Abstract
Stimuli-responsive hydrogels are materials with great potential for development of active functionalities in fluidics and micro-fluidics. Based on the current state of research on pH sensors, hydrogel sensors are described qualitatively and quantitatively for the first time. The review introduces the physical background of the special properties of stimuli-responsive hydrogels. Following, transducers are described which are able to convert the non-electrical changes of the physical properties of stimuli-responsive hydrogels into an electrical signal. Finally, the specific sensor properties, design rules and general conditions for sensor applications are discussed.
Keywords: pH sensor, microsensor, stimuli-responsive hydrogel, phase transition
1. Introduction
Stimuli-responsive polymers or hydrogels can change their volume significantly in response to small alterations of certain environmental parameters. The changes in volume can be more than hundredfold, based on absorption or on release of aqueous solution accompanied by considerable swelling forces if an external force is applied. Therefore, about 1950 the discoverers W. KUHN, A. KATCHALSKY, and J.W. BREITENBACH [1-4] wrote about “muscle-like working” and predicted a great potential of these materials. At the beginning of the eighties the work of T. TANAKA inspired to the development of manifold stimuli-sensitive hydrogels with sensitivities across temperature [5], electrical field values [6], light [7], pH, solvent composition and specific ions [8-11]. Approximately 2000 the development of the fundamentals for technical applications started. To fabricate functional hydrogel structures at the micro-scale size photo-patterning technologies [12-15] and principles based on microgels [16] were developed. In fluidics and microfluidics outstanding multi-purpose functionalities have been emerged involving microvalves [16-20], automatic valves [15,16,18, 21-24], pumps [25-27], and chemostat valves performing feedback control to keep an adjustable concentration on a constant level, e.g. salts and alcohols [28,29]. Some of these devices are already commercialised, e.g. the Hydrogel Valve of the company GeSiM, Germany [17,30].
Remarkable efforts have been carried out to realise chemical sensors, which use the manifold sensitivities and enormous changes in properties of hydrogels [31]. Such sensors can have a straightforward design. They are directly working devices which can have a high selectivity and sensitivity. Many of these developments are addressed to pH sensors. The current state of research enables for the first time to describe the characteristics of hydrogel based sensors both qualitatively and quantitatively.
This review is aimed to give a basic understanding of the behaviour of stimuli-responsive hydrogels, to describe why these gels are used as sensor materials, what for sensor properties can be obtained, and which special features have to be respected.
2. Hydrogel Behaviour
2.1. Thermodynamics
Stimuli-responsive polymers are plastic materials with molecule chains cross-linked to a three dimensional network. They are synthesised by a cross-linking reaction between polymer molecules [32] or by a cross-linking polymerisation, which is simultaneously synthesising polymer chains and linking them concomitantly [5]. Polymer molecules consist of small molecular units, the so-called monomers, which can be arranged in a sequence to form a long polymer chain or to form branched polymer molecules with side chains. Generally, all polymers are solvophilic to certain solvents. Not cross-linked polymers are soluble in presence of these solvents. Due to the interconnections between the polymer chains cross-linked polymers are insoluble but swell by solvent absorption. If they can swell in water they are called hydrogels.
a) Hydrogel behaviour in solvents
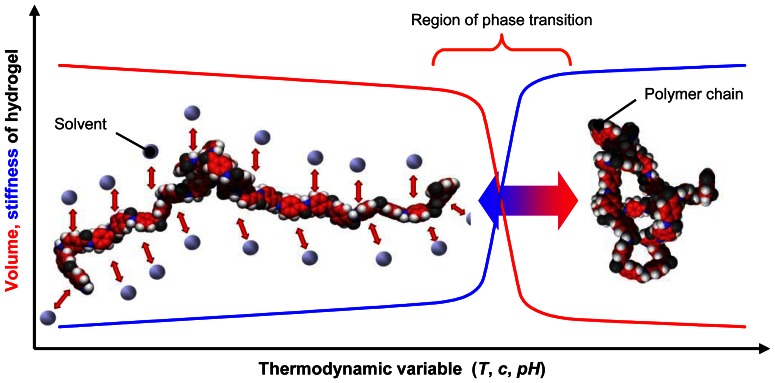
Unlike “normal” solvophilic polymers stimuli-responsive hydrogels exhibit a first-order- or a continuous (also called second-order) phase transition behaviour. As illustrated in Figure 1 they exhibit two phases. A separated phase of the gel is dominated by polymer-polymer interactions. In this case the gel reaches its maximal value of hydrophobicity and shrinks. The second phase, a mixed phase, is characterised by solvent-polymer-interactions, which aspire the best mixing of polymer and aqueous solution. Therefore, within the mixed phase the hydrogel gains its maximum of hydrophilicity and swells. Close by the phase interface a small alteration of a thermodynamic variable, namely a solvent concentration, results in a change of the phase characterised by an abrupt change in physical properties of hydrogel, especially in volume, mass, stiffness and more.
Figure 1. Phase transition behaviour of stimuli-responsive hydrogels.
The swollen phase of the gel (left) is dominated by polymer-solvent interactions obtaining the best mixing of the polymer chains and the aqueous solution. The shrunken phase of the hydrogel (right) is determined by polymer-polymer-interactions, which remove solution out of the gel. Near the phase interface, within the range of phase transition, small alterations of a thermodynamic value result in a change of the phase of the hydrogel.
The polymer-solvent-interactions of the mixing phase generate osmotic pressures Δπmix acting expansively. Due to the polymer-polymer-interactions the polymer network counteracts this expansion by an elastic force respected by Δπelast . The hydrogel obtains its swelling equilibrium at the balance of the pressures, which can be described by
| (1) |
The FLORY-REHNER theory [33,34] and the FLORY-HUGGINS theory [35,36] describe these processes in detail.
Special solvent-responsive hydrogels can be additionally temperature-sensitive. Such gels have a slightly hydrophobic nature and contain groups, which preferably interact with water molecules by hydrogen bonds which cause the hydrogel swelling. These hydrogen bonds depend on the temperature. Exceeding a critical temperature, the so-called lower critical solution temperature, the hydrogen bonds between polymer and water break apart. Now, the hydrophobic nature of the gel can dominate resulting in a shrinking of the gel.
b) Behaviour of polyelectrolyte hydrogels
Polyelectrolyte hydrogels comprise weak acidic and weak basic groups, respectively, which can be ionised. For example, gels containing acidic groups are deprotonated in basic surrounding conditions as following:
| (2) |
Therefore, the density of likewise charged groups within the network strongly increases accompanied by an adequate generation of mobile counterions inside the gel, which induces the phase transition due to electrostatic repulsion. In an acidic ambient the acidic gel protonates
| (3) |
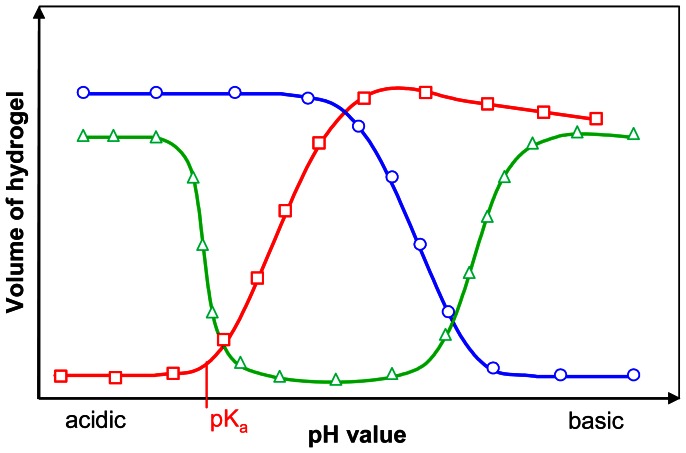
resulting in a decrease of both the charge density and the content of mobile counterions within the hydrogel leading to gel shrinking [37]. The phase transition of the gels occurs in a small range close by the apparent acid dissociation constant pKa of the hydrogel which is mostly identical with the pKa of the ionisable group. Approximately at the apparent pKa of the gel the ionisation begins accompanied by a drastic swelling of the hydrogel. If the ionisation of the ionisable component is completed the swelling process stops. Further pH increase only increases the ionic strength. This decreases the osmotic pressure and leads to shrinking of the gel (see Figure 2, acidic hydrogel). That figure shows the general behaviours of the three types of polyelectrolyte gels.
