Abstract
Background
Several studies have documented a significant association between vasomotor symptoms (VMS) and a decrement in health outcomes among postmenopausal women, but these studies have mostly focused on the US. The aim of the current study was to broaden this investigation by examining the burden of VMS symptoms in the European Union with respect to both humanistic and economic outcomes.
Methods
All women aged 40–75 years who completed the 2010 5EU (France, Germany, Italy, Spain, and the UK) National Health and Wellness Survey were identified as potential respondents and invited to participate in an additional cross-sectional, Internet-based survey. Only postmenopausal women from 5EU were included in the current analyses (n = 3801). VMS was assessed using the Menopausal Rating Scale, and was used in multiple regression models as the primary predictor of health status (EQ-5D-3L), work productivity loss, and the number of physician visits due to menopause.
Results
Over half (50.3%) of postmenopausal women experienced either mild (24.6%), moderate (17.6%), or severe (8.1%) VMS. Controlling for confounding variables, mild (b = −0.03, P < 0.05), moderate (b = −0.07, P < 0.05), and severe VMS (b = −0.17, P < 0.05) were each associated with worse health utilities relative to women without VMS. Similarly, increased resource use (b = 1.04–2.39, all P < 0.05), overall work impairment (b = 8.71–19.69, all P < 0.05), and activity impairment (b = 11.22–24.54, all P < 0.05) were also observed as VMS severity increased (with each b representing the difference between each level of severity and the reference category).
Conclusion
These results suggest a high prevalence of VMS in Western Europe. These symptoms are also associated with both humanistic and economic outcomes. Improved management of VMS may be able to increase the health status and ability to work productively as well as reduce societal direct costs.
Keywords: menopause, vasomotor symptoms, health status, work productivity
Introduction
The transition into menopause is frequently associated with a number of symptoms. Women often experience anxiety, depression, decreased libido, vaginal dryness, insomnia, difficulty concentrating, hot flashes (also referred to as hot flushes), and night sweats, among others.1–3 Hot flushes/flashes and night sweats, often collectively referred to as vasomotor symptoms (VMS), are among the most common symptoms experienced by postmenopausal women in Europe, with some studies suggesting a prevalence of approximately 75%.4 The presence of these symptoms can often linger for several years.5,6
A number of studies have documented a significant association between the presence of VMS and a decrement in health outcomes.7–14 Karaçam and Seker found that higher self-reported frequency of VMS was associated with worse quality of life.8 In the US, the Study of Women’s Health Across the Nation reported that many symptoms associated with menopause, including VMS, were also associated with significantly lower levels of health-related quality of life among postmenopausal women.9,10
Much less research has investigated the relationship between VMS and economic-related outcomes, including both work productivity losses and health care resource utilization. Results from the STRIDE (Do Stage Transitions Result in Detectable Effects?) study conducted in the US indicated that women experiencing VMS reported over $1600 more in direct health care-related costs than postmenopausal women not experiencing VMS.15 However, few other studies have been conducted and none, to our knowledge, outside the US. The aim of the current study was to investigate further the burden of VMS in the European Union with respect to health status, as well as work productivity losses and the number of physician visits. This research would better inform the broader influence of these symptoms on women who are transitioning into menopause.
Materials and methods
Data source
Women who completed the National Health and Wellness Survey (NHWS) were identified as potential respondents. The NHWS is a self-reported, Internet-based health survey conducted in the US, 5EU (France, Germany, Italy, Spain, and the UK), Japan, urban People’s Republic of China, urban Russia, and Brazil. Additional details on the survey methodology for each country have been reported previously.16–18 To overview, members of a global Internet panel were recruited using a random stratified sampling framework to ensure the final sample of each country was comparable with that country’s demographic profile. Comparisons between the NHWS and other established sources have been made elsewhere.16,18,19
All women aged 40–75 years who completed the 2010 US, 5EU, and Japan NHWS were identified as potential respondents and invited to participate in an additional cross-sectional, Internet-based survey. Although these respondents came from representative data sources, there was no imposed sampling frame on this follow-up survey. A total of 15,305, 10,015, and 3628 women from the US, 5EU, and Japan, respectively, were invited to participate, with a respective response rate of 4517 (29.5%), 5678 (56.7%), and 2005 (55.3%).
