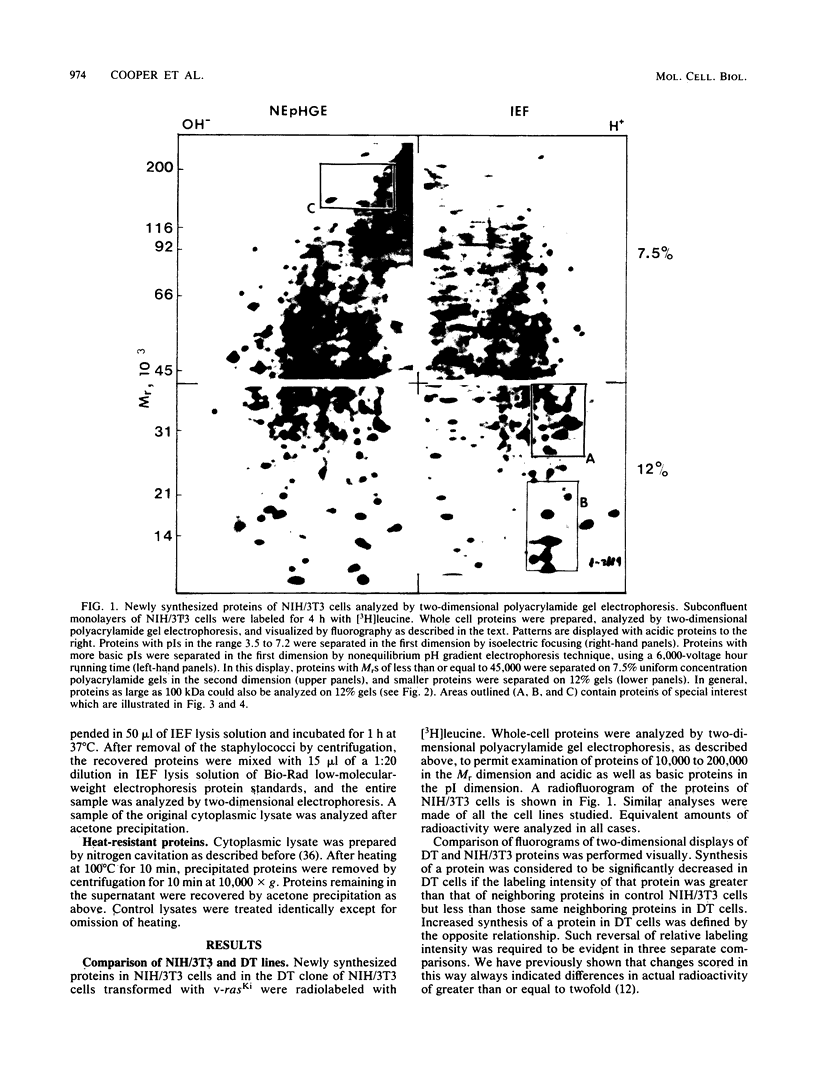
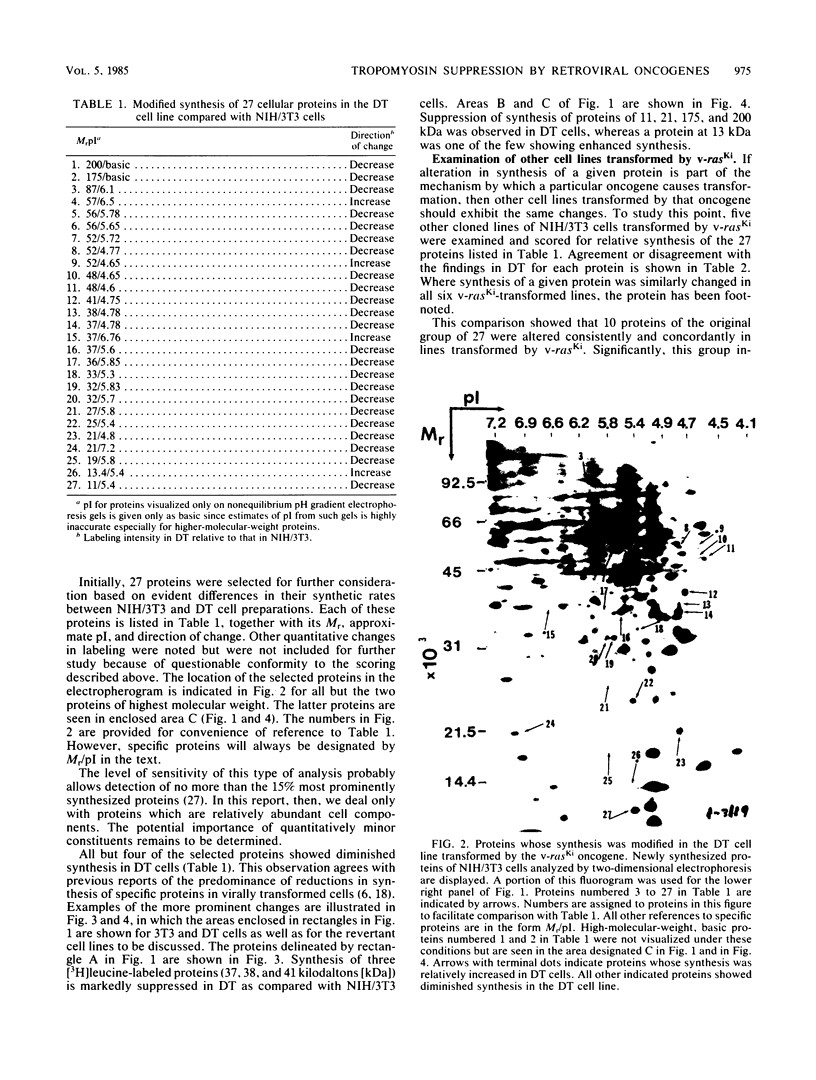
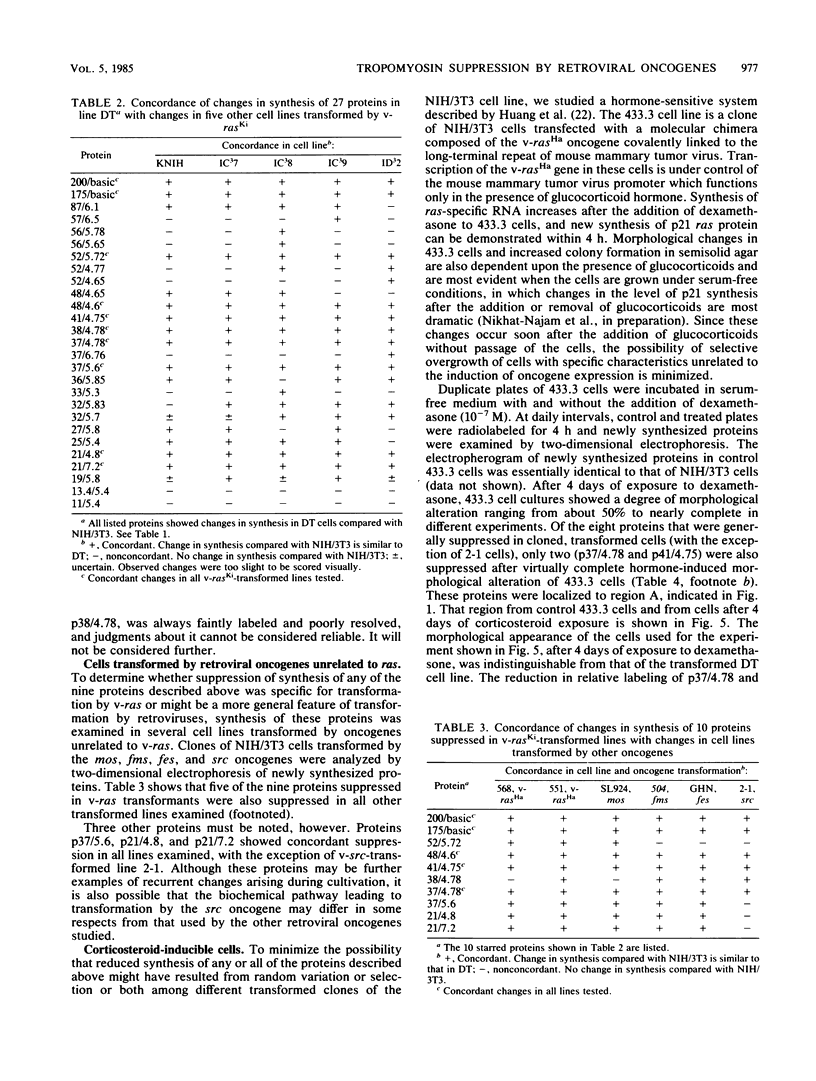
Abstract
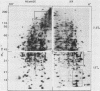
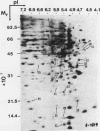
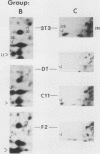
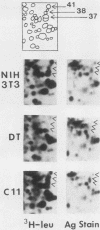
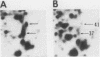
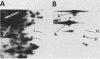
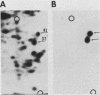
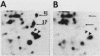
To identify proteins whose production may be altered as a common event in the expression of structurally diverse oncogenes, we compared two-dimensional electropherograms of newly synthesized proteins from NIH/3T3 cell lines transformed by a variety of retroviral oncogenes, from cellular revertant lines, and from a line (433.3) which expresses the v-ras oncogene in response to corticosteroids. Most alterations in the synthesis of specific proteins detected by this approach appeared to be the result of selection during prolonged cultivation and were probably unrelated to the transformation process. However, we detected seven proteins whose synthesis was strongly suppressed in cell lines transformed by each of the six retroviral oncogenes we studied and whose production was fully or partially restored in two cellular revertant lines. Suppression of two of these proteins was also correlated with the initial appearance of morphological alteration during corticosteroid-induced oncogene expression in 433.3 cells. These proteins (p37/4.78 and p41/4.75) were identified as tropomyosins, a group of at least five cytoskeletal proteins. Transformation by the papovaviruses simian virus 40 and polyomavirus caused no suppression of synthesis of these tropomyosins. This indicates that suppression of tropomyosin synthesis is not a nonspecific response by cells to being forced to grow with the transformed phenotype but is specifically associated with oncogenesis by diverse retroviral oncogenes. The results are consistent with the hypothesis that the different biochemical processes initiated by expression of structurally diverse retroviral oncogenes may converge on a limited number of common targets, one of which is the mechanism which regulates the synthesis of tropomyosins.