Abstract
The protein synthesis elongation factor EF-1 alpha of Mucor racemosus hyphae contained eight or nine methylated amino acids per molecule, whereas the factor from sporangiospores was nonmethylated. During the course of spore germination, the specific activity of the factor in crude extracts increased sixfold. This increase in activity was accompanied by a constant level of EF-1 alpha-specific mRNA and a constant level of EF-1 alpha protein. Methylation of the protein, however, accelerated during the germination process, in parallel with the increase in specific activity of the factor. We propose that the activity of EF-1 alpha is regulated during germination through methylation of the protein and does not involve transcriptional regulation.
Full text
PDF
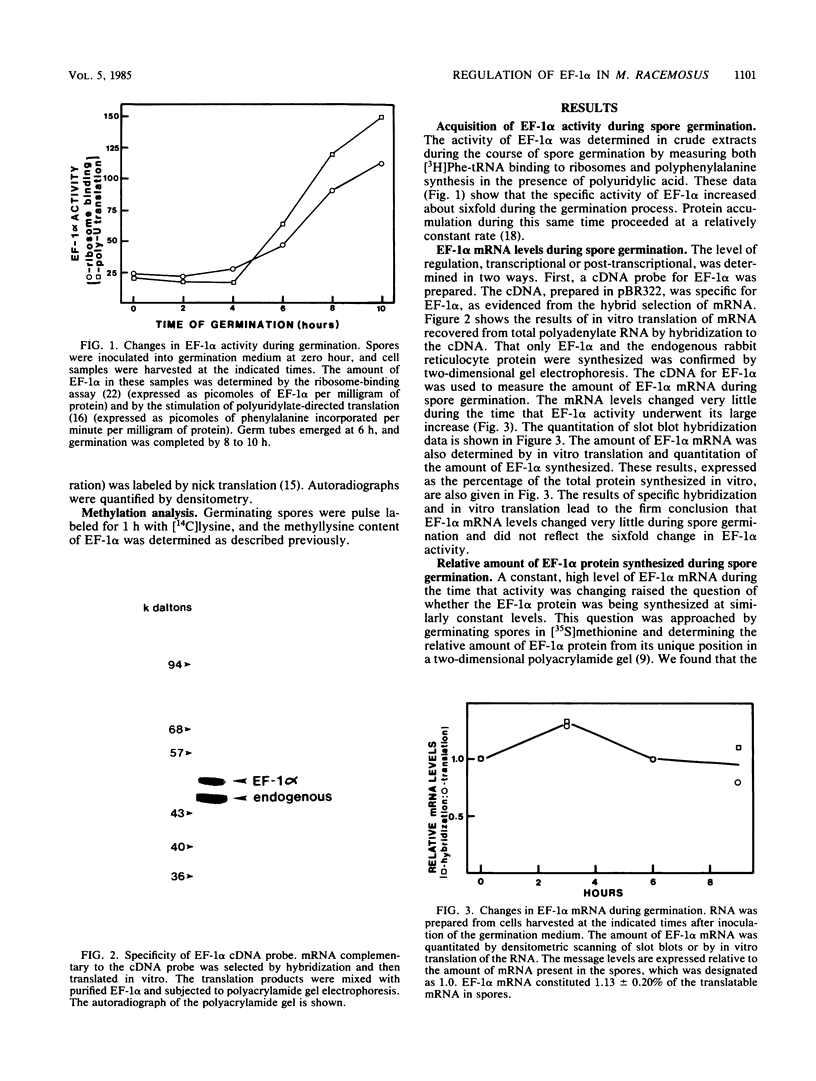
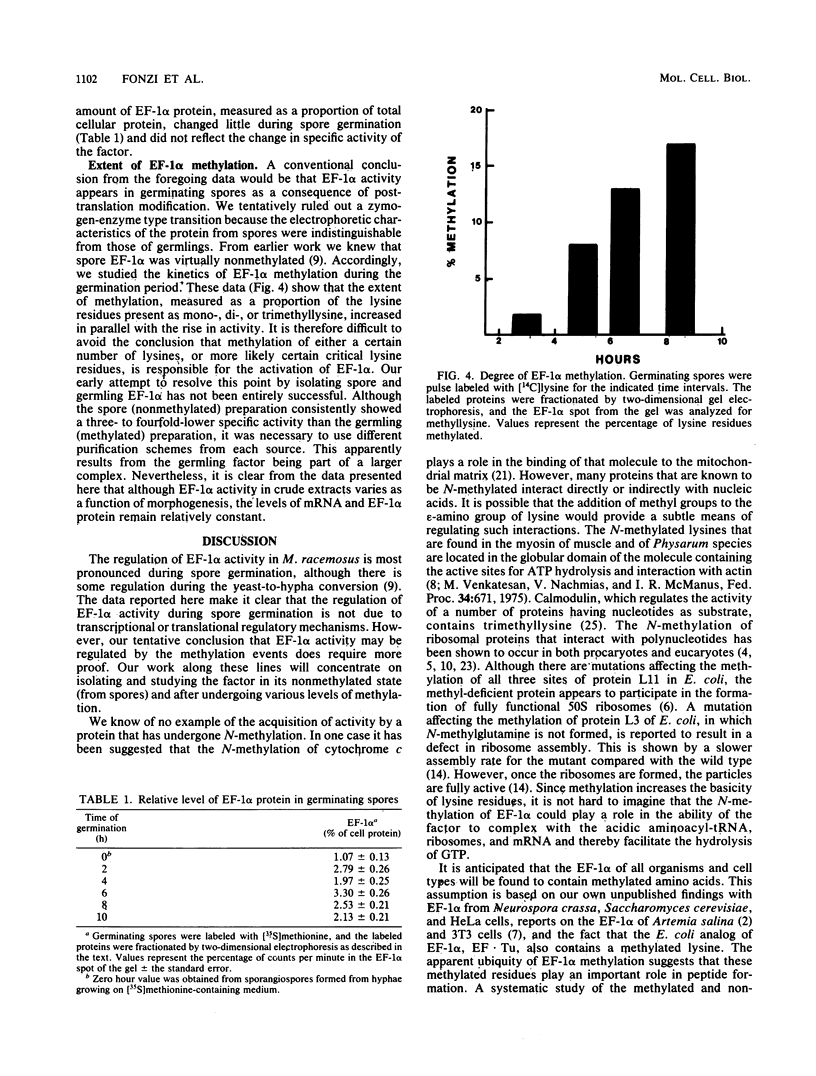

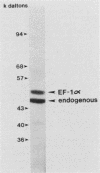
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton T. H., Lodish H. F. Developmental changes in messenger RNAs and protein synthesis in Dictyostelium discoideum. Dev Biol. 1977 Oct 1;60(1):180–206. doi: 10.1016/0012-1606(77)90118-x. [DOI] [PubMed] [Google Scholar]
- Amons R., Pluijms W., Roobol K., Möller W. Sequence homology between EF-1 alpha, the alpha-chain of elongation factor 1 from Artemia salina and elongation factor EF-Tu from Escherichia coli. FEBS Lett. 1983 Mar 7;153(1):37–42. doi: 10.1016/0014-5793(83)80115-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang C. N., Chang N. Methylation of the ribosomal proteins in Escherichia coli. Nature and stoichiometry of the methylated amino acids in 50S ribosomal proteins. Biochemistry. 1975 Feb 11;14(3):468–477. doi: 10.1021/bi00674a002. [DOI] [PubMed] [Google Scholar]
- Chang F. N., Navickas I. J., Chang C. N., Dancis B. M. Methylation of ribosomal proteins in HeLa cells. Arch Biochem Biophys. 1976 Feb;172(2):627–633. doi: 10.1016/0003-9861(76)90117-x. [DOI] [PubMed] [Google Scholar]