Sample
All women who were invited were asked “when was the last time you had any menstrual bleeding or spotting?”, with the following response choices: “still having regular menstrual bleeding”, “still having menstrual bleeding but it is irregular (changes in frequency, duration, or heaviness of flow)”, “stopped within the last 6 months”, “stopped 7 to 12 months ago”, “stopped over 12 months ago”, or “decline to answer”). Of the 5678 women in the 5EU who completed the survey, only those who reported that they has stopped menstrual bleeding over 12 months earlier (post-menopause) were included in the analyses (n = 3801).
Measures
Demographics
Demographic variables in the analysis included the following: country of residence (France, Germany, Italy, Spain, or the UK), age (40–49 years, 50–59 years, 60–75 years), marital status (married, single/never married, divorced, separated, widowed, or living with partner), education (university education versus less than university education), employment (full-time, part-time, self-employed versus not currently working), annual household income (less than 20,000€, 20,000€–39,999€, ≥40,000€, or decline to answer; British Pound Sterling values were assessed in the UK, but were converted to the Euro ranges mentioned above), and health insurance (public only versus private).
Health characteristics
Current exercise behavior (exercising at least once in the previous month for 20 minutes versus not), smoking habits (currently smoking versus not currently smoking), alcohol consumption (drinking alcohol once or more in the last month versus not), and height and weight (which were used to calculate a body mass index category, ie, underweight, normal, overweight, obese, or decline to provide weight) information was included and assessed consistently across all regions. The Charlson comorbidity index20 was also calculated to account for the comorbid burden experienced by each respondent. The Charlson comorbidity index is calculated by weighting the presence of the following conditions, as reported by the respondent as ever having experienced them, and summing the result: human immunodeficiency virus/acquired immune deficiency syndrome, metastatic tumor, lymphoma, leukemia, any tumor, moderate/severe renal disease, hemiplegia, diabetes, mild liver disease, ulcer disease, connective tissue disease, chronic pulmonary disease, dementia, cerebrovascular disease, peripheral vascular disease, myocardial infarction, and congestive heart failure. The greater the total index score, the greater the comorbid burden on the patient. Because of skew, the Charlson comorbidity index score was dichotomized into 0 versus 1 or more.
Vasomotor symptoms
The Menopause Rating Scale21 was used as the instrument to assess the presence and severity of 11 menopause-related symptoms. The Menopause Rating Scale asks “which of the following symptoms apply to you at this time?” and include “none”, “mild”, “moderate”, “severe”, and “extremely severe” response options. Respondents were categorized as not experiencing VMS (responding “none” to the single item of “hot flashes, sweating [episodes of sweating]”), experiencing mild VMS (responding “mild”), experiencing moderate vasomotor symptoms (responding “moderate”), or experiencing severe VMS (responding to “severe” or “extremely severe”).
Health status
Health status was assessed using the EQ-5D-3L instrument, which assesses the health state of the respondent at the time of completion based on five dimensions, ie, mobility, self-care, usual activities, pain/discomfort, and anxiety/depression.22 The responses to each dimension (no problems, some problems, or extreme problems), which constitute a description of the respondent’s health state, are then converted to an index score. This index score, which provides a measure of the utility of that health state, was used in the analyses. Health utilities vary conceptually from 0 (a health state equivalent to death) to 1 (a health state equivalent to perfect health); however, it is possible for health utilities derived from the EQ-5D-3L to be negative, which would be interpreted as a health state worse than death. Because not all countries have their own algorithm for creating a health utility score from the EQ-5D-3L responses, the UK algorithm was used for each respondent, regardless of their country. Past research has suggested that a difference of 0.03 or more would constitute a clinically meaningful one.23
Work productivity loss
Work productivity was assessed using the Work Productivity and Activity Impairment questionnaire specific to VMS.24 This scale is an instrument with evidence of validity which is used to measure lost work productivity and impairment in daily activities in the past seven days. Four subscales (absenteeism, presenteeism, overall work impairment, and activity impairment) are generated in the form of percentages (from 0% to 100%), with higher values indicating greater impairment. Absenteeism represents the percentage of work time missed due to VMS in the past seven days. Presenteeism represents the percentage of impairment while at work due to VMS in the past seven days. Overall work impairment represents the total percentage of impairment due to either VMS-related absenteeism or VMS-related presenteeism. Activity impairment represents the percentage of impairment during daily activities due to VMS. Only employed respondents reporting VMS provided data on absenteeism, presenteeism, and overall work impairment, but all respondents with VMS provided data on activity impairment. To date, no research to our knowledge has provided guidance on what a clinically relevant difference would represent on the Work Productivity and Activity Impairment questionnaire. However, the scale has intrinsic meaning in that each point increase represents an additional 1% in impairment.