Figure 2. Phase transition behaviour of polyelectrolyte hydrogels.
Acidic hydrogels (
 ) are ionised by deprotonation in basic solutions, which have an excess of hydroxyl groups. Basic hydrogels (
) are ionised by deprotonation in basic solutions, which have an excess of hydroxyl groups. Basic hydrogels (
 ) swell in acidic solutions due to the ionisation of their basic groups by protonation. Amphiphilic hydrogels (
) swell in acidic solutions due to the ionisation of their basic groups by protonation. Amphiphilic hydrogels (
 ) contain both acidic and basic groups. Therefore they show two phase transitions.
) contain both acidic and basic groups. Therefore they show two phase transitions.
The contribution of the electrostatic interaction of polyelectrolyte hydrogels to the balance of the osmotic pressure has to be respected as an expansive pressure Δπion, so that Eq. 1 must be rewritten to
| (4) |
The mobility of the counterions of the gel should be high enough to make their release into the surrounding solution possible. However, because the hydrogel keeps the charge neutrality inside itself, it can't release its counterions but only exchange them with an adequate ion from the surrounding medium. Such exchange can affect the balance of the osmotic pressures of the gel and of the surrounding solvent leading to a change of swelling equlibrium of the gel. At low ionic strengths the ion exchange is marginal and its influence on the swelling is negligible. Increasing the surrounding ion concentration to a medium ionic strength results in an exchange of the mobile counterions of the gel by the surrounding ions and in an arise of the osmotic pressure inside the hydrogel. This is associated with a swelling. High ionic strength results in a polyelectrolyte shielding reducing the osmotic pressure accompanied by a hydrogel shrinkage [38]. The contribution of the ionic strength Δπbath to the balance of osmotic pressure has to be considered additive [39]:
| (5) |
2.2. Swelling kinetics
The swelling and shrinking of hydrogels requires a transport of matter, which is time-consuming. To initiate a volume phase transition two transport mechanisms have to be considered.
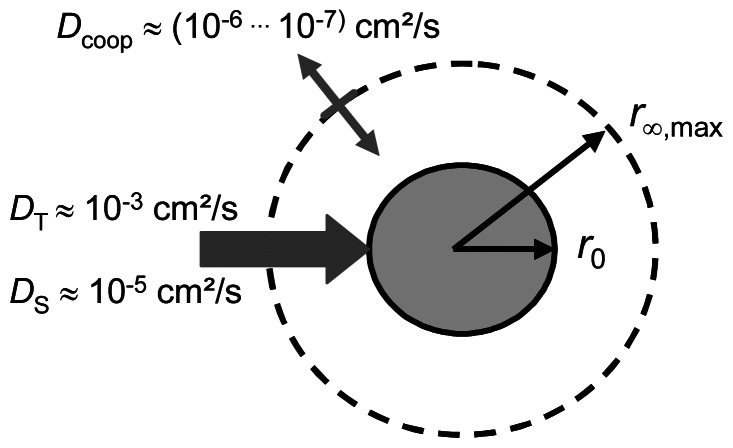
First, the initiating stimulus has to be transferred into the hydrogel, such as temperature difference, solvents or ions, which is associated with a change of the balance of the osmotic pressure. The transport occurs either energetically by heat transfer (described by the thermal transfer coefficient, DT) or by continuous mass diffusion of a solvent into the hydrogel (described by the spontaneous mass transfer coefficient, DS) shown in Figure 3. Then as a second mechanism, to obtain the swelling equilibrium of the changed osmotic pressure balance, the hydrogel swells or shrinks absorbing or releasing swelling agent. Concomitantly, the polymer chains of the network have to be moved to obtain their new positions. Comprising both the solvent diffusion and the net chain motion TANAKA and FILLMORE developed the model of “collective” or “cooperative” diffusion characterised by the cooperative diffusion coefficient Dcoop [40-42].
Figure 3. Transport processes at swelling and shrinking of hydrogels.
r0 - initial radius, r∞,max - maximal radius in the swelling equilibrium.
For a spherical gel as shown in Figure 3, this theory predicts a characteristic time constant of swelling process as following:
| (6) |
where r is the final radius and the characteristic dimension, respectively. The radius of the hydrogel during the swelling process is expressed as
| (7) |
whereas the shrinking process follows
| (8) |
The TANAKA-FILLMORE theory describes the unloaded and free swelling behaviour without influence of the surrounding area. Swelling times obtained using this theory correlate excellently with the experimental data. Unfortunately the uninfluenced free swelling of gels without an externally applied force can hardly be used for functional sensor and actuator elements.
The characteristics of the swelling kinetics of polyelectrolyte gel in presence of buffer ions was investigated by LESHO and SHEPPARD [43]. In this case the characteristic time constant describing a “buffer-mediated diffusion reaction” is given by:
| (9) |
(δ- gel thickness, DHB - diffusivity of the buffer molecule into the gel, H0 - hydration, βgel - buffer capacity of the hydrogel, βHB - buffer capacity of the buffer solution).
Summarizing Eq. 6 to 9, to obtain small characteristic time constants - due to the square dependency - the characteristic hydrogel dimension should be as small as possible. For swelling in ion containing liquids a characteristic time constant can be expected which is increasing with rising buffer capacity of the hydrogel. The time constant decreases with an increasing capacity of the buffer solution.
It should be mentioned that in the case of swelling, τ is increasing with an increasing counter pressure. Furthermore, with increasing counter pressure the maximal volume of the hydrogel V∞,max decreases in the swelling equilibrium non-linearly and strongly disproportional. Limitations of the swelling agent supply additionally increase the characteristic time constant of swelling. In principle, τ could be increased to infinity by corresponding restriction of swelling agent supply.
3. Sensor Transducers
Sensor transducers are components, which convert the non-electrical changes of properties of the stimuli-responsive hydrogel into an evaluable signal, in most cases an electrical signal. Two basic principles can be used in gel sensors:
-
-
transducers based on mechanical work performed by hydrogel swelling and shrinking, and
-
-
transducers observing changes in properties (e.g. densities, mass, volume, stiffness) of free swelling gels.
This chapter describes fundamental transducer principles, which were used for hydrogel sensors.
3.1. Transducers of free swelling gels
Transducers using free swelling gels as sensor materials have to directly observe changes in one or more hydrogel properties. Currently, optical, oscillating and conductimetric transducer principles are used.
