Abstract
Objective
The role of androgens in chronic disease pathogenesis, cognitive function and libido during menopause is of increasing interest. The aim of this study was to characterize the distribution and expression of androgenic proteins in the macaque ovary and to investigate the relationship between serum androgen concentrations, follicle number, and the persistence of androgenesis in the aging macaque ovary.
Methods
The subjects were 26 adult female cynomolgus macaques. Ovaries were immunostained for cytochrome P450 17α-hydroxylase/17–20 lyase (P450c17), 3β-hydroxysteroid dehydrogenase (3βHSD), and cytochrome b5 (cytb5). Based on primordial follicle counts, animals were divided into tertiles (low (≤200), intermediate (226–1232), and high (2372–4356)) to evaluate differences in androgen staining and changes in serum androgen concentrations following ovariectomy.
Results
Positive immunostaining for P450c17 and cytb5 within the theca interna layer of growing follicles persisted in advanced atretic follicles and secondary interstitial cells (residual stromal cells). Ovaries with low follicle numbers had less staining for all androgenic proteins compared to ovaries with higher numbers of growing follicles. Immunostaining for cytb5 was the most reliable marker for persistent androgenesis in ovaries with minimal primordial follicle numbers (<100) and residual stromal cells. Following ovariectomy, a significant decrease in testosterone (−27.7%, −30.8%, −27.5%; p < 0.01) and androstenedione (−33.4%, −35.7%, −46.0%; p < 0.01) was observed in monkeys with low, intermediate, and high primordial follicle counts, respectively.
Conclusions
Despite low follicle numbers, the aging macaque ovary retains the necessary proteins for androgenesis within residual stromal cells and contributes to peripheral androgen concentrations.
Keywords: MENOPAUSE, MONKEY, ANIMAL MODEL, OVARIAN RESERVE, FOLLICLES, AGING, IMMUNOHISTOCHEMISTRY
INTRODUCTION
Several cross-sectional studies have demonstrated a substantial decrease (approximately 50%) in plasma testosterone and androstenedione concentrations among women between the ages of 20 and 45 years of age, with no change in androgen levels across the menopausal transition and postmenopausally1,2. However, whether and to what extent this steady decline in androgen levels relates to aging ovarian function and follicle number remain unclear2.
In the ovary, testosterone and androstenedione are synthesized in the theca interna cell layer within the periphery of growing follicles. Following their synthesis, testosterone and androstenedione diffuse into the adjacent granulosa cell layer and are aromatized into estradiol and estrone, respectively3,4. From puberty, the total number of ovarian follicles decreases gradually until approximately 37 years of age, at which time the rate of decline increases exponentially5. By the onset of menopause, the ovary is atrophic and depleted of estrogen-producing follicles; however, androgenesis is thought to persist in secondary interstitial cells scattered throughout the ovarian stroma3,6. Originally derived from theca interna cells of atretic follicles3, these secondary interstitial cells are thought to remain responsive to luteinizing hormone and produce measurable amounts of testosterone and androstenedione postmenopausally6. A few in vitro studies have investigated the expression of androgenic proteins in these residual stromal cells of postmenopausal women, but results have been inconsistent and difficult to relate to the aforementioned clinical studies investigating peripheral androgen concentrations7–9.
The efficient formation of androgens within the ovary requires the expression and coordination of steroidogenic enzymes and accessory proteins. The cytochrome P450 enzyme 17α-hydroxylase/17–20 lyase (P450c17) is a bifunctional enzyme involved in the production of both progesterone and androgens within the ovary through the differential metabolism of pregnenolone, a cholesterol metabolite10,11. Whether pregnenolone is utilized predominately for the generation of testosterone and androstenedione depends on the expression level of two other proteins, cytochrome b5 (cytb5)10–14 and 3β-hydroxysteroid dehydrogenase (3βHSD)15,16. The 17α-hydroxylation reaction of P450c17 in the ovary is involved in the formation of both progesterone and androgens, whereas the 17–20 lyase reaction of P450c17 is unique to androgen production. Experiments have shown that cytb5 selectively promotes androgen synthesis by augmenting the 17–20 lyase activity of P450c1712,13. The expression level of 3βHSD is also important in the regulation of ovarian androgenesis. 3βHSD competes with P450c17 to catalyze the synthesis of progesterone from pregnenolone, which could otherwise be metabolized to dehydroepiandrosterone (DHEA) by the 17α-hydroxylation/17–20 lyase activity of P450c17. The low expression level of 3βHSD relative to P450c17 promotes androgen synthesis, whereas a high expression level of 3βHSD increases progesterone production11. The cytb5 protein has been co-localized with the P450c17 enzyme within the theca interna cell layer of human premenopausal ovaries17; however, little information is known about the cellular localization and expression of P450c17, cytb5 and 3βHSD in the aging ovary.
A clear understanding of the androgenic function of the aging ovary is of clinical importance for several reasons. In premenopausal women, endogenous androgens help to maintain bone density, muscle mass, and cognitive function as well as sexual well-being18–23; therefore, there has been a growing interest in the role of androgens in chronic disease pathogenesis (osteoporosis, cardiovascular disease, metabolic syndrome) and quality-of-life issues (memory loss, decreased libido) during menopause18,23–25. Also, since androgens appear to decline with age, there has been increasing interest in androgen replacement therapy for older women, especially for those with a history of ovariectomy and low sexual desire26,27.
Female macaques and women share many reproductive endocrine characteristics, including a 28-day menstrual cycle28, peripheral aromatization of androgens to estrogens29, and natural menopause30,31, with a similar pattern of primordial follicle decline32 and hormonal profile31,33 to that of women. In order to further characterize the macaque model for use in translational studies, the immunolocalization and relative expression of P450c17, cytb5 and 3βHSD within secondary interstitial cells and all stages of folliculogenesis and atresia were investigated within this animal model. Monkeys were then divided into tertiles according to primordial follicle number (a marker of ovarian age) in order to characterize the effect of declining follicle numbers on androgenic expression patterns, morphological features, and the contribution of residual stromal cells to serum androgen concentrations. We hypothesized that aging macaque ovaries with low primordial follicle counts (<200) would have less immunostaining for all three proteins and a smaller percentage change in androgen levels following ovariectomy compared to those with higher follicle counts. The findings of this study will provide important information regarding the relationship between serum androgen concentrations, follicle number, and the persistence of androgenesis in the aging ovary, thus increasing the importance of the macaque model for use in translational studies of health issues critical to postmenopausal women.