Health care resource use
All respondents were presented with a list of physician types (eg, general practitioner, internist) and asked to select which ones they have seen in the past six months for any of their menopausal symptoms. For each physician they have seen in the past six months for their symptoms, respondents were asked to indicate the number of visits. These visits were then summed to represent the total number of menopause-related physician visits.
Statistical analyses
Descriptive analyses were conducted on all demographic and health characteristic variables. The relationship between VMS severity and health outcomes was examined using multiple regressions controlling for the following set of covariates: age (40–54 years served as the reference category), education (less than a university education served as the reference), annual household income (the lowest income category served as the reference), Charlson comorbidity index (an index score of 0 served as the reference), health insurance (public only served as the reference), and body mass index (normal category served as the reference). The models predicting health status and the number of menopause-specific physician visits included all women. Because the Work Productivity and Activity Impairment questionnaire is VMS-specific, only women who reported experiencing VMS were included in those models. Absenteeism, presenteeism, and overall work impairment were also only asked among those women who were currently employed. Overall models were conducted on all 5EU countries combined and also separately by country. Additional models were run to test for the interaction between country and VMS. Nonstandardized regression coefficients (b), standard errors of measurement, and 95% confidence intervals around those coefficients, and statistical significance (P) are reported for each predictor in these models.
Results
Descriptive results
Across all five countries, postmenopausal women were mostly aged 60–75 years (57.9%, see Table 1), although a number of them remained in the workforce (36.0%). Over half (53.5%) of these women were either overweight or obese, with nearly a quarter (23.1%) having a Charlson comorbidity index score ≥1. A total of 72.9% reported sleep problems, 71.3% reported urogenital symptoms, 59.2% reported psychological problems, and 18.3% reported a diagnosis of osteoporosis. VMS, being either mild (24.6%), moderate (17.6%), or severe (8.1%), was experienced by over half of the respondents.
Table 1.
Descriptive data of the post-menopausal women in the 5EU
| EU n = 3,801 |
||
|---|---|---|
|
|
||
| n | Weighted % | |
| Country | ||
| Germany | 970 | 29.7% |
| Spain | 294 | 14.3% |
| France | 1054 | 20.5% |
| Italy | 387 | 15.6% |
| United Kingdom | 1096 | 19.9% |
| Age | ||
| 40 to 49 years | 183 | 5.2% |
| 50 to 59 years | 1491 | 36.9% |
| 60 to 75 years | 2127 | 57.9% |
| University educated | 1630 | 43.0% |
| Married | 2295 | 59.7% |
| Household income* | ||
| Low income | 1151 | 28.6% |
| Medium income | 1640 | 44.1% |
| High income | 364 | 9.6% |
| Decline to answer income | 646 | 17.6% |
| Employed | 1398 | 36.0% |
| BMI | ||
| BMI <18.5 | 70 | 1.7% |
| BMI 18.5 to <25 | 1504 | 40.8% |
| BMI 25 to <30 | 1239 | 32.8% |
| BMI 30 or over | 828 | 20.7% |
| Decline to answer BMI | 160 | 4.0% |
| Currently smoke | 933 | 25.2% |
| Currently exercise | 1990 | 52.9% |
| Currently drink alcohol | 2738 | 69.1% |
| CCI >0 | 887 | 23.1% |
Notes:
Low household income refers to <20,000€, medium household income refers to 20,000€ to <40,000€, and high household income refers to ≥40,000€.
Effect of VMS on health outcomes
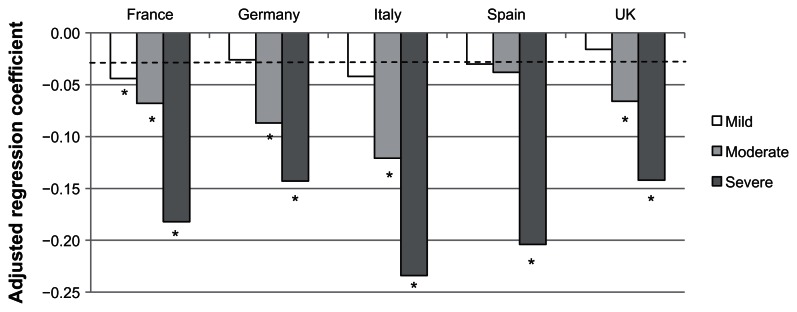
Controlling for confounding variables, women in the 5EU with VMS reported significantly worse health utility scores than women without VMS. These effects increased concomitantly with severity (mild VMS, b = −0.03, P = 0.002; moderate VMS, b = −0.07, P < 0.001; severe VMS, b = −0.17, P < 0.001; see Table 2). All of these values exceeded the cutoff for clinical significance. When looking at country-specific models, the pattern was similar across countries (see Figure 1). Indeed, a subsequent 5EU combined model testing for the interaction between country and VMS severity was not significant (all P > 0.11). The effect of mild VMS varied between b = −0.02 and b = −0.04 across countries, although only significant in France. The effects of moderate VMS varied between b = −0.04 and b = −0.09 (all of which were clinically significant), with all effects being statistically significant except for Spain. Within each country, the effects of severe VMS on health utility scores were both statistically and clinically significant (b = −0.14 to −0.23).
