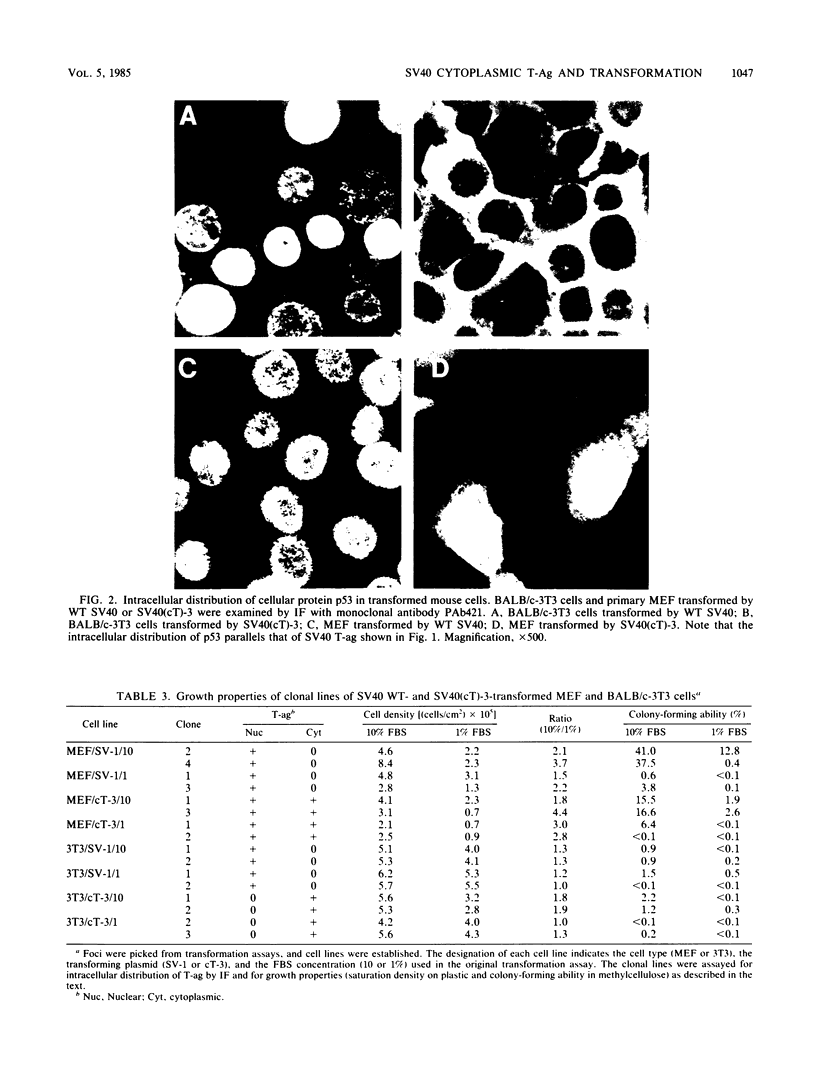
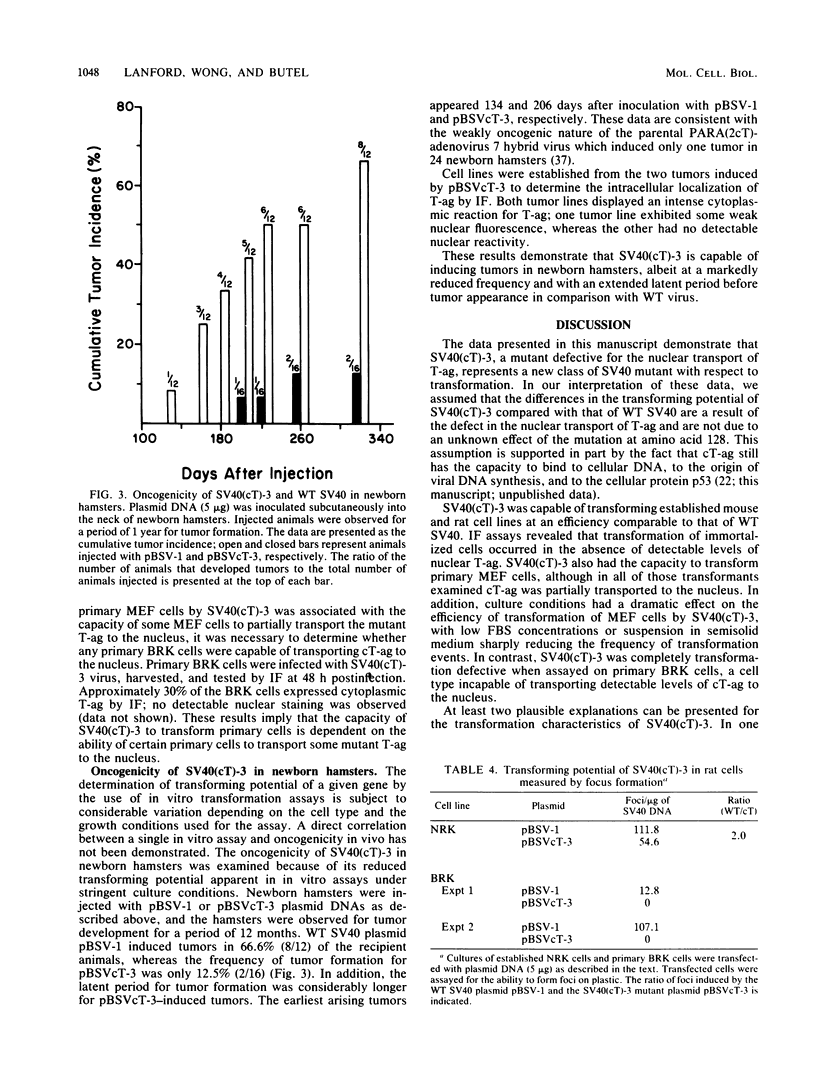
Abstract
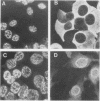
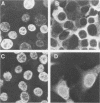
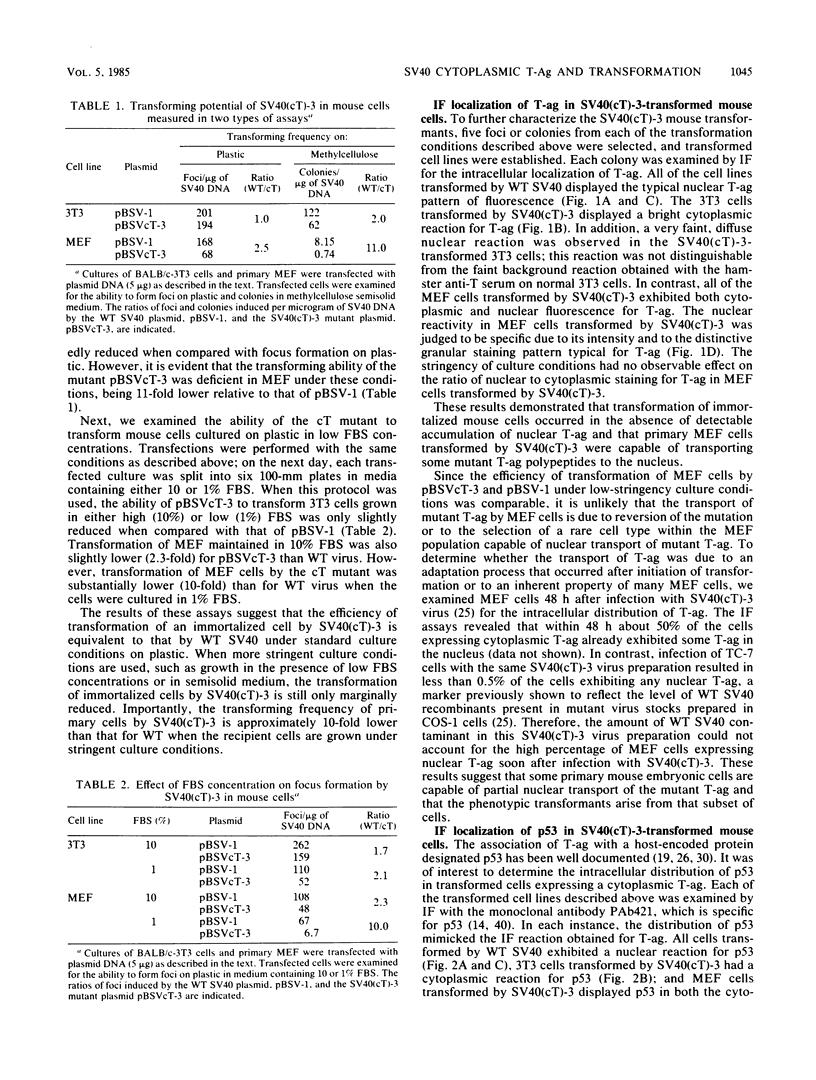
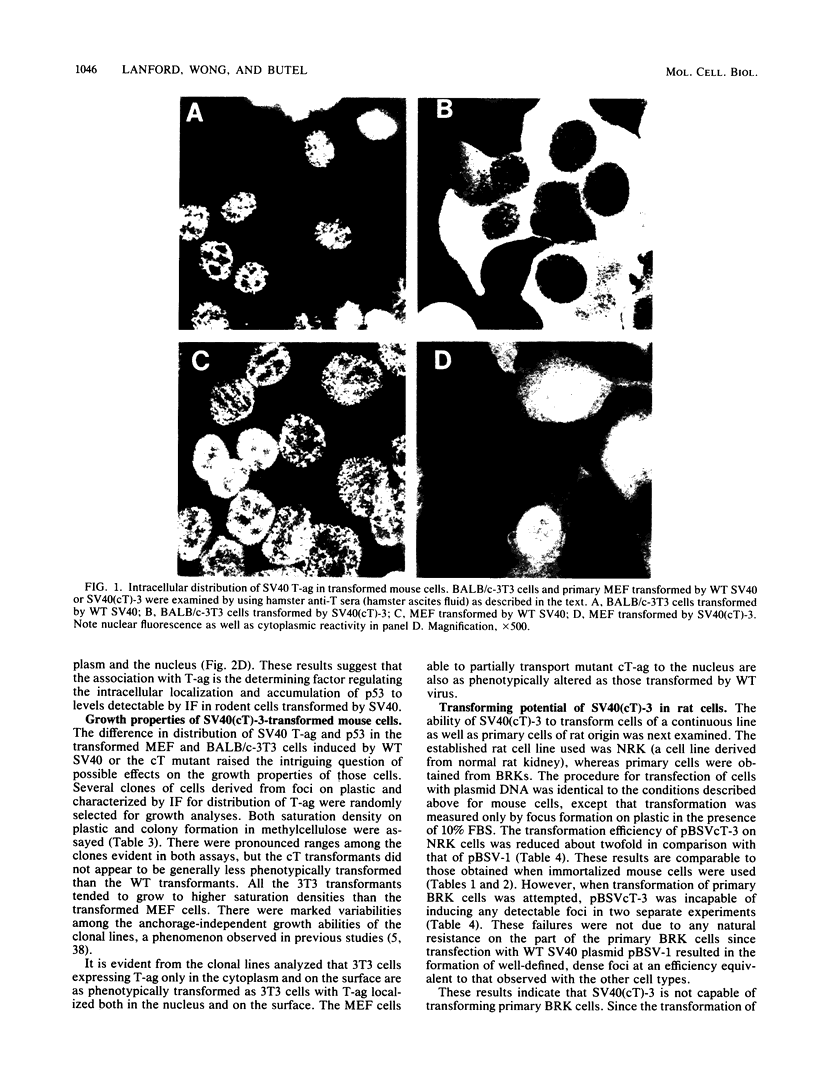
The transforming potential and oncogenicity of a simian virus 40 (SV40) mutant affecting T-antigen (T-ag), SV40(cT)-3, was examined in an effort to dissect T-ag functions in transformation. SV40(cT)-3 has a point mutation at nucleotide 4434 that abolishes the transport of T-ag to the nucleus but does not affect its association with the cell surface. Transfection-transformation assays were performed with primary cells and established cell lines of mouse and rat origin. The efficiency of transformation for established cell lines by SV40(cT)-3 was comparable to that of wild-type SV40, indicating that transformation of established cell lines can occur in the absence of detectable amounts of nuclear T-ag. Transformation of primary mouse embryo fibroblasts by SV40(cT)-3 was markedly influenced by culture conditions; the relative transforming frequency was dramatically reduced in assays involving focus formation in low serum concentrations or anchorage-independent growth. Immunofluorescence tests revealed that the transformed mouse embryo fibroblasts partially transport the mutant cT-ag to the cell nucleus. Transformed cell lines induced by SV40(cT)-3 did not differ in growth properties from wild-type transformants. SV40(cT)-3 was completely defective for the transformation of primary baby rat kidney cells, a primary cell type unable to transport the mutant T-ag to the nucleus. The intracellular localization of cellular protein p53 was found to mimic T-ag distribution in all the transformants analyzed. The mutant virus was weakly oncogenic in vivo: the induction of tumors in newborn hamsters by SV40(cT)-3 was reduced in incidence and delayed in appearance in comparison to wild-type SV40. These observations suggest that cellular transformation is regulated by both nuclear and surface-associated forms of SV40 T-ag.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouchard L., Gelinas C., Asselin C., Bastin M. Tumorigenic activity of polyoma virus and SV40 DNAs in newborn rodents. Virology. 1984 May;135(1):53–64. doi: 10.1016/0042-6822(84)90116-8. [DOI] [PubMed] [Google Scholar]
