Abstract
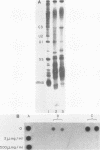
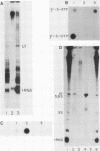
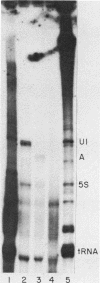
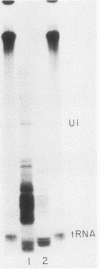
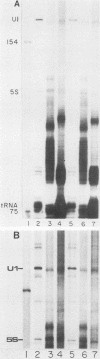
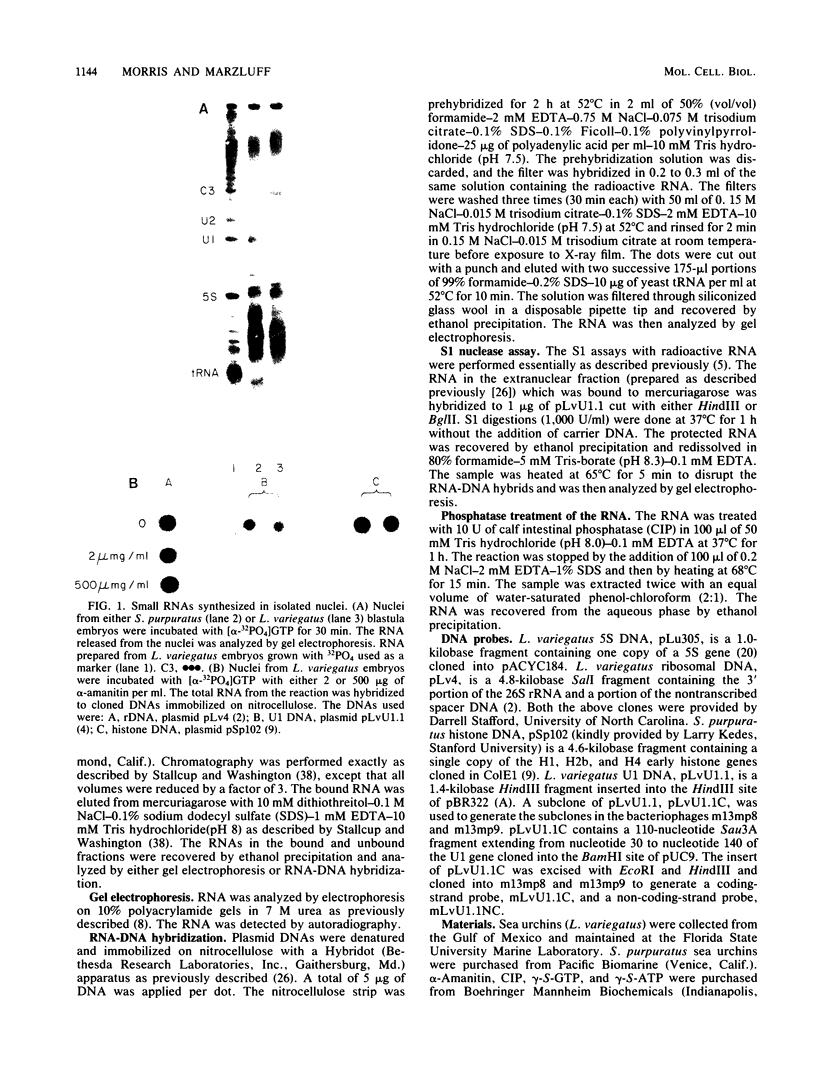
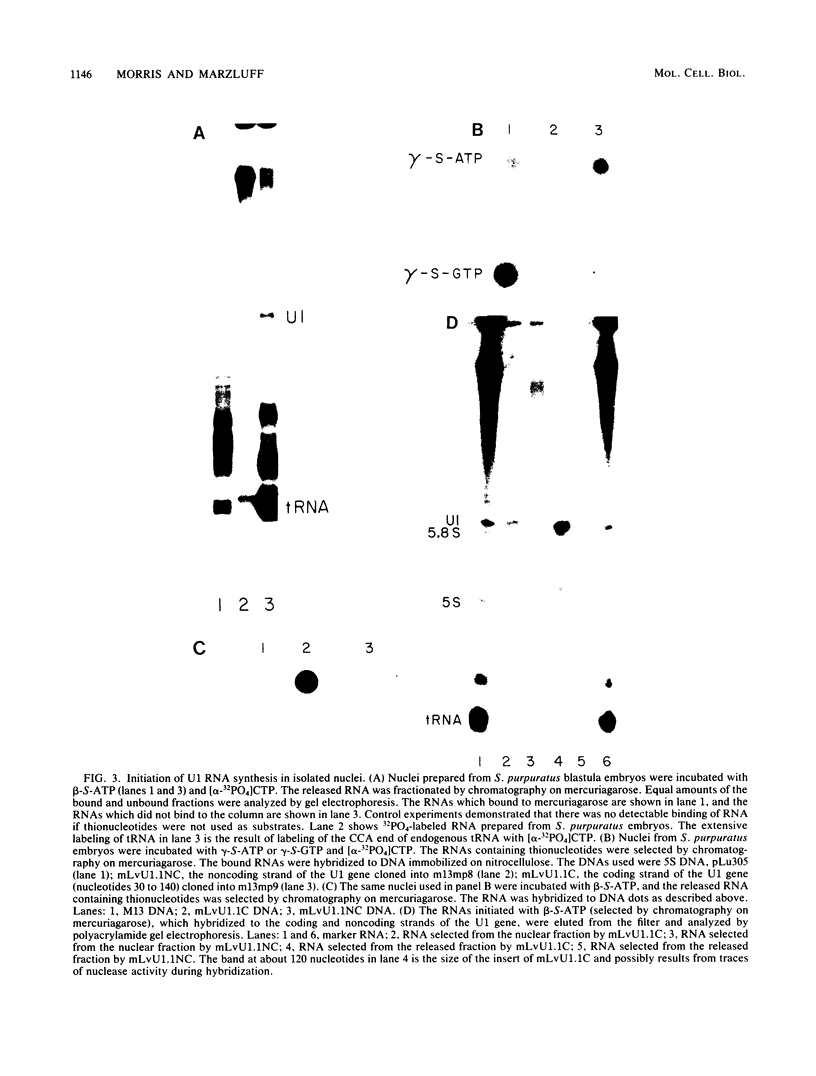
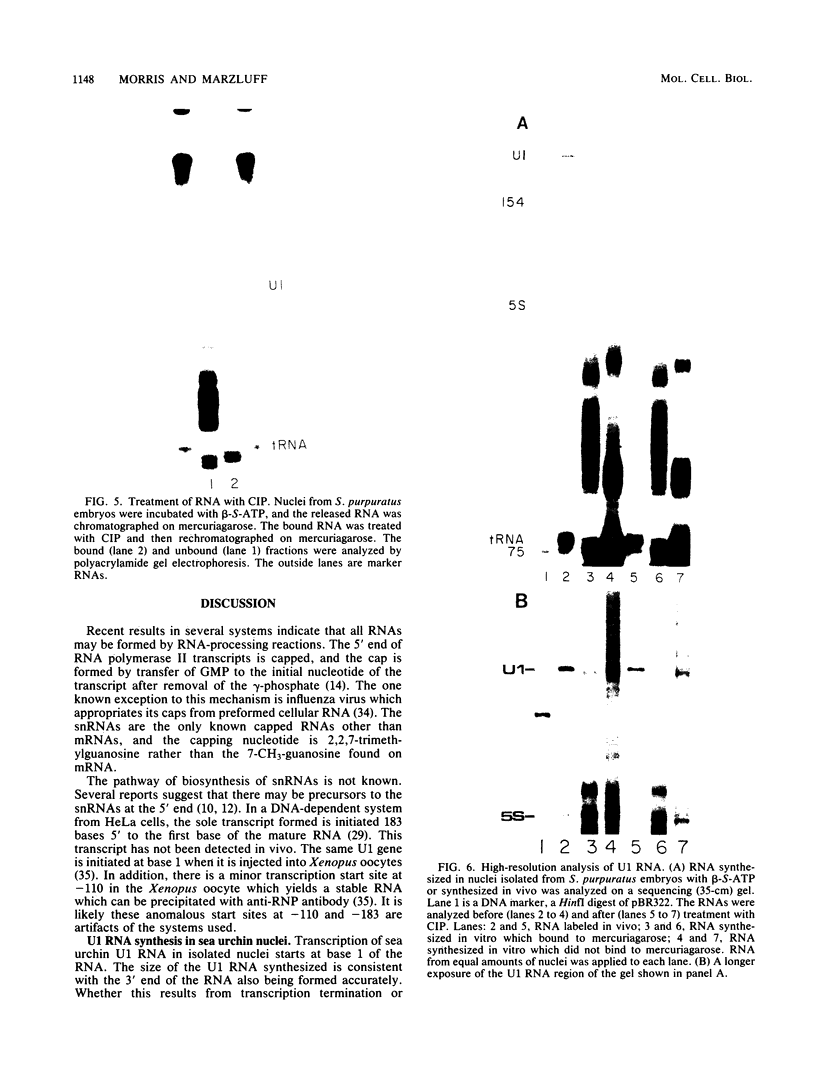
Nuclei from sea urchin blastula embryos synthesize a variety of small RNAs, one of which has identical mobility with sea urchin U1 RNA. This RNA is synthesized by RNA polymerase II and, in a hybridization-selection experiment, was selected by the cloned sea urchin U1 gene. The U1 RNA was initiated with ATP, but not GTP, in isolated nuclei with beta-S- and gamma-S-ribonucleotide triphosphates as substrates. The U1 RNA containing thiophosphate at the 5' end was not capped but accumulated as an uncapped transcript from which the thiophosphate could be removed with calf intestinal phosphatase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birchmeier C., Folk W., Birnstiel M. L. The terminal RNA stem-loop structure and 80 bp of spacer DNA are required for the formation of 3' termini of sea urchin H2A mRNA. Cell. 1983 Dec;35(2 Pt 1):433–440. doi: 10.1016/0092-8674(83)90176-9. [DOI] [PubMed] [Google Scholar]
- Blin N., Sperrazza J. M., Wilson F. E., Bieber D. G., Mickel F. S., Stafford D. W. Organization of the ribosomal RNA gene cluster in Lytechinus variegatus. Restriction analysis and cloning of restriction fragments. J Biol Chem. 1979 Apr 25;254(8):2716–2721. [PubMed] [Google Scholar]
- Branlant C., Krol A., Ebel J. P., Lazar E., Gallinaro H., Jacob M., Sri-Widada J., Jeanteur P. Nucleotide sequences of nuclear U1A RNAs from chicken, rat and man. Nucleic Acids Res. 1980 Sep 25;8(18):4143–4154. doi: 10.1093/nar/8.18.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. T., Morris G. F., Chodchoy N., Sprecher C., Marzluff W. F. Structure of the sea urchin U1 RNA repeat. Nucleic Acids Res. 1985 Jan 25;13(2):537–556. doi: 10.1093/nar/13.