Abstract
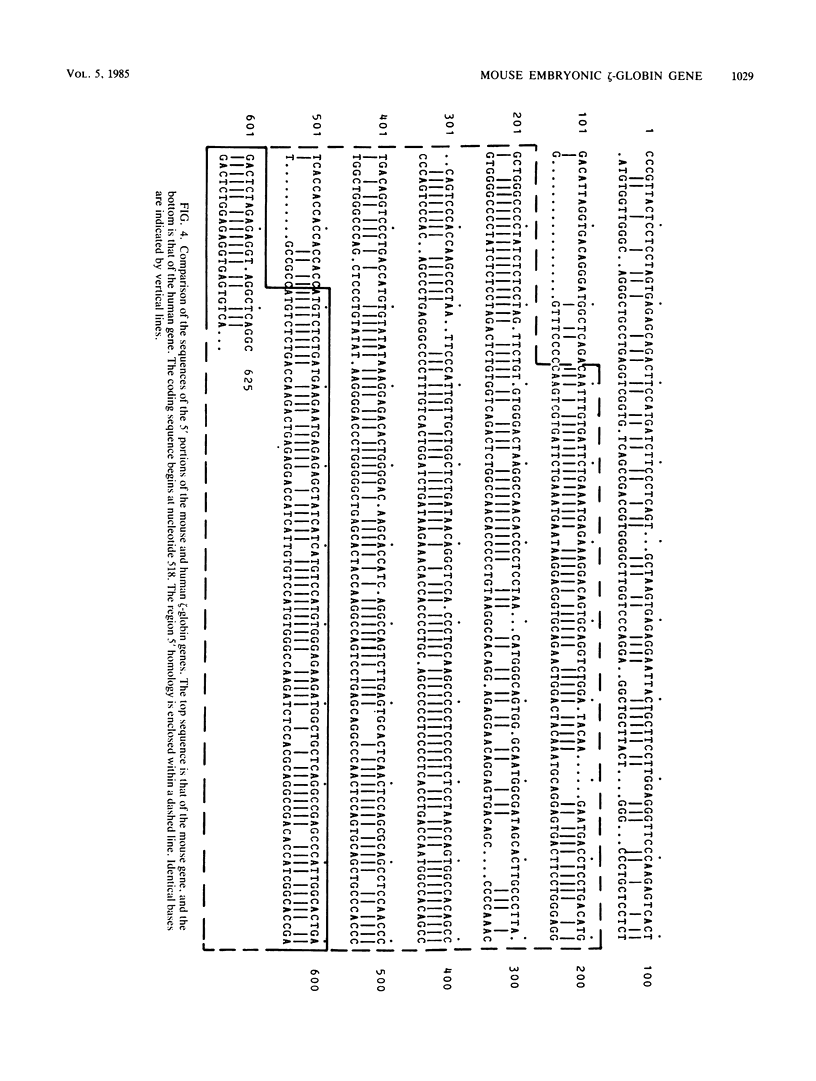
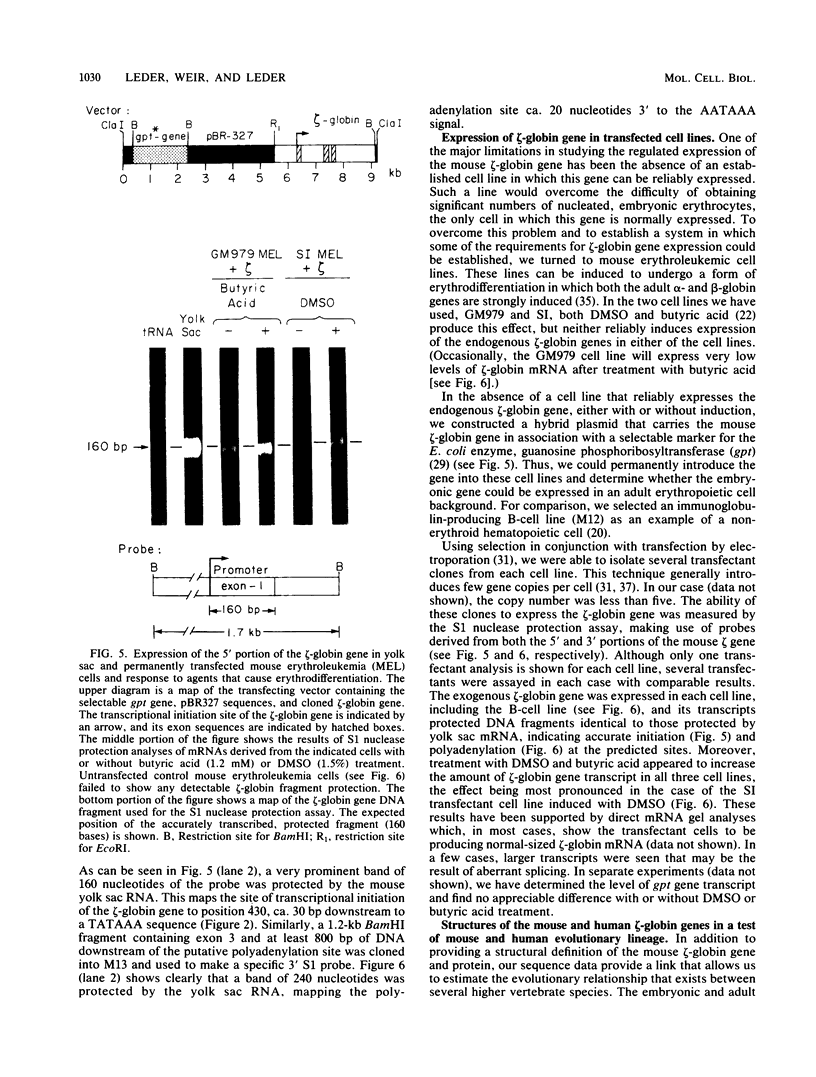
We have determined the complete sequence of the embryonic alpha-like, zeta (zeta)-globin gene of the BALB/c mouse. The structure of this gene establishes the amino acid sequence of the mouse embryonic zeta-globin polypeptide chain and allows us to identify sequences within the gene that may be important for its expression. One of these is a 300-base segment that is tightly conserved between mice and humans and is located at the 5' end of the zeta-globin gene. By introducing the cloned gene into permanently transfected mouse erythroleukemic cell lines and comparing its transcript with that of zeta-globin mRNA derived from embryonic yolk sac erythrocytes, we are able to show that the cloned gene is transcriptionally active and that its transcript is correctly initiated and processed. Interestingly, the zeta-globin gene is also active when permanently transfected into an immunoglobulin-producing B-cell, a cell that presumably has tissue-specific requirements for gene expression. Further, a comparison of the amino acid coding sequence of the mouse zeta-globin gene to that of zeta-like globin genes of other species supports a revised evolutionary lineage in which goats and humans are closely related, whereas mice are further removed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Barker J. E. Development of the mouse hematopoietic system. I. Types of hemoglobin produced in embryonic yolk sac and liver. Dev Biol. 1968 Jul;18(1):14–29. doi: 10.1016/0012-1606(68)90020-1. [DOI] [PubMed] [Google Scholar]
- Battey J., Moulding C., Taub R., Murphy W., Stewart T., Potter H., Lenoir G., Leder P. The human c-myc oncogene: structural consequences of translocation into the IgH locus in Burkitt lymphoma. Cell. 1983 Oct;34(3):779–787. doi: 10.1016/0092-8674(83)90534-2. [DOI] [PubMed] [Google Scholar]
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Brotherton T. W., Chui D. H., Gauldie J., Patterson M. Hemoglobin ontogeny during normal mouse fetal development. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2853–2857. doi: 10.1073/pnas.76.6.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAIG M. L., RUSSELL E. S. A DEVELOPMENTAL CHANGE IN HEMOGLOBINS CORRELATED WITH AN EMBRYONIC RED CELL POPULATION IN THE MOUSE. Dev Biol. 1964 Oct;10:191–201. doi: 10.1016/0012-1606(64)90040-5. [DOI] [PubMed] [Google Scholar]
