Abstract
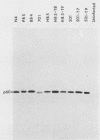
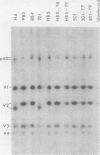
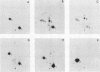
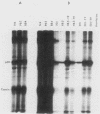
We have previously found that Rous sarcoma virus variants in which the viral src (v-src) gene is replaced by the cellular src (c-src) gene have no transforming activity. In this study, we analyzed the basis for the inability of the p60c-src overproduced by these variants to transform cells. Phosphorylations of tyrosine residues in total cell protein or in cellular 34K protein are known to be markedly enhanced upon infection with wild-type Rous sarcoma virus. We found that these tyrosine phosphorylations were only slightly increased in the c-src-containing virus-infected cells, whereas both levels were significantly increased by infection with wild-type Rous sarcoma virus, or transforming mutant viruses which are derived from c-src-containing viruses by spontaneous mutation. Phosphorylation at tyrosine 416 of p60 itself was also extremely low in overproduced p60c-src and high in p60s of transforming mutant viruses. In immunoprecipitates with monoclonal antibody, the overproduced p60c-src had much lower casein tyrosine kinase activity than did p60v-src. We previously showed that p60 myristylation and plasma membrane localization may be required for cell transformation. p60c-src was similar to transforming p60s in these properties. These results strongly suggest that the low level of tyrosine phosphorylation by overproduced p60c-src accounts for its inability to transform cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolen J. B., Thiele C. J., Israel M. A., Yonemoto W., Lipsich L. A., Brugge J. S. Enhancement of cellular src gene product associated tyrosyl kinase activity following polyoma virus infection and transformation. Cell. 1984 Oct;38(3):767–777. doi: 10.1016/0092-8674(84)90272-1. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson E., Erikson R. L. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981 Aug;25(2):363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Brugge J., Yonemoto W., Darrow D. Interaction between the Rous sarcoma virus transforming protein and two cellular phosphoproteins: analysis of the turnover and distribution of this complex. Mol Cell Biol. 1983 Jan;3(1):9–19. doi: 10.1128/mcb.3.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton G. M., Roskoski R., Jr Tyrosyl and cyclic AMP-dependent protein kinase activities in BHK cells that express viral pp60src. Mol Cell Biol. 1984 May;4(5):973–977. doi: 10.1128/mcb.4.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Belzer S. K., Purchio A. F. Structurally and functionally modified forms of pp60v-src in Rous sarcoma virus-transformed cell lysates. Mol Cell Biol. 1984 Jul;4(7):1213–1220. doi: 10.1128/mcb.4.7.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Brugge J. S., Erikson R. L. Characterization of a normal avian cell protein related to the avian sarcoma virus transforming gene product. Cell. 1978 Dec;15(4):1363–1369. doi: 10.1016/0092-8674(78)90061-2. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Erikson R. L. Structural analysis of the avian sarcoma virus transforming protein: sites of phosphorylation. J Virol. 1979 Feb;29(2):770–781. doi: 10.1128/jvi.29.2.770-781.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Changes in protein phosphorylation in Rous sarcoma virus-transformed chicken embryo cells. Mol Cell Biol. 1981 Feb;1(2):165–178. doi: 10.1128/mcb.1.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Four different classes of retroviruses induce phosphorylation of tyrosines present in similar cellular proteins. Mol Cell Biol. 1981 May;1(5):394–407. doi: 10.1128/mcb.1.5.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton P. C., Brugge J. S. Neural tissues express high levels of the cellular src gene product pp60c-src. Mol Cell Biol. 1983 Jun;3(6):1157–1162. doi: 10.1128/mcb.3.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Bishop J. M. Transit of pp60v-src to the plasma membrane. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7117–7121. doi: 10.1073/pnas.79.23.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Levinson A. D., Bishop J. M. The protein encoded by the transforming gene of avian sarcoma virus (pp60src) and a homologous protein in normal cells (pp60proto-src) are associated with the plasma membrane. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3783–3787. doi: 10.1073/pnas.77.7.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983 Jun 2;303(5916):435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Pellman D., Hanafusa H. A short sequence in the p60src N terminus is required for p60src myristylation and membrane association and for cell transformation. Mol Cell Biol. 1984 Sep;4(9):1834–1842. doi: 10.1128/mcb.4.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Hanafusa H. Local mutagenesis of Rous sarcoma virus: the major sites of tyrosine and serine phosphorylation of pp60src are dispensable for transformation. Cell. 1983 Sep;34(2):597–607. doi: 10.1016/0092-8674(83)90392-6. [DOI] [PubMed] [Google Scholar]