METHODS
Animal subjects
Data presented here are from a retrospective study consisting of 26 adult female cynomolgus macaques (Macaca fascicularis) used originally for a larger randomized trial studying the effects of an atherogenic diet, type of dietary protein (soy vs. casein-lactalbumin), reproductive stage, and social stress on atherosclerosis progression34–37. Following 32 months of exposure to an atherogenic diet containing either soy or casein– lactalbumin, all animals were ovariectomized. Data from those animals consuming the casein–lactalbumin diet are presented here. The effect of dietary protein source and cardiovascular risk on ovarian aging has been reported elsewhere36.
All monkeys were imported from Indonesia and housed in stable social groups of four to six animals per group. Adult status was confirmed radiographically by complete epiphyseal closure at the distal radius, ulna and the proximal tibia. At the time of the study reported here, the estimated age range (based on dentition, radiographs, and other physical characteristics) was 13–23 years of age. All animal procedures were approved by the Wake Forest University Animal Care and Use Committee and conducted in compliance with state and federal laws. Wake Forest University is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Study design
One ovary from each monkey was used to determine the total number of primordial follicles36. The contralateral ovary was used for immunohistochemical staining for key androgenic proteins: P450c17, 3βHSD, and cytb5. One ovary per animal was used for each analysis based on findings by Miller and colleagues and Appt and colleagues that follicle numbers between ovaries are symmetrical in macaques38,39. Based on the observation that the number of primordial follicles differed substantially (range 0–4356) among individuals, the 26 animals were divided into tertiles (low, intermediate, and high) for subsequent morphological descriptions and serum hormone analyses.
Immunohistochemistry
Fixed ovaries were bisected prior to embedding and slides with thin sections (5 μm) were sent to the University of Alabama at Birmingham (CRP) for immunohistochemistry using techniques similar to those utilized previously with human ovarian tissues17. After deparaffinization and hydration of the tissue sections, the endogenous peroxidase activity was quenched with 3% hydrogen peroxide. Then, the slides were incubated in 5% normal goat serum for 10 min at 25°C to decrease non-specific binding. The slides were drained and incubated with primary antiserum diluted in phosphate buffer. Rabbit antihuman cytochrome b5 antiserum was kindly provided by Dr Alan Conley, University of California at Davis. The chicken anti-human P450c17 polyclonal antibody was kindly provided by Dr Michael Waterman, Vanderbilt University. The rabbit antihuman placental 3βHSD antiserum was developed by Dr Parker at the University of Alabama at Birmingham. Slides were treated with biotinylated second antibodies followed by incubation with avidin–biotin–conjugated peroxidase (Bio Genex). Immunoreactivity was detected by incubation of the tissue sections in DAB as the chromagen (Bio Genex). Slides were then counterstained with hematoxylin. Immunopositive corpora lutea (producing progesterone during the luteal phase) were considered positive controls for 3βHSD immunoreactivity. Sections of rhesus adrenal glands were used as positive controls for P450c17 and cytb5. Negative controls consisted of ovarian and adrenal tissue sections incubated as above in the absence of primary antiserum. No immunostaining was noted in any instance in the negative control sections.
Ovarian follicle counts
Each ovary was fixed in Bouin’s solution (75 ml picric acid solution (1.3%), 25 ml of formaldehyde (37%), and 5 ml glacial acetic acid), transferred to 70% ethanol 24 h later, and then embedded whole in paraffin for follicle counting. Follicle counting was conducted at the University of Arizona as previously described36. Briefly, the ovaries were sectioned serially (4–5 μm) and stained with hematoxylin and eosin. Follicles containing an oocyte nucleus were identified (primordial, primary, secondary, and antral) and counted in every 100th section. The total number of sections per ovary varied from five to ten depending on individual differences in ovarian size and the presence of a corpus luteum.
Ovarian follicle classification
Follicle classification for morphology was determined based on criteria previously described in Buse and colleagues40. Follicles were classified as primordial (oocyte surrounded by a single layer of flattened granulosa cells), primary (oocyte surrounded by a single layer of cuboidal granulosa cells), and early secondary (oocyte surrounded by more than one layer of cuboidal granulosa cells, but no theca interna cells). Advanced secondary follicles were slightly larger and had a developing theca cell layer. Follicles with an antrum lined by granulosa cells and well developed internal and external theca cell layers were classified as antral follicles. Follicles with a detached and apoptotic granulosa cell layer, as indicated by cell shrinkage, chromatin condensation and nuclear fragmentation, were classified as atretic follicles. Early atretic follicles retained an antrum whereas advanced atretic follicles had a collapsed antrum with hypertrophic theca interstitial cells surrounding an apoptotic granulosa cell center. Secondary interstitial cells, presumed to be derived from the hypertrophic theca interstitial cells of advanced atretic follicles, were also identified scattered throughout the stroma3. Photomicrographs were taken using an Axioplan 2 upright light microscope.
Menstrual cyclicity
As part of a larger randomized study, the average cycle length for each animal was determined from the vaginal bleeding history37. Seventeen to 20 months prior to ovariectomy, monkeys were subjected to daily vaginal swabs for 10 months. More than 250 cycles were evaluated and used to determine mean cycle length for this study population.
Serum androgens
Serum concentrations for total testosterone and androstenedione were determined from pre- and post-ovariectomy samples collected within 5 and 11 months of surgery, respectively. For blood collection, animals were sedated with ketamine HCl (15 mg/kg, intramuscular) and blood samples were obtained between 09.00 and 12.00 following an overnight fast. All samples were stored at −20°C or below until assayed. Serum hormone assays were performed at the Biomarkers Core Lab, Yerkes National Primate Research Center of Emory University. Total testosterone and androstenedione concentrations were measured from serum using commercially prepared kits (DSL, Webster, TX, USA). The intra-assay coefficients of variation (CV) were less than 7% for both assays. The inter-assay CVs were 12.06% at 1.07 ng/ml and 13.34% at 5.48 ng/ml for androstenedione while the inter-assay CVs for total testosterone were 5.95% at 0.68 ng/ml and 4.14% at 5.67 ng/ml.
Statistical analysis
Since the primordial follicle count data were skewed, a non-parametric Kruskal–Wallis test and a post-hoc Wilcoxon rank sum test were used to examine the differences in primordial follicle counts among the tertiles. In order to investigate the effect of ovariectomy on peripheral androgen concentrations within each tertile with differential primordial follicle counts, a mixed model approach was used for both total testosterone and androstenedione (primary outcomes). The model included the main effect of primordial follicle tertile, pre- and post-ovariectomy timepoints, and their interaction. This model allowed the comparison of pre- and post-ovariectomy androgen concentrations within each tertile as well as the comparison of serum androgen concentrations among tertiles within the pre- and post-ovariectomy periods. A similar mixed model was used to investigate any change in body weight following ovariectomy among the tertiles. In order to determine any differences in the magnitude of change in circulating androgens among the tertiles, percentage changes in total testosterone and androstenedione were calculated and analyzed by a one-way ANOVA. A one-way ANOVA was also used to determine any differences in average menstrual cycle length among the follicle tertiles. A two-tailed significance level of 0.05 was selected for all comparisons, and all analyses were done using JMP statistical software (version 8.0.2; SAS Institute, Inc, Cary, NC, USA).