3.1.1. Optical transducers
Most of these transducers work optically. Optical transducers can directly measure changes in optical properties of hydrogels. A different approach is based on the observation of special fillings or surface coatings, which are changed or moved due to hydrogel swelling.
a) Optical transmission
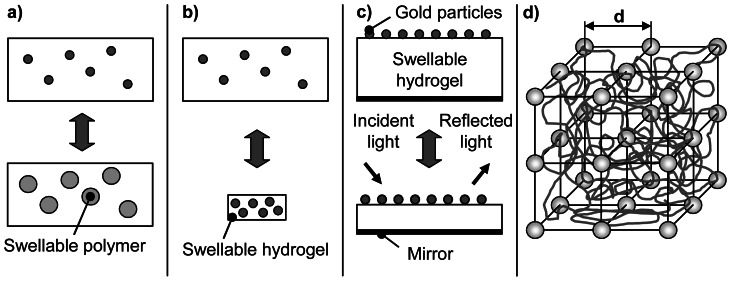
Within the phase separated shrunken state hydrogels are often opaque accompanied by a low optical transmission coefficient. In the swollen state hydrogels with homogeneous structure are clear and transparent. ODEH et al. used a similar principle to realize a sensor based on changes in optical transmission [44]. Coloured microspheres shown in Figure 4a increase their transmission by an increase of water absorption. Shrinking of the microspheres results in an increase of the turbidity of the sensing element. The changes of optical properties can be monitored as a transmission measurement using a conventional spectrophotometer or a miniature fibre optic spectrometer [45].
Figure 4. Basic functions of the sensor material used in optical transducers.
a) Swellable hydrogel particles, entrapped into a membrane, change the optical transmission of the sensing element (according to [44]); b) Changes in hydrogel swelling change the density and spacing of entrapped particles; c) Changes in thickness of a hydrogel structure displace reflective layers. d) Changes in hydrogel volume change the lattice spacing d of a crystalline colloidal array.
b) Refractive index
The change in transmission of the hydrogel is related to the change in the refractive index between swollen and shrunken state. Water has a lower refractive index than the shrunken hydrogel. During the process of swelling the water content increases decreasing the refractive index of the hydrogel. This behaviour was used in a dual-channel chirped grating pad membrane sensor. One channel of the sensor is used for pH detection by a hydrogel membrane, the other channel as an on-chip refractometer to determine the refractive index of the test solutions [46]. SEITZ and co-workers investigated both changes in optical transmission and in refractive index [47,48].
c) Reflection
The SEITZ group studied the diffuse reflected light of swellable hydrogels and found a significant change of the reflection intensity while a volume change [49]. A diffuse reflection occurs on interfaces between regions with a different refractive index, especially between the bulk polymer and the aqueous solution in the pores [50]. During a polymer swelling the bulk polymer dilutes with water causing its refractive index to decrease and become closer to the refractive index of the water in the pore space. This reduces the reflectance at the polymer-pore interface resulting in a decrease of the reflected intensity. Beside the refractive index, transmission, size, shape and position of such micro-structures are further factors which affect the reflection intensity.
Changes in reflected intensity were used for a single fibre sensor shown in Figure 5a[49]. The transducer device consists of a single optical fibre coated on the end with a drop of the sensitive polymer. The system includes a light emitting diode as a light source, a photodiode detector, and a fibre-optic coupler, which serves as beamsplitter. As further transducer elements a bifurcated bundle of optical fibres [51] and an optical reflective device [50] were reported.
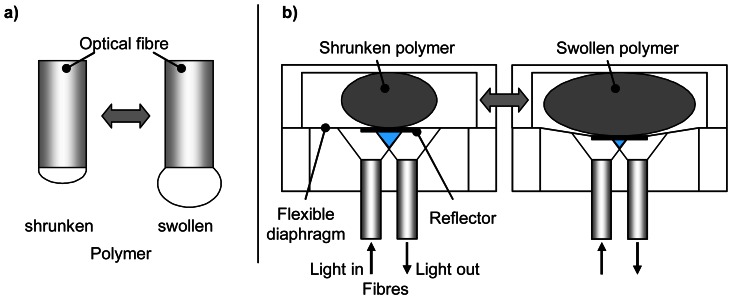
Figure 5. Reflective fibre optic chemical sensors based on swelling polymers.
a) Single fibre sensor based on changes in diffuse reflection of polymers (according to [49]). b) Dual-fibre sensor basing on the displacement of a reflector (according to [68,69]).
A reflection interference system was realised consisting of a gold particle coated hydrogel layer which is placed on top of a mirror (see Figure 4c). Gold particles and the mirror act as an optical thin-film resonance system with reflection properties depending on the thickness of the hydrogel layer. Changes in thickness of the hydrogel layer were monitored by the slope of the characteristic reflection minimum of the device [52,53].
d) Optical wavelength diffraction
ASHER et al. incorporated a colloidal crystalline array (CCA) of spheres into a stimuli-responsive hydrogel [54-57]. The crystalline colloidal array diffracts the light at visible wavelengths determined by the lattice spacing d (see Figure 4d), which gives rise to an intense colour. By swelling the mean separation between the colloidal spheres increases shifting the BRAGG peak of the diffracted light to longer wavelengths. A change of 0.5% in the hydrogel volume shifts the diffraction wavelength by ∼1 nm.
Another principle was described as holographic sensor by LOWE et al. [58,59]. Here, the holographic diffraction wavelength or color, respectively, of the holograms is used to characterize shrinkage and swelling behaviour of the hydrogel as a function of the analyte.
e) Fluorescence intensity
Fluorophore labeled hydrogels, that undergo a change in swelling degree, modify their fluorescence intensity [60,61]. Swelling of the gel decreases the intensity while a shrinking increases it. The change of fluorescence intensity can be of more than 30 %.
3.1.2. Conductometric transducer
SHEPPARD and co-workers introduced a conductometric sensor, which is an interdigitated electrode array coated with a hydrogel layer [62,63]. Measured at frequencies in the range 100 Hz to 100 kHz the electrode impedance is primarily resistive. A swelling of the hydrogel layer results in an increase of the conductivity of the hydrogel accompanied by a decrease of the resistance.
3.1.3. Oscillating transducers
Oscillating transducers are devices changing their resonance frequency. Changes in properties of a load result in a shift of this resonance frequency. This can be accompanied by a change of the signal amplitude.
a) Quartz crystal micro balance
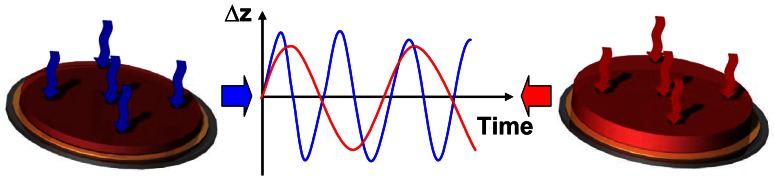
Applying electric field quartz crystals can be stimulated to oscillate stable in their resonance frequency. Loading of the quartz resonator results in changes of the resonance frequency (Figure 6). This frequency shift is the base of the quartz crystal micro balance principle. In our own work quartz crystal micro balance is used as a transduction element to monitor changes in the properties of thin hydrogel layers [64]. An increase in the surface load of a quartz crystal should theoretically induce a decrease of the resonance frequency. However, it was observed a frequency shift increasing with increasing mass and volume of hydrogel. Essentially, both the stiffness and the density of the hydrogel strongly decrease by swelling and thus increase its volume and mass. As a consequence, the effective surface load which is swinging decreases during the swelling process. Changes in mechanical properties of the hydrogel coating of the quartz cause changes in damping of the signal amplitude. This damping can be used as second data acquisition channel. The damping decreases with increasing hydrogel swelling.
Figure 6. Principle of the quartz crystal micro balance sensor.