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. M., Scolnick E. M. Construction and isolation of a transforming murine retrovirus containing the src gene of Rous sarcoma virus. J Virol. 1983 May;46(2):594–605. doi: 10.1128/jvi.46.2.594-605.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey K. Tropomyosin: a new asymmetric protein component of the muscle fibril. Biochem J. 1948;43(2):271–279. doi: 10.1042/bj0430271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein B. W., Bamburg J. R. Tropomyosin binding to F-actin protects the F-actin from disassembly by brain actin-depolymerizing factor (ADF). Cell Motil. 1982;2(1):1–8. doi: 10.1002/cm.970020102. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. Gene expression in normal and virally transformed mouse 3T3b and hamster BHK21 cells. Exp Cell Res. 1980 Jun;127(2):249–260. doi: 10.1016/0014-4827(80)90430-9. [DOI] [PubMed] [Google Scholar]
- Brown S., Levinson W., Spudich J. A. Cytoskeletal elements of chick embryo fibroblasts revealed by detergent extraction. J Supramol Struct. 1976;5(2):119–130. doi: 10.1002/jss.400050203. [DOI] [PubMed] [Google Scholar]
- Byers H. R., White G. E., Fujiwara K. Organization and function of stress fibers in cells in vitro and in situ. A review. Cell Muscle Motil. 1984;5:83–137. doi: 10.1007/978-1-4684-4592-3_2. [DOI] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Varmus H. E., Bishop J. M., Essex M., Hardy W. D., Jr, Martin G. S., Rosenberg N. E., Scolnick E. M., Weinberg R. A., Vogt P. K. Proposal for naming host cell-derived inserts in retrovirus genomes. J Virol. 1981 Dec;40(3):953–957. doi: 10.1128/jvi.40.3.953-957.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Cohen C. A tropomyosin-like protein from human platelets. J Mol Biol. 1972 Jul 21;68(2):383–387. doi: 10.1016/0022-2836(72)90220-3. [DOI] [PubMed] [Google Scholar]
- Cooper H. L., Fagnani R., London J., Trepel J., Lester E. P. Effect of interferons on protein synthesis in human lymphocytes: enhanced synthesis of eight specific peptides in T cells and activation-dependent inhibition of overall protein synthesis. J Immunol. 1982 Feb;128(2):828–833. [PubMed] [Google Scholar]