- Camper S. A., Luck D. N., Yao Y., Woychik R. P., Goodwin R. G., Lyons R. H., Jr, Rottman F. M. Characterization of the bovine prolactin gene. DNA. 1984 Jun;3(3):237–249. doi: 10.1089/dna.1.1984.3.237. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cudennec C. A., Thiery J. P., Le Douarin N. M. In vitro induction of adult erythropoiesis in early mouse yolk sac. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2412–2416. doi: 10.1073/pnas.78.4.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czelusniak J., Goodman M., Hewett-Emmett D., Weiss M. L., Venta P. J., Tashian R. E. Phylogenetic origins and adaptive evolution of avian and mammalian haemoglobin genes. Nature. 1982 Jul 15;298(5871):297–300. doi: 10.1038/298297a0. [DOI] [PubMed] [Google Scholar]
- Dodgson J. B., Engel J. D. The nucleotide sequence of the adult chicken alpha-globin genes. J Biol Chem. 1983 Apr 10;258(7):4623–4629. [PubMed] [Google Scholar]
- Dodgson J. B., McCune K. C., Rusling D. J., Krust A., Engel J. D. Adult chicken alpha-globin genes alpha A and alpha D: no anemic shock alpha-globin exists in domestic chickens. Proc Natl Acad Sci U S A. 1981 Oct;78(10):5998–6002. doi: 10.1073/pnas.78.10.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Engel J. D., Rusling D. J., McCune K. C., Dodgson J. B. Unusual structure of the chicken embryonic alpha-globin gene, pi'. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1392–1396. doi: 10.1073/pnas.80.5.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris J. S. Definitions of taxa. Syst Zool. 1967 Jun;16(2):174–175. [PubMed] [Google Scholar]
- Ferris S. D., Wilson A. C., Brown W. M. Evolutionary tree for apes and humans based on cleavage maps of mitochondrial DNA. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2432–2436. doi: 10.1073/pnas.78.4.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour R. S., Spandidos D. A., Vass J. K., Gow J. W., Paul J. A negative regulatory sequence near the mouse beta-maj globin gene associated with a region of potential Z-DNA. EMBO J. 1984 Jun;3(6):1263–1272. doi: 10.1002/j.1460-2075.1984.tb01961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher L. H., Kim K. J., Green I., Paul W. E. Ia antigen-bearing B cell tumor lines can present protein antigen and alloantigen in a major histocompatibility complex-restricted fashion to antigen-reactive T cells. J Exp Med. 1982 Feb 1;155(2):445–459. doi: 10.1084/jem.155.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer J., Shen C. K., Maniatis T. The chromosomal arrangement of human alpha-like globin genes: sequence homology and alpha-globin gene deletions. Cell. 1980 May;20(1):119–130. doi: 10.1016/0092-8674(80)90240-8. [DOI] [PubMed] [Google Scholar]
- Leder A., Leder P. Butyric acid, a potent inducer of erythroid differentiation in cultured erythroleukemic cells. Cell. 1975 Jul;5(3):319–322. doi: 10.1016/0092-8674(75)90107-5. [DOI] [PubMed] [Google Scholar]