Changes in hydrogel properties cause changes of the complex resonance frequency of a quartz crystal micro balance.
b) Magnetoelastic sensors
GRIMES et al. describe thick-film devices, which are stimulated to mechanically oscillate in their resonance frequency by a magnetic field impulse [65-67]. In turn the mechanical oscillation of the sensor induces magnetic flux that can be detected remotely. The frequency decreases with an increasing of hydrogel mass. Therefore, small changes in mass of a hydrogel coating can be detected by monitoring the shift in the resonance frequency of the sensor. The shift in resonance frequency is also affected by the elasticity of the sensor coating, temperature, viscosity and density of the test solution.
3.2. Transducers based on mechanical work of the hydrogel
Such transducers use the ability of hydrogels to deform or to strain mechanically a transduction element resulting in a change of a special property of that element or in a change of a detectable distance.
3.2.1. Optical transducers
a) Reflective diaphragms
By such devices the swellable polymer is coupled to a reflector (see Figure 5b). Changes in polymer volume cause the reflecting diaphragm to move, which in turn changes the intensity of light reflected back into the optical fibre [68,69].
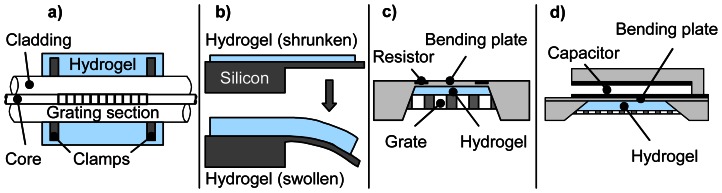
b) Fibre BRAGG grating sensors
ZHANG et al. presented hydrogel based fibre optic BRAGG grating sensors [70,71]. By swelling, the hydrogel pushes the clamps fixed on the fibre BRAGG grating (Figure 7a). This results in a physical stretch of the fibre which expands the grating period. When the hydrogel shrinks the BRAGG wavelength decreases.
Figure 7. Sensor transducers basing on mechanical work on stimuli-responsive hydrogel.
a) Fibre BRAGG grating sensor, b) Microcantilever, c) Piezoresistive bending plate sensor, d) Capacitive bending plate sensor.
3.2.2. Mechanical transducers
a) Microcantilevers
Microcantilevers can transduce changes of mass, temperature, heat, or stress, into bending (static mode) or a change in resonance frequency (dynamic mode). To measure in liquids the static mode is more preferred than the dynamic mode. The microcantilever is commonly coupled to an optical or piezo-resistive read-out system [72]. The groups of PEPPAS [73,74] and JI [75] used devices with an optical read-out system as transducer element to monitor changes in swelling of a hydrogel coating (Figure 7b).
b) Bending plate transducers
These transducers usually include a piezo-resistive WHEATSTONE-bridge and are commonly used as pressure sensors. Several groups developed hydrogel-based sensors using bending plate transducers [76-81]. Principally, the stimuli-sensitive hydrogel is placed in a fixed volume between a stiff/rigid grate, which is permeable for the stimulus, and the bending plate (see Figure 7c). If the hydrogel swells the plate deflects resulting in a change of the resistance of the piezo-resistive bridge. HERBER et al. extended this principle to a device able to measure contents of carbon dioxide [80,81].
A capacitive bending plate sensor as shown in Figure 7d is reported in [82]. Here the bending of the plate changes the distance of the capacitor plates accompanied by a change of its capacity.
To avoid a perturbation of the process pressure bending plate sensors should be pressure compensated.
4. Sensitive Hydrogel
Usually, pH sensitive polymer networks consist of a backbone polymer carrying weak acidic or basic groups. The backbone polymer provides a mechanical stability of the gel whereas the ionisable group contributes to the pH sensitivity. As mentioned such syntheses can be performed as a cross-linking reaction between polymer molecules or as a cross-linking polymerisation, which is simultaneously synthesising polymer chains and linking them concomitantly.
Poly(vinyl alcohol)-poly(acrylic acid) networks have been synthesised by cross-linking poly(vinyl alcohol) and poly(acrylic acid). Both water-solved components must be mixed, dried and finally cross-linked by heating [64]. By variation of the ratio between the backbone polymer poly(vinyl alcohol) and poly(acrylic acid) or by the cross-linking parameters both mechanical properties and sensitivity can be adjusted [83].
Most of the other hydrogels are prepared by free radical polymerisation. We explain the general synthesis on the example of the photo-patterned hydrogel used in [74]. This hydrogel consists of methacrylic acid (MAA) as ionisable component and poly(ethylene glycol) dimethacrylate (PEGDMA) as a backbone polymer. It has to be prepared as a mixture containing the monomers with a defined mole ratio MAA:PEGDMA and a photo-initiator. Following the mixture can be spin-coated onto the substrate, which is typically pre-treated with an adhesion promoter. Finally the polymerisation has to be performed by UV exposure through a mask.
Generally, the composition of the hydrogel determines the pKa value and the nature (acidic or basic) of the ionisable component necessary for the special sensor application. After defining that component a backbone material has to be found which can be copolymerised or cross-linked with the other component. Further conditions influencing the choice of hydrogel are adhesion properties onto the substrate and compatibility to the ambient.
5. Sensor Properties
The properties of hydrogel-based pH-sensors are summarised in Table 1. Due to author specific interpretations of the sensor characteristic we standardise the results by the evaluation of the characteristic curves.
Table 1.
Comparison of hydrogel based pH sensors .* estimated by characteristic curves
| Sensor type | Data channel | Transducer resolution | Sensitivity per pH unit | Accuracy of measurement | pH working range | Hydrogel | Minimal response time | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| overall | best point | ||||||||||
|
|
|
||||||||||
| Transducer signal | pH unit | Transducer signal | pH unit | Material | Thickness | ||||||
| Refractometric sensor [46] | refractive index | 2.5x10−4 10−5/4x10−2 | 3.11x10−2 | ± 3.75x10- * | ± 1.64x10-3 * | ± 1.1x10-4 | ± 4.8x10-4 | 7-7.75 | HEMA-DMAEMA | 300 nm (dry) | ∼ 80 s |
| Holographic sensor [58] | wavelength | 6x10−3 (1nm/165nm) | 165 nm | ± 5.3 nm * | ± 0.032 * | ± 3.1 nm * | ± 0.019 * | 5 - 7 | PHEMA-co-MAA | 10 μm | ∼ 250 s |
| CCA sensor[55] (Braggdiffraction) | wavelength | 3,3x10−3 (1nm/300nm) | 74.2 nm | - | - | - | - | 4.3-8.5 | PAAm-PCCA | 125 μm | 10 min |
| Conductimetric sensor [63] | resistance | - | 100 Ω | - | - | ± 5.2Ω | ± 0.052 | 7 - 8 | HEMA-DMAEMA | 8 μm | 350 s |
| Quartz crystalmicro balance[64] | frequency damping | 3.3x10−6 (0.1Hz/30kHz) | 13.2 kHz 25.467 kHz | ± 0.62 kHz ± 1.085 kHz | ± 0.047 ± 0.042 | ± 179 Hz ± 89.5 Hz | ± 0.013 ± 3.5x10-3 | 2.55 – 3.45 | PVA-PAA | 390 nm (dry) | 500 ms |
| Magnetoelastic sensor [67] | frequency | 2,4x10−4 0.4Hz/1.7kHz | 506 Hz | ± 66 Hz | ± 0.13 | - | - | 4,4 – 8,5 | poly(AA-co-IOA) | 1.4 μm | 120 s |
| Microcantilever [74] | deflexion | 5x105 (1nm/20μm) | 20.3 μm | ± 1.15 μm * | ± 0.057 * | ± 0.3 μm * | ± 0.015 * | 6 - 6,8 | PMAA-PEGDMA | 2.2 μm | few minutes |
| Microcantilever [75] | deflexion | 2,9x105 (1nm/35μm) | 1000 nm | ± 5 % | ± 0.05 | - | - | 6 - 9 | AAm-DMAEMA | 15 μm | 15 min |
| Bending plate sensor [79] | resistance | 1,67x10-5 (1μV/60mV) | 14.55 mV * | ± 3.5 mV * | ± 0.24 * | ± 1.9 mV * | ± 0.13 * | 5.5 - 11 | PVA-PAA | 40 μm | ∼ 12 min |
HEMA:DMAEMA: 2-hydroxyethyl methacrylate - N,N-dimethylaminoethyl methacrylate
PHEMA-co-MAA: poly(hydroxyethyl methacrylate-co- methacrylic acid) different acidic and basic monomers where also used
PAAm-PCCA: poly(acrylamide) - polymerised crystalline colloidal array
PVA-PAA: poly(vinyl alcohol) – poly(acrylic acid)
poly(AA-co-IOA): poly(acrylic acid-co-isooctyl acrylate)
PMAA-PEGDMA: poly (methacrylic acid)-Poly(ethylene glycol) dimethacrylate
AAm-DMAEMA: acrylamide - N,N-dimethylaminoethyl methacrylate
5.1. Transducer resolution
The transducer resolution describes the capability of the transduction element to resolve a transduction signal related to the application specific working range. All reported transducers do not restrict the sensor sensitivity. Optical transducers show a resolution between 10−3 and 10−4, while oscillating and micromechanical transducers resolve the working range with 10−4 and 10−6.