- Cooper H. L., Park M. H., Folk J. E. Posttranslational formation of hypusine in a single major protein occurs generally in growing cells and is associated with activation of lymphocyte growth. Cell. 1982 Jul;29(3):791–797. doi: 10.1016/0092-8674(82)90441-x. [DOI] [PubMed] [Google Scholar]
- Der C. J., Krontiris T. G., Cooper G. M. Transforming genes of human bladder and lung carcinoma cell lines are homologous to the ras genes of Harvey and Kirsten sarcoma viruses. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3637–3640. doi: 10.1073/pnas.79.11.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. W., Defeo D., Shih T. Y., Gonda M. A., Young H. A., Tsuchida N., Lowy D. R., Scolnick E. M. The p21 src genes of Harvey and Kirsten sarcoma viruses originate from divergent members of a family of normal vertebrate genes. Nature. 1981 Aug 6;292(5823):506–511. doi: 10.1038/292506a0. [DOI] [PubMed] [Google Scholar]
- Even J., Anderson S. J., Hampe A., Galibert F., Lowy D., Khoury G., Sherr C. J. Mutant feline sarcoma proviruses containing the viral oncogene (v-fes) and either feline or murine control elements. J Virol. 1983 Mar;45(3):1004–1016. doi: 10.1128/jvi.45.3.1004-1016.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattoum A., Hartwig J. H., Stossel T. P. Isolation and some structural and functional properties of macrophage tropomyosin. Biochemistry. 1983 Mar 1;22(5):1187–1193. doi: 10.1021/bi00274a031. [DOI] [PubMed] [Google Scholar]
- Fransen L., Van Roy F., Fiers W. Changes in gene expression and protein phosphorylation in murine cells, transformed or abortively infected with wild type and mutant simian virus 40. J Biol Chem. 1983 Apr 25;258(8):5276–5290. [PubMed] [Google Scholar]
- Goldfarb M., Shimizu K., Perucho M., Wigler M. Isolation and preliminary characterization of a human transforming gene from T24 bladder carcinoma cells. Nature. 1982 Apr 1;296(5856):404–409. doi: 10.1038/296404a0. [DOI] [PubMed] [Google Scholar]