- Brockman W. W. Transformation of BALB/c-3T3 cells by tsA mutants of simian virus 40: temperature sensitivity of the transformed phenotype and retransofrmation by wild-type virus. J Virol. 1978 Mar;25(3):860–870. doi: 10.1128/jvi.25.3.860-870.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Butel J. S. Role of simian virus 40 gene A function in maintenance of transformation. J Virol. 1975 Mar;15(3):619–635. doi: 10.1128/jvi.15.3.619-635.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butel J. S., Guentzel M. J., Rapp F. Variants of defective simian papovavirus 40 (PARA) characterized by cytoplasmic localization of simian papovavirus 40 tumor antigen. J Virol. 1969 Nov;4(5):632–641. doi: 10.1128/jvi.4.5.632-641.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V., Cole C. N., Smith A. E., Paucha E., Tegtmeyer P., Rundell K., Berg P. Organization and expression of early genes of simian virus 40. Proc Natl Acad Sci U S A. 1978 Jan;75(1):117–121. doi: 10.1073/pnas.75.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W., Hanke K., Henning R. Simian virus 40 T-antigen-related cell surface antigen: serological demonstration on simian virus 40-transformed monolayer cells in situ. J Virol. 1980 Aug;35(2):505–518. doi: 10.1128/jvi.35.2.505-518.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W., Walter G. Domains of simian virus 40 large T-antigen exposed on the cell surface. Virology. 1982 Oct 15;122(1):56–70. doi: 10.1016/0042-6822(82)90377-4. [DOI] [PubMed] [Google Scholar]
- Duff R., Rapp F. Transformation of hamster cells by variants of PARA-adenovirus 7 Able to induce SV40 tumor antigen in the cytoplasm. Virology. 1970 Sep;42(1):273–275. doi: 10.1016/0042-6822(70)90269-2. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Friedmann T., Esty A., LaPorte P., Deininger P. The nucleotide sequence and genome organization of the polyoma early region: extensive nucleotide and amino acid homology with SV40. Cell. 1979 Jul;17(3):715–724. doi: 10.1016/0092-8674(79)90278-2. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Brocklehurst J. R., Dulbecco R. Virus-specific proteins in the plasma membrane of cells lytically infected or transformed by pol-oma virus. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4666–4670. doi: 10.1073/pnas.74.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D., Richardson W. D., Markham A. F., Smith A. E. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature. 1984 Sep 6;311(5981):33–38. doi: 10.1038/311033a0. [DOI] [PubMed] [Google Scholar]