- Colson C., Lhoest J., Urlings C. Genetics of ribosomal protein methylation in Escherichia coli. III. Map position of two genes, prmA and prmB, governing methylation of proteins L11 and L3. Mol Gen Genet. 1979 Feb 1;169(3):245–250. doi: 10.1007/BF00382270. [DOI] [PubMed] [Google Scholar]
- Coppard N. J., Clark B. F., Cramer F. Methylation of elongation factor 1 alpha in mouse 3T3B and 3T3B/SV40 cells. FEBS Lett. 1983 Dec 12;164(2):330–334. doi: 10.1016/0014-5793(83)80311-1. [DOI] [PubMed] [Google Scholar]
- Hardy M. F., Harris C. I., Perry S. V., Stone D. Occurrence and formation of the N epsilon-methyl-lysines in myosin and the myofibrillar proteins. Biochem J. 1970 Dec;120(3):653–660. doi: 10.1042/bj1200653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt W. R., Garcia R., Merrick W. C., Sypherd P. S. Methylation of elongation factor 1 alpha from the fungus Mucor. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3433–3437. doi: 10.1073/pnas.79.11.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klagsbrun M., Furano A. V. Methylated amino acids in the proteins of bacterial and mammalian cells. Arch Biochem Biophys. 1975 Aug;169(2):529–539. doi: 10.1016/0003-9861(75)90196-4. [DOI] [PubMed] [Google Scholar]
- Larsen A., Sypherd P. S. Physiological control of phosphorylation ribosomal protein S6 in Mucor racemosus. J Bacteriol. 1980 Jan;141(1):20–25. doi: 10.1128/jb.141.1.20-25.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen A., Sypherd P. Ribosomal proteins of the dimorphic fungus, Mucor racemosus. Mol Gen Genet. 1979 Aug;175(1):99–109. doi: 10.1007/BF00267861. [DOI] [PubMed] [Google Scholar]
- Legocki A. B. Elongation factor EF-2 from wheat germ: purification and properties. Methods Enzymol. 1979;60:703–712. doi: 10.1016/s0076-6879(79)60065-4. [DOI] [PubMed] [Google Scholar]