5.2. Sensitivity and accuracy of measurement
a) Sensitivity
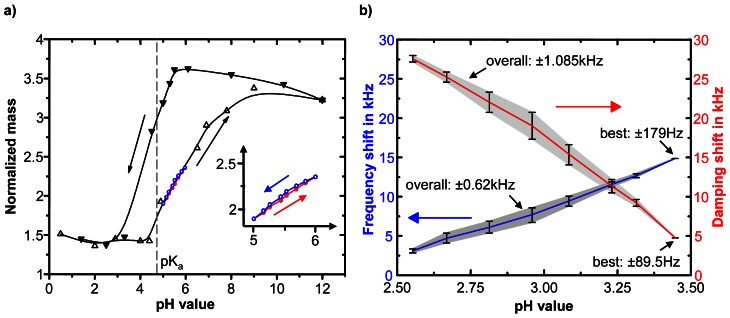
Most sensors work within the phase transition range of the used hydrogel. As shown in Figure 8a for poly(vinyl alcohol)-poly(acrylic acid) the range is between the pKa ≈ 4.7 of the poly(acrylic acid) and pH of 9 because at pH 9 the ionisation of the acidic groups is completed. The drastic volume change of the hydrogel within the phase transition results in an extraordinary sensitivity per pH unit which is in the order of 10−3 to 10−5. As Figure 8b illustrates within that range the sensor characteristics is approximately linear. pH measurements outside of the hydrogel phase transition range are not recommendable. Below that range the gel is shrunken accompanied by marginal sensitivity. As shown in Figure 8a above the range the gel is swollen, there is a poor sensitivity and a big influence by the ionic strength within the solution.
Figure 8. pH sensitivity of a poly(vinyl alcohol) – poly(acrylic acid) hydrogel.
a) Swelling behaviour of a bulk gel. b) Characteristics of a quartz crystal micro balance pH sensor (according [64]).
b) Accuracy of measurement
However, the overall measurement accuracy of most sensors (see Figure 8b), which is identical with the standard deviation of typically 95% confidence level, is in the order of ±10−2 pH units. Causes of this adverse effect are complicated phenomena resulting in a distinctive hysteretic swelling behaviour shown in Figure 8a[83]. The characteristic basic to acidic curve is quite different from the acidic to basic curve. This is reflected in mechanical and optical hydrogel properties [84]. SUZUKI explains that phenomenon with both a replacement of the H+ counterion by an adequate ion such as Na+ and an excess of these ions inside the gel causing a shielding or screening of the ionised gel groups [85].
As shown in the inset of Figure 8a the hysteresis can be significantly lowered by strong restriction of the working range within the phase transition range. For example, the working range of the quartz crystal micro balance sensor (Figure 8b) spans 0.9 pH units accompanied with an overall accuracy of ± 1.085 kHz (± 0.042 pH units) for damping shift. Further decrease of the working range to 0.14 pH units (3.45 to 3.31) results in an increase of the accuracy to ± 89.5 Hz (± 3.5x10−3 pH units).
As mentioned counterforces affect the sensitivity of a sensor by lowering the volume change of the hydrogel. The results of the bending plate sensor (Table 1, [79]) also indicate a decrease of the measurement accuracy.
c) Working range
The working range of the pH sensor can be defined by selection of the ionisable hydrogel component. In many cases the working range is directly corresponding to the pKa of the ionic group. As it can be observed in Figure 8 the bulk gel show an apparent pKa of 4.7, which strongly accords to the pKa of the poly(acrylic acid). But in special cases the apparent pKa of the hydrogel sensors can be shifted as shown in Figure 8b on the example of the hydrogel-coated quartz crystal micro balance sensor with an apparent pKa of 2.2 [64]. Cause of this shift is a dependency of the phase transition condition on the thickness of very thin hydrogel layers, which are bonded to a rigid surface. For poly(vinyl alcohol)-poly(acrylic acid) this phenomenon can be observed below 400 nm, for poly(N-isopropylacrylamide) the shift was also reported for thicknesses below 500 nm [86,87].
5.3. Response time
The response time of sensors depends on the values as anticipated in the chapter swelling kinetics. Characteristic dimensions in the nm range allow obtaining a sensor response within the upper millisecond range, whereas dimensions in the order of 100 μm results in response times of several minutes.
The influence of the ionic strength on the response time can be examined on both sensors with thicknesses in the order of 300 nm. The quartz crystal micro balance sensor was investigated in the acidic range at a high ionic strength and obtained a shortest response time of 500 ms [64]. The refractometric sensor works within the neutral pH range characterised by a low ionic strength. Therefore, the response is time consuming with about 80 s.
Furthermore, the response time of the sensors is strongly affected by forces which influence the swelling process of the hydrogel. Sensors using the mechanical work of the hydrogels tend to show a significant difference between time of swelling and shrinking. At the bending plate sensor [79] the plate constrains the swelling due to the elastic counterforce resulting in a response time of 7.6 h after an increase of pH 1 to pH 6. The vice versa shrinking process is assisted by the elastic force of the bending plate, which leads to a response time of about 40 min. The shrinking is more than eleven times faster than the swelling.
In contrast devices based on free swelling gels tend to be quicker and show no significant difference between swelling and shrinking time. For example, the quartz crystal micro balance sensor possesses a 500 ms response time of swelling and 800 ms for shrinking by a change of pH 1.84 to 3.19 and vice versa [64].
5.4. Calibration and offset correction
The good linearity of the sensors within their working range allows the application of a simple two-point calibration [63]. It should be noted that a calibration must be performed separately for each process solution.
Besides such calibration an offset can be corrected by adjusting the function-defining dimension, e.g. a plate distance to the hydrogel. The basic principle for a mechanical adjustment is described in [29]. For special hydrogels with a double-sensitivity an adjustment of the offset can be performed electronically [28].
5.5. Long-term stability
During the first operation a hydrogel sensor often shows poor accuracy of measurement. This is caused by changes in the microscopic structure of the polymer network because too short polymer chains have to be broken and other chains have to find their optimal arrangement. Performing a number of conditioning cycles (mostly less than 10 cycles), the polymer network can be “warmed up” resulting in a significant increase of the measurement accuracy [88].