- Czernilofsky A. P., Levinson A. D., Varmus H. E., Bishop J. M., Tischer E., Goodman H. Corrections to the nucleotide sequence of the src gene of Rous sarcoma virus. Nature. 1983 Feb 24;301(5902):736–738. doi: 10.1038/301736b0. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Konopka J. B., Witte O. N. Activation of the c-abl oncogene by viral transduction or chromosomal translocation generates altered c-abl proteins with similar in vitro kinase properties. Mol Cell Biol. 1985 Jan;5(1):204–213. doi: 10.1128/mcb.5.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Identification of a cellular protein substrate phosphorylated by the avian sarcoma virus-transforming gene product. Cell. 1980 Oct;21(3):829–836. doi: 10.1016/0092-8674(80)90446-8. [DOI] [PubMed] [Google Scholar]
- Erikson R. L., Collett M. S., Erikson E., Purchio A. F. Evidence that the avian sarcoma virus transforming gene product is a cyclic AMP-independent protein kinase. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6260–6264. doi: 10.1073/pnas.76.12.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber E. A., Krueger J. G., Hanafusa H., Goldberg A. R. Only membrane-associated RSV src proteins have amino-terminally bound lipid. Nature. 1983 Mar 10;302(5904):161–163. doi: 10.1038/302161a0. [DOI] [PubMed] [Google Scholar]
- Gilmer T. M., Erikson R. L. Development of anti-pp60src serum with antigen produced in Escherichia coli. J Virol. 1983 Jan;45(1):462–465. doi: 10.1128/jvi.45.1.462-465.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H., Halpern C. C., Buchhagen D. L., Kawai S. Recovery of avian sarcoma virus from tumors induced by transformation-defective mutants. J Exp Med. 1977 Dec 1;146(6):1735–1747. doi: 10.1084/jem.146.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba H., Takeya T., Cross F. R., Hanafusa T., Hanafusa H. Rous sarcoma virus variants that carry the cellular src gene instead of the viral src gene cannot transform chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4424–4428. doi: 10.1073/pnas.81.14.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karess R. E., Hanafusa H. Viral and cellular src genes contribute to the structure of recovered avian sarcoma virus transforming protein. Cell. 1981 Apr;24(1):155–164. doi: 10.1016/0092-8674(81)90511-0. [DOI] [PubMed] [Google Scholar]
- Karess R. E., Hayward W. S., Hanafusa H. Cellular information in the genome of recovered avian sarcoma virus directs the synthesis of transforming protein. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3154–3158. doi: 10.1073/pnas.76.7.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. G., Garber E. A., Goldberg A. R. Subcellular localization of pp60src in RSV-transformed cells. Curr Top Microbiol Immunol. 1983;107:51–124. [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Lipsich L. A., Lewis A. J., Brugge J. S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983 Nov;48(2):352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. L., Branton P. E. Identification, purification, and characterization of phosphotyrosine-specific protein phosphatases from cultured chicken embryo fibroblasts. Mol Cell Biol. 1984 Jun;4(6):1003–1012. doi: 10.1128/mcb.4.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppermann H., Levinson A. D., Levintow L., Varmus H. E., Bishop J. M., Kawai S. Two cellular proteins that immunoprecipitate with the transforming protein of Rous sarcoma virus. Virology. 1981 Sep;113(2):736–751. doi: 10.1016/0042-6822(81)90202-6. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Levinson A. D., Varmus H. E., Levintow L., Bishop J. M. Uninfected vertebrate cells contain a protein that is closely related to the product of the avian sarcoma virus transforming gene (src). Proc Natl Acad Sci U S A. 1979 Apr;76(4):1804–1808. doi: 10.1073/pnas.76.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppermann H., Levinson W., Bishop J. M. A cellular protein that associates with the transforming protein of Rous sarcoma virus is also a heat-shock protein. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1067–1071. doi: 10.1073/pnas.78.2.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R. C., Varmus H. E., Bishop J. M. Expression of v-src and chicken c-src in rat cells demonstrates qualitative differences between pp60v-src and pp60c-src. Cell. 1984 May;37(1):131–139. doi: 10.1016/0092-8674(84)90308-8. [DOI] [PubMed] [Google Scholar]