Table 2.
Multiple regression results predicting health utility scores for all of 5EU combined
| Variable | b | SE b | 95% LCL |
95% UCL |
P-value |
|---|---|---|---|---|---|
| Intercept | 0.835 | 0.020 | 0.797 | 0.874 | <0.0001 |
| No VMS (reference) | – | – | – | – | – |
| Mild VMS | −0.030 | 0.009 | −0.048 | −0.011 | 0.0017 |
| Moderate VMS | −0.074 | 0.011 | −0.095 | −0.052 | <0.0001 |
| Severe VMS | −0.170 | 0.014 | −0.197 | −0.142 | <0.0001 |
| University educated | 0.007 | 0.008 | −0.008 | 0.022 | 0.3742 |
| Income: <20,000€ (reference) | – | – | – | – | – |
| Income: 20,000€ to <40,000€ | 0.035 | 0.009 | 0.018 | 0.053 | 0.0001 |
| Income: 40,000€ or more | 0.080 | 0.014 | 0.051 | 0.108 | <0.0001 |
| Income: decline to answer | 0.040 | 0.012 | 0.017 | 0.063 | 0.0007 |
| Private insurance | 0.011 | 0.010 | −0.009 | 0.031 | 0.2781 |
| BMI: underweight | −0.070 | 0.029 | −0.126 | −0.014 | 0.015 |
| BMI: normal weight (reference) | – | – | – | – | – |
| BMI: overweight | −0.022 | 0.009 | −0.039 | −0.004 | 0.0156 |
| BMI: obese | −0.102 | 0.010 | −0.122 | −0.082 | <0.0001 |
| BMI: decline to answer | −0.069 | 0.020 | −0.107 | −0.030 | 0.0005 |
| CCI >0 | −0.105 | 0.009 | −0.122 | −0.087 | <0.0001 |
| Age 40–49 (reference) | – | – | – | – | – |
| Age 50–59 | 0.000 | 0.018 | −0.036 | 0.036 | 0.9989 |
| Age 60–75 | 0.016 | 0.018 | −0.019 | 0.052 | 0.3707 |
Abbreviations: b, regression coefficient; SE b, standard error of the regression coefficient; 95% LCL, 95% lower confidence limit; 95% UCL, 95% upper confidence limit.
Figure 1.
Adjusted regression coefficients of severity of vasomotor symptoms predicting health status as measured by the EQ-5D.
Notes: *P < 0.05; Covariates included age, education, income, health insurance, BMI and the Charlson comorbidity index. Dotted line represents clinically-meaningful cutoff for the EQ-5D.
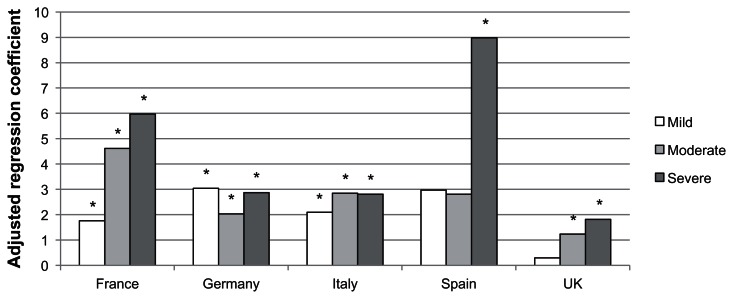
Pooling 5EU countries together, the severity of VMS was associated with an increased number of menopause-specific physician visits. Women with mild VMS (b = 1.04, P < 0.001), moderate VMS (b = 1.63, P < 0.001), and severe VMS (b = 2.39, P < 0.001) reported significantly more menopause-specific physician visits than women without VMS (see Table 3). The interaction term of country by VMS severity was not significant (P = 0.67). Means from the within-country models are reported in Figure 2. For Germany and Italy, it appeared that the presence versus absence of VMS rather than the degree of severity had the most influence on physician visits. The effects of mild, moderate, and severe VMS in those countries were generally similar. Conversely, France, Spain, and the UK demonstrated variability in the effects of mild, moderate, and severe VMS on the number of physician visits, with increasing severity associated with an increasing number of visits (although only severe VMS was significantly associated with physician visits in Spain).