RESULTS
Immunolocalization and expression of steroidogenic enzymes and accessory protein
The immunohistochemical localizations of P450c17, cytb5 and 3βHSD in the macaque ovary are presented in Table 1 and Figure 1. The intensity of immunostaining in various stages of folliculogenesis and atresia, including primordial, primary, secondary, antral and atretic follicles as well as secondary interstitial cells within the stroma, are represented in Table 1. Beginning with advanced secondary follicles (≥150 μm in diameter), thecal cells were immunopositive for P450c17 and cytb5. Immunopositive staining for 3βHSD was only observed in antral and atretic follicles.
Table 1.
Immunohistochemical localization of androgenic enzymes (P450c17, 3βHSD) and accessory protein (cytb5) in ovarian follicles
| Stain | Primordial/primary | Early secondary (<150 μm) | Advanced secondary (>150 μm)
|
Antral
|
Early atretic
|
Advanced atretic
|
Secondary interstitial cells | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GC | TC | GC | TC | GC | TC | GC | TC | ||||
| P450c17 | − | − | − | + | − | ++ | − | ++ | − | + | + |
| Cytb5 | − | − | − | ++ | − | +++ | − | +++ | − | ++ | ++ |
| 3βHSD | − | − | − | − | − | + | − | +/− | − | +/− | − |
GC, granulosa cells; TC, theca interna cells
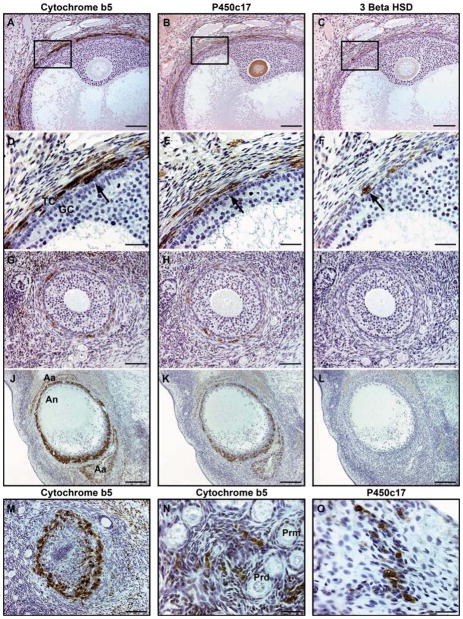
Figure 1.
Co-localization of androgenic enzymes, P450c17 and 3βHSD, and accessory protein, cytochrome b5, within the theca interna cell layer (arrows) of (A–C) antral [(D–F) box inset], (G–I) advanced secondary, (J–L) early atretic, (M) late atretic follicles, and (N, O) secondary interstitial cells in the ovarian stroma. Bars represent 30 μm for D–F, N, O; 40 μm for G–I; 100 μm for A–C, M; 200 μm for J–L. TC, theca interna cells; GC, granulosa cells; Aa, advanced atretic follicle; An, antrum; Prd, primordial follicles; Prm, primary follicles
The co-localization of P450c17, cytb5 and 3βHSD within the theca interna cell layer of growing and atretic follicles is depicted in Figure 1. Cytoplasmic immunolabeling for P450c17 and cytb5 was observed within the theca interna cell layer of antral follicles (Figure 1A, B, D, E), advanced secondary follicles (Figure 1G, H), early atretic follicles (Figure 1J, K), and late atretic follicles (Figure 1M) as well as secondary interstitial cells scattered throughout the ovarian stroma (Figure 1N, O). Compared to P450c17, cytb5 was the most highly expressed androgenic protein in the macaque ovary. The number of immunopositive cells and intensity of the immunoreactivity were greater for cytb5 than P450c17 in all stages of folliculogenesis and atresia examined. In addition, secondary interstitial cells immunopositive for cytb5 were more abundant than those immunopositive for P450c17 (Figure 1N, O). Generally, as the degree of apoptosis progressed within the theca interna cell layer of the atretic follicle, the number and intensity of P450c17 and cytb5 immunopositive cells decreased. 3βHSD was localized within the cytoplasm of theca interna cells of antral follicles (Figure 1C, F); however, the intensity and number of cells were significantly less than for both P450c17 and cytb5 (Figure 1A, B, D, E). Expression of 3βHSD in early and late atretic follicles varied. Some early and late atretic follicles were lightly stained positive for 3βHSD while other atretic follicles were immunonegative (Figure 1L). Newly formed corpora lutea, used as positive control tissue for 3βHSD immunoreactivity, were found to have strong cytoplasmic staining (not shown).
In addition to the theca interna cell layer, the ooplasma of oocytes within primary, secondary and antral follicles were commonly immunopositive for P450c17 (but not cytb5 and 3βHSD) (Figure 1B). Within the corpora lutea, both the theca and granulosa lutein cell populations were immunopositive for 3βHSD, whereas only the luteinizing theca cells stained positive for P450c17 and cytb5 (not shown). Negative controls used to detect non-specific staining had no immunoreactivity for all three markers.
Expression pattern of androgenic enzymes and accessory protein
The total number of primordial follicles per ovary varied substantially among the ovaries examined (range 0–4356), indicating the presence of a wide range of ovarian ages. Based on this finding, the ovaries were divided into tertiles (low, intermediate, high) according to their primordial follicle counts, for subsequent analyses. The lowest tertile (Low, n =9) had a primordial follicle count that ranged from 0 to 209 (median= 52), the intermediate tertile (Int, n = 9) ranged from 226 to 1232 follicles (median = 641), and the highest tertile (High, n = 8) had follicle counts ranging from 2372 to 4356 (median= 3054). A significant difference in primordial follicle counts was present among all tertiles (Low vs. Int, p <0.0004; Int vs. High, p < 0.0001; High vs. Low, p < 0.0001). All animals in this population had regular menstrual cycles and no animals had a history of amenorrhea. The average cycle length did not differ significantly among the tertiles (ANOVA p > 0.05). The average cycle length was 29.01 ± 0.67 days for the Low tertile, 30.04 ± 0.67 for the Int tertile, and 29.63 ± 0.71 days for the High tertile (mean ± standard equivalent of the mean, SEM).