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunick D., Zandomeni R., Ackerman S., Weinmann R. Mechanism of RNA polymerase II--specific initiation of transcription in vitro: ATP requirement and uncapped runoff transcripts. Cell. 1982 Jul;29(3):877–886. doi: 10.1016/0092-8674(82)90449-4. [DOI] [PubMed] [Google Scholar]
- Busch H., Reddy R., Rothblum L., Choi Y. C. SnRNAs, SnRNPs, and RNA processing. Annu Rev Biochem. 1982;51:617–654. doi: 10.1146/annurev.bi.51.070182.003153. [DOI] [PubMed] [Google Scholar]
- Card C. O., Morris G. F., Brown D. T., Marzluff W. F. Sea urchin small nuclear RNA genes are organized in distinct tandemly repeating units. Nucleic Acids Res. 1982 Dec 11;10(23):7677–7688. doi: 10.1093/nar/10.23.7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn R. H., Lowry J. C., Kedes L. H. Histone genes of the sea urchin (S. purpuratus) cloned in E coli: order, polarity, and strandedness of the five histone-coding and spacer regions. Cell. 1976 Sep;9(1):147–161. doi: 10.1016/0092-8674(76)90060-x. [DOI] [PubMed] [Google Scholar]
- Eliceiri G. L., Sayavedra M. S. Small RNAs in the nucleus and cytoplasm of HeLa cells. Biochem Biophys Res Commun. 1976 Sep 20;72(2):507–512. doi: 10.1016/s0006-291x(76)80070-8. [DOI] [PubMed] [Google Scholar]
- Eliceiri G. L. Sensitivity of low molecular weight RNA synthesis to UV radiation. Nature. 1979 May 3;279(5708):80–81. doi: 10.1038/279080a0. [DOI] [PubMed] [Google Scholar]
- Eliceiri G. L., Smith J. H. Sensitivity to UV radiation of small nuclear RNA synthesis in mammalian cells. Mol Cell Biol. 1983 Dec;3(12):2151–2155. doi: 10.1128/mcb.3.12.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney T., Jr, Eliceiri G. L. Intracellular distribution of low molecular weight RNA species in HeLa cells. J Cell Biol. 1980 Nov;87(2 Pt 1):398–403. doi: 10.1083/jcb.87.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbüchle O., Schibler U. Mouse beta-globin and adenovirus-2 major late transcripts are initiated at the cap site in vitro. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2283–2286. doi: 10.1073/pnas.78.4.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschel C. C., Birnstiel M. L. The organization and expression of histone gene families. Cell. 1981 Aug;25(2):301–313. doi: 10.1016/0092-8674(81)90048-9. [DOI] [PubMed] [Google Scholar]
- Hofer E., Darnell J. E., Jr The primary transcription unit of the mouse beta-major globin gene. Cell. 1981 Feb;23(2):585–593. doi: 10.1016/0092-8674(81)90154-9. [DOI] [PubMed] [Google Scholar]
- Krämer A., Keller W., Appel B., Lührmann R. The 5' terminus of the RNA moiety of U1 small nuclear ribonucleoprotein particles is required for the splicing of messenger RNA precursors. Cell. 1984 Aug;38(1):299–307. doi: 10.1016/0092-8674(84)90551-8. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A. L., Blin N., Stafford D. W. Cloning and organization of genes for 5S ribosomal RNA in the sea urchin. Lytechinus variegatus. Gene. 1981 Jun-Jul;14(1-2):51–62. doi: 10.1016/0378-1119(81)90147-5. [DOI] [PubMed] [Google Scholar]
- Madore S. J., Wieben E. D., Pederson T. Intracellular site of U1 small nuclear RNA processing and ribonucleoprotein assembly. J Cell Biol. 1984 Jan;98(1):188–192. doi: 10.1083/jcb.98.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser T., Gesteland R. F. Human U1 loci: genes for human U1 RNA have dramatically similar genomic environments. Cell. 1982 May;29(1):257–264. doi: 10.1016/0092-8674(82)90110-6. [DOI] [PubMed] [Google Scholar]
- Marzluff W. F., Brown D. T., Lobo S., Wang S. S. Isolation and characterization of two linked mouse U1b small nuclear RNA genes. Nucleic Acids Res. 1983 Sep 24;11(18):6255–6270. doi: 10.1093/nar/11.18.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzluff W. F., Jr, Murphy E. C., Jr, Huang R. C. Transcription of the genes for 5S ribosomal RNA and transfer RNA in isolated mouse myeloma cell nuclei. Biochemistry. 1974 Aug 27;13(18):3689–3696. doi: 10.1021/bi00715a011. [DOI] [PubMed] [Google Scholar]