Table 3.
Multiple regression results predicting physician visits for all of 5EU combined
| Variable | b | SE b | 95% LCL |
95% UCL |
P-value |
|---|---|---|---|---|---|
| Intercept | 0.517 | 0.466 | −0.396 | 1.43 | 0.2666 |
| No VMS (reference) | – | – | – | – | – |
| Mild VMS | 1.035 | 0.224 | 0.596 | 1.474 | <0.0001 |
| Moderate VMS | 1.626 | 0.259 | 1.119 | 2.134 | <0.0001 |
| Severe VMS | 2.391 | 0.336 | 1.732 | 3.051 | <0.0001 |
| University educated | −0.079 | 0.185 | −0.442 | 0.285 | 0.6715 |
| Income: <20,000€ (reference) | – | – | – | – | – |
| Income: 20,000€ to <40,000€ | 0.014 | 0.216 | −0.409 | 0.436 | 0.9495 |
| Income: 40,000€ or more | 0.691 | 0.342 | 0.021 | 1.362 | 0.0433 |
| Income: decline to answer | 0.131 | 0.277 | −0.411 | 0.674 | 0.6348 |
| Private insurance | 0.915 | 0.242 | 0.441 | 1.389 | 0.0002 |
| BMI: underweight | −0.594 | 0.678 | −1.923 | 0.734 | 0.3806 |
| BMI: normal weight (reference) | – | – | – | – | – |
| BMI: overweight | −0.022 | 0.213 | −0.44 | 0.395 | 0.9167 |
| BMI: obese | −0.199 | 0.244 | −0.677 | 0.28 | 0.416 |
| BMI: decline to answer | −0.847 | 0.468 | −1.764 | 0.07 | 0.0702 |
| CCI >0 | 0.627 | 0.215 | 0.205 | 1.049 | 0.0036 |
| Age 40–49 (reference) | – | – | – | – | – |
| Age 50–59 | 0.378 | 0.434 | −0.474 | 1.229 | 0.3847 |
| Age 60–75 | 0.578 | 0.429 | −0.262 | 1.419 | 0.1773 |
Abbreviations: b, regression coefficient; SE b, standard error of the regression coefficient; 95% LCL, 95% lower confidence limit; 95% UCL, 95% upper confidence limit; BMI, body mass index; VMS, vasomotor symptoms.
Figure 2.
Adjusted regression coefficients of severity of vasomotor symptoms predicting the number of physician visits in the past six months.
Notes: *P < 0.05; Covariates included age, education, income, health insurance, BMI and the Charlson comorbidity index.
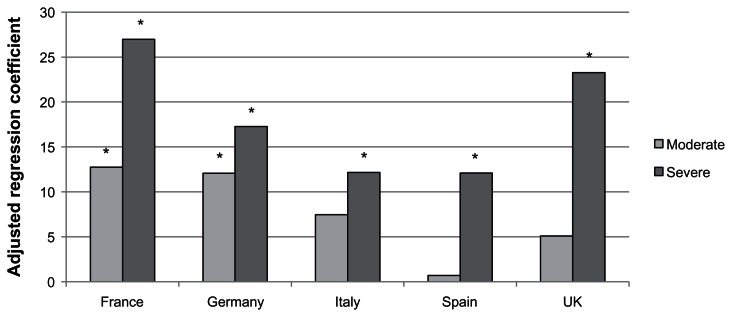
Among women in the 5EU who were currently employed and had experienced VMS, the percentage of work impairment increased concomitantly with symptom severity. Severe VMS was significantly associated with more VMS-related absenteeism (b = 2.34, P < 0.001) than mild VMS, but moderate VMS was not (b = 0.34, P = 0.55). Both moderate (b = 8.77–8.71) and severe (b = 18.41–19.69) VMS were associated with increasing rates of both VMS-related presenteeism and VMS-related overall work impairment, respectively (all P < 0.001), compared with mild VMS. Because overall work impairment is a combination of both absenteeism and presenteeism, country-level differences were examined just on this variable (see Figure 3). Although, within each country, severe VMS was significantly associated with greater overall VMS-related work impairment (b = 12.17–26.98), a significant country by VMS severity interaction was uncovered (P < 0.05). Specifically, moderate VMS had a minimal effect in Spain and was only significantly associated with greater impairment in France (b = 12.75) and Germany (b = 12.07).
