Definition and History
Unfractionated heparin (UFH) and its derivatives, the low-molecular weight heparins (LMWHs; henceforth, collectively referred to as heparin), remain the most commonly prescribed anticoagulants for the prophylaxis and treatment of venous thromboembolism (VTE) in hospitalized patients.1 In a subset of treated patients (< 5%), heparin elicits a life-threatening immune complication, heparin-induced thrombocytopenia (HIT). HIT is a self-limited hypercoagulable disorder occurring predominantly in hospitalized patients. The cardinal manifestations of HIT are declining platelet counts within 5-14 days after heparin exposure and a predilection for arterial and venous thrombosis.2
The clinical syndrome of HIT was first described in the 1950’s by Weissman & Tobin.3 Subsequent studies revealed the immune origins of this syndrome4 with the identification of antibodies directed to antigenic complexes of platelet factor 4 (PF4) and heparin (H).5 With the advent of immunoassays for detection of PF4/H antibodies, it is now recognized that an asymptomatic immune response to PF4/H occurs far more commonly than clinical complications of disease (thrombocytopenia and/or thrombosis). This chapter reviews our current understanding of the pathogenesis, clinical features, laboratory testing and therapeutic options for patients with HIT.
Etiology and Pathogenesis
PF4/H complexes and the immune response in HIT
The primary physiologic role of PF4 is to neutralize the antithrombotic effect of heparin and heparin-like molecules (heparan sulfate, chondroitin sulfate) on cell-surfaces. Upon platelet activation, PF4, a positively charged protein residing in platelet α-granules, is released in large amounts, binds to endothelial heparin sulfate and displaces antithrombin (AT) from the cell-surface. When patients are administered pharmacologic doses of heparin for thromboprophylaxis or for treatment, cell-bound PF4 dissociates from endothelial sites to form ultra-large complexes with circulating heparin through electrostatic interactions. Recent murine studies have shown that these circulating and/or cell bound PF4/H complexes are highly immunogenic in vivo.6 Once formed, immune complexes containing IgG antibody and antigen are capable of engaging cellular Fc receptors on platelets,7 monocytes,8,9 and neutrophils10 to promote cellular activation and thrombin generation.11
Epidemiology of HIT
In recent prospective investigations employing UFH and/or LMWH, the overall incidence of HIT is estimated at 0.5-0.8% of treated patients.12,13 Drug and host characteristics contribute to the risk of developing HIT. Of the various drug dependent characteristics influencing immunogenicity: chain-length (UFH > LMWH ≥ fondaparinux), animal source of heparin (bovine > porcine)14 and route (intravenous > subcutaneous),15 heparin chain length appears to be the most clinically significant. The incidence of HIT is approximately ten fold higher with UFH (~3%) as compared to LMWH (0.2%)16 in patients receiving thromboprophylactic doses. These differences in UFH and LMWH subside, however, when treatment doses are administered. In a meta-analysis involving 13 studies and > 5,000 patients, the rates of HIT were comparable in patients receiving UFH or LMWH (LMWH 1.2% vs UFH 1.5%).13 Surprisingly, no increase in HIT incidence has been reported17,18 despite the increased utilization of LMWHs in recent years for the prevention of hospital-acquired VTE. Although rates of seroconversion are similar for fondaparinux and LMWH,19 the occurrence of HIT appears to be infrequent with fondaparinux.20
Host risk factors include clinical context of heparin exposure and patient characteristics (age, gender and race). Patients on the general medical, cardiology and surgical services (orthopedic and trauma) are at higher risk than patients on obstetric, pediatric or renal (chronic hemodialysis) services.2,21 The reasons for this variable risk are presently unknown, but are thought to arise from differences in basal levels of platelet activation and circulating PF4 levels. Consistent with observations of a low incidence of HIT in pediatric and obstetric patients, a recent large analysis of hospital discharges of ~ 270,000 inpatient records showed that HIT was exceedingly rare in patients less than 40 years of age.13 In this same study, among patients with VTE, the incidence of secondary thrombocytopenia, presumably due to HIT, was higher among blacks (relative risk or RR 1.3) as compared to whites. Although one recent study showed a higher incidence of HIT among females (odds ratio or OR of 2.422), other studies have found a slightly higher risk among males (RR 1.1).13 Several genetic polymorphisms, including homozygozity of the FcγRIIIa-158V allele,23 the protein tyrosine phosphatase CD14824 and the interleukin-10 promoter25 have been described in single center studies of patients with and without HIT. The clinical significance of these findings remains to be established in larger studies.
Clinical Elements of Diagnosis
Because of the high incidence of asymptomatic PF4/H conversion (see section on Laboratory Elements of Diagnosis) in patients exposed to heparin, it is essential to understand the clinical features associated with disease presentation. Three essential elements comprise the clinical evaluation of patients suspected of HIT: 1) Documenting the presence of thrombocytopenia and/or thrombosis 2) Establishing the temporal course of thrombocytopenia relative to heparin exposure and 3) Excluding other causes of thrombocytopenia. A detailed discussion of these clinical diagnostic elements and commonly used diagnostic algorithms is provided below. Table 1 summarizes the clinical features commonly or infrequently seen in HIT.
Table 1. Clinical Features Consistent/Not Consistent with HIT.
| Consider HIT | HIT Unlikely |
|---|---|
| Following clinical symptoms within 4-14 days of new heparin therapy or within 24 hours of heparin re- exposure*
|
|
Re-exposure within three months of prior heparin therapy
Abbreviations: DIC, disseminated intravascular thrombosis
1) Documenting the presence of thrombocytopenia and/or thrombosis
Thrombocytopenia in HIT
Thrombocytopenia is an essential diagnostic feature of HIT and is reported to occur in ~95% of HIT patients during the course of illness.26-28 Patients who develop skin necrosis are a notable exception to this diagnostic rule, as thrombocytopenia frequently does not accompany this atypical manifestation.29,30 Thrombocytopenia in HIT can present as an absolute drop in platelet count below the normal range (platelet count < 150 × 109/L) or as a relative decrease of 30-50% from baseline counts. Absolute thrombocytopenia results in a moderate thrombocytopenia, with mean platelet counts of 50-70 × 109/L. In the postoperative period, where platelet counts typically rebound to a higher number than the pre-operative count, the immediate post-operative platelet count should be considered as the baseline platelet count for determining the change in platelet count. This revised definition of thrombocytopenia has been shown to be sensitive and specific for diagnosing HIT.31
Less than 5% of patients with HIT will have a platelet count < 20 × 109/L.29 The presence of petechiae or extensive ecchymoses in the absence of disseminated intravascular coagulation (DIC) should prompt a search for an alternative diagnoses (see Table 1).29 Severe thrombocytopenia as a manifestation of HIT is associated with a high risk of thrombotic complications, likely due to platelet consumption.28 In a retrospective series of 408 patients, patients with severe thrombocytopenia (defined as > 90% decline from baseline counts) were noted to have an 8-fold higher risk for thrombotic complications as compared to patients with a < 30% platelet count decline.28
Several retrospective and prospective studies have shown that isolated thrombocytopenia is a harbinger of subsequent thromboses in patients (20-50%).28,32-34 In one-third of patients, the thromboembolic complication (TEC) can occur concurrently or precede the development of thrombocytopenia.28,35,36 Because of the therapeutic implications of finding a VTE in HIT patients with isolated thrombocytopenia, patients diagnosed with isolated HIT should undergo routine screening for subclinical TEC (such as lower extremity ultrasound).34
Thrombosis in HIT
Thrombosis is the most feared complication of HIT. In prospective and retrospective series, thrombotic complications have been reported to occur in 29%-57%28,37 of HIT patients. In one registry, 25% of patients developed 3 or more thromboembolic complications.28 Prior to the availability of current therapies, 16% of all thrombotic complications were fatal and 9% of all thrombotic events resulted in limb amputation.37 In relation to thrombocytopenia, a large retrospective study of patients with HIT found that in 34% of patients, thrombotic complications will precede or occur concurrently with a major decrease in platelets.28
Thrombotic events involving the venous circulation occur far more commonly than arterial thrombotic events, with reported frequencies of 2.4:1-4:1.28,33 Lower limb deep venous thrombosis (DVT) and pulmonary embolism comprise the vast majority of venous thrombotic events.28 Upper limb DVTs are also common but are reported to occur almost exclusively at central venous catheter sites.38 The postoperative period has also been strongly associated with venous thrombosis in HIT.33,37,39
Arterial thromboses occur in 7-14%33,37 of patients affected with HIT. In one series of patients with HIT, a history of cardiovascular events, including myocardial infarction, and a history of cardiovascular surgery were associated with a significantly increased incidence of arterial thrombosis.39 In order of decreasing frequency, common sites of arterial thrombosis include: limb artery thrombosis, thrombotic stroke and myocardial infarction.28 Atypical sites of presentation including bilateral adrenal hemorrhage,40 venous limb gangrene, cerebral venous thrombosis,41 spinal ischemia,41 and skin necrosis should warrant consideration of HIT in the differential diagnosis.42
Presently, there are no definitive means for predicting the risk of thrombosis in patients who develop isolated thrombocytopenia in HIT. Studies have shown that established risk factors for hypercoagulability, such as protein C, protein S, antithrombin clotting factor mutations and/or platelet polymorphisms do not contribute significantly to thrombotic tendency.39,43 Certain common serologic features occur at a higher frequency among patients with thrombotic HIT as compared to those with isolated thrombocytopenia in HIT, including IgG isotype,44 antibodies capable of platelet activation44-46 and high antibody levels (as gauged by optical density (OD) and/or titer).47-49 Risk factors for thrombosis development are outlined in Table 2.