- Moore C. L., Sharp P. A. Site-specific polyadenylation in a cell-free reaction. Cell. 1984 Mar;36(3):581–591. doi: 10.1016/0092-8674(84)90337-4. [DOI] [PubMed] [Google Scholar]
- Morris G. F., Marzluff W. F. A factor in sea urchin eggs inhibits transcription in isolated nuclei by sea urchin RNA polymerase III. Biochemistry. 1983 Feb 1;22(3):645–653. doi: 10.1021/bi00272a019. [DOI] [PubMed] [Google Scholar]
- Mount S. M., Pettersson I., Hinterberger M., Karmas A., Steitz J. A. The U1 small nuclear RNA-protein complex selectively binds a 5' splice site in vitro. Cell. 1983 Jun;33(2):509–518. doi: 10.1016/0092-8674(83)90432-4. [DOI] [PubMed] [Google Scholar]
- Mount S. M., Steitz J. A. Sequence of U1 RNA from Drosophila melanogaster: implications for U1 secondary structure and possible involvement in splicing. Nucleic Acids Res. 1981 Dec 11;9(23):6351–6368. doi: 10.1093/nar/9.23.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. T., Burgess R. R., Dahlberg J. E., Lund E. Transcription of a gene for human U1 small nuclear RNA. Cell. 1982 May;29(1):265–274. doi: 10.1016/0092-8674(82)90111-8. [DOI] [PubMed] [Google Scholar]
- Nijhawan P., Marzluff W. F. Metabolism of low molecular weight ribonucleic acids in early sea urchin embryos. Biochemistry. 1979 Apr 3;18(7):1353–1360. doi: 10.1021/bi00574a035. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Mount S. M., Steitz J. A., Sharp P. A. Splicing of messenger RNA precursors is inhibited by antisera to small nuclear ribonucleoprotein. Cell. 1983 Nov;35(1):101–107. doi: 10.1016/0092-8674(83)90212-x. [DOI] [PubMed] [Google Scholar]
- Price D. H., Parker C. S. The 3' end of drosophila histone H3 mRNA is produced by a processing activity in vitro. Cell. 1984 Sep;38(2):423–429. doi: 10.1016/0092-8674(84)90497-5. [DOI] [PubMed] [Google Scholar]
- Reeve A. E., Shatkin A. J., Huang R. C. Guanosine 5'-O-(3-thiotriphosphate) inhibits capping of reovirus mRNA. J Biol Chem. 1982 Jun 25;257(12):7018–7022. [PubMed] [Google Scholar]