An important problem is the delamination of the hydrogel layer of the sensor. This can be avoided by using an adhesion promoter with long spacers [89], very thin hydrogel layers [64], film-developing microgels [90] or enclosing of the hydrogel element [77].
Prior in solutions with high ionic strength hardly soluble complexes or salts can be accumulated inside the hydrogels resulting in an irreversible malfunction of the sensor. The development of such agglomerates can occur within few hours. Only very thin hydrogel films do not tend to such effects. To avoid this the sensor should be regularly purged and stored in deionised water. A correctly maintained hydrogel-based sensor can be used several years.
6. Conclusions
Hydrogel-based pH sensors exhibit an extraordinary sensitivity in the order of up to 10−5 pH units. The statistically proven accuracy of measurement is typically 10−2 to 10−3. They are able to measure in real-time. The working range of sensors spans two or three pH units. Therefore the hydrogel-based technology does not provide universal sensors operating in a large pH range. Rather, the advantage of that sensors is a wide diversity of usable hydrogels providing tailored sensor solution for many special applications. Furthermore, hydrogel-based sensors can be miniaturised and are integrable into Microsystems. They can be realized using commercialised transducer principles.
Acknowledgments
The Deutsche Forschungsgemeinschaft is gratefully acknowledged for financing the collaborative research centre 287 “Reactive Polymers in Inhomogeneous Systems, in Melts, and at Interfaces”, RI 1294/2 and RI 1294/4.
References and Notes
- 1.Kuhn W. Reversible Dehnung und Kontraktion bei Änderung der Ionisation eines Netzwerks polyvalenter Fadenmolekülionen. Experientia. 1949;5(8):318–319. doi: 10.1007/BF02172635. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn W., Hargitay B., Katchalsky A., Eisenberg H. Reversible dilation and contraction by changing the state of ionization of high-polymer acid networks. Nature. 1950;165:514–516. [Google Scholar]
- 3.Katchalsky A. Rapid swelling and deswelling of reversible gels of polymeric acids by ionization. Experientia. 1949;5(8):319–320. doi: 10.1007/BF02172636. [DOI] [PubMed] [Google Scholar]
- 4.Breitenbach J.W., Karlinger H. Über Quellung von vernetzter Polymethacrylsäure. Monatsh. Chem. 1949;80(2):312–313. [Google Scholar]
- 5.Tanaka T. Collapse of gels and the critical endpoint. Phys. Rev. Lett. 1978;40(12):820–823. [Google Scholar]
- 6.Tanaka T., Nishio I., Sun S.-T., Ueno-Nishio S. Collapse of gels in an electric-field. Science. 1982;218:467–469. doi: 10.1126/science.218.4571.467. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki A., Tanaka T. Phase-transition in polymer gels induced by visible-light. Nature. 1990;346:345–347. [Google Scholar]
- 8.Zhang Y., Ji H.F., Brown G.M., Thundat T. Detection of CrO42- using a hydrogel swelling microcantilever sensor. Anal. Chem. 2003;75(18):4773–4777. doi: 10.1021/ac0343026. [DOI] [PubMed] [Google Scholar]
- 9.Irie M., Misumi Y., Tanaka T. Stimuli-responsive polymers: chemical induced reversible phase separation of an aqueous solution of poly(N-isopropylacrylamide) with pendent crown ether groups. Polymer. 1993;34(21):4531–4535. [Google Scholar]
- 10.Tanaka T., Wang C., Pande V., Grosberg A.Y., English A., Masamune S., Gold H., Levy R., King K. Polymer gels that can recognize and recover molecules. Faraday Discuss. 1995;101:201–206. [Google Scholar]
- 11.Li W., Zhao H., Teasdale P.R., John R., Zhang S. Synthesis and characterisation of a polyacrylamide-polyacrylic acid copolymer hydrogel for environmental analysis of Cu and Cd. React. Funct. Polym. 2002;52:31–41. [Google Scholar]
- 12.Hoffmann J., Plötner M., Kuckling D., Fischer W.J. Photopatterning of thermally sensitive hydrogels useful for microactuators. Sens. Actuat. A. 1999;77(2):139–144. [Google Scholar]
- 13.Kuckling D., Adler H.J.P., Arndt K.F., Wolff T., Hoffman J., Fischer W.J. Photocrosslinking of thin polymer films - Materials for sensors and actuators. Macromol. Symp. 1999;142:111–120. [Google Scholar]
- 14.Kuckling D., Adler H.J.P., Arndt K.F., Hoffmann J., Plötner M., Wolff T. Photocrosslinking of thin films of temperature-sensitive polymers. Polym. Adv. Technol. 1999;10(6):345–352. [Google Scholar]
- 15.Beebe D.J., Moore J.S., Bauer J.M., Yu Q., Liu R.H., Devadoss C., Jo B.H. Functional hydrogel structures for autonomous flow control inside microfluidic channels. Nature. 2000;404:588–590. doi: 10.1038/35007047. [DOI] [PubMed] [Google Scholar]
- 16.Arndt K.-F., Kuckling D., Richter A. Application of sensitive hydrogels in flow control. Polym. Adv. Techn. 2000;11(8-12):496–505. [Google Scholar]
- 17.Richter A., Kuckling D., Howitz S., Gehring T., Arndt K.F. Electronically controllable microvalves based on smart hydrogels: magnitudes and potential applications. J. Microelectromech. Syst. 2003;12(5):748–753. [Google Scholar]
- 18.Richter A., Howitz S., Kuckling D., Kretschmer K., Arndt K.F. Automatically and electronically controllable hydrogel based valves and microvalves – design and operating performance. Macromol. Symp. 2004;210:447–456. [Google Scholar]
- 19.Yu C., Mutlu S., Selvaganapathy P., Mastrangelo C.H., Svec F., Fréchet J.M.J. Flow control valves for analytical microfluidic chips without mechanical parts based on thermally responsive monolithic polymers. Anal. Chem. 2003;75(8):1958–1961. doi: 10.1021/ac026455j. [DOI] [PubMed] [Google Scholar]
- 20.Wang J., Chen Z., Mauk M., Hong K.S., Li M., Yang S., Bau1 H.H. Self-actuated, thermo-responsive hydrogel valves for lab on a chip. Biomed. Microdev. 2005;7(4):313–322. doi: 10.1007/s10544-005-6073-z. [DOI] [PubMed] [Google Scholar]
- 21.Richter A., Arndt K.F. 5th Internat. Symp. on Polym. Adv. Technol. Tokyo: 1999. Design and properties of a chemomechanical valve; pp. 31.8.–5.9.1999.156. [Google Scholar]
- 22.Baldi A., Gu Y., Loftness P.E., Siegel R.A., Ziaie B. A hydrogel-actuated environmentally sensitive microvalve for active flow control. J. Microelectromech. Syst. 2003;12(5):613–621. [Google Scholar]
- 23.Kuckling D., Arndt K.F., Richter A. Temperature and pH dependent swelling behavior of poly(N-isopropylacrylamide)-copolymer and their use in flow control. Macromol. Mater. Eng. 2003;288:144–151. [Google Scholar]
- 24.Harmon M.E., Tang M., Frank C.W. A microfluidic actuator based on thermoresponsive hydrogels. Polymer. 2003;44:4547–4556. [Google Scholar]
- 25.Suzuki H., Tokuda T., Kobayashi K. A disposable “intelligent mosquito” with a reversible sampling mechanism using the volume-phase transition of a gel. Sens. Actuat. B. 2002;83(1-3):53–59. [Google Scholar]
- 26.Eddington D.T., Beebe D.J. A valved responsive hydrogel microdispensing device with integrated pressure source. J. Microelectromech. Syst. 2004;13(4):586–593. [Google Scholar]
- 27.Richter A., Klenke C., Arndt K.F. Adjustable low dynamic pumps based on hydrogels. Macromol. Symp. 2004;210:377–384. [Google Scholar]
- 28.Richter A., Türke A., Pich A. Controlled double-sensitivity of microgels applied to electronically adjustable chemostats. Adv. Mater. 2007;19:1109–1112. [Google Scholar]
- 29.Richter A., Wenzel J., Kretschmer K. Mechanically adjustable chemostats based on stimuli-responsive polymers. Sens. Actuat. B. 2007;125:569–573. [Google Scholar]
- 30.Hydrogel Valves – Dead-Volume-Free Microfluidic Switches Data sheet 2007. [downloaded at 10/22/2007]. http://www.gesim.de/upload/PDFs/Hydrogel_en.pdf.
