrectomy for cystoid macular oedema with attached posterior hyaloid membrane in patients with diabetes. Br J Ophthalmol. 1999;83(1):12–14. doi: 10.1136/bjo.83.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yanyali A, Horozoglu F, Celik E, Nohutcu AF. Long-term outcomes of pars plana vitrectomy with internal limiting membrane removal in diabetic macular edema. Retina. 2007;27(5):557–566. doi: 10.1097/01.iae.0000249390.61854.d5. [DOI] [PubMed] [Google Scholar]
- 51.Dillinger P, Mester U. Vitrectomy with removal of the internal limiting membrane in chronic diabetic macular oedema. Graefes Arch Clin Exp Ophthalmol. 2004;242(8):630–637. doi: 10.1007/s00417-003-0849-8. [DOI] [PubMed] [Google Scholar]
- 52.Kumagai K, Furukawa M, Ogino N, Larson E, Iwaki M, Tachi N. Long-term follow-up of vitrectomy for diffuse nontractional diabetic macular edema. Retina. 2009;29(4):464–472. doi: 10.1097/IAE.0b013e31819c632f. [DOI] [PubMed] [Google Scholar]
- 53.Flaxel CJ, Edwards AR, Aiello LP, et al. Factors associated with visual acuity outcomes after vitrectomy for diabetic macular edema: diabetic retinopathy clinical research network. Retina. 2010;30(9):1488–1495. doi: 10.1097/IAE.0b013e3181e7974f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor SR, Isa H, Joshi L, Lightman S. New developments in corticosteroid therapy for uveitis. Ophthalmologica. 2010;224( Suppl 1):S46–S53. doi: 10.1159/000318021. [DOI] [PubMed] [Google Scholar]
- 55.Fluocinolone acetonide ophthalmic–Bausch and Lomb: fluocinolone actinide Envision TD implant. Drugs R D. 2005;6(2):116–119. doi: 10.2165/00126839-200506020-00007. [DOI] [PubMed] [Google Scholar]
- 56.Pearson PA, Comstock TL, Ip M, et al. Fluocinolone acetonide intravitreal implant for diabetic macular edema: a 3-year multicenter, randomized, controlled clinical trial. Ophthalmology. 2011;118(8):1580–1587. doi: 10.1016/j.ophtha.2011.02.048. [DOI] [PubMed] [Google Scholar]
- 57.Pearson PA, Levy B, Comstock T Fluocinolone Acetonide Implant Study Group. Fluocinolone acetonide intravitreal implant to treat diabetic macular edema: a 3–year results of a multi–center clinical trial. Invest Ophthalmol Vis Sci. 2006;47 E-Abstract 5442. [Google Scholar]
- 58.Campochiaro PA, Hafiz G, Shah SM, et al. Famous Study Group. Sustained ocular delivery of fluocinolone acetonide by an intravitreal insert. Ophthalmology. 2010;117(7):1393–1399. doi: 10.1016/j.ophtha.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 59.Campochiaro PA, Brown DM, Pearson A, et al. FAME Study Group. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118(4):626–635. doi: 10.1016/j.ophtha.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 60.Campochiaro PA, Brown DM, Pearson A, et al. FAME Study Group. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119(10):2125–2132. doi: 10.1016/j.ophtha.2012.04.030. [DOI] [PubMed] [Google Scholar]