Given that cytb5 was the most highly expressed androgenic protein, cytb5 immunoreactivity was used to identify differences in androgen staining patterns and morphological changes among the tertiles (Figure 2). Specifically, among those ovaries in the High primordial follicle tertile (Figure 2A), all stages of folliculogenesis and atresia were present including a large population of developing and atretic follicles immunopositive for cytb5. All stages of developing and atretic follicles were also present within the Int tertile ovaries (Figure 2B); however, the number of follicles, and therefore the amount of immunoreactivity, was qualitatively lower compared to that in the High tertile. In the Low tertile (<200 primordial follicles, Figure 2C), the ovaries had thin, dense cortices containing a large quantity of fibrous connective tissue (corpora atretica and albicantia). Some ovaries had a small population of growing and atretic follicles with moderate immunostaining, while others with a primordial follicle count <100 had only a few immunopositive atretic follicles and secondary interstitial cells scattered within the ovarian stroma.
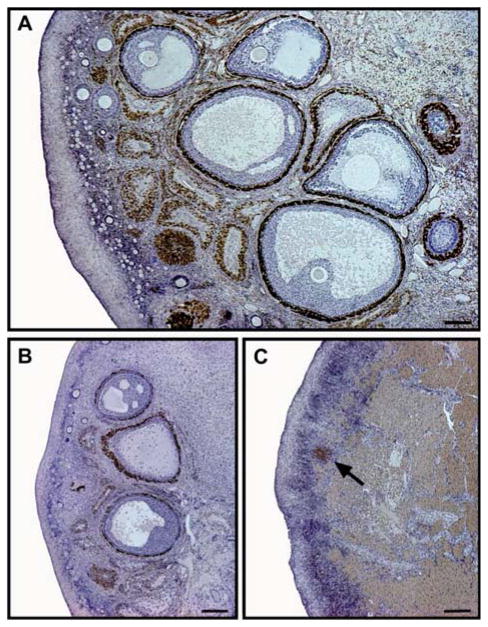
Figure 2.
Immunoreactivity of cytochrome b5, an accessory protein for androgen synthesis, within aging macaque ovaries with high (A), intermediate (B) and low (C) primordial follicle counts. Distinct changes in morphology and abundance of immunostaining with declining follicle populations. (A) Large population of developing and atretic follicles with abundant immunostaining; primordial follicle count 3546. Bar represents 400 μm. (B) Small population of immunopositive growing follicles with numerous atretic follicles; primordial follicle count 600. Bar represents 200 μm. (C) Few immunopositive atretic follicles (arrow) and secondary interstitial cells within the residual ovarian stromal tissue; primordial follicle count 0. Bar represents 200 μm
Effect of ovariectomy and ovarian aging on serum androgen concentrations
Pre- and post-ovariectomy serum concentrations of total testosterone and androstenedione (mean ± SEM) for each tertile (Low, Int, High) are depicted in Table 2. The absolute means (± SEM) of the preoperative and postoperative androgen concentrations were within the range of those reported for women41,42. Within each tertile, both total testosterone and androstenedione serum concentrations were significantly reduced following ovariectomy (p < 0.01 for all). The mean percentage change in total testosterone and androstenedione concentrations for each tertile and the entire cohort are depicted in Figure 3. The mean percentage change in total testosterone following ovariectomy for the entire cohort (including all tertiles) was −28.7 ± 3.3%, however, there was no difference in total testosterone reduction among the tertiles (Low −27.7 ± 6.2%, Int −30.8 ± 4.7%, High −27.5 ± 6.7%, ANOVA p = 0.90). Similarly, the mean percentage change in androstenedione for the entire cohort was significantly reduced following ovariectomy (−38.0 ± 4.9%), but the reduction did not differ by tertile (Low −33.4 ± 9.7%, Int −35.7 ± 9.5%, High −46.0 ± 5.5%, ANOVA p =0.57).
Table 2.
Pre- and post-ovariectomy serum androgens and body weights stratified by primordial follicle tertiles. The preoperative samples were collected within 5 months of ovariectomy whereas postoperative samples were collected within 11 months of surgery. Data are given as median (range) or mean (standard error of the mean)
| Entire cohort (n = 26) | Low (n = 9) | Intermediate (n = 9) | High (n = 8) | p Value | |
|---|---|---|---|---|---|
| Primordial follicle count | 620 (0–4356) | 52 (0–209) | 641 (226–1232) | 3054 (2372–4356) | <0.0001 |
| Total testosterone (ng/ml) | |||||
| Pre-ovariectomy | 1.04 (0.07) | 0.94 (0.08) | 1.14 (0.15) | 1.05 (0.16) | 0.5386 |
| Post-ovariectomy | 0.74 (0.06) | 0.68 (0.1) | 0.76 (0.07) | 0.78 (0.16) | 0.7956 |
| Androstenedione (ng/ml) | |||||
| Pre-ovariectomy | 2.45 (0.20) | 2.32 (0.25) | 2.47 (0.36) | 2.59 (0.45) | 0.8662 |
| Post-ovariectomy | 1.43 (0.12) | 1.41 (0.15) | 1.51 (0.26) | 1.36 (0.22) | 0.8846 |
| Body weight (kg) | |||||
| Pre-ovariectomy | 3.63 (0.09) | 3.57 (0.13) | 3.69 (0.16) | 3.64 (0.22) | 0.8745 |
| Post-ovariectomy | 3.59 (0.12) | 3.50 (0.17) | 3.71 (0.20) | 3.54 (0.28) | 0.7681 |
Figure 3.
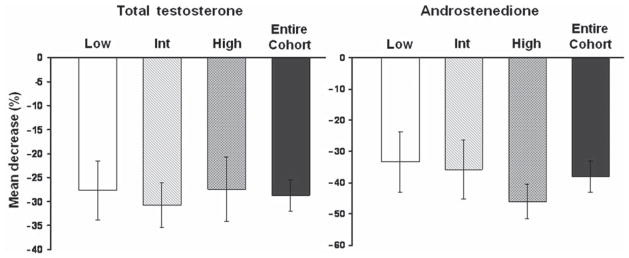
Mean percentage change in circulating total testosterone and androstenedione concentrations following ovariectomy in monkeys with low (Low, n = 9), intermediate (Int, n = 9) and high (High, n = 8) primordial follicle counts and the entire cohort. Error bars represent standard error of the mean. Ovariectomy significantly decreased circulating testosterone and androstenedione serum levels (p < 0.01), but the percentage change was not significantly different (p > 0.05) among the tertiles
DISCUSSION
Despite considerable research on the androgenic capacity of the ovary7–9,17,41–47, there still remains much uncertainty regarding the relationship between peripheral androgen concentrations, follicle number, and the persistence of androgenesis in the aging ovary containing few growing follicles and residual stromal cells. Using a cohort of macaques with primordial follicle counts ranging from normal to depleted, the current study demonstrates that expression of androgenic proteins (P450c17 and cytb5) within the theca interna cell layer of growing follicles persists in advanced atretic follicles and secondary interstitial cells (residual stromal cells). Additionally, a significant decrease in serum testosterone and androstenedione concentrations was observed following the removal of ovaries with low primordial follicle numbers (≤200), suggesting that aging macaque ovaries contribute significantly to serum androgen concentrations. It is important to note, however, that a decrease in the number of immunopositive cells (theca interna and secondary interstitial cells) was observed as the growing and atretic follicle populations were depleted.