- Hendricks M., Weintraub H. Multiple tropomyosin polypeptides in chicken embryo fibroblasts: differential repression of transcription by Rous sarcoma virus transformation. Mol Cell Biol. 1984 Sep;4(9):1823–1833. doi: 10.1128/mcb.4.9.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks M., Weintraub H. Tropomyosin is decreased in transformed cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5633–5637. doi: 10.1073/pnas.78.9.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. L., Ostrowski M. C., Berard D., Hager G. L. Glucocorticoid regulation of the Ha-MuSV p21 gene conferred by sequences from mouse mammary tumor virus. Cell. 1981 Dec;27(2 Pt 1):245–255. doi: 10.1016/0092-8674(81)90408-6. [DOI] [PubMed] [Google Scholar]
- Leavitt J., Bushar G., Kakunaga T., Hamada H., Hirakawa T., Goldman D., Merril C. Variations in expression of mutant beta actin accompanying incremental increases in human fibroblast tumorigenicity. Cell. 1982 Feb;28(2):259–268. doi: 10.1016/0092-8674(82)90344-0. [DOI] [PubMed] [Google Scholar]
- Leavitt J., Kakunaga T. Expression of a variant form of actin and additional polypeptide changes following chemical-induced in vitro neoplastic transformation of human fibroblasts. J Biol Chem. 1980 Feb 25;255(4):1650–1661. [PubMed] [Google Scholar]
- Leonardi C. L., Warren R. H., Rubin R. W. Lack of tropomyosin correlates with the absence of stress fibers in transformed rat kidney cells. Biochim Biophys Acta. 1982 Apr 29;720(2):154–162. doi: 10.1016/0167-4889(82)90007-6. [DOI] [PubMed] [Google Scholar]
- Lester E. P., Lemkin P., Lipkin L., Cooper H. L. Computer-assisted analysis of two-dimensional electrophoreses of human lymphoid cells. Clin Chem. 1980 Sep;26(10):1392–1402. [PubMed] [Google Scholar]
- Matsumura F., Lin J. J., Yamashiro-Matsumura S., Thomas G. P., Topp W. C. Differential expression of tropomyosin forms in the microfilaments isolated from normal and transformed rat cultured cells. J Biol Chem. 1983 Nov 25;258(22):13954–13964. [PubMed] [Google Scholar]