- Leder A., Swan D., Ruddle F., D'Eustachio P., Leder P. Dispersion of alpha-like globin genes of the mouse to three different chromosomes. Nature. 1981 Sep 17;293(5829):196–200. doi: 10.1038/293196a0. [DOI] [PubMed] [Google Scholar]
- Leder P., Tiemeier D., Enquist L. EK2 derivatives of bacteriophage lambda useful in the cloning of DNA from higher organisms: the lambdagtWES system. Science. 1977 Apr 8;196(4286):175–177. doi: 10.1126/science.322278. [DOI] [PubMed] [Google Scholar]
- Ley T. J., Anagnou N. P., Pepe G., Nienhuis A. W. RNA processing errors in patients with beta-thalassemia. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4775–4779. doi: 10.1073/pnas.79.15.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebhaber S. A., Goossens M., Kan Y. W. Homology and concerted evolution at the alpha 1 and alpha 2 loci of human alpha-globin. Nature. 1981 Mar 5;290(5801):26–29. doi: 10.1038/290026a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson A. M., Orkin S. H. The 3' untranslated regions of the duplicated human alpha-globin genes are unexpectedly divergent. Cell. 1980 Nov;22(2 Pt 2):371–377. doi: 10.1016/0092-8674(80)90347-5. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Leder P. The complete sequence of a chromosomal mouse alpha--globin gene reveals elements conserved throughout vertebrate evolution. Cell. 1979 Nov;18(3):875–882. doi: 10.1016/0092-8674(79)90139-9. [DOI] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Gil A., Maniatis T. The structure of the human zeta-globin gene and a closely linked, nearly identical pseudogene. Cell. 1982 Dec;31(3 Pt 2):553–563. doi: 10.1016/0092-8674(82)90311-7. [DOI] [PubMed] [Google Scholar]
- Rich A. Right-handed and left-handed DNA: conformational information in genetic material. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):1–12. doi: 10.1101/sqb.1983.047.01.003. [DOI] [PubMed] [Google Scholar]
- Ross J., Aviv H., Leder P. Characterization of synthetic rabbit globin DNA. Arch Biochem Biophys. 1973 Oct;158(2):494–502. doi: 10.1016/0003-9861(73)90541-9. [DOI] [PubMed] [Google Scholar]
- Tiemeier D. C., Tilghman S. M., Leder P. Purification and cloning of a mouse ribosomal gene fragment in coliphage lambda. Gene. 1977;2(3-4):173–191. doi: 10.1016/0378-1119(77)90016-6. [DOI] [PubMed] [Google Scholar]
- Wong T. K., Neumann E. Electric field mediated gene transfer. Biochem Biophys Res Commun. 1982 Jul 30;107(2):584–587. doi: 10.1016/0006-291x(82)91531-5. [DOI] [PubMed] [Google Scholar]
- Yoshida A., Lin M. NH 2 -terminal formylmethionine- and NH 2 -terminal methionine-cleaving enzymes in rabbits. J Biol Chem. 1972 Feb 10;247(3):952–957. [PubMed] [Google Scholar]