Table 2. Risk Factors for Thrombosis in HIT.
| Predictors of Thrombosis in HIT | |
|---|---|
| Correlated with Thrombotic Risk | No Correlation |
Despite these serologic features, the presence of platelet activating IgG antibodies in some patients with asymptomatic PF4/heparin antibodies50 or the occurrence of low titer antibodies in other patients with a clinically confirmed diagnosis of thrombotic HIT47 does not permit unambiguous segregation of these risk factors. Discontinuing heparin therapy after early recognition of HIT does not appear to lower the risk of subsequent thrombosis.51
2) Establishing the temporal course of thrombocytopenia relative to heparin exposure
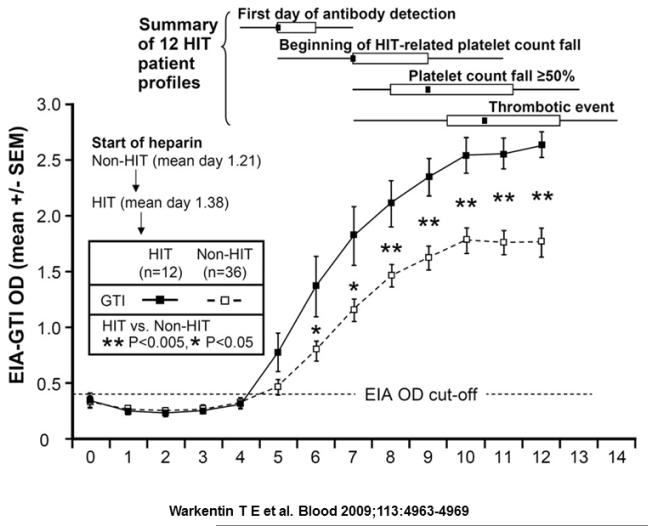
In heparin-naïve patients, platelet counts classically decline within 5-10 days of heparin initiation. As shown in a recent study of the evolution of the HIT immune response, 12 patients with HIT were examined serially for PF4/H antibody levels and platelet counts. As shown in Figure 1, seroconversions occurred at a median of 4 days from start of heparin therapy, with a fall in platelet count occurring 2 days after seroconversion (~ 6 days from start of heparin therapy). The interval time to when the platelet count decline met diagnostic criteria for HIT in this study (> 50% platelet count fall) occurred 4 days after seroconversion (median time interval of 8 days from the start of heparin). Thrombosis also occurred after seroconversion, but often was coincident with changes in platelet counts.36 These observations, coupled with studies of murine models,52 suggest that patients’ PF4/H seroconversions must precede thrombocytopenia and or thrombosis, and clinical events predating seroconversion are unlikely to be related to HIT.36 In 30% of patients with HIT, an atypical, rapid fall in the platelet count, occurring at a median of 10 hours after beginning heparin therapy can occur from pre-existing PF4/H antibodies caused by recent heparin exposure (within 3 months).26 A small-subset of patients develops thrombocytopenia days to weeks after heparin exposure,53 a clinical variant called “Delayed-Onset HIT.” Delayed onset HIT is frequently associated with complications of DIC and/or extensive thrombosis54 and should be considered in patients presenting with new onset thrombocytopenia within 2-4 weeks of a recent hospitalization.
Figure 1. Evolution of the immune response relative to clinical manifestations of HIT.
12 patients with HIT and 36 seropositive non-HIT control patients were monitored for PF4/heparin antibodies, thrombocytopenia and thrombosis after orthopedic surgery. HIT patients are indicated by ■, and seropositive non-HIT controls by □. Time course of seroconversions are shown on the x-axis and OD levels between the patients with HIT and the seropositive non-HIT controls (P < 0.05 by nonpaired t test) are shown on the y-axis. At the top of the figure, summary data for 12 HIT patient profiles are shown for 4 key events (first day of antibody detection, beginning of HIT-related platelet count fall, platelet count fall ≥ 50%, and thrombotic event), summarized as median (small black squares within rectangles), interquartile range (open rectangles), and range (ends of thin black lines). Adapted from Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. The New England journal of medicine 1995;332:1330-5.with permission.
Discontinuation of heparin should allow prompt resolution of thrombocytopenia. In clinical practice, platelet counts typically increase within 48 hours of heparin discontinuation and thrombocytopenia usually resolves within 4-14 days.29 A prolonged duration of thrombocytopenia (>7 days) after heparin discontinuation has been linked with disease severity.51 Despite platelet count recovery, thrombotic risk remains high for 4-6 weeks, due to the presence of circulating PF4/H antibodies33 and these antibodies likely contribute to the development of delayed onset HIT.54 The median time to antibody clearance is 85-90 days,26,49 although in one series, ~35% of patients were noted to be seropositive for up to one year.49 To what extent PF4/H seropositivity, in the absence of thrombocytopenia and/or thrombosis, predisposes patients to thrombotic complications remains controversial.49,55-57 Unlike other drug-induced thrombocytopenias, the risk of recurrent HIT with subsequent heparin re-exposure appears to be low, but these findings have not been prospectively investigated. Although several retrospective analyses and case reports suggest that the risk of recurrence may be low in patients who become seronegative for PF4/H antibodies,58 current guidelines recommend avoiding routine heparin re-exposure in these patients.59
3) Excluding other causes of thrombocytopenia
The majority of patients suspected of HIT are not likely to have disease.60,61 With the routine implementation of heparin thromboprophylaxis in most hospitals as well as the frequent occurrence of thrombocytopenia in hospitalized patients,62 the statistical likelihood that these two clinical scenarios will converge is far more likely than the occurrence of HIT. Illustrating this point was a recent study by Oliveira and colleagues of 2420 patients treated with heparin63 who were assessed for development of thrombocytopenia (defined as a platelet count less than 150 × 109/L, reduction in platelet count of 50% or more from the admission level, or both). In this study, 881 patients or 36.4% (95% confidence interval [CI], 34.5%-38.3%) met the definition for thrombocytopenia while receiving heparin therapy; 13% of patients met both criteria of a decreased absolute platelet count as well as a reduction in platelet count of > 50%. In this study, ~ 0.7% of patients were diagnosed with HIT.63
The differential diagnosis of acute thrombocytopenia in a hospitalized patient is extensive as shown in Table 1 (HIT Unlikely column). Thrombocytopenia is common in the intensive care units (ICU) occurring in 38-46% of patients.64 Thrombocytopenia is particularly problematic in the cardiac surgery setting, where patients have a number of risk factors for HIT, including recent heparin exposure, the inflammatory milieu of surgery and high rates of PF4/H seroconversion.50,55,65,66 In one recent study of cardiac surgery patients requiring > 7 days in the cardiac ICU, 21% of patients (70/329) developed thrombocytopenia, with 67/70 patients (95%) having alternative or non-HIT related causes for thrombocytopenia.67 Adding to the complexity of evaluation of cardiac surgery patients is the relatively frequent use of mechanical devices, such as intra-aortic balloon pumps (IABP). Thrombocytopenia is frequently encountered in patients with IABP, occurring at a frequency of 30-50% of cases.68
Clinical Algorithms in assessing likelihood of HIT
Given the broad differential diagnosis and frequency of thrombocytopenia in hospitalized patients, clinical algorithms have been developed to assist clinicians in tabulating the risk of HIT in a given patient.
4T’s Scoring System
The most widely-used clinical scoring system is the 4T’s, developed by Dr. Warkentin at McMaster University. The 4T scoring system assesses the clinical diagnostic elements discussed above and assigns a score (0, 1, or 2; maximum total score of 8) for the following features: the magnitude of Thrombocytopenia, Timing of platelet count fall or complication in relation to heparin use, Thrombosis or other HIT-associated sequelae, and absence of anoTher explanation for thrombocytopenia.29 A 4T score of 6-8 is consistent with a high pretest probability of HIT, a score of 4-5 is consistent with an intermediate probability of HIT, and a score of 0-3 is consistent with a low probability of HIT.29 The diagnostic utility of the 4T score has been examined in numerous prospective and retrospective studies.46,69-73 In all studies to date, the 4T’s has consistently demonstrated excellent negative predictive value (NPV), with a 4T score < 3 reliably translating into a low likelihood of serologically confirmed HIT.46,69-74 On the other hand, the positive predictive value (PPV) of the 4T scoring system is variable and highly dependent on the practitioner’s background.69 To demonstrate the effect of a practitioner’s experience in utilizing the 4T’s scoring system, Lo and colleagues tested this algorithm at two medical centers, in Hamilton, Canada and Greifswald, Germany (GW). The practitioner applying the 4T’s at the Hamilton General Hospital (HGH) was Dr. Warkentin, the developer of the 4T’s scoring system. In Greifswald, general practitioners utilized the 4T’s for diagnosing HIT. When the clinical scores were correlated with laboratory testing, the NPV was high at both medical centers (98% at HGH and 100% at GW). However, the predictive value of intermediate scores [HGH: 8/28 (28.6%), GW: 11/139 (7.9%)] and high scores [HGH: 8/8 (100%), GW: 9/42 (21.4%)] markedly differed by institution. The clinical utility of the 4T’s in predicting the likelihood of HIT was low in Germany, where primary care providers were using the algorithm, but much higher in Canada in the hands of an experienced HIT diagnostician. This study, as well as others, 70-73 confirms that the PPV of intermediate and high scores is far less reliable than the NPV.
HIT Expert Probability (HEP) Score
In an effort to improve on the specificity and the PPV of the 4T’s, the HIT Expert Probability (HEP) score was developed using expert opinion to refine the clinical scoring system. In this model, 26 experts were asked to assign points to 8 clinical features of HIT based on diagnostic relevance (magnitude of fall in platelet count, timing of fall in platelet count, nadir platelet count, thrombosis, skin necrosis, acute systemic reaction, bleeding, and other causes of thrombocytopenia).75 Based on the median score, each clinical feature was then assigned a point ranging from −3 to +3 and the HEP score, a pretest probability model, was created. In a validation study at a single institution, the HEP score demonstrated improved interobserver agreement and improved correlation with serologic HIT testing when compared to the 4T’s score. In this study, the HEP score was 100% sensitive and 60% specific for diagnosing HIT.75 However, unlike the 4T’s which is fairly simple to perform, the HEP score is more complex and cumbersome to use. Additional prospective studies are needed to validate the HEP Scoring system.