- Kimura G., Itagaki A. Initiation and maintenance of cell transformation by simian virus 40: a viral genetic property. Proc Natl Acad Sci U S A. 1975 Feb;72(2):673–677. doi: 10.1073/pnas.72.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Butel J. S. Antigenic relationship of SV40 early proteins to purified large T polypeptide. Virology. 1979 Sep;97(2):295–306. doi: 10.1016/0042-6822(79)90341-6. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Butel J. S. Biochemical characterization of nuclear and cytoplasmic forms of SV40 tumor antigens encoded by parental and transport-detective mutant SV40-adenovirus 7 hybrid viruses. Virology. 1980 Sep;105(2):314–327. doi: 10.1016/0042-6822(80)90033-1. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Butel J. S. Construction and characterization of an SV40 mutant defective in nuclear transport of T antigen. Cell. 1984 Jul;37(3):801–813. doi: 10.1016/0092-8674(84)90415-x. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Butel J. S. Effect of nuclear localization of large tumor antigen on growth potential of SV40-transformed cells. Virology. 1981 Apr 15;110(1):147–158. doi: 10.1016/0042-6822(81)90016-7. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Butel J. S. Inhibition of nuclear migration of wild-type SV40 tumor antigen by a transport-defective mutant of SV40-adenovirus 7 hybrid virus. Virology. 1980 Sep;105(2):303–313. doi: 10.1016/0042-6822(80)90032-x. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Butel J. S. Intracellular transport of SV40 large tumor antigen: a mutation which abolishes migration to the nucleus does not prevent association with the cell surface. Virology. 1982 May;119(1):169–184. doi: 10.1016/0042-6822(82)90074-5. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Levine A. J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979 May;17(1):43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G., Chou J. Y. Simian virus 40 functions required for the establishment and maintenance of malignant transformation. J Virol. 1975 Mar;15(3):599–612. doi: 10.1128/jvi.15.3.599-612.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G., Setlow V. P., Edwards C. A., Vembu D. The roles of the simian virus 40 tumor antigens in transformation of Chinese hamster lung cells. Cell. 1979 Jul;17(3):635–643. doi: 10.1016/0092-8674(79)90271-x. [DOI] [PubMed] [Google Scholar]