The current study provides experimental evidence that the aging ovary is still androgenically active after menopause, based on the co-localization of cytb5 and P450c17 within the degenerating theca interna cell layer of advanced atretic follicles and secondary interstitial cells within the stroma. In contrast, two previous in vitro studies have not detected the presence of P450c17 mRNA and protein7,8 within postmenopausal human ovarian stromal cells, leading the authors to conclude that the senescent ovary is not steroidogenically active. The lack of evidence of androgen production in the aforementioned studies may relate to the heterogeneous cell population in the ovarian stroma, which consists of interstitial cells scattered throughout the stroma3. Consequently, P450c17 expression among individual stromal cells would be highly variable and may yield negative results. In the present study, immunostaining for P450c17 was observed within atretic follicles and secondary interstitial cells of macaque ovaries with low, intermediate, and high follicle numbers.
Immunolabeling for cytb5 appeared to be the most reliable indicator for persistent androgen production in all of the ovaries, most importantly in ovaries with very low (<100) primordial follicle counts. The number and intensity of theca interna and secondary interstitial cells immunopositive for cytb5 were consistently greater than those immunopositive for P450c17. These observations are in agreement with findings reported by Havelock and colleagues who demonstrated persistent cytb5 mRNA production in isolated ovarian stromal cells from postmenopausal women9.
In the present study, P450c17 protein was localized within the ooplasma of oocytes during most stages of follicular development from primary to antral follicles. Other studies have reported similar results in normal human and monkey ovaries. Tamura and colleagues co-localized P450c17 and cytochrome P450 aromatase (which converts androgens to estrogens in granulosa cells) within the oocytes of human ovaries while Hild-Petito and colleagues localized androgen receptors within the oocyte of monkey ovarian follicles48,49. The significance of these findings is unknown, but the authors suggest that oocytes may have the capacity to produce androgen and estrogen early in follicular development, prior to synthesis within the theca interna and granulosa cell layers48.
The relative expression level of 3βHSD is also important in the regulation of ovarian androgenesis. Low expression of 3βHSD relative to P450c17 promotes androgen synthesis, whereas a high expression level of 3βHSD increases progesterone production11. The mRNA transcript50 and enzyme activity of 3βHSD29, as well as the expression level of 3βHSD in the corpus luteum during pregnancy and across the menstrual cycle51, has been characterized in rhesus macaques; however, the expression level of 3βHSD in various stages of follicular development and atresia in a macaque ovary in regards to androgenesis and ovarian aging has not been described previously. Within the cynomolgus macaque ovary, we observed a low level of immunoreactivity for 3βHSD compared to P450c17 and cytb5 within the antral follicles, with minimal to no immunostaining within the advanced secondary and early atretic follicles. Low expression levels of 3βHSD in this study are not likely to be due to inefficient antibody detection because newly formed corpora lutea (positive controls) were consistently immunopositive (not shown). Taken together, these findings are consistent with those reported in women, although some studies have also observed 3βHSD expression within the membrana granulosa during late stages of folliculogenesis in addition to the theca interna cell layer of developing follicles52,53.
Studies that provide the most convincing evidence that the aging ovary maintains its steroidogenic function postmenopausally are those that report lower circulating androgen levels in surgically menopausal women compared to naturally menopausal women2,43,54–57. Davison and colleagues showed that both total and free testosterone concentrations in ovariectomized women were approximately half of those reported in age matched controls2. Similarly, Vermeulen and colleagues demonstrated that surgically postmenopausal women had approximately 35% lower androstenedione levels compared to naturally postmenopausal women (mean ± SEM, 64 ± 9 and 99 ± 13 ng/100 ml, respectively)43. A few other studies2,42 have reported lower androstenedione concentrations in women with a history of an ovariectomy; however, this finding was not statistically significant. In the present study, significant decreases in both total testosterone and androstenedione were observed in all follicle tertiles of statistically equal magnitude, indicating that the aging macaque ovary, with declining follicle numbers, still contributes significantly to circulating androgen concentrations.
In the current study, a distinct morphological difference in the amount of immunostaining, particularly for cytb5, was observed among ovaries with differential follicle counts. Due to the substantial decrease in the number of growing follicles among the ovaries with < 100 primordial follicles, only immunopositive atretic follicles and scattered interstitial cells were present within the ovarian cortex. Based on this observation, we hypothesize that androgen production will continue to decrease after ovarian senescence and that the postmenopausal ovary may eventually become hormonally inactive in the later decades of life. Since many studies have been done with women in their forties, fifties and sixties, some authors have concluded that serum testosterone and androstenedione levels do not fluctuate with menopause1,58. However, based on our morphological observations of decreased staining with decreasing follicle numbers, we hypothesize that circulating testosterone and androstenedione levels may not be independent of follicle depletion, and studies are needed in older women > 70 years of age that only have interstitial cells remaining within their ovarian cortices. In addition, more work is needed to further investigate the relationship between follicle counts, reproductive hormones, quantitative immunohistochemical, and gene expression measures within ovaries of various reproductive ages across the menopausal transition and beyond menopause. Previous studies, including the present investigation, are limited by small sample size, small age range and a lack of well-defined reproductive status to make such correlation analyses.
An understanding of the androgenic capacity of the aging ovary is of considerable clinical importance. Several studies have suggested that the decrease in estradiol production by the ovary during the menopausal transition creates a relative androgen excess (increased testosterone to estradiol ratio) that may hasten the development of chronic diseases such as cardiovascular disease24,59,60, metabolic syndrome25,61, and possibly endometrial cancer through the peripheral aromatization of androgens to estrogens62. A decline in adrenal-derived DHEA and DHEA sulfate serum levels is associatedwithaginginwomenthatisindependentofmenopause63,64; however, the extent to which declining follicle counts affect circulating testosterone and androstenedione levels and their role in chronic disease development and progression is still unclear. Estradiol concentrations were not evaluated in this retrospective study; however, we found that monkeys with aged ovaries, containing residual stromal cells and few growing follicles, had comparable pre-ovariectomy serum androgen concentrations to macaque ovaries with numerous developing follicles, suggesting that the former had a larger androgen to estradiol ratio than the latter due to fewer estradiol-producing follicles. Since postmenopausal women have a higher risk for chronic disease than their premenopausal counterparts, our data would tend to suggest that the androgen to estrogen ratio may be an important indicator of risk.