Cardiac Surgery (Lillo-Le Louet) Scoring System
Nowhere is the challenge of distinguishing HIT from other causes of thrombocytopenia more difficult than in the clinical setting of cardiac surgery. Cardiac surgery is associated with a number of comorbidities that confound the diagnosis of HIT, including several risk factors for thrombocytopenia (dilutional effect, infection/DIC, cardiogenic shock, mechanical devices, multiple medications), increased rates of thrombosis (20% in one recent retrospective study of non-HIT patients)76 and a high prevalence of PF4/H seroconversion (see Laboratory Elements of Diagnosis). Despite the high-risk features of this clinical setting, retrospective and prospective series have demonstrated that the post-operative risk of HIT after cardiac surgery is low (0.6-2%).77,78 Due to the difficulty in recognizing HIT post-cardiac surgery, Lillo-Le Louet and colleagues identified three independent clinical variables (platelet count pattern, time from cardiopulmonary bypass (CPB) to suspicion of HIT and CPB duration) based on a clinical cohort suspected of HIT.79 In patients with HIT, a characteristic biphasic pattern of platelet count recovery was observed. Platelet counts initially decline for 2-4 days after surgery, then rebound into the normal range or beyond, and then fall once again79 due to antibody development and HIT. Based on these observations, scores were assigned for platelet count time course or pattern (biphasic = 2, persistent thrombocytopenia = 1), time from CPB to date of HIT suspicion (≥ 5 days = 2, < 5 days = 0) and CPB duration (≤ 118 min = 1, > 118 min = 0). In their retrospective study, a score of ≥ 2 was associated with a high probability of HIT (PPV of 62%), whereas a score ≥ 5 was associated with a markedly higher PPV of 95%.79 In a recent prospective study of 1,722 patients undergoing cardiac surgery, the Lillo-Le Louet scoring system was compared to that of the 4T’s in predicting the likelihood of HIT.78 In this study, both scoring systems were found to have a low PPV (56% for the 4T’s and 41% for Lillo-Le Louet) and low concordance (kappa coefficient = 0.39). The Lillo-Le Louet scoring system also had a lower NPV (78%) than the 4T’s (91%). The authors concluded that the diagnostic performance of both scoring systems were low. However, the authors found that the biphasic pattern of platelet count recovery in the post-cardiac surgery setting remained a strong predictor for HIT.78
Laboratory Elements of Diagnosis
In clinical practice, the majority of patients suspected of HIT are likely to have an intermediate clinical probability for HIT. In these patients, establishing the presence or absence of PF4/H antibodies by laboratory methods comprises an essential element of the diagnostic evaluation. This section will discuss the types of immunologic and functional assays for diagnosing HIT.
Immunoassays
Immunoassays for the detection of PF4/H antibodies are widely available. These assays detect binding of antibodies from plasma or serum to immobilized PF4/H complexes. Bound antibody is then detected by secondary labeled antibodies using a colorimetric endpoint. Detailed descriptions or comparisons of the strengths and limitations of the various assays are beyond the scope of this chapter. The reader is referred to Table 3 with test-specific information and references.
Table 3. Immunoassays available for the detection of PF4/H Abs.
| Assay | Vendor | Polyclonal v. IgG Specific |
Principle | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Asserachrom IgGAM | Diagnostica Stago | Polyclonal | ELISA | 100%71,89 | 64%-86.46%71,89 |
| GTI PF4 IgG | GTI Diagnostics | IgG specific | ELISA | 100%89,123 | 42%-95.92%89,123 |
|
HemosIL AcuStar HIT- Ab(PF4-H) |
Instrumentation laboratory |
Polyclonal | Latex enhanced immunoturbidi metric assay |
100%124 | 81.2%124 |
|
HemosIL AcuStar HIT- IgG(PF4-H) |
Instrumentation laboratory |
IgG specific | Latex enhanced immunoturbidi metric assay |
100%124 | 96.5%124 |
|
ID-heparin/PF4 PaGIA |
Diamed | Polyclonal | particle gel immunoassay |
94%-100%71,72,125 | 61%-95%71,72,125 |
| Poly-ELISA | GTI Diagnostics | Polyclonal | ELISA | 100%72 | 80.8%72 |
| Zymutest HIA IgG | Hyphen Biomed Research |
IgG specific | ELISA | 100%89,123 | 44%-95.92%89,123 |
| Zymutest HIA IgGAM | Hyphen Biomed Research |
Polyclonal | ELISA | 100%89 | 87.50%89 |
Enzyme linked immunoassay, ELISA
Commercial immunoassays are routinely used at most medical centers due to technical ease, rapid turnaround time and high sensitivity of the assays (> 99%44,72,80,81). However, the main shortcoming of these assays is their lack of specificity (40-70%44,65) due to the frequency of asymptomatic seroconversions. Seropositivity, without HIT, can be seen in ~8-17% of general medical and surgical patients treated with UFH,82-85 2-8% of those treated with LMWH19,82 and 1-2% of patients treated with fondaparinux.19,21 Heparin exposure during cardiac surgery remains the highest risk factor for asymptomatic seroconversions. Depending on the immunoassay, asymptomatic seroconversions can be demonstrated in 27% to 61% of patients after cardiac surgery.55,83,86
Because of these constraints in specificity, several test modifications have been introduced to improve on the diagnostic performance of immunoassays. These include the use of IgG specific assays, quantitative measurement of optical density (OD), and utilization of high heparin concentration to demonstrate heparin-dependent binding. Several studies have shown that the IgG-specific ELISAs improve diagnostic specificity.87-89 In a pooled analysis of studies examining the sensitivity and specificity of the polyclonal v. IgG specific ELISAs, Cuker et.al showed that the specificity of the IgG ELISA was increased compared to the polyspecific ELISA (94% for IgG specific v. 89% for the polyclonal ELISA), but occurred at a small expense to the sensitivity of the assay (96% for IgG specific v. 98% for the polyclonal ELISA).87 This translates into a small number of patients who truly have HIT but who will have a false negative result on the IgG-specific ELISA. Studies have also shown that the quantitative assessment of ODs or expression of titers in particle-based ELISAs also improves the diagnostic accuracy of the ELISAs. These studies confirm a strong correlation of the OD/titers with platelet activating properties48,90 and thrombotic risk.47,56 Several investigators have examined the utility of using higher OD cut-offs in ELISA’s for determining the likelihood of HIT.72,91 In these studies, based on the type of immunoassay and the cut-off values, a change in the cut-off value was uniformly associated with a loss in sensitivity (17%-91% sensitivity reported using an IgG-specific ELISA with cut-off OD > 1).72,91
HIT antibodies show heparin-dependent binding over a range of physiologic heparin concentrations (0.1-1U/mL). The presence of excess heparin (10-100 U/mL) significantly attenuates the binding of HIT antibodies to antigen. This principle is the basis of using high heparin concentrations in serologic and functional assays to confirm the presence of heparin-dependent antibodies. Studies have shown that the use of a high heparin step improves the specificity from 72% to 89%.91,92 The high heparin step, however, can fail to show inhibition in instances where the OD is extremely high.91,92 Recent studies have also shown that combined use of two91,92 or all three maneuvers (IgG, ODs and high heparin step) can be used to improve the diagnostic utility of the ELISAs. While such an approach, theoretically, should markedly improve the assay’s specificity, there are many examples/reports of HIT patients whose serologic profiles do not conform to these criteria.47,87,91 Until prospective evaluation and validation of these laboratory modifications occurs, it should be stressed that laboratory testing must accompany a clinical evaluation to avoid serious adverse outcomes from under- or over-diagnosis of HIT.
Functional assays
Functional assays such as the serotonin release assay (SRA; in North America) and the heparin induced platelet activation test (HIPA; in Europe) utilize washed platelets to measure HIT antibody induced platelet activation.93 A positive result is established when heparin-dependent platelet activation is demonstrated along with inhibition of platelet activation in the presence of excess heparin (100 units/mL) and in the presence of an antibody which blocks platelet Fc receptors.29
Functional assays are more specific for HIT 44 and more predictive for thrombocytopenia when compared to ELISA based testing. Whereas functional assays, in particular the SRA, have high specificity (> 95%)44 and are associated with a high positive predictive values (89-100%),21 the sensitivity of functional assays are less robust (62-100%)44,65,94,95 due to a number of technical variables affecting platelet reactivity.96 Other important drawbacks to these assays include lack of standardization,97 complexity of the assays and the use of radioactive isotopes with the SRA. For these reasons, many medical centers do not offer this test on-site, and testing is often referred to specialized commercial laboratories. Consequently, functional assays are rarely available to clinicians at the time of initial evaluation and are often used to confirm the diagnosis of HIT post-hoc.
Normalization of laboratory testing
After discontinuing heparin, the median time to platelet recovery is approximately 4 days, although it can take up to 4 weeks for platelets to fully recover to > 150 × 109/L.29 However, both ELISA testing for HIT antibodies and functional assays, such as the SRA, require longer to normalize. In a retrospective study of 243 patients with serologically confirmed HIT, the median time for the antigen assay to normalize was 85 days and the median time for the activation assay to normalize was 50 days.26
Therapeutic Options and Prognosis
General principles
Management of HIT requires that the clinician navigate between the Scylla of overdiagnosis/overtreatment and Charybdis of withholding therapy in patients with true HIT. With the widespread use of ELISAs and the false-positive detection rate of PF4/H antibodies in many clinical settings, current clinical practices have leaned towards the overdiagnosis of HIT.60 Overtreatment with potent non-heparin anticoagulants, such as the direct thrombin inhibitors, is associated with a high risk of bleeding (1% risk for major bleeding per treatment day).98 Similarly, disastrous consequences can be expected if the clinical manifestations of HIT are not promptly recognized (5-10% daily risk of thrombosis).11 To avoid these extremes, physicians need to apply clinical algorithms (described above) and initiate treatment based on the strength of the clinical suspicion of HIT, even prior to availability of laboratory results.
Patients with a low-clinical suspicion of HIT should not undergo laboratory testing, nor have their heparin discontinued, as the NPV of clinical scoring systems approaches 100% in numerous studies.46,69-74 Patients who are deemed to have an intermediate or high clinical suspicion for HIT should have all heparin products (including heparin flushes) discontinued and an alternative anticoagulant, ideally a parenteral direct thrombin inhibitor (DTI), started. Simply discontinuing heparin alone or starting a vitamin K antagonist alone is not adequate to prevent the development or progression of thrombotic complications. In a retrospective review of patients with serologically confirmed HIT, 47.6% of patients who had heparin substituted for warfarin suffered a subsequent thrombosis.33
Of the three agents available in the United States for the treatment of HIT, two belong to the DTI family (argatroban and bivalirudin; lepirudin production has been discontinued by the manufacturer as of May 2013) and the other, fondaparinux, a synthetic pentasaccharide belongs to the heparin family but has minimal cross-reactivity with heparin. Refer to Table 4 for recommendations on dosing and monitoring of the alternative anticoagulants.