- McCormick F., Harlow E. Association of a murine 53,000-dalton phosphoprotein with simian virus 40 large-T antigen in transformed cells. J Virol. 1980 Apr;34(1):213–224. doi: 10.1128/jvi.34.1.213-224.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. Simian virus 40 gene A function and maintenance of transformation. J Virol. 1975 Mar;15(3):636–644. doi: 10.1128/jvi.15.3.636-644.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp F., Pauluzzi S., Butel J. S. Variation in properties of plaque progeny of PARA (defective simian papovavirus 40)-adenovirus 7. J Virol. 1969 Nov;4(5):626–631. doi: 10.1128/jvi.4.5.626-631.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulzadegan M., Cowie A., Carr A., Glaichenhaus N., Kamen R., Cuzin F. The roles of individual polyoma virus early proteins in oncogenic transformation. Nature. 1982 Dec 23;300(5894):713–718. doi: 10.1038/300713a0. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Perbal B., Cuzin F. Growth control in simian virus 40-transformed rat cells: temperature-independent expression of the transformed phenotype in tsA transformants derived by agar selection. J Virol. 1978 Oct;28(1):1–5. doi: 10.1128/jvi.28.1.1-5.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulzadegan M., Seif R., Cuzin F. Conditions leading to the establishment of the N (a gene dependent) and A (a gene independent) transformed states after polyoma virus infection of rat fibroblasts. J Virol. 1978 Nov;28(2):421–426. doi: 10.1128/jvi.28.2.421-426.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Richardson L. S., Butel J. S. Properties of transformed hamster cells containing SV40 tumor antigen in the cytoplasm. Int J Cancer. 1971 Jan 15;7(1):75–85. doi: 10.1002/ijc.2910070109. [DOI] [PubMed] [Google Scholar]
- Risser R., Pollack R. A nonselective analysis of SV40 transformation of mouse 3T3 cells. Virology. 1974 Jun;59(2):477–489. doi: 10.1016/0042-6822(74)90457-7. [DOI] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Santos M., Butel J. S. Association of SV40 large tumor antigen and cellular proteins on the surface of SV40-transformed mouse cells. Virology. 1982 Jul 15;120(1):1–17. doi: 10.1016/0042-6822(82)90002-2. [DOI] [PubMed] [Google Scholar]