CONCLUSIONS
Our results suggest that the aging macaque ovary, despite declining follicle numbers, retains the necessary enzymes and accessory proteins for androgenesis. Significant immunostaining for androgenic proteins cytb5 and P450c17 was observed within the theca interna cell layer of growing follicles, but most importantly in the atretic follicles and secondary interstitial cells throughout the stroma. As growing follicle populations are depleted, androgen-producing secondary interstitial cells and atretic follicles remain and contribute to circulating androgen pools, as indicated by a significant decrease in serum total testosterone and androstenedione concentrations following the removal of ovaries containing a minimal number of follicles. Taken together, our data indicate that the steroidogenic capacity of the aging macaque ovary appears to be similar to that of women. Consequently, this model has the potential to provide important information about the relationship among ovarian androgens and the trajectory for chronic disease risk (cardiovascular, osteoporosis, metabolic syndrome), cognitive decline, and sexual dysfunction in post-menopausal women.
Acknowledgments
The authors would like to thank the following: Melissa Ayers, Dewayne Cairnes, Patty Christian, Laurie Custer, Barbara Staton, Debbie Golden, Andrea Grantham, Margaret May, Hermina Borgerink, Jean Gardin, and Maryanne Post for their technical contributions; Alan J. Conley, BVSc, MS, PhD, Professor of Population Health and Reproduction, University of California-Davis, School of Veterinary Medicine and Michael R. Waterman, PhD, Professor of Biochemistry, Vanderbilt University School of Medicine for generously providing antiserum for cytochrome b5 and P450c17, respectively. For serum androgen measurements, the authors would like to thank the staff of the Endocrine Core Laboratory at Yerkes Primate Center. Finally, the authors would like to express their gratitude to Thomas B. Clarkson, DVM, Professor of Pathology (Comparative Medicine) for manuscript review and his continued support and guidance.
Source of funding This work was supported by grants from the National Institutes of Health (NIH) National Center for Research Resources (NCRR) (K01 RR 021322-05 to CEW and T32 RR07009-32 to KE), National Heart, Lung, and Blood Institute (NHLBI) (R01 HL079421 to JRK) and the National Institute of Aging (NIA) (RO1 AG 027847 to SEA, R03-AG20290 to CRP). The contents are solely the responsibility of the authors and do not necessarily represent the view of the NCRR, NHLBI, NIA or NIH.
Footnotes
Conflict of interest The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Zumoff B, Strain GW, Miller LK, Rosner W. Twenty-four-hour mean plasma testosterone concentration declines with age in normal premenopausal women. J Clin Endocrinol Metab. 1995;80:1429–30. doi: 10.1210/jcem.80.4.7714119. [DOI] [PubMed] [Google Scholar]
- 2.Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90:3847–53. doi: 10.1210/jc.2005-0212. [DOI] [PubMed] [Google Scholar]
- 3.Erickson GF, Magoffin DA, Dyer CA, Hofeditz C. The ovarian androgen producing cells: a review of structure/function relationships. Endocrine Rev. 1985;6:371–99. doi: 10.1210/edrv-6-3-371. [DOI] [PubMed] [Google Scholar]
- 4.Magoffin DA. Ovarian theca cell. Int J Biochem. 2005;37:1344–9. doi: 10.1016/j.biocel.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopause transition: evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab. 1987;65:1231–7. doi: 10.1210/jcem-65-6-1231. [DOI] [PubMed] [Google Scholar]
- 6.Adashi EY. The climacteric ovary as a functional gonadotropin-driven androgen-producing gland. Fertil Steril. 1994;62:20–7. doi: 10.1016/s0015-0282(16)56810-1. [DOI] [PubMed] [Google Scholar]
- 7.Couzinet B, Meduri G, Lecce MG, et al. The postmenopausal ovary is not a major androgen-producing gland. J Clin Endocrinol Metab. 2001;86:5060–6. doi: 10.1210/jcem.86.10.7900. [DOI] [PubMed] [Google Scholar]
- 8.Jabara S, Christenson LK, Wang CY, et al. Stromal cells of the human postmenopausal ovary display a distinctive biochemical and molecular phenotype. J Clin Endrocrinol Metab. 2003;88:484–92. doi: 10.1210/jc.2002-021274. [DOI] [PubMed] [Google Scholar]
- 9.Havelock JC, Rainey WE, Bradshaw KD, Carr BR. The post-menopausal ovary displays a unique pattern of steroidogenic enzyme expression. Hum Reprod. 2006;21:309–17. doi: 10.1093/humrep/dei373. [DOI] [PubMed] [Google Scholar]
- 10.Hall PF. Cytochrome P-450 c21scc: one enzyme with two actions: hydroxylase and lyase. J Steriod Biochem Molec Biol. 1991;40:527–32. doi: 10.1016/0960-0760(91)90272-7. [DOI] [PubMed] [Google Scholar]
- 11.Conley AJ, Bird IM. The role of cytochrome P450 17α-hydroxylase and 3β-hydroxysteroid dehydrogenase in the integration of gondal and adrenal steroidogenesis via the Δ5 and Δ4 pathways of steroidogenesis in mammals. Biol Reprod. 1997;56:789–99. doi: 10.1095/biolreprod56.4.789. [DOI] [PubMed] [Google Scholar]
- 12.Katagiri M, Kagawa N, Waterman MR. The role of cytochrome b5 in the biosynthesis of androgens by human P450c17. Arch Biochem Biophys. 1995;317:343–7. doi: 10.1006/abbi.1995.1173. [DOI] [PubMed] [Google Scholar]
- 13.Lee-Robichaud P, Wright JN, Akhtar ME, Akhtar M. Modulation of the activity of human 17α-hydroxylase-17,20-lyase (CYP17) by cytochrome b5: endocrinological and mechanistic implications. Biochem J. 1995;308:901–8. doi: 10.1042/bj3080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auchus RJ, Lee TC, Miller WL. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem. 1998;273:3158–65. doi: 10.1074/jbc.273.6.3158. [DOI] [PubMed] [Google Scholar]
- 15.Doody KJ, Lorence MC, Mason JI, Simpson ER. Expression of messenger ribonucleic acid species encoding steroidogenic enzymes in human follicles and corpora lutea throughout the menstrual cycle. J Clin Endocrinol Metab. 1990;70:1041–5. doi: 10.1210/jcem-70-4-1041. [DOI] [PubMed] [Google Scholar]