Table 4. Alternative Anticoagulants for Treatment of HIT.
| Argatroban | Bivalirudin | Fondaparinux | |
|---|---|---|---|
| Approval | HIT or HIT patients undergoing PCI with or at risk for HIT |
Patients with or at risk of developing HIT during PCI |
Has not received FDA approval for use in HIT |
|
| |||
| Bolus | None | None | N/A |
|
| |||
|
Initial dose for isolated HIT or HIT with thrombosis |
1.5-2 mcg/kg/min100 | 0.15-0.2 mg/kg/h59 | < 50 kg: 5 mg SC daily |
| 50-100 kg: 7.5 mg SC daily | |||
| > 100 kg: 10 mg SC daily59 | |||
|
| |||
|
Initial dose for renal impairment |
No adjustment necessary126 | 0.08-0.1 mg/kg/h (CrCl 30-60 mL/min) |
Use with caution if CrCl 30-50 mL/min |
| 0.03-0.05 mg/kg/h (CrCl < 30 mL/min or for patients receiving renal replacement therapy)109 |
Contraindicated if CrCl < 30 mL/min116 | ||
|
| |||
|
Initial dose for hepatic impairment |
0.5-1.2 mcg/kg/min100 | No adjustment necessary127 | No adjustment necessary116 |
|
| |||
| Monitoring | Obtain baseline aPTT and 2 hours after starting infusion126 |
Obtain baseline aPTT and 2-3 hours after starting infusion |
Anti-FXa activity can be monitored in renal insufficiency115 |
|
| |||
| Target | aPTT 1.5-3 times the baseline aPTT. Max goal aPTT=100 sec100 |
aPTT 1.5-2.5 times the baseline aPTT59 |
N/A |
|
| |||
|
Dosage Adjustment (HIT/HITTS only) |
Adjust infusion by 0.25-0.5 mg/kg/min to achieve goal aPTT. Recheck aPTT 2-4 hours after each dosage change.126 |
Adjust infusion by 20-25% to achieve goal aPTT. Recheck aPTT 2 hours after each dosage change. |
N/A |
|
| |||
| Maximum dose | 10 mcg/kg/min | 0.25 mg/kg/h127 | 10 mg daily116 |
|
| |||
|
Transition to warfarin |
Monitor chromogenic FXa level while on combined therapy.101 |
Monitor INR and/or chromogenic FXa while on combined therapy. |
Monitor INR while on combined therapy. Discontinue therapy when INR ≥ 2 for 2 consecutive days116 |
| Chromogenic FXa:102 24-45% (INR 2-3) 15-35% (INR 2.5-3.5) |
Chromogenic FXa: 24-45% (INR 2-3) 15-35% (INR 2.5-3.5) |
||
| Confirm INR 4-6 hours after infusion is stopped |
|||
|
| |||
|
Cost of daily
therapy 107 |
$1313 | $742 | $103 |
|
| |||
|
Special considerations |
No antidote is available126 | No antidote is available127 | No antidote is available116 |
|
HIT in PCI: 0.75 mg/kg bolus followed by a 1.75 mg/kg/h infusion for the duration of the procedure (all subgroups) |
|||
| After PCI: 1.75 mg/kg/h (CrCl ≥ 30 mL/min) 1 mg/kg/h (CrCl < 30 mL/min) 0.25 mg/kg/h (hemodialysis) Optional infusion after PCI 0.2 mg/kg/h for 20 hours127 |
|||
Not applicable, N/A; creatinine clearance, CrCl; factor Xa, FX
Argatroban
Argatroban is the only DTI approved by the US FDA for the prevention and treatment of thrombosis in patients with HIT as well as for patients undergoing percutaneous coronary intervention with, or at risk for, HIT. Argatroban was approved based on two prospective, open-label multicenter studies enrolling a total of 373 patients with HIT.32,99 In these trials, patients treated with argatroban were compared to historical controls and were found to have a reduced incidence of new thrombosis, the need for amputation, and death (34% to 35%) as compared to controls (43%).32,99 More rapid platelet recovery was also seen in the argatroban arm and major bleeding rates were not different between the two study arms.99
In patients with normal hepatic function, argatroban should be infused at an initial rate of 1.5-2 mcg/kg/min without an initial bolus. Initial infusion rates should be reduced to 0.5-1.2 mcg/kg/min for patients with heart failure, anasarca, or other conditions resulting in hepatic dysfunction.100 An activated partial thromboplastin time (aPTT) should be obtained 2 hours after starting the infusion, and the infusion should be adjusted by 0.25-0.5 mcg/kg/min to achieve a goal aPTT 1.5-3 times the baseline aPTT value, with a maximum goal aPTT of 100 seconds. The infusion rate should not exceed 10 mcg/kg/min.100 Argatroban is hepatically cleared, and, therefore is the agent of choice for patients with HIT and renal insufficiency.59
Once a patient is stably anticoagulated on argatroban and the platelet count has fully recovered (> 150 × 109/L), warfarin can be initiated at a low dose (≤ 5 mg) and overlapped with argatroban for at least 5 days.59 Because argatroban prolongs the International Normalized Ratio (INR),101 the transition to warfarin requires close monitoring of factor X levels, as measured by chromogenic assays. A chromogenic factor X level ≤ 45% at the time of argatroban discontinuation has been shown to be predictive of a therapeutic INR.102
Reported major bleeding rates with argatroban range from 0%-10%.103,104_ENREF_76 Identified risk factors for major bleeding on argatroban include: the presence of a HIT associated TEC, pulmonary impairment, and an aPTT > 100 seconds.105 If bleeding occurs, the infusion rate should be decreased or the infusion should be entirely discontinued. Once argatroban is discontinued, the aPTT will typically normalize within 2-4 hours.106 No specific antidote for argatroban is available at this time. The reported wholesale acquisition cost of one vial of argatroban (100 mg/mL; 2.5 mL vial) required for one day’s treatment is $1313.107
Bivalirudin
Bivalirudin is a DTI approved for use by the FDA only in patients who have or are at risk of developing HIT during percutaneous coronary intervention (PCI), a setting where its safety and efficacy have been demonstrated.108 Bivalirudin has not been approved by the FDA for other clinical settings of HIT, but several case series have reported the safety of this agent for HIT in non-PCI settings.109,110
If used for treatment of HIT, bivalirudin should be given without a bolus and infused at an initial rate of 0.15-0.2 mg/kg/h for a goal aPTT which is 1.5-2.5 times the patient’s baseline aPTT.59 Bivalirudin has a short half-life of 25 minutes and is cleared by both renal (20%) and plasma enzymatic (80%) mechanisms.109 For patients with renal insufficiency, bivalirudin should be dose reduced (0.08-0.1 mg/kg/h for creatinine clearance 30-60 mL/minute; 0.03-0.05 mg/kg/h for creatinine clearance < 30 mL/minute or for patients receiving renal replacement therapy).109 The reported wholesale acquisition cost of one vial bivalirudin (250 mg vial) required for one day’s treatment is $742.107
Fondaparinux
Fondaparinux belongs to the heparin family and is a long-acting (half-life = 17 hours), selective inhibitor of factor Xa.93 This drug has not received FDA approval for use in HIT. However, emerging data suggests a role for fondaparinux in HIT, as HIT antibodies show minimal cross-reactivity with this agent in vitro < 5%.111 In several small series of HIT patients treated with fondaparinux (n=55), no recurrent TEC were reported.112-115 Therapy with fondaparinux was well tolerated and only one patient, who had developed renal dysfunction, developed a major bleed.115 These studies demonstrated that fondaparinux may be an effective anticoagulant for the treatment of HIT.
If used for the treatment of HIT, guidelines for therapeutic dosing should be followed (see Table 4). 5 mg subcutaneously (SC) daily should be given for patients who weigh < 50 kg, 7.5 mg SC daily for patients who weigh 50-100 kg, and 10 mg SC daily for patients who weigh > 100 kg.59 Fondaparinux is primarily eliminated in the urine as unchanged drug (77%).116 Therefore, the use of fondaparinux is contraindicated in patients with severe renal impairment (creatinine clearance < 30 mL/min) due to the increased risk of bleeding in patients when renal clearance is reduced.116 The reported wholesale acquisition cost of one vial of generic fondaparinux (7.5 mg vial) required for one day’s treatment is $103.107
Warfarin therapy
Once the patient is stably anticoagulated on an alternative, non-heparin anticoagulant and the platelet count has fully recovered (> 150 × 109/L), warfarin can be initiated at a low dose (≤ 5 mg).59 Administration of the alternative anticoagulant and warfarin should overlap for at least 5 days, as premature discontinuation of the alternative anticoagulant may lead to thrombotic events.117 Up to 4 weeks of anticoagulation with warfarin have been recommended for patients with isolated HIT who do not suffer a TEC.21,59 For patients with HIT who develop a thrombotic complication, extending anticoagulation with warfarin for a total of 3-6 months is recommended.59
Platelet transfusions
Despite the frequency of thrombocytopenia in patients with HIT, bleeding complications remain very rare29 and historically, routine platelet transfusions were not advised due to concern for an increased risk of thrombosis.118 Although recent reports indicate that platelet transfusions may be safe and are not associated with TEC,119 there is considerable theoretical concern that platelet transfusions may heighten the prothrombotic state in HIT due to the high concentrations of the antigen PF4 in platelets.120 Thus, at this time, platelet transfusions are only recommended for patients with HIT who have active bleeding or for those patients who need to undergo procedures associated with a high risk of bleeding.59
Heparin re-exposure
HIT is thought to be a self-limited disorder, based on the transience of circulating PF4/H antibodies,26 and isolated case series of inadvertent exposure demonstrate lack of disease recurrence. In a small case series, seven patients with a history of HIT who were now serologically negative did not experience recurrent thrombocytopenia or thrombotic events after subsequent courses of heparin.26 Likewise, patients with a history of HIT who become seronegative for PF4/H antibodies have been successfully and safely anticoagulated with heparin during hemodialysis121 and during cardiopulmonary bypass surgery.58,122
In the absence of prospective studies examining the safety of heparin re-exposure in HIT patients, current guidelines recommend avoidance of heparin in patients with recent HIT who still have detectable PF4/H antibodies, as they are at risk for developing rapid-onset HIT and thrombosis.59 However, once HIT antibodies become undetectable by serologic assays, short-term re-exposure to heparin can be considered.59 In this scenario, administration of heparin should be restricted to the procedure itself and unnecessary heparin exposure should be avoided._ENREF_4759
Summary
Heparin-induced thrombocytopenia is a prothrombotic disorder caused by antibodies to PF4/H complexes. It classically presents with declining platelet counts 5-14 days after heparin administration and results in a predisposition to arterial and venous thrombosis. Establishing the diagnosis of HIT can be extremely challenging, especially in patients with multiple medical comorbidities. Therefore, it is essential to conduct a thorough clinical evaluation in addition to laboratory testing to confirm the presence of PF4/H antibodies. Multiple clinical algorithms have been developed to aid the clinician in predicting the likelihood of HIT. Once HIT is recognized, an alternative anticoagulant (DTI or fondaparinux) should be initiated to prevent further complications.