- Lhoest J., Colson C. Cold-sensitive ribosome assembly in an Escherichia coli mutant lacking a single methyl group in ribosomal protein L3. Eur J Biochem. 1981 Dec;121(1):33–37. doi: 10.1111/j.1432-1033.1981.tb06425.x. [DOI] [PubMed] [Google Scholar]
- Merrick W. C. Assays for eukaryotic protein synthesis. Methods Enzymol. 1979;60:108–123. doi: 10.1016/s0076-6879(79)60011-3. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Sypherd P. S. Regulation of macromolecular synthesis during hyphal germ tube emergence from Mucor racemosus sporangiospores. J Bacteriol. 1978 Apr;134(1):76–83. doi: 10.1128/jb.134.1.76-83.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M., Sypherd P. S. Regulation of translation rate during morphogenesis in the fungus Mucor. Biochemistry. 1978 Feb 21;17(4):569–575. doi: 10.1021/bi00597a002. [DOI] [PubMed] [Google Scholar]
- Paznokas J. L., Sypherd P. S. Respiratory capacity, cyclic adenosine 3',5'-monophosphate, and morphogenesis of Mucor racemosus. J Bacteriol. 1975 Oct;124(1):134–139. doi: 10.1128/jb.124.1.134-139.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott W. A., Mitchell H. K. Secondary modification of cytochrome c by Neurospora crassa. Biochemistry. 1969 Nov;8(11):4282–4289. doi: 10.1021/bi00839a009. [DOI] [PubMed] [Google Scholar]
- Slobin L. I., Möller W. Purification of elongation factor 1 from embryos of Artemia salina. Methods Enzymol. 1979;60:685–703. doi: 10.1016/s0076-6879(79)60064-2. [DOI] [PubMed] [Google Scholar]
- Terhorst C., Wittmann-Liebold B., Möller W. 50-S ribosomal proteins. Peptide studies on two acidic proteins, A 1 and A 2 , isolated from 50-S ribosomes of Escherichia coli. Eur J Biochem. 1972 Jan 31;25(1):13–19. doi: 10.1111/j.1432-1033.1972.tb01661.x. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Sharief F., Vanaman T. C. The complete amino acid sequence of the Ca2+-dependent modulator protein (calmodulin) of bovine brain. J Biol Chem. 1980 Feb 10;255(3):962–975. [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]