Figure 3.
Adjusted regression coefficients of severity of vasomotor symptoms predicting the percentage of overall work impairment in the past seven days.
Notes: *P < 0.05; Covariates included age, education, income, health insurance, BMI and the Charlson comorbidity index.
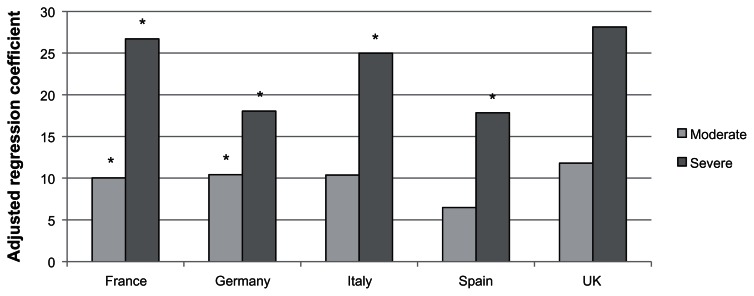
Pooling all countries together, among all women experiencing VMS (not just those employed), both moderate (b = 11.22, P < 0.001) and severe VMS (b = 24.54, P < 0.001) were associated with more VMS-related activity impairment than mild VMS. The overall country by VMS severity interaction term was not significant (P = 0.18). Within each country (except the UK), severe VMS was associated with significantly more activity impairment than mild VMS (see Figure 4; b = 17.86–28.13). However, only in France (b = 10.04) and Germany (b = 10.43) did women with moderate VMS report significantly more activity impairment than women with mild VMS.
Figure 4.
Adjusted regression coefficients of severity of vasomotor symptoms predicting the percentage of activity impairment in the past seven days.
Notes: *P < 0.05; Covariates included age, education, income, health insurance, BMI and the Charlson comorbidity index.
Discussion
Although VMS have been previously reported as one of the most common menopausal symptoms, few studies have been conducted outside the US. Further, few studies have examined economic outcomes associated with the presence and severity of VMS. The aim of the current study was to address this gap by quantifying the burden of VMS in the 5EU. As reported previously, VMS are highly prevalent symptoms, experienced by more than half of women in the 5EU. Although our estimates are lower than previous studies,4 this may have to do with the different countries included.
Consistent with prior research,7–14 our results suggest a significant relationship between VMS and health status, even after accounting for sociodemographic and comorbidity variables. Health status did vary by severity level, suggesting that it is not just the mere presence of the symptom that affects the mental and physical functioning of postmenopausal women. The health status decrements by each severity level were fairly uniform across countries although, because of different sample sizes across countries, not all differences for women with mild VMS were statistically significant. Regardless of country, women with either moderate or severe VMS reported both statistically and clinically meaningful differences in health state utilities compared with those without VMS.
Although direct comparisons with the literature are difficult, these results suggest a substantial burden on postmenopausal women, particularly for those with severe VMS. Indeed, the health utilities observed among these women (mean 0.63) are lower than those observed among patients with obesity, hypertension, hip/knee pain, and depression, and are similar to those observed among patients with asthma and diabetes.25
A significant effect of VMS was also observed with respect to the number of physician visits. In the case of France and, to a lesser extent Spain and the UK, as severity increased, so did the number of visits. Conversely, no differences were observed across severity levels in Germany and Italy, although women with mild, moderate, and severe VMS all reported significantly more physician visits than women without VMS. These data suggest that, for women in Germany and Italy, it is the presence of these symptoms rather than severity that is most strongly associated with physician visits. Given the coefficient levels of the mild group in these two countries (which were higher than France and the UK), it is possible that women in Germany and Italy have a lower threshold for tolerating VMS with respect to initiating visits with their physician. It is also possible that these country differences are merely the result of different health care systems. However, the overall interaction term was not significant, suggesting that further research is required to test these hypotheses accurately and whether these differences are merely a sampling error artifact. Although the health care resource use variables in this study were limited in scope to the number of physician visits, these findings do suggest additional direct costs, on a societal level. Other components of direct cost may also be related to the presence of VMS, and its severity should be included in future research.