Heparin-induced thrombocytopenia is a prothrombotic disorder caused by antibodies to PF4/H complexes. It classically presents with declining platelet counts 5-14 days after heparin administration and results in a predisposition to arterial and venous thrombosis.
Establishing the diagnosis of HIT can be extremely challenging, especially in patients with multiple medical comorbidities.
Once HIT is recognized, an alternative anticoagulant (DTI or fondaparinux) should be initiated to prevent further complications.
Acknowledgments
Supported by the National Institutes of Health HL110860, HL109825 and AI101992 (GMA) and 2T32HL007057-36 (GML).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Grace M. Lee, Division of Hematology, Department of Medicine, DUMC Box 3841, Duke University Medical Center, Room 301 Sands Building, Durham, NC 27710, Phone: 919-668-1550, Fax: 919-684-2420, grace.lee@duke.edu.
Gowthami M. Arepally, Division of Hematology, Department of Medicine, Duke University Medical Center, DUMC Box 3486, Room 301 Sands Building, Durham, NC 27710.
References
- 1.Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e195S–226S. doi: 10.1378/chest.11-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuker A, Cines DB. How I treat heparin-induced thrombocytopenia. Blood. 2012;119:2209–18. doi: 10.1182/blood-2011-11-376293. [DOI] [PubMed] [Google Scholar]
- 3.Weismann RE, Tobin RW. Arterial embolism occurring during systemic heparin therapy. AMA archives of surgery. 1958;76:219–25. doi: 10.1001/archsurg.1958.01280200041005. discussion 25-7. [DOI] [PubMed] [Google Scholar]
- 4.Rhodes GR, Dixon RH, Silver D. Heparin induced thrombocytopenia: eight cases with thrombotic-hemorrhagic complications. Annals of surgery. 1977;186:752–8. doi: 10.1097/00000658-197712000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amiral J, Bridey F, Dreyfus M, et al. Platelet factor 4 complexed to heparin is the target for antibodies generated in heparin-induced thrombocytopenia. Thrombosis and haemostasis. 1992;68:95–6. [PubMed] [Google Scholar]
- 6.Suvarna S, Espinasse B, Qi R, et al. Determinants of PF4/heparin immunogenicity. Blood. 2007;110:4253–60. doi: 10.1182/blood-2007-08-105098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelton JG, Sheridan D, Santos A, et al. Heparin-induced thrombocytopenia: laboratory studies. Blood. 1988;72:925–30. [PubMed] [Google Scholar]
- 8.Rauova L, Hirsch JD, Greene TK, et al. Monocyte-bound PF4 in the pathogenesis of heparin-induced thrombocytopenia. Blood. 2010;116:5021–31. doi: 10.1182/blood-2010-03-276964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasthuri RS, Glover SL, Jonas W, et al. PF4/heparin-antibody complex induces monocyte tissue factor expression and release of tissue factor positive microparticles by activation of FcgammaRI. Blood. 2012;119:5285–93. doi: 10.1182/blood-2011-06-359430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao Z, Visentin GP, Dayananda KM, Neelamegham S. Immune complexes formed following the binding of anti-platelet factor 4 (CXCL4) antibodies to CXCL4 stimulate human neutrophil activation and cell adhesion. Blood. 2008;112:1091–100. doi: 10.1182/blood-2008-04-153288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greinacher A, Eichler P, Lubenow N, Kwasny H, Luz M. Heparin-induced thrombocytopenia with thromboembolic complications: meta-analysis of 2 prospective trials to assess the value of parenteral treatment with lepirudin and its therapeutic aPTT range. Blood. 2000;96:846–51. [PubMed] [Google Scholar]
- 12.Prandoni P, Siragusa S, Girolami B, Fabris F. The incidence of heparin-induced thrombocytopenia in medical patients treated with low-molecular-weight heparin: a prospective cohort study. Blood. 2005;106:3049–54. doi: 10.1182/blood-2005-03-0912. [DOI] [PubMed] [Google Scholar]
- 13.Stein PD, Hull RD, Matta F, Yaekoub AY, Liang J. Incidence of thrombocytopenia in hospitalized patients with venous thromboembolism. The American journal of medicine. 2009;122:919–30. doi: 10.1016/j.amjmed.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 14.Francis JL, Palmer GJ, 3rd, Moroose R, Drexler A. Comparison of bovine and porcine heparin in heparin antibody formation after cardiac surgery. The Annals of thoracic surgery. 2003;75:17–22. doi: 10.1016/s0003-4975(02)04349-7. [DOI] [PubMed] [Google Scholar]
- 15.Ban-Hoefen M, Francis C. Heparin induced thrombocytopenia and thrombosis in a tertiary care hospital. Thrombosis research. 2009;124:189–92. doi: 10.1016/j.thromres.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood. 2005;106:2710–5. doi: 10.1182/blood-2005-04-1546. [DOI] [PubMed] [Google Scholar]
- 17.Zhou A, Winkler A, Emamifar A, et al. Is the Incidence of Heparin-Induced Thrombocytopenia Affected by the Increased Use of Heparin for the Prevention of Deep Venous Thrombosis? Chest. 2012 doi: 10.1378/chest.11-2926. [DOI] [PubMed] [Google Scholar]
- 18.Rothberg MB, Pekow PS, Lahti M, Lindenauer PK. Comparative effectiveness of low-molecular-weight heparin versus unfractionated heparin for thromboembolism prophylaxis for medical patients. Journal of hospital medicine: an official publication of the Society of Hospital Medicine. 2012;7:457–63. doi: 10.1002/jhm.1938. [DOI] [PubMed] [Google Scholar]
- 19.Warkentin TE, Cook RJ, Marder VJ, et al. Anti-platelet factor 4/heparin antibodies in orthopedic surgery patients receiving antithrombotic prophylaxis with fondaparinux or enoxaparin. Blood. 2005;106:3791–6. doi: 10.1182/blood-2005-05-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warkentin TE, Maurer BT, Aster RH. Heparin-induced thrombocytopenia associated with fondaparinux. The New England journal of medicine. 2007;356:2653–5. doi: 10.1056/NEJMc070346. discussion -5. [DOI] [PubMed] [Google Scholar]
- 21.Arepally GM, Ortel TL. Clinical practice. Heparin-induced thrombocytopenia. The New England journal of medicine. 2006;355:809–17. doi: 10.1056/NEJMcp052967. [DOI] [PubMed] [Google Scholar]
- 22.Warkentin TE, Sheppard JA, Sigouin CS, Kohlmann T, Eichler P, Greinacher A. Gender imbalance and risk factor interactions in heparin-induced thrombocytopenia. Blood. 2006;108:2937–41. doi: 10.1182/blood-2005-11-012450. [DOI] [PubMed] [Google Scholar]
- 23.Gruel Y, Pouplard C, Lasne D, Magdelaine-Beuzelin C, Charroing C, Watier H. The homozygous FcgammaRIIIa-158V genotype is a risk factor for heparin-induced thrombocytopenia in patients with antibodies to heparin-platelet factor 4 complexes. Blood. 2004;104:2791–3. doi: 10.1182/blood-2004-01-0058. [DOI] [PubMed] [Google Scholar]
- 24.Rollin J, Pouplard C, Gratacap MP, et al. Polymorphisms of protein tyrosine phosphatase CD148 influence FcgammaRIIA-dependent platelet activation and the risk of heparin-induced thrombocytopenia. Blood. 2012;120:1309–16. doi: 10.1182/blood-2012-04-424044. [DOI] [PubMed] [Google Scholar]
- 25.Pouplard C, Cornillet-Lefebvre P, Attaoua R, et al. Interleukin-10 promoter microsatellite polymorphisms influence the immune response to heparin and the risk of heparin-induced thrombocytopenia. Thrombosis research. 2012;129:465–9. doi: 10.1016/j.thromres.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 26.Warkentin TE, Kelton JG. Temporal aspects of heparin-induced thrombocytopenia. The New England journal of medicine. 2001;344:1286–92. doi: 10.1056/NEJM200104263441704. [DOI] [PubMed] [Google Scholar]
- 27.Warkentin TE. Heparin-induced thrombocytopenia: pathogenesis and management. British journal of haematology. 2003;121:535–55. doi: 10.1046/j.1365-2141.2003.04334.x. [DOI] [PubMed] [Google Scholar]
- 28.Greinacher A, Farner B, Kroll H, Kohlmann T, Warkentin TE, Eichler P. Clinical features of heparin-induced thrombocytopenia including risk factors for thrombosis. A retrospective analysis of 408 patients. Thrombosis and haemostasis. 2005;94:132–5. doi: 10.1160/TH04-12-0825. [DOI] [PubMed] [Google Scholar]
- 29.Warkentin TE, Greinacher A. Heparin-Induced Thrombocytopenia. 3rd ed. Marcel Dekker, Inc.; New York: 2004. [Google Scholar]
- 30.Schindewolf M, Lindhoff-Last E, Ludwig RJ, Boehncke WH. Heparin-induced skin lesions. Lancet. 2012 doi: 10.1016/S0140-6736(12)60409-7. [DOI] [PubMed] [Google Scholar]
- 31.Warkentin TE, Roberts RS, Hirsh J, Kelton JG. An improved definition of immune heparin-induced thrombocytopenia in postoperative orthopedic patients. Archives of internal medicine. 2003;163:2518–24. doi: 10.1001/archinte.163.20.2518. [DOI] [PubMed] [Google Scholar]