- Matsumura F., Yamashiro-Matsumura S., Lin J. J. Isolation and characterization of tropomyosin-containing microfilaments from cultured cells. J Biol Chem. 1983 May 25;258(10):6636–6644. [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Noda M., Selinger Z., Scolnick E. M., Bassin R. H. Flat revertants isolated from Kirsten sarcoma virus-transformed cells are resistant to the action of specific oncogenes. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5602–5606. doi: 10.1073/pnas.80.18.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R., Osborn M., Weber K. Patterns of organization of actin and myosin in normal and transformed cultured cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):994–998. doi: 10.1073/pnas.72.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulciani S., Santos E., Lauver A. V., Long L. K., Aaronson S. A., Barbacid M. Oncogenes in solid human tumours. Nature. 1982 Dec 9;300(5892):539–542. doi: 10.1038/300539a0. [DOI] [PubMed] [Google Scholar]
- Resch K., Schneider S., Szamel M. Separation of right-side-out-oriented subfractions from purified thymocyte plasma membranes by affinity chromatography on concanavalin A-sepharose. Anal Biochem. 1981 Nov 1;117(2):282–292. doi: 10.1016/0003-2697(81)90724-7. [DOI] [PubMed] [Google Scholar]
- Schloss J. A., Goldman R. D. Isolation of a high molecular weight actin-binding protein from baby hamster kidney (BHK-21) cells. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4484–4488. doi: 10.1073/pnas.76.9.4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C., Weinberg R. A. Isolation of a transforming sequence from a human bladder carcinoma cell line. Cell. 1982 May;29(1):161–169. doi: 10.1016/0092-8674(82)90100-3. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O. Oncogenes and cancer: the p21 ras genes. Cancer Invest. 1984;2(2):109–123. doi: 10.3109/07357908409020294. [DOI] [PubMed] [Google Scholar]
- Stone D., Smillie L. B. The amino acid sequence of rabbit skeletal alpha-tropomyosin. The NH2-terminal half and complete sequence. J Biol Chem. 1978 Feb 25;253(4):1137–1148. [PubMed] [Google Scholar]
- Talbot K., MacLeod A. R. Novel form of non-muscle tropomyosin in human fibroblasts. J Mol Biol. 1983 Feb 15;164(1):159–174. doi: 10.1016/0022-2836(83)90091-8. [DOI] [PubMed] [Google Scholar]