Our results also suggest that among women employed who experience VMS, the severity of their symptoms is significantly associated with the degree to which their work is impaired. This was particularly true for women with severe VMS who, after adjusting for sociodemographics and comorbidities, reported approximately 12%–27% more impairment in the previous week relative to women who only experienced mild VMS. For women in France and Germany, similarly strong effects were observed for women with moderate VMS (13% and 12%, respectively, more impairment than women with mild symptoms). Although women who experienced VMS were generally 60–75 years of age, nearly one third remained in the workforce, suggesting a sizeable number of women are affected. Economic calculations were not within the scope of the current project, but these findings would suggest that, from a societal perspective, there are significant indirect costs associated with VMS (particularly with severe VMS) as up to a quarter of work time is impacted from these symptoms.
Very similar findings were observed with impairment of activity. Among all women who experienced VMS (regardless of employment status), severity of VMS symptoms was associated with level of impairment in daily activities. As with work impairment, this was particularly true for women with severe VMS, who experienced an additional 13%–26% impairment (depending upon the country) in their day-to-day activities compared with women with mild VMS. Although prior research has shown an effect of VMS on health status, this is the first study to our knowledge to demonstrate an effect of VMS on the ability of postmenopausal women to engage in leisure activities.
The results of this study complement the existing literature which have, until now, largely focused on the effect of menopausal symptoms on health status in the US. Our findings support the significant and clinically relevant effects that VMS have on health status in France, Germany, Italy, Spain, and the UK. Yet, our results also suggest additional economic effects in Europe which have previously gone unreported. Women with VMS use significantly more health care resources and, among women with VMS, increasing severity is associated with more impairment in work and daily activities. These findings suggest a broader impact of VMS than previously thought, and highlight the need for improved management of these common symptoms to alleviate the health status burden experienced by these women and to reduce both direct and indirect costs from a societal perspective.
Limitations
Because the survey is self-reported, without any clinical verification of comorbidities or physician visits, additional measurement error could have been introduced. One of the key predictors in this study was severity of VMS, but it is unclear whether other aspects of this symptom (eg, frequency) may have a stronger relationship with health outcomes than severity per se. The study was cross-sectional, so the causal relationship between symptoms and health outcomes is only theoretical and cannot be directly supported by the data. Although an attempt was made to rule out alternative explanations, these relationships may be explained by unmeasured confounding variables. For example, time since menopause was not available for the analysis and may help to explain additional variability. It is also possible that the sample from the current study differs meaningfully from the population of women experiencing menopause across these five countries. Although women in the NHWS (the sample source) are demographically representative of each country, it is unclear the extent to which the women who participated in this follow-up survey are representative.
Conclusion
These results suggest a high prevalence of VMS in the 5EU. These symptoms are associated with both humanistic and economic outcomes. Improved management of these symptoms may increase health status in postmenopausal women and their ability to work productively, as well as reducing direct societal costs.
Footnotes
Disclosure
This study was sponsored by Pfizer Inc. Dr. DiBonaventura is an employee of Kantar Health, who were paid consultants to Pfizer in connection with the development of this manuscript.
References
- 1.Feldman BM, Voda A, Gronseth E. The prevalence of hot flash and associated variables among perimenopausal women. Res Nurs Health. 1985;8(3):261–268. doi: 10.1002/nur.4770080308. [DOI] [PubMed] [Google Scholar]
- 2.Freeman EW, Sammel MD, Lin H, Garcia CR, Kapoor S, Ferdousi T. The role of anxiety and hormonal changes in menopausal hot flashes. Menopause. 2005;12(3):258–266. doi: 10.1097/01.gme.0000142440.49698.b7. [DOI] [PubMed] [Google Scholar]
- 3.NIH State-of-the-Science conference statement on management of menopause-related symptoms. NIH Consens State Sci Statements. 2005;22(1):1–38. [No authors listed] [PubMed] [Google Scholar]