- 32.Lewis BE, Wallis DE, Leya F, Hursting MJ, Kelton JG. Argatroban anticoagulation in patients with heparin-induced thrombocytopenia. Archives of internal medicine. 2003;163:1849–56. doi: 10.1001/archinte.163.15.1849. [DOI] [PubMed] [Google Scholar]
- 33.Warkentin TE, Kelton JG. A 14-year study of heparin-induced thrombocytopenia. The American journal of medicine. 1996;101:502–7. doi: 10.1016/s0002-9343(96)00258-6. [DOI] [PubMed] [Google Scholar]
- 34.Tardy B, Tardy-Poncet B, Fournel P, Venet C, Jospe R, Dacosta A. Lower limb veins should be systematically explored in patients with isolated heparin-induced thrombocytopenia. Thrombosis and haemostasis. 1999;82:1199–200. [PubMed] [Google Scholar]
- 35.Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. The New England journal of medicine. 1995;332:1330–5. doi: 10.1056/NEJM199505183322003. [DOI] [PubMed] [Google Scholar]
- 36.Warkentin TE, Sheppard JA, Moore JC, Cook RJ, Kelton JG. Studies of the immune response in heparin-induced thrombocytopenia. Blood. 2009;113:4963–9. doi: 10.1182/blood-2008-10-186064. [DOI] [PubMed] [Google Scholar]
- 37.Nand S, Wong W, Yuen B, Yetter A, Schmulbach E, Gross Fisher S. Heparin-induced thrombocytopenia with thrombosis: incidence, analysis of risk factors, and clinical outcomes in 108 consecutive patients treated at a single institution. American journal of hematology. 1997;56:12–6. doi: 10.1002/(sici)1096-8652(199709)56:1<12::aid-ajh3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 38.Hong AP, Cook DJ, Sigouin CS, Warkentin TE. Central venous catheters and upper-extremity deep-vein thrombosis complicating immune heparin-induced thrombocytopenia. Blood. 2003;101:3049–51. doi: 10.1182/blood-2002-05-1448. [DOI] [PubMed] [Google Scholar]
- 39.Boshkov LK, Warkentin TE, Hayward CP, Andrew M, Kelton JG. Heparin-induced thrombocytopenia and thrombosis: clinical and laboratory studies. British journal of haematology. 1993;84:322–8. doi: 10.1111/j.1365-2141.1993.tb03072.x. [DOI] [PubMed] [Google Scholar]
- 40.Ernest D, Fisher MM. Heparin-induced thrombocytopaenia complicated by bilateral adrenal haemorrhage. Intensive Care Med. 1991;17:238–40. doi: 10.1007/BF01709885. [DOI] [PubMed] [Google Scholar]
- 41.Pohl C, Harbrecht U, Greinacher A, et al. Neurologic complications in immune-mediated heparin-induced thrombocytopenia. Neurology. 2000;54:1240–5. doi: 10.1212/wnl.54.6.1240. [DOI] [PubMed] [Google Scholar]
- 42.Warkentin TE, Roberts RS, Hirsh J, Kelton JG. Heparin-induced skin lesions and other unusual sequelae of the heparin-induced thrombocytopenia syndrome: a nested cohort study. Chest. 2005;127:1857–61. doi: 10.1378/chest.127.5.1857. [DOI] [PubMed] [Google Scholar]
- 43.Carlsson LE, Lubenow N, Blumentritt C, et al. Platelet receptor and clotting factor polymorphisms as genetic risk factors for thromboembolic complications in heparin-induced thrombocytopenia. Pharmacogenetics. 2003;13:253–8. doi: 10.1097/00008571-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Warkentin TE, Sheppard JA, Moore JC, Moore KM, Sigouin CS, Kelton JG. Laboratory testing for the antibodies that cause heparin-induced thrombocytopenia: how much class do we need? The Journal of laboratory and clinical medicine. 2005;146:341–6. doi: 10.1016/j.lab.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Alberio L, Kimmerle S, Baumann A, Taleghani BM, Biasiutti FD, Lammle B. Rapid determination of anti-heparin/platelet factor 4 antibody titers in the diagnosis of heparin-induced thrombocytopenia. The American journal of medicine. 2003;114:528–36. doi: 10.1016/s0002-9343(03)00080-9. [DOI] [PubMed] [Google Scholar]
- 46.Pouplard C, Gueret P, Fouassier M, et al. Prospective evaluation of the ‘4Ts’ score and particle gel immunoassay specific to heparin/PF4 for the diagnosis of heparin-induced thrombocytopenia. Journal of thrombosis and haemostasis: JTH. 2007;5:1373–9. doi: 10.1111/j.1538-7836.2007.02524.x. [DOI] [PubMed] [Google Scholar]
- 47.Zwicker JI, Uhl L, Huang WY, Shaz BH, Bauer KA. Thrombosis and ELISA optical density values in hospitalized patients with heparin-induced thrombocytopenia. Journal of thrombosis and haemostasis: JTH. 2004;2:2133–7. doi: 10.1111/j.1538-7836.2004.01039.x. [DOI] [PubMed] [Google Scholar]
- 48.Warkentin TE, Sheppard JI, Moore JC, Sigouin CS, Kelton JG. Quantitative interpretation of optical density measurements using PF4-dependent enzyme-immunoassays. Journal of thrombosis and haemostasis: JTH. 2008;6:1304–12. doi: 10.1111/j.1538-7836.2008.03025.x. [DOI] [PubMed] [Google Scholar]
- 49.Mattioli AV, Bonetti L, Zennaro M, Ambrosio G, Mattioli G. Heparin/PF4 antibodies formation after heparin treatment: temporal aspects and long-term follow-up. American heart journal. 2009;157:589–95. doi: 10.1016/j.ahj.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 50.Bauer TL, Arepally G, Konkle BA, et al. Prevalence of heparin-associated antibodies without thrombosis in patients undergoing cardiopulmonary bypass surgery. Circulation. 1997;95:1242–6. doi: 10.1161/01.cir.95.5.1242. [DOI] [PubMed] [Google Scholar]
- 51.Wallis DE, Workman DL, Lewis BE, Steen L, Pifarre R, Moran JF. Failure of early heparin cessation as treatment for heparin-induced thrombocytopenia. The American journal of medicine. 1999;106:629–35. doi: 10.1016/s0002-9343(99)00124-2. [DOI] [PubMed] [Google Scholar]
- 52.Reilly MP, Taylor SM, Hartman NK, et al. Heparin-induced thrombocytopenia/thrombosis in a transgenic mouse model requires human platelet factor 4 and platelet activation through FcgammaRIIA. Blood. 2001;98:2442–7. doi: 10.1182/blood.v98.8.2442. [DOI] [PubMed] [Google Scholar]
- 53.Warkentin TE, Kelton JG. Delayed-onset heparin-induced thrombocytopenia and thrombosis. Annals of internal medicine. 2001;135:502–6. doi: 10.7326/0003-4819-135-7-200110020-00009. [DOI] [PubMed] [Google Scholar]
- 54.Rice L, Attisha WK, Drexler A, Francis JL. Delayed-onset heparin-induced thrombocytopenia. Annals of internal medicine. 2002;136:210–5. doi: 10.7326/0003-4819-136-3-200202050-00009. [DOI] [PubMed] [Google Scholar]
- 55.Everett BM, Yeh R, Foo SY, et al. Prevalence of heparin/platelet factor 4 antibodies before and after cardiac surgery. The Annals of thoracic surgery. 2007;83:592–7. doi: 10.1016/j.athoracsur.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 56.Baroletti S, Hurwitz S, Conti NA, Fanikos J, Piazza G, Goldhaber SZ. Thrombosis in suspected heparin-induced thrombocytopenia occurs more often with high antibody levels. The American journal of medicine. 2012;125:44–9. doi: 10.1016/j.amjmed.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 57.Yusuf AM, Warkentin TE, Arsenault KA, Whitlock R, Eikelboom JW. Prognostic importance of preoperative anti-PF4/heparin antibodies in patients undergoing cardiac surgery. A systematic review. Thrombosis and haemostasis. 2012;107:8–14. doi: 10.1160/TH11-07-0480. [DOI] [PubMed] [Google Scholar]
- 58.Potzsch B, Klovekorn WP, Madlener K. Use of heparin during cardiopulmonary bypass in patients with a history of heparin-induced thrombocytopenia. The New England journal of medicine. 2000;343:515. doi: 10.1056/NEJM200008173430718. [DOI] [PubMed] [Google Scholar]
- 59.Linkins LA, Dans AL, Moores LK, et al. Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e495S–530S. doi: 10.1378/chest.11-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cuker A. Heparin-induced thrombocytopenia (HIT) in 2011: an epidemic of overdiagnosis. Thrombosis and haemostasis. 2011;106:993–4. doi: 10.1160/TH11-09-0677. [DOI] [PubMed] [Google Scholar]
- 61.Lo GK, Sigouin CS, Warkentin TE. What is the potential for overdiagnosis of heparin-induced thrombocytopenia? American journal of hematology. 2007;82:1037–43. doi: 10.1002/ajh.21032. [DOI] [PubMed] [Google Scholar]
- 62.Stephan F, Hollande J, Richard O, Cheffi A, Maier-Redelsperger M, Flahault A. Thrombocytopenia in a surgical ICU. Chest. 1999;115:1363–70. doi: 10.1378/chest.115.5.1363. [DOI] [PubMed] [Google Scholar]
- 63.Oliveira GB, Crespo EM, Becker RC, et al. Incidence and prognostic significance of thrombocytopenia in patients treated with prolonged heparin therapy. Archives of internal medicine. 2008;168:94–102. doi: 10.1001/archinternmed.2007.65. [DOI] [PubMed] [Google Scholar]
- 64.Baughman RP, Lower EE, Flessa HC, Tollerud DJ. Thrombocytopenia in the intensive care unit. Chest. 1993;104:1243–7. doi: 10.1378/chest.104.4.1243. [DOI] [PubMed] [Google Scholar]