- 31.van der Linden H.J., Herber S., Olthuis W., Bergveld P. Stimulus-sensitive hydrogels and their applications in chemical (micro)analysis. Analyst. 2003;128:325–331. doi: 10.1039/b210140h. [DOI] [PubMed] [Google Scholar]
- 32.Arndt K.F., Schmidt T., Richter A., Kuckling D. High response smart gels: synthesis and application. Macromol. Symp. 2004;207:257–268. [Google Scholar]
- 33.Flory P.J., Rehner J. Statistical mechanics of cross-linked polymer networks I. Rubber elasticity. J. Chem. Phys. 1943;11:512–520. [Google Scholar]
- 34.Flory P.J., Rehner J. Statistical mechanics of cross-linked polymer networks II. Swelling. J. Chem. Phys. 1943;11:521–526. [Google Scholar]
- 35.Huggins M.L. Solutions of long chain compounds. J. Chem. Phys. 1941;9(5):440. [Google Scholar]
- 36.Flory P.J. Thermodynamics of high polymer solutions. J. Chem. Phys. 1941;9(8):660–661. [Google Scholar]
- 37.Siegel R.A., Firestone B.A. pH-dependent equilibrium swelling properties of hydrophobic polyelectrolyte copolymer gels. Macromolecules. 1988;21(11):3254–3259. [Google Scholar]
- 38.English A.E., Tanaka T., Edelman E.R. Equilibrium and non-equilibrium phase transitions in copolymer polyelectrolyte hydrogels. J. Chem. Phys. 1997;107(5):1645–1654. [Google Scholar]
- 39.English A.E., Edelman E.R., Tanaka T. Polymer hydrogel phase transitions. In: Tanaka T., editor. Experimental Methods in Polymer Science. Academic Press Cambridge Burlington; London: 2000. p. 547. [Google Scholar]
- 40.Tanaka T., Hocker L., Benedek G.B. Spectrum of light scattered from a viscoelastic gel. J. Chem. Phys. 1973;59:5151–5159. [Google Scholar]
- 41.Tanaka T., Fillmore D.J. Kinetics of swelling of gels. J. Chem. Phys. 1979;70(3):1214–1218. [Google Scholar]
- 42.Li Y., Tanaka T. Kinetics of swelling and shrinking of gels. J. Chem. Phys. 1990;92(2):1365–1371. [Google Scholar]
- 43.Lesho M.J., Sheppard N.F. A method for studying swelling kinetics based on measurement of electrical conductivity. Polym. Gels Networks. 1997;5:503–523. [Google Scholar]
- 44.Shakhsher Z.M., Odeh I., Jabr S., Seitz W.R. An optical chemical sensor based on swellable dicarboxylate functionalized polymer microspheres for pH copper and calcium determination. Microchim. Acta. 2004;144:147–153. [Google Scholar]
- 45.Oktar O., Caglar P., Seitz W.R. Chemical modulation of thermosensitive poly(N-isopropylacryl-amide) microsphere swelling: a new strategy for chemical sensing. Sens. Actuat. B. 2005;104:179–185. [Google Scholar]
- 46.Dübendorfer J., Kunz R.E., Jobst G., Moser I., Urban G. Integrated optical pH sensor using replicated chirped grating coupler sensor chips. Sens. Actuat. B. 1998;50(3):210–219. doi: 10.1364/ao.37.001890. [DOI] [PubMed] [Google Scholar]
- 47.Rooney M.T.V., Seitz W.R. An optically sensitive membrane for pH based on swellable polymer microspheres in a hydrogel. Anal. Comm. 1999;36:267–270. [Google Scholar]
- 48.Seitz W.R., Rooney M.T.V., Miele E.W., Wang H., Kaval N., Zhang L., Doherty S., Milde S., Lenda J. Derivatized, swellable polymer microspheres for chemical transduction. Anal. Chim. Acta. 1999;400:55–64. [Google Scholar]
- 49.Shakhsher Z., Seitz W.R., Legg K.D. Single fiber-optic pH sensor based on changes in reflection accompanying polymer swelling. Anal. Chem. 1994;66:1731–1735. doi: 10.1021/ac00082a021. [DOI] [PubMed] [Google Scholar]
- 50.Zhang L., Langmuir M.E., Bai M., Seitz W.R. A sensor for pH based on an optical reflective device coupled to the swelling of an aminated polystyrene membrane. Talanta. 1997;44:1691–1698. doi: 10.1016/s0039-9140(97)00080-5. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Z., Shakhser Z., Seitz W.R. Aminated polystyrene membranes for a fiber optic pH sensor based on reflectance changes accompanying polymer swelling. Microchim. Acta. 1995;121:41–50. [Google Scholar]
- 52.Schalkhammer T., Lobmaier C., Pittner F., Aussenegg A.F.R. The use of metal-island-coated pH-sensitive swelling polymers for biosensor applications. Sens. Actuat B. 1995;24-25:166–172. [Google Scholar]
- 53.Aussenegg F.R., Brunner H., Leitner A., Lobmaier C., Schalkhammer T. Metal island coated polymer swelling over mirror system (MICSPOMS): a new principle for measure ionic strength. Sens. Actuat. B. 1995;29:204–209. [Google Scholar]
- 54.Holtz J.H., Asher S.A. Polymerized colloidal crystal hydrogel films as intelligent chemical sensing materials. Nature. 1997;389:829–832. doi: 10.1038/39834. [DOI] [PubMed] [Google Scholar]
- 55.Lee K., Asher A.S. Photonic crystal chemical sensors: pH and ionic strength. J. Am. Chem. Soc. 2000;122:9534–9537. [Google Scholar]
- 56.Yan F., Asher S.A. Cation identity dependence of crown ether photonic crystal Pb2+ sensing. Anal. Bioanal. Chem. 2007;387:2121–2130. doi: 10.1007/s00216-006-1088-8. [DOI] [PubMed] [Google Scholar]
- 57.Ben-Moshe M., Alexeev V.L., Asher S.A. Fast responsive crystalline colloidal array photonic crystal glucose sensors. Anal. Chem. 2006;78:5149–5157. doi: 10.1021/ac060643i. [DOI] [PubMed] [Google Scholar]
- 58.Marshall A.J., Blyth J., Davidson C.A.B., Lowe C.R. PH-sensitive holographic sensors. Anal. Chem. 2003;75(17):4423–4431. doi: 10.1021/ac020730k. [DOI] [PubMed] [Google Scholar]
- 59.Sartain F.K., Yang X., Lowe C.R. Holographic lactate sensor. Anal. Chem. 2006;78:5664–5670. doi: 10.1021/ac060416g. [DOI] [PubMed] [Google Scholar]
- 60.McCurley M.F. An optical biosensor using a fluorescent, swelling sensing element. Biosens. Bioelectronics. 1994;9:527–533. [Google Scholar]
- 61.Saunders B.R., Vincent B. Osmotic de-swelling of polystyrene microgel particles. Colloid Polym. Sci. 1997;275:9–17. [Google Scholar]