- Parsons S. J., McCarley D. J., Ely C. M., Benjamin D. C., Parsons J. T. Monoclonal antibodies to Rous sarcoma virus pp60src react with enzymatically active cellular pp60src of avian and mammalian origin. J Virol. 1984 Aug;51(2):272–282. doi: 10.1128/jvi.51.2.272-282.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patschinsky T., Hunter T., Esch F. S., Cooper J. A., Sefton B. M. Analysis of the sequence of amino acids surrounding sites of tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1982 Feb;79(4):973–977. doi: 10.1073/pnas.79.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier F., Calothy G., Karess R. E., Erikson E., Hanafusa H. Role of p60src kinase activity in the induction of neuroretinal cell proliferation by rous sarcoma virus. J Virol. 1982 Jun;42(3):780–789. doi: 10.1128/jvi.42.3.780-789.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F. Evidence the pp60src, the product of the Rous sarcoma virus src gene, undergoes autophosphorylation. J Virol. 1982 Jan;41(1):1–7. doi: 10.1128/jvi.41.1.1-7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Henderson L. E., Oroszlan S., Garber E. A., Hanafusa H. Amino terminal myristylation of the protein kinase p60src, a retroviral transforming protein. Science. 1985 Jan 25;227(4685):427–429. doi: 10.1126/science.3917576. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K., Eckhart W. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma virus. Cell. 1980 Jul;20(3):807–816. doi: 10.1016/0092-8674(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Patschinsky T., Berdot C., Hunter T., Elliott T. Phosphorylation and metabolism of the transforming protein of Rous sarcoma virus. J Virol. 1982 Mar;41(3):813–820. doi: 10.1128/jvi.41.3.813-820.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Trowbridge I. S., Cooper J. A., Scolnick E. M. The transforming proteins of Rous sarcoma virus, Harvey sarcoma virus and Abelson virus contain tightly bound lipid. Cell. 1982 Dec;31(2 Pt 1):465–474. doi: 10.1016/0092-8674(82)90139-8. [DOI] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa H., Balduzzi P. C. Cellular sequences related to three new onc genes of avian sarcoma virus (fps, yes, and ros) and their expression in normal and transformed cells. J Virol. 1982 Apr;42(1):143–152. doi: 10.1128/jvi.42.1.143-152.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart J. E., Oppermann H., Czernilofsky A. P., Purchio A. F., Erikson R. L., Bishop J. M. Characterization of sites for tyrosine phosphorylation in the transforming protein of Rous sarcoma virus (pp60v-src) and its normal cellular homologue (pp60c-src). Proc Natl Acad Sci U S A. 1981 Oct;78(10):6013–6017. doi: 10.1073/pnas.78.10.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M. A., Bishop J. M., Colby W. W., Levinson A. D. Phosphorylation of tyrosine-416 is not required for the transforming properties and kinase activity of pp60v-src. Cell. 1983 Mar;32(3):891–901. doi: 10.1016/0092-8674(83)90074-0. [DOI] [PubMed] [Google Scholar]
- Sorge L. K., Levy B. T., Maness P. F. pp60c-src is developmentally regulated in the neural retina. Cell. 1984 Feb;36(2):249–257. doi: 10.1016/0092-8674(84)90218-6. [DOI] [PubMed] [Google Scholar]
- Takeya T., Feldman R. A., Hanafusa H. DNA sequence of the viral and cellular src gene of chickens. 1. Complete nucleotide sequence of an EcoRI fragment of recovered avian sarcoma virus which codes for gp37 and pp60src. J Virol. 1982 Oct;44(1):1–11. doi: 10.1128/jvi.44.1.1-11.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Nishida T., Miyajima N., Kawai S., Ooi T., Toyoshima K. The erbB gene of avian erythroblastosis virus is a member of the src gene family. Cell. 1983 Nov;35(1):71–78. doi: 10.1016/0092-8674(83)90209-x. [DOI] [PubMed] [Google Scholar]
- Ziemiecki A., Friis R. R., Bauer H. Half-life of the Rous sarcoma virus transforming protein pp60src and its associated kinase activity. Mol Cell Biol. 1982 Apr;2(4):355–360. doi: 10.1128/mcb.2.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]