- 65.Pouplard C, May MA, Iochmann S, et al. Antibodies to platelet factor 4-heparin after cardiopulmonary bypass in patients anticoagulated with unfractionated heparin or a low-molecular-weight heparin: clinical implications for heparin-induced thrombocytopenia. Circulation. 1999;99:2530–6. doi: 10.1161/01.cir.99.19.2530. [DOI] [PubMed] [Google Scholar]
- 66.Trossaert M, Gaillard A, Commin PL, Amiral J, Vissac AM, Fressinaud E. High incidence of anti-heparin/platelet factor 4 antibodies after cardiopulmonary bypass surgery. British journal of haematology. 1998;101:653–5. doi: 10.1046/j.1365-2141.1998.00750.x. [DOI] [PubMed] [Google Scholar]
- 67.Selleng S, Selleng K, Wollert HG, et al. Heparin-induced thrombocytopenia in patients requiring prolonged intensive care unit treatment after cardiopulmonary bypass. Journal of thrombosis and haemostasis: JTH. 2008;6:428–35. doi: 10.1111/j.1538-7836.2007.02870.x. [DOI] [PubMed] [Google Scholar]
- 68.Bream-Rouwenhorst HR, Hobbs RA. Heparin-dependent antibodies and thrombosis without heparin-induced thrombocytopenia. Pharmacotherapy. 2008;28:1401–7. doi: 10.1592/phco.28.11.1401. [DOI] [PubMed] [Google Scholar]
- 69.Lo GK, Juhl D, Warkentin TE, Sigouin CS, Eichler P, Greinacher A. Evaluation of pretest clinical score (4 T’s) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. Journal of thrombosis and haemostasis: JTH. 2006;4:759–65. doi: 10.1111/j.1538-7836.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- 70.Crowther MA, Cook DJ, Albert M, et al. The 4Ts scoring system for heparin-induced thrombocytopenia in medical-surgical intensive care unit patients. Journal of critical care. 2010;25:287–93. doi: 10.1016/j.jcrc.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 71.Denys B, Stove V, Philippe J, Devreese K. A clinical-laboratory approach contributing to a rapid and reliable diagnosis of heparin-induced thrombocytopenia. Thrombosis research. 2008;123:137–45. doi: 10.1016/j.thromres.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 72.Bakchoul T, Giptner A, Najaoui A, Bein G, Santoso S, Sachs UJ. Prospective evaluation of PF4/heparin immunoassays for the diagnosis of heparin-induced thrombocytopenia. Journal of thrombosis and haemostasis: JTH. 2009;7:1260–5. doi: 10.1111/j.1538-7836.2009.03465.x. [DOI] [PubMed] [Google Scholar]
- 73.Nellen V, Sulzer I, Barizzi G, Lammle B, Alberio L. Rapid exclusion or confirmation of heparin-induced thrombocytopenia: a single-center experience with 1,291 patients. Haematologica. 2012;97:89–97. doi: 10.3324/haematol.2011.048074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bryant A, Low J, Austin S, Joseph JE. Timely diagnosis and management of heparin-induced thrombocytopenia in a frequent request, low incidence single centre using clinical 4T’s score and particle gel immunoassay. British journal of haematology. 2008;143:721–6. doi: 10.1111/j.1365-2141.2008.07401.x. [DOI] [PubMed] [Google Scholar]
- 75.Cuker A, Arepally G, Crowther MA, et al. The HIT Expert Probability (HEP) Score: a novel pre-test probability model for heparin-induced thrombocytopenia based on broad expert opinion. Journal of thrombosis and haemostasis: JTH. 2010;8:2642–50. doi: 10.1111/j.1538-7836.2010.04059.x. [DOI] [PubMed] [Google Scholar]
- 76.Trehel-Tursis V, Louvain-Quintard V, Zarrouki Y, Imbert A, Doubine S, Stephan F. Clinical and biological features of patients suspected or confirmed to have heparin-induced thrombocytopenia in a cardiothoracic surgical ICU. Chest. 2012 doi: 10.1378/chest.11-3074. [DOI] [PubMed] [Google Scholar]
- 77.Kuitunen A, Suojaranta-Ylinen R, Kukkonen S, Niemi T. A comparison of the haemodynamic effects of 4% succinylated gelatin, 6% hydroxyethyl starch (200/0.5) and 4% human albumin after cardiac surgery. Scand J Surg. 2007;96:72–8. doi: 10.1177/145749690709600114. [DOI] [PubMed] [Google Scholar]
- 78.Piednoir P, Allou N, Provenchere S, et al. Heparin-induced thrombocytopenia after cardiac surgery: an observational study of 1,722 patients. Journal of cardiothoracic and vascular anesthesia. 2012;26:585–90. doi: 10.1053/j.jvca.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 79.Lillo-Le Louet A, Boutouyrie P, Alhenc-Gelas M, et al. Diagnostic score for heparin-induced thrombocytopenia after cardiopulmonary bypass. Journal of thrombosis and haemostasis: JTH. 2004;2:1882–8. doi: 10.1111/j.1538-7836.2004.00949.x. [DOI] [PubMed] [Google Scholar]
- 80.Greinacher A, Juhl D, Strobel U, et al. Heparin-induced thrombocytopenia: a prospective study on the incidence, platelet-activating capacity and clinical significance of antiplatelet factor 4/heparin antibodies of the IgG, IgM, and IgA classes. Journal of thrombosis and haemostasis: JTH. 2007;5:1666–73. doi: 10.1111/j.1538-7836.2007.02617.x. [DOI] [PubMed] [Google Scholar]
- 81.McFarland J, Lochowicz A, Aster R, Chappell B, Curtis B. Improving the specificity of the PF4 ELISA in diagnosing heparin-induced thrombocytopenia. American journal of hematology. 2012;87:776–81. doi: 10.1002/ajh.23248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Amiral J, Peynaud-Debayle E, Wolf M, Bridey F, Vissac AM, Meyer D. Generation of antibodies to heparin-PF4 complexes without thrombocytopenia in patients treated with unfractionated or low-molecular-weight heparin. American journal of hematology. 1996;52:90–5. doi: 10.1002/(SICI)1096-8652(199606)52:2<90::AID-AJH4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 83.Warkentin TE, Sheppard JA, Horsewood P, Simpson PJ, Moore JC, Kelton JG. Impact of the patient population on the risk for heparin-induced thrombocytopenia. Blood. 2000;96:1703–8. [PubMed] [Google Scholar]
- 84.Arepally G, Reynolds C, Tomaski A, et al. Comparison of PF4/heparin ELISA assay with the 14C-serotonin release assay in the diagnosis of heparin-induced thrombocytopenia. American journal of clinical pathology. 1995;104:648–54. doi: 10.1093/ajcp/104.6.648. [DOI] [PubMed] [Google Scholar]
- 85.Schmitt BP, Adelman B. Heparin-associated thrombocytopenia: a critical review and pooled analysis. Am J Med Sci. 1993;305:208–15. doi: 10.1097/00000441-199304000-00003. [DOI] [PubMed] [Google Scholar]
- 86.Visentin GP, Malik M, Cyganiak KA, Aster RH. Patients treated with unfractionated heparin during open heart surgery are at high risk to form antibodies reactive with heparin:platelet factor 4 complexes. The Journal of laboratory and clinical medicine. 1996;128:376–83. doi: 10.1016/s0022-2143(96)80009-6. [DOI] [PubMed] [Google Scholar]
- 87.Cuker A, Ortel TL. ASH evidence-based guidelines: is the IgG-specific anti-PF4/heparin ELISA superior to the polyspecific ELISA in the laboratory diagnosis of HIT? Hematology/the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2009;250:2. doi: 10.1182/asheducation-2009.1.250. [DOI] [PubMed] [Google Scholar]
- 88.Denys B, Devreese K. A clinical-laboratory approach contributing to a rapid and reliable diagnosis of heparin-induced thrombocytopenia: An update. Thrombosis research. 2009;124:642–3. doi: 10.1016/j.thromres.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 89.Morel-Kopp MC, Aboud M, Tan CW, Kulathilake C, Ward C. Heparin-induced thrombocytopenia: evaluation of IgG and IgGAM ELISA assays. Int J Lab Hematol. 2011;33:245–50. doi: 10.1111/j.1751-553X.2010.01276.x. [DOI] [PubMed] [Google Scholar]
- 90.Pouplard C, Leroux D, Regina S, Rollin J, Gruel Y. Effectiveness of a new immunoassay for the diagnosis of heparin-induced thrombocytopenia and improved specificity when detecting IgG antibodies. Thrombosis and haemostasis. 2010;103:145–50. doi: 10.1160/TH09-04-0253. [DOI] [PubMed] [Google Scholar]
- 91.Althaus K, Strobel U, Warkentin TE, Greinacher A. Combined use of the high heparin step and optical density to optimize diagnostic sensitivity and specificity of an anti-PF4/heparin enzyme-immunoassay. Thrombosis research. 2011;128:256–60. doi: 10.1016/j.thromres.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 92.Whitlatch NL, Kong DF, Metjian AD, Arepally GM, Ortel TL. Validation of the high-dose heparin confirmatory step for the diagnosis of heparin-induced thrombocytopenia. Blood. 2010;116:1761–6. doi: 10.1182/blood-2010-01-262659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Warkentin TE. How I diagnose and manage HIT. Hematology/the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2011;2011:143–9. doi: 10.1182/asheducation-2011.1.143. [DOI] [PubMed] [Google Scholar]
- 94.Walenga JM, Jeske WP, Wood JJ, Ahmad S, Lewis BE, Bakhos M. Laboratory tests for heparin-induced thrombocytopenia: a multicenter study. Seminars in hematology. 1999;36:22–8. [PubMed] [Google Scholar]