- 62.Sheppard N.F., Tucker R.C., Salehi-Had S. Design of a conductimetric pH microsensor swelling hydrogels. Sens. Actuat. B. 1993;10:73–77. [Google Scholar]
- 63.Sheppard N.F., Lesho M.J., McNally P., Francomacaro S. Microfabricated conductimetric pH sensor. Sens. Actuat. B. 1995;28:95–102. [Google Scholar]
- 64.Richter A., Bund A., Keller M., Arndt K.F. Characterization of a microgravimetric sensor based on pH sensitive hydrogels. Sens. Actuat. B. 2004;99(2-3):579–585. [Google Scholar]
- 65.Stojanov P.G., Doherty S.A., Grimes C.A., Seitz W.R. A remotely interogatable sensor for chemical monitoring. IEEE Transactions on Magnetics. 1998;34(4):1315–1317. doi: 10.1109/20.706533. [DOI] [PubMed] [Google Scholar]
- 66.Grimes C.A., Stojanov P.G., Liu Y., Tong C., Ong K.G., Loiselle K., Shaw M., Doherty S.A, Seitz W.R. A magnetostatic-coupling basedremote query sensor for environmental monitoring. J. Phys. D. 1999;32:1329–1335. doi: 10.1088/0022-3727/32/12/308. [DOI] [PubMed] [Google Scholar]
- 67.Ruana C., Zenga K., Grimes C.A. A mass-sensitive pH sensor based on a stimuli-responsive polymer. Anal. Chimica Acta. 2003;497:123–131. [Google Scholar]
- 68.Seitz W.R. New directions in fiber optic chemical sensors: sensors based on polymer swelling. J. Mol. Structure. 1993;292:105–114. [Google Scholar]
- 69.McCurley M.F., Seitz W.R. Swelling of a polymer membrane for use in a glucose biosensor. (ACS Symposium Series).1992;487:301–309. [Google Scholar]
- 70.Cong J., Zhang X., Chen K., Xu J. Fiber optic Bragg grating sensor based on hydrogels for measuring salinity. Sens. Actuat. B. 2002;87:487–490. [Google Scholar]
- 71.Liu X., Zhang X., Cong J., Xu J., Chen K. Demonstration of etched cladding fiber Bragg grating-based sensors with hydrogel coating. Sens. Actuat. B. 2003;96:468–472. [Google Scholar]
- 72.Carrascosa L.G., Moreno M., Àlvarez M., Lechuga L.M. Nanomechanical biosensors: a new sensing tool. Trends in Analytical Chemistry. 2006;25(3):196–206. [Google Scholar]
- 73.Bashir R., Hilt J.Z., Elibol O., Gupta A.M., Peppas N.A. Micromechanical cantilever as an ultrasensitive pH microsensor. Appl. Phys. Lett. 2002;81:3091–3093. [Google Scholar]
- 74.Hilt J.Z, Gupta A.M., Bashir R., Peppas N.A. Ultrasensitive biomems sensors based on microcantilevers patterned with environmentally responsive hydrogels. Biomed. Microdev. 2003;5(3):177–184. [Google Scholar]
- 75.Zhang Y., Ji H.F., Snow D., Sterling R., Brown G.M. A pH sensor based on a microcantilever coated with intelligent hydrogel. Instrumentation Sci. Technol. 2004;32(4):361–369. [Google Scholar]
- 76.Han I.S., Han M.H., Kim J., Lew S., Lee Y.J., Horkay F., Magda J.J. Constant-volume hydrogel osmometer: a new device concept for miniature biosensors. Biomacromol. 2002;3:1271–1275. doi: 10.1021/bm0255894. [DOI] [PubMed] [Google Scholar]
- 77.Gerlach G., Günther M., Suchaneck G., Sorber J., Arndt K.F., Richter A. Application of sensitive hydrogels in chemical and pH sensors. Macromol. Symp. 2004;210:403–410. [Google Scholar]
- 78.Gerlach G., Guenther M., Sorber J., Suchaneck G., Arndt K.-F., Richter A. Chemical and pH sensors based on the swelling behavior of hydrogels. Sens. Actuat. B. 2005;111-112:555–561. [Google Scholar]
- 79.Trinh Q.T., Gerlach G., Sorber J., Arndt K.-F. Hydrogel-based piezoresistive pH sensors: design, simulation and output characteristics. Sens. Actuat. B. 2006;117:17–26. [Google Scholar]
- 80.Herber S., Olthuis W., Bergveld P. A swelling hydrogel-based PCO2 sensor. Sens. Actuat. B. 2003;91:378–382. [Google Scholar]
- 81.Herber S., Eijkel J., Olthuis W., Bergveld P., van den Berg A. Study of chemically induced pressure generation of hydrogels under isochoric conditions using a microfabricated device. J Chem. Phys. 2004;121(6):2746–2751. doi: 10.1063/1.1773153. [DOI] [PubMed] [Google Scholar]
- 82.Strong Z.A., Wang A.W., Conaghy C.F. Hydrogel-actuated capacitive transducer for wireless biosensors. Biomed. Microdev. 2002;4(2):97–103. [Google Scholar]
- 83.Arndt K.F., Richter A., Ludwig S., Zimmermann J., Kressler J., Kuckling D., Adler H.J. Poly(vinyl alcohol)/(poly(acrylic acid) hydrogels: FT-IR spectroscopic characterization of crosslinking reaction and work at transition point. Acta Polym. 1999;50:383–390. [Google Scholar]
- 84.Kretschmer K., Kuckling D., Richter A. SPR investigations of pH dependent swelling of thin poly(vinyl alcohol)/poly(acrylic acid) layers. unpublished. [Google Scholar]
- 85.Suzuki A., Suzuki H. Hysteretic behavior and irreversibility of polymer gels by pH change. J. Chem. Phys. 1995;103(11):4706–4710. [Google Scholar]
- 86.Harmon M.E., Kuckling D., Frank C.W. Photo-cross-linkable PNIPAAm copolymers. 2. Effects of constraint on temperature and pH-responsive hydrogel layers. Macromolecules. 2003;36:162–172. [Google Scholar]
- 87.Harmon M.E., Kuckling D., Pareek P., Frank C.W. Photo-cross-linkable PNIPAAm copolymers. 4. Effects of copolymerization and cross-linking on the volume-phase transition in constrained hydrogel layers. Langmuir. 2003;19:10947–10956. [Google Scholar]
- 88.Richter A., Howitz S., Kuckling D., Arndt K.F. Influence of phenomena of volume phase transition at the behavior of hydrogel based valves. Sens. Actuat. B. 2004;99(2-3):451–458. [Google Scholar]
- 89.Kuckling D., Hoffmann J., Plötner M., Ferse D., Kretschmer K., Adler H.J.P., Arndt K.F., Reichelt R. Photo cross-linkable poly(N-isopropylacrylamide) copolymers III: micro-fabricated temperature responsive hydrogels. Polymer. 2003;44(16):4455–4462. [Google Scholar]
- 90.Bhattacharya S., Eckert F., Boyko V., Pich A. Temperature-, pH-, and magnetic-field-sensitive hybrid microgels. Small. 2007;4:650–657. doi: 10.1002/smll.200600590. [DOI] [PubMed] [Google Scholar]