- Robertson H. D., Dickson E., Plotch S. J., Krug R. M. Identification of the RNA region transferred from a representative primer, beta-globin mRNA, to influenza mRNA during in vitro transcription. Nucleic Acids Res. 1980 Mar 11;8(5):925–942. doi: 10.1093/nar/8.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuzeski J. M., Lund E., Murphy J. T., Steinberg T. H., Burgess R. R., Dahlberg J. E. Synthesis of human U1 RNA. II. Identification of two regions of the promoter essential for transcription initiation at position +1. J Biol Chem. 1984 Jul 10;259(13):8345–8352. [PubMed] [Google Scholar]
- Smith M. M., Reeve A. E., Huang R. C. Analysis of RNA initiated in isolated mouse myeloma nuclei using purine nucleoside 5'[gamma-S]triphosphates as affinity probes. Cell. 1978 Oct;15(2):615–626. doi: 10.1016/0092-8674(78)90030-2. [DOI] [PubMed] [Google Scholar]
- Smith M. M., Reeve A. E., Huang R. C. Transcription of bacteriophage lambda DNA in vitro using purine nucleoside 5'-[gamma-S]triphosphates as affinity probes for RNA chain initiation. Biochemistry. 1978 Feb 7;17(3):493–500. doi: 10.1021/bi00596a019. [DOI] [PubMed] [Google Scholar]
- Stallcup M. R., Washington L. D. Region-specific initiation of mouse mammary tumor virus RNA synthesis by endogenous RNA polymerase II in preparations of cell nuclei. J Biol Chem. 1983 Mar 10;258(5):2802–2807. [PubMed] [Google Scholar]
- Washington L. D., Stallcup M. R. A comparison of nucleoside (beta-S)triphosphates and nucleoside (gamma-S)triphosphates as suitable substrates for measuring transcription initiation in preparations of cell nuclei. Nucleic Acids Res. 1982 Dec 20;10(24):8311–8322. doi: 10.1093/nar/10.24.8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe-Nagasu N., Itoh Y., Tani T., Okano K., Koga N., Okada N., Ohshima Y. Structural analysis of gene loci for rat U1 small nuclear RNA. Nucleic Acids Res. 1983 Mar 25;11(6):1791–1801. doi: 10.1093/nar/11.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]