- 95.Tomer A, Masalunga C, Abshire TC. Determination of heparin-induced thrombocytopenia: a rapid flow cytometric assay for direct demonstration of antibody-mediated platelet activation. American journal of hematology. 1999;61:53–61. doi: 10.1002/(sici)1096-8652(199905)61:1<53::aid-ajh10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 96.Walenga JM, Jeske WP, Fasanella AR, Wood JJ, Bakhos M. Laboratory tests for the diagnosis of heparin-induced thrombocytopenia. Seminars in thrombosis and hemostasis. 1999;25(Suppl 1):43–9. [PubMed] [Google Scholar]
- 97.Price EA, Hayward CP, Moffat KA, Moore JC, Warkentin TE, Zehnder JL. Laboratory testing for heparin-induced thrombocytopenia is inconsistent in North America: a survey of North American specialized coagulation laboratories. Thrombosis and haemostasis. 2007;98:1357–61. doi: 10.1160/th07-06-0401. [DOI] [PubMed] [Google Scholar]
- 98.Warkentin TE, Greinacher A, Koster A, Lincoff AM. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:340S–80S. doi: 10.1378/chest.08-0677. [DOI] [PubMed] [Google Scholar]
- 99.Lewis BE, Wallis DE, Berkowitz SD, et al. Argatroban anticoagulant therapy in patients with heparin-induced thrombocytopenia. Circulation. 2001;103:1838–43. doi: 10.1161/01.cir.103.14.1838. [DOI] [PubMed] [Google Scholar]
- 100.Hursting MJ, Soffer J. Reducing harm associated with anticoagulation: practical considerations of argatroban therapy in heparin-induced thrombocytopenia. Drug safety: an international journal of medical toxicology and drug experience. 2009;32:203–18. doi: 10.2165/00002018-200932030-00003. [DOI] [PubMed] [Google Scholar]
- 101.Hursting MJ, Zehnder JL, Joffrion JL, Becker JC, Knappenberger GD, Schwarz RP., Jr The International Normalized Ratio during concurrent warfarin and argatroban anticoagulation: differential contributions of each agent and effects of the choice of thromboplastin used. Clinical chemistry. 1999;45:409–12. [PubMed] [Google Scholar]
- 102.Arpino PA, Demirjian Z, Van Cott EM. Use of the chromogenic factor X assay to predict the international normalized ratio in patients transitioning from argatroban to warfarin. Pharmacotherapy. 2005;25:157–64. doi: 10.1592/phco.25.2.157.56950. [DOI] [PubMed] [Google Scholar]
- 103.Bartholomew JR, Pietrangeli CE, Hursting MJ. Argatroban anticoagulation for heparin-induced thrombocytopenia in elderly patients. Drugs & aging. 2007;24:489–99. doi: 10.2165/00002512-200724060-00005. [DOI] [PubMed] [Google Scholar]
- 104.Hoffman WD, Czyz Y, McCollum DA, Hursting MJ. Reduced argatroban doses after coronary artery bypass graft surgery. The Annals of pharmacotherapy. 2008;42:309–16. doi: 10.1345/aph.1K434. [DOI] [PubMed] [Google Scholar]
- 105.Hursting MJ, Verme-Gibboney CN. Risk factors for major bleeding in patients with heparin-induced thrombocytopenia treated with argatroban: a retrospective study. Journal of cardiovascular pharmacology. 2008;52:561–6. doi: 10.1097/FJC.0b013e3181926928. [DOI] [PubMed] [Google Scholar]
- 106.Swan SK, Hursting MJ. The pharmacokinetics and pharmacodynamics of argatroban: effects of age, gender, and hepatic or renal dysfunction. Pharmacotherapy. 2000;20:318–29. doi: 10.1592/phco.20.4.318.34881. [DOI] [PubMed] [Google Scholar]
- 107.Choice of drugs for heparin-induced thrombocytopenia The Medical letter on drugs and therapeutics. 2012;54:43–4. [PubMed] [Google Scholar]
- 108.Mahaffey KW, Lewis BE, Wildermann NM, et al. The anticoagulant therapy with bivalirudin to assist in the performance of percutaneous coronary intervention in patients with heparin-induced thrombocytopenia (ATBAT) study: main results. The Journal of invasive cardiology. 2003;15:611–6. [PubMed] [Google Scholar]
- 109.Kiser TH, Burch JC, Klem PM, Hassell KL. Safety, efficacy, and dosing requirements of bivalirudin in patients with heparin-induced thrombocytopenia. Pharmacotherapy. 2008;28:1115–24. doi: 10.1592/phco.28.9.1115. [DOI] [PubMed] [Google Scholar]
- 110.Dang CH, Durkalski VL, Nappi JM. Evaluation of treatment with direct thrombin inhibitors in patients with heparin-induced thrombocytopenia. Pharmacotherapy. 2006;26:461–8. doi: 10.1592/phco.26.4.461. [DOI] [PubMed] [Google Scholar]
- 111.Savi P, Chong BH, Greinacher A, et al. Effect of fondaparinux on platelet activation in the presence of heparin-dependent antibodies: a blinded comparative multicenter study with unfractionated heparin. Blood. 2005;105:139–44. doi: 10.1182/blood-2004-05-2010. [DOI] [PubMed] [Google Scholar]
- 112.Lobo B, Finch C, Howard A, Minhas S. Fondaparinux for the treatment of patients with acute heparin-induced thrombocytopenia. Thrombosis and haemostasis. 2008;99:208–14. doi: 10.1160/TH07-04-0252. [DOI] [PubMed] [Google Scholar]
- 113.Grouzi E, Kyriakou E, Panagou I, Spiliotopoulou I. Fondaparinux for the treatment of acute heparin-induced thrombocytopenia: a single-center experience. Clinical and applied thrombosis/hemostasis: official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2010;16:663–7. doi: 10.1177/1076029609347900. [DOI] [PubMed] [Google Scholar]
- 114.Goldfarb MJ, Blostein MD. Fondaparinux in acute heparin-induced thrombocytopenia: a case series. Journal of thrombosis and haemostasis: JTH. 2011;9:2501–3. doi: 10.1111/j.1538-7836.2011.04489.x. [DOI] [PubMed] [Google Scholar]
- 115.Warkentin TE, Pai M, Sheppard JI, Schulman S, Spyropoulos AC, Eikelboom JW. Fondaparinux treatment of acute heparin-induced thrombocytopenia confirmed by the serotonin-release assay: a 30-month, 16-patient case series. Journal of thrombosis and haemostasis: JTH. 2011;9:2389–96. doi: 10.1111/j.1538-7836.2011.04487.x. [DOI] [PubMed] [Google Scholar]
- 116.GlaxoSmithKline . ARIXTRA PRESCRIBING INFORMATION. 2011. [Google Scholar]
- 117.Bartholomew JR, Hursting MJ. Transitioning from argatroban to warfarin in heparin-induced thrombocytopenia: an analysis of outcomes in patients with elevated international normalized ratio (INR) Journal of thrombosis and thrombolysis. 2005;19:183–8. doi: 10.1007/s11239-005-1849-9. [DOI] [PubMed] [Google Scholar]
- 118.Babcock RB, Dumper CW, Scharfman WB. Heparin-induced immune thrombocytopenia. The New England journal of medicine. 1976;295:237–41. doi: 10.1056/NEJM197607292950501. [DOI] [PubMed] [Google Scholar]
- 119.Refaai MA, Chuang C, Menegus M, Blumberg N, Francis CW. Outcomes after platelet transfusion in patients with heparin-induced thrombocytopenia. Journal of thrombosis and haemostasis: JTH. 2010;8:1419–21. doi: 10.1111/j.1538-7836.2010.03861.x. [DOI] [PubMed] [Google Scholar]
- 120.Zucker MB, Katz IR. Platelet factor 4: production, structure, and physiologic and immunologic action. Proc Soc Exp Biol Med. 1991;198:693–702. doi: 10.3181/00379727-198-43309. [DOI] [PubMed] [Google Scholar]
- 121.Wanaka K, Matsuo T, Matsuo M, et al. Re-exposure to heparin in uremic patients requiring hemodialysis with heparin-induced thrombocytopenia. Journal of thrombosis and haemostasis: JTH. 2010;8:616–8. doi: 10.1111/j.1538-7836.2009.03734.x. [DOI] [PubMed] [Google Scholar]
- 122.Nuttall GA, Oliver WC, Jr., Santrach PJ, et al. Patients with a history of type II heparin-induced thrombocytopenia with thrombosis requiring cardiac surgery with cardiopulmonary bypass: a prospective observational case series. Anesthesia and analgesia. 2003;96:344–50. doi: 10.1097/00000539-200302000-00009. table of contents. [DOI] [PubMed] [Google Scholar]
- 123.Bakchoul T, Giptner A, Bein G, Santoso S, Sachs UJ. Performance characteristics of two commercially available IgG-specific immunoassays in the assessment of heparin-induced thrombocytopenia (HIT) Thrombosis research. 2011;127:345–8. doi: 10.1016/j.thromres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 124.Legnani C, Cini M, Pili C, Boggian O, Frascaro M, Palareti G. Evaluation of a new automated panel of assays for the detection of anti-PF4/heparin antibodies in patients suspected of having heparin-induced thrombocytopenia. Thrombosis and haemostasis. 2010;104:402–9. doi: 10.1160/TH10-01-0002. [DOI] [PubMed] [Google Scholar]
- 125.Eichler P, Raschke R, Lubenow N, Meyer O, Schwind P, Greinacher A. The new ID-heparin/PF4 antibody test for rapid detection of heparin-induced antibodies in comparison with functional and antigenic assays. British journal of haematology. 2002;116:887–91. doi: 10.1046/j.0007-1048.2002.03363.x. [DOI] [PubMed] [Google Scholar]
- 126.GlaxoSmithKline . Argatroban Prescribing Information. Apr, 2012. [Google Scholar]
- 127.Company TM. Angiomax Prescribing Information. 2012. [Google Scholar]