- 16.Rhéaume E, Lachance Y, Zhao HF, et al. Structure and expression of a new complementary DNA encoding the almost exclusive 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4-isomerase in human adrenals and gonads. Mol Endocrinol. 1991;5:1147–57. doi: 10.1210/mend-5-8-1147. [DOI] [PubMed] [Google Scholar]
- 17.Dharia S, Slane A, Jian M, Conner M, Conley AJ, Parker CP. Colocalization of P450c17 and cytochrome b5 in androgen-synthesizing tissues of the human. Biol Reprod. 2004;71:83–8. doi: 10.1095/biolreprod.103.026732. [DOI] [PubMed] [Google Scholar]
- 18.Davis SR, McCloud P, Strauss BJ, Burger H. Testosterone enhances estradiol’s effects on postmenopausal bone density and sexuality. Maturitas. 1995;21:227–36. doi: 10.1016/0378-5122(94)00898-h. [DOI] [PubMed] [Google Scholar]
- 19.Žofková I, Bahbouh R, Hill M. The pathophysiological implications of circulating androgens on bone mineral density in a normal female population. Steroids. 2000;65:857–61. doi: 10.1016/s0039-128x(00)00136-7. [DOI] [PubMed] [Google Scholar]
- 20.Notelovitz M. Androgen effects on bone and muscle. Fertil Steril. 2002;77:S34–40. doi: 10.1016/s0015-0282(02)02968-0. [DOI] [PubMed] [Google Scholar]
- 21.Bachmann GA, Leiblum SR. The impact of hormones on menopausal sexuality: a literature review. Menopause. 2004;11:120–30. doi: 10.1097/01.GME.0000075502.60230.28. [DOI] [PubMed] [Google Scholar]
- 22.Tok EC, Ertunc D, Oz U, Camdeviren H, Ozdemir G, Dilek S. The effect of circulating androgens on bone mineral density in postmenopausal women. Maturitas. 2004;48:235–42. doi: 10.1016/j.maturitas.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Will MA, Randolph JF. The influence of reproductive hormones on brain function in the menopausal transition. Minerva Ginecol. 2009;61:469–81. [PubMed] [Google Scholar]
- 24.Liu Y, Ding J, Bush TL, et al. Relative androgen excess and increased cardiovascular risk after menopause: a hypothesized relation. Am J Epidemiol. 2001;154:489–94. doi: 10.1093/aje/154.6.489. [DOI] [PubMed] [Google Scholar]
- 25.Torréns JI, Sutton-Tyrrell K, Zhao X, et al. Relative androgen excess during the menopausal transition predicts incident metabolic syndrome in midlife women: Study of Women’s Health Across the Nation. Menopause. 2008;16:257–64. doi: 10.1097/gme.0b013e318185e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shifren JL, Braunstein GD, Simon JA, et al. Transdermal testerosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343:682–8. doi: 10.1056/NEJM200009073431002. [DOI] [PubMed] [Google Scholar]
- 27.Shifren JL. Androgen deficiency in the oophorectomized women. Fertil Steril. 2002;77:S60–2. doi: 10.1016/s0015-0282(02)02970-9. [DOI] [PubMed] [Google Scholar]
- 28.Corner GW. Ovulation and menstruation in Macacus rhesus. Contrib Embryol. 1923;15:73–102. [Google Scholar]
- 29.Martel C, Melner MH, Gagné D, Simard J, Labrie F. Widespread tissue distribution of steroid sulfatase, 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4 isomerase (3 beta-HSD), 17 beta-HSD 5 alpha-reductase and aromatase activities in the rhesus monkey. Mol Cell Endocrinol. 1994;104:103–11. doi: 10.1016/0303-7207(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 30.Gilardi KVK, Shideler SE, Valverde CR, Roberts JA, Lasley BL. Characterization of the onset of menopause in the rhesus macaque. Biol Reprod. 1997;57:335–40. doi: 10.1095/biolreprod57.2.335. [DOI] [PubMed] [Google Scholar]
- 31.Kavanagh K, Williams JK, Wagner JD. Naturally occurring menopause in cynomolgus monkeys: changes in hormone, lipid, and carbohydrate measures with hormonal status. J Med Primatol. 2005;34:171–7. doi: 10.1111/j.1600-0684.2005.00114.x. [DOI] [PubMed] [Google Scholar]
- 32.Nichols SM, Bavister BD, Brenner CA, Didier PJ, Harrison RM, Kubisch HM. Ovarian senescence in the rhesus monkey (Macaca mulatta) Hum Reprod. 2005;20:79–83. doi: 10.1093/humrep/deh576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shideler SE, Gee NA, Chen J, Lasley BL. Estrogen and progesterone metabolites and follicle stimulating hormone in the aged macaque female. Biol Reprod. 2001;65:1718–25. doi: 10.1095/biolreprod65.6.1718. [DOI] [PubMed] [Google Scholar]
- 34.Lees CJ, Kaplan JR, Chen H, Jerome CP, Register TC, Franke AA. Bone mass and soy isoflavones in socially housed, premenopausal macaques. Am J Clin Nutr. 2007;86:245–50. doi: 10.1093/ajcn/86.1.245. [DOI] [PubMed] [Google Scholar]
- 35.Walker SE, Register TC, Appt SE, et al. Plasma lipid-dependent and -independent effects of dietary soy protein and social status on atherogenesis in premenopausal monkeys: implications for postmenopausal atherosclerosis burden. Menopause. 2008;15:950–7. doi: 10.1097/gme.0b013e3181612cef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Appt SE, Chen H, Goode AK, et al. The effect of diet and cardiovascular risk on ovarian aging in cynomolgus monkeys (Macaca fascicularis) Menopause. 2010;17:741–8. doi: 10.1097/gme.0b013e3181d20cd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaplan JR, Chen H, Appt SE, et al. Impairment of ovarian function and associated health-related abnormalities are attributed to low social status in premenopausal monkeys and not mitigated by a high-isoflavone soy diet. Hum Reprod. 2010;25:3083–94. doi: 10.1093/humrep/deq288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller PB, Charleston JS, Battaglia DE, Klein NA, Soules MR. Morphometric analysis of primordial follicle number in pigtailed monkey ovaries: symmetry and relationship with age. Biol Reprod. 1999;61:553–6. doi: 10.1095/biolreprod61.2.553. [DOI] [PubMed] [Google Scholar]
- 39.Appt SE, Clarkson TB, Hoyer PB, et al. Experimental induction of reduced ovarian reserve in a nonhuman primate model. Comp Med. 2010;60:380–8. [PMC free article] [PubMed] [Google Scholar]
- 40.Buse E, Zöller M, Van Esch E. The macaque ovary with special reference to the cynomolgus macaque (Macaca fascicularis) Toxicol Pathol. 2008;36:24–66S. [Google Scholar]