- Santos M., Butel J. S. Surface T-antigen expression in simian virus 40-transformed mouse cells: correlation with cell growth rate. Mol Cell Biol. 1985 May;5(5):1051–1057. doi: 10.1128/mcb.5.5.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif R., Cuzin F. Temperature-sensitive growth regulation in one type of transformed rat cells induced by the tsa mutant of polyoma virus. J Virol. 1977 Dec;24(3):721–728. doi: 10.1128/jvi.24.3.721-728.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif R., Martin R. G. Simian virus 40 small t antigen is not required for the maintenance of transformation but may act as a promoter (cocarcinogen) during establishment of transformation in resting rat cells. J Virol. 1979 Dec;32(3):979–988. doi: 10.1128/jvi.32.3.979-988.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. J., Topp W. C., Hanich R., Sambrook J. F. Mutants of SV40 with an altered small t protein are reduced in their ability to transform cells. Cell. 1978 May;14(1):79–88. doi: 10.1016/0092-8674(78)90303-3. [DOI] [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Smolar N., Walsh J. E., Griffin B. E. Coding potential and regulatory signals of the polyoma virus genome. Nature. 1980 Jan 31;283(5746):445–453. doi: 10.1038/283445a0. [DOI] [PubMed] [Google Scholar]
- Soule H. R., Butel J. S. Subcellular Localization of simian virus 40 large tumor antigen. J Virol. 1979 May;30(2):523–532. doi: 10.1128/jvi.30.2.523-532.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule H. R., Lanford R. E., Butel J. S. Detection of simian virus 40 surface-associated large tumor antigen by enzyme-catalyzed radioiodination. Int J Cancer. 1982 Mar 15;29(3):337–344. doi: 10.1002/ijc.2910290318. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J Virol. 1975 Mar;15(3):613–618. doi: 10.1128/jvi.15.3.613-618.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Epithelioid and fibroblastic rat kidney cell clones: epidermal growth factor (EGF) receptors and the effect of mouse sarcoma virus transformation. J Cell Physiol. 1978 Mar;94(3):335–342. doi: 10.1002/jcp.1040940311. [DOI] [PubMed] [Google Scholar]