- 4.Genazzani AR, Schneider HPG, Panay N, Nijland EA. The European Menopause Survey 2005: women’s perceptions on menopause and postmenopause hormone therapy. Gynecol Endocrinol. 2006;22(7):369–375. doi: 10.1080/09513590600842463. [DOI] [PubMed] [Google Scholar]
- 5.North American Menopause Society. Menopause Practice: A Clinician’s Guide. 4th ed. Mayfield Heights, OH: North American Menopause Society; 2010. [Google Scholar]
- 6.Berecki-Gisolf J, Begum N, Dobson AJ. Symptoms reported by women in midlife: menopausal transition or aging? Menopause. 2009;16(5):1021–1029. doi: 10.1097/gme.0b013e3181a8c49f. [DOI] [PubMed] [Google Scholar]
- 7.Williams RE, Kalilani L, DiBenedetti DB, Zhou X, Fehnel SE, Clark RV. Healthcare seeking and treatment for menopausal symptoms in the United States. Maturitas. 2007;58(4):348–358. doi: 10.1016/j.maturitas.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Karaçam Z, Seker SE. Factors associated with menopausal symptoms and their relationship with the quality of life among Turkish women. Maturitas. 2007;58(1):75–82. doi: 10.1016/j.maturitas.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Avis NE, Ory M, Matthews KA, Schocken M, Bromberger J, Colvin A. Health-related quality of life in a multiethnic sample of middle-aged women: Study of Women’s Health Across the Nation (SWAN) Med Care. 2003;41(11):1262–1276. doi: 10.1097/01.MLR.0000093479.39115.AF. [DOI] [PubMed] [Google Scholar]
- 10.Avis NE, Colvin A, Bromberger JT, et al. Change in health-related quality of life over the menopausal transition in a multiethnic cohort of middle-aged women: Study of Women’s Health Across the Nation (SWAN) Menopause. 2009;16(5):860–869. doi: 10.1097/gme.0b013e3181a3cdaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantril H. The Pattern of Human Concerns. New Brunswick, NJ: Rutgers University Press; 1965. [Google Scholar]
- 12.Gallicchio L, Miller S, Zacur H, Flaws JA. Race and health-related quality of life in midlife women in Baltimore, Maryland. Maturitas. 2009;63(1):67–72. doi: 10.1016/j.maturitas.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams RE, Levine KB, Kalilani L, Lewis J, Clark RV. Menopause-specific questionnaire assessment in US population-based study shows negative impact on health-related quality of life. Maturitas. 2009;62(2):153–159. doi: 10.1016/j.maturitas.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Daly E, Gray A, Barlow D, McPherson K, Roche M, Vessey M. Measuring the impact of menopausal symptoms on quality of life. BMJ. 1993;307(6908):836–840. doi: 10.1136/bmj.307.6908.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hess R, Chang C-C, Ness RB, Hays RD, Kapoor WN, Bryce CL. The associations of menopause and health-related quality of life with health services utilization: results from the STRIDE study. Abstract presented at the 32nd Annual Meeting of the Society of Medical Decision Making; Toronto, Ontario, Canada. October 24–27, 2010. [Google Scholar]
- 16.DiBonaventura MD, Wagner JS, Yuan Y, L’Italien G, Langley P, Ray Kim W. Humanistic and economic impacts of hepatitis C infection in the United States. J Med Econ. 2010;13(4):709–718. doi: 10.3111/13696998.2010.535576. [DOI] [PubMed] [Google Scholar]
- 17.Langley P, Muller-Schwerfe G, Nicolaou A, Liedgens H, Pergolizzi J, Varrassi G. The impact of pain on labor force participation, absenteeism and presenteeism in the European Union. J Med Econ. 2010;13(4):662–672. doi: 10.3111/13696998.2010.529379. [DOI] [PubMed] [Google Scholar]
- 18.Liu GG, DiBonaventura MD, Yuan Y, et al. The burden of illness for patients with viral hepatitis C: evidence from a national survey in Japan. Value Health. 2012;15(Suppl 1):S65–S72. doi: 10.1016/j.jval.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Finkelstein EA, Allaire BT, DiBonaventura MD, Burgess SM. Direct and indirect costs and potential cost savings of laparoscopic adjustable gastric banding among obese patients with diabetes. J Occup Environ Med. 2011;53(9):1025–1029. doi: 10.1097/JOM.0b013e318229aae4. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Hauser GA, Huber IC, Keller PJ, Lauritzen C, Schneider HPG. Evaluation of the Menopause Rating Scale. Zentralbl Gynakol. 1994;116(1):16–23. German. [PubMed] [Google Scholar]
- 22.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 23.Walters SJ, Brazier JE. What is the relationship between the minimally important difference and health state utility values? The case of the SF-6D. Health Qual Life Outcomes. 2003;1:4. doi: 10.1186/1477-7525-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 25.Kontodimopoulos N, Pappa E, Papadopoulos AA, Tountas Y, Niakas D. Comparing SF-6D and EQ-5D utilities across groups differing in health status. Qual Life Res. 2009;18(1):87–97. doi: 10.1007/s11136-008-9420-8. [DOI] [PubMed] [Google Scholar]