- 41.Judd HL, Lucas WE, Yen SSC. Effect of oophorectomy on circulating testosterone and androstenedione levels in patients with endometrial cancer. Am J Obstet Gynecol. 1974;118:793–8. doi: 10.1016/0002-9378(74)90490-6. [DOI] [PubMed] [Google Scholar]
- 42.Fogle RH, Stanczyk FZ, Zhang X, Paulson RJ. Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab. 2007;92:3040–3. doi: 10.1210/jc.2007-0581. [DOI] [PubMed] [Google Scholar]
- 43.Judd HL, Judd GE, Lucas WE, Yen SSC. Endocrine function of the postmenopausal ovary: concentration of androgens and estrogens in ovarian and peripheral vein blood. J Clin Endocrinol Metab. 1974;39:1020–4. doi: 10.1210/jcem-39-6-1020. [DOI] [PubMed] [Google Scholar]
- 44.Vermeulen A. The hormonal activity of the postmenopausal ovary. J Clin Endocrinol Metab. 1976;42:247–53. doi: 10.1210/jcem-42-2-247. [DOI] [PubMed] [Google Scholar]
- 45.Longcope C, Hunter R, Franz C. Steroid secretion by the postmenopausal ovary. Am J Obstet Gynecol. 1980;138:564–8. doi: 10.1016/0002-9378(80)90287-2. [DOI] [PubMed] [Google Scholar]
- 46.Lucisano A, Acampora MG, Russo N, Maniccia E, Montemurro A, Dell’Acqua S. Ovarian and peripheral plasma levels of progestogens, androgens and oestrogens in postmenopausal women. Maturitas. 1984;6:45–53. doi: 10.1016/0378-5122(84)90064-1. [DOI] [PubMed] [Google Scholar]
- 47.Aiman J, Forney JP, Parker CR., Jr Secretion of androgens and estrogens by normal and neoplastic ovaries in postmenopausal women. Obstet Gynecol. 1986;68:1–5. [PubMed] [Google Scholar]
- 48.Tamura T, Kitawaki J, Yamamoto T, et al. Immunohistochemical localization of 17α-hydroxylase/C17–20 lyase and aromatase cytochrome P-450 in the human ovary during the menstrual cycle. J Endrocinol. 1992;135:589–95. doi: 10.1677/joe.0.1350589. [DOI] [PubMed] [Google Scholar]
- 49.Hild-Petito S, West NB, Brenner RM, Stouffer RL. Localization of androgen receptor in the follicle and corpus luteum of the primate ovary during the menstrual cycle. Biol Reprod. 1991;44:561–8. doi: 10.1095/biolreprod44.3.561. [DOI] [PubMed] [Google Scholar]
- 50.Simard J, Melner MH, Breton N, et al. Characterization of macaque 3β-hydroxy-5-ene steroid dehydrogenas/Δ5-Δ4 isomerase: structure and expression in steroidogenic and peripheral tissues in primate. Mol Cell Endocrinol. 1991;75:101–10. doi: 10.1016/0303-7207(91)90224-g. [DOI] [PubMed] [Google Scholar]
- 51.Sanders SL, Stouffer RL. Localization of steroidogenic enzymes in macaque luteal tissue during the menstrual cycle and stimulated early pregnancy: immunohistochemical evidence supporting the two-cell model for estrogen production in the primate corpus luteum. Biol Reprod. 1997;56:1077–87. doi: 10.1095/biolreprod56.5.1077. [DOI] [PubMed] [Google Scholar]
- 52.Sasano H, Mori T, Sasano N, Nagura H, Mason JI. Immunolocalization of 3β-hydroxysteroid dehydrogenase in human ovary. J Reprod Fertil. 1990;89:743–51. doi: 10.1530/jrf.0.0890743. [DOI] [PubMed] [Google Scholar]
- 53.Dupont E, Labrie F, Luu-The V, Pelletier G. Immunocytochemical localization of 3β-hydroxysteroid dehydrogenase/Δ5-Δ4-isomerase in human ovary. J Clin Endocrinol Metab. 1992;74:994–8. doi: 10.1210/jcem.74.5.1569177. [DOI] [PubMed] [Google Scholar]
- 54.Laughlin GA, Barrett-Connor E, Kritz-Silverstein D, von Mühlen D. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2000;85:645–51. doi: 10.1210/jcem.85.2.6405. [DOI] [PubMed] [Google Scholar]
- 55.Cappola AR, Ratcliffe SJ, Bhasin S, et al. Determinants of serum total and free testosterone levels in women over the age of 65 years. J Clin Endocrinol Metab. 2007;92:509–16. doi: 10.1210/jc.2006-1399. [DOI] [PubMed] [Google Scholar]
- 56.Korse CM, Bonfrer JMG, van Beurden M, Verheijen RHM, Rookus MA. Estradiol and testosterone levels are lower after oophorectomy than after natural menopause. Tumor Biol. 2009;30:37–42. doi: 10.1159/000199449. [DOI] [PubMed] [Google Scholar]
- 57.McTiernan A, Wu L, Barnabei VM, et al. Relation of demographic factors, menstrual history, reproduction and medication use to sex hormone levels in postmenopausal women. Breast Cancer Res Treat. 2008;108:217–31. doi: 10.1007/s10549-007-9588-6. [DOI] [PubMed] [Google Scholar]
- 58.Burger HG, Dudley EC, Cui J, Dennerstein L, Hopper JL. A prospective longitudinal study of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone-binding globulin levels through the menopause transition. J Clin Endocrinol Metab. 2000;85:2832–8. doi: 10.1210/jcem.85.8.6740. [DOI] [PubMed] [Google Scholar]
- 59.Sutton-Tyrrell K, Wildman RP, Matthews KA, et al. Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN) Circulation. 2005;111:1242–9. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- 60.Sowers MR, Jannausch M, Randolph JF, et al. Androgens are associated with hemostatic and inflammatory factors among women at the mid-life. J Clin Endocrinol Metab. 2005;90:6064–71. doi: 10.1210/jc.2005-0765. [DOI] [PubMed] [Google Scholar]
- 61.Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome. Arch Intern Med. 2008;168:1568–75. doi: 10.1001/archinte.168.14.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jongen VH, Sluijmer AV, Heineman MJ. The postmenopausal ovary as an androgen-producing gland; hypothesis on the etiology of endometrial cancer. Maturitas. 2002;43:77–85. doi: 10.1016/s0378-5122(02)00140-8. [DOI] [PubMed] [Google Scholar]
- 63.Zumoff B, Rosenfeld RS, Strain GW, et al. Sex differences in the twenty-four hour mean plasma concentrations of dehydroisoandrosterone (DHA) and dehydroiandrosterone sulfate (DHAS) and the DHA to DHAS ratio in normal adults. J Clin Endocrinol Metab. 1980;51:330–3. doi: 10.1210/jcem-51-2-330. [DOI] [PubMed] [Google Scholar]
- 64.Labrie F, Bélanger A, Cusan L, Gomez J, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997;82:2396–402. doi: 10.1210/jcem.82.8.4160. [DOI] [PubMed] [Google Scholar]