Abstract
Background
Infantile hemangiomas (IHs) can cause significant morbidity during proliferation, yet there is no FDA-approved treatment. IHs are believed to form from stem cells (HemSCs), which differentiate towards an endothelial cell (HemECs) phenotype. Recently, propranolol has demonstrated effectiveness in the treatment of complicated IHs. We hypothesize that propranolol facilitates IH involution by altering cellular behavior in both HemECs and HemSCs.
Methods
HemECs and HemSCs were isolated from resected IH specimens. Cells were treated with 100μM propranolol for 48 hours, and apoptosis determined by presence of Annexin V antibody. Proliferation of HemSCs and HemECs were assessed after treatment with 50μM or 100μM propranolol, or vehicle for 72 and 96 hours respectively. Adipogenesis was induced in HemSCs with and without propranolol. Pro-adipogenic genes PPARδ, PPARγ, C/EBPα, C/EBPβ, C/EBPδ, RXRα and RXRγ were analyzed by quantitative PCR (qPCR).
Results
Annexin V levels were increased in propranolol-treated HemECs, but not in HemSCs. Proliferation of HemSCs and HemECs was inhibited by propranolol in a dose-dependent manner. Propranolol-treated HemSCs demonstrated accelerated adipogenesis when compared to untreated controls. Transcript levels of adipogenesis-associated genes C/EBPβ (p<0.05), RXRγ (p<0.05), and PPARγ (p<0.02) were significantly increased when treated with 50μM or 100μM propranolol, and C/EBPδ (p<0.05), RXRα (p<0.05), PPARδ (p<0.01) transcripts were increased when treated with 100μM propranolol. C/EBPα transcript levels remained unchanged at either dose.
Conclusions
These results show that propranolol increased apoptosis of HemECs but not HemSCs, and accelerated adipogenesis of HemSC. Thus, propranolol likely accelerates involution to fibrofatty residuum.
Introduction
Infantile hemangiomas (IHs) are benign tumors of infancy affecting approximately 5% of infants. The natural history of an IH can be divided into three phases: rapid proliferation shortly after birth, followed by stabilization for months to years, and finally involution throughout childhood. A fibrofatty residuum can result after involution is complete (1). During the proliferative phase, IH can cause serious morbidity and even mortality depending on its location. Complications include bleeding, obstructive amblyopia and astigmatism, respiratory distress, and congestive heart failure (2-9). Despite the prevalence and predictable natural history of IHs, their origin remains unknown and regulatory mechanisms that act at different phases are only recently being elucidated.
Currently, the leading candidate for the cellular origin of IHs is the hemangioma stem cell (HemSC) (10). HemSCs have been demonstrated to be highly proliferative in culture and capable of differentiating into multiple cell types including endothelial cells, adipocytes, chondrocytes, osteocytes, and neuroglial cells. Furthermore, when resuspended in Matrigel and implanted subcutaneously, HemSCs can form functional, GLUT1+ endothelial cell-lined blood vessels. After four weeks, Matrigel plugs also contain HemSC-derived adipocytes consistent with the natural history of IHs in an accelerated time frame.
Propranolol, a sympatholytic non-selective beta-blocker used to treat hypertension and anxiety, was recently discovered to be an effective drug for the treatment of IH, causing rapid involution (11). The molecular mechanisms behind this effect are unknown. In vitro studies using human umbilical vein endothelial cells (HUVECs) and human brain microvascular endothelial cells (HBMECs) have demonstrated that propranolol has anti-angiogenic properties. Propranolol treatment of HUVECs inhibited proliferation, chemotactic migration, and tube formation. Propranolol also inhibited VEGF-A-induced tyrosine phosphorylation of VEGFR-2, leading to reduced phosphorylation of the extracellular signal-related kinases-1/2 and secretion of pro-matrix metalloproteinase-2 and matrix metalloproteinase-9 (12, 13). However, the effects of propranolol on HemECs and HemSCs isolated from IHs remains unknown, and therefore, the mechanism behind propranolol's effect on IHs remains to be elucidated.
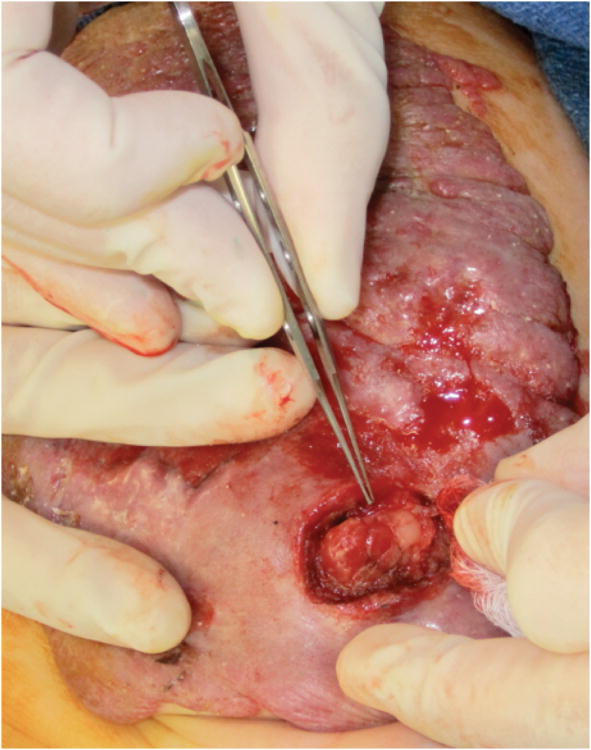
Recently, in our clinic, a 6-month-old child developed persistent ulceration and bleeding of a large abdominal wall IH despite several months of propranolol therapy. The patient was taken to the operating room for surgical debulking of the ulcerated and bleeding areas. Intra-operative findings showed that the superficial layers had the typical red, raised, bosselated appearance, while the deeper tissue of the IH consisted mainly of fatty tissue, similar to that observed in an involuting IH (Figure 1).
Figure 1.
Ulcerated abdominal segmental hemangioma on a 6-month-old female patient. Note the typical red, raised, bosselated appearance of the superficial portion of the infantile hemangioma with ulcerating and bleeding areas, whereas the deeper areas, at the end of the forceps, consisted of fibrofatty tissue typically seen in an involuting or involuted IH.
Based on this clinical observation and published studies, we hypothesized that propranolol possesses the ability to promote adipogenic differentiation (involution) of HemSCs in addition to its known anti-angiogenic properties.
Methods
Preparation of hemangioma specimens
IRB approval for collection of resected human hemangiomas was obtained from Columbia University College of Physicians & Surgeons (IRB #AAAA9976). Tissues were used immediately for cell isolation for in vitro experiments.
Cell Isolation
HemECs and HemSCs were isolated from clinically resected specimens as previously described (10). Briefly, the hemangioma samples were minced into small pieces with a scalpel, digested using collagenase (Roche), and filtered through a 100-micron cell strainer. From this single cell suspension, cells expressing CD133 were selected using a magnetic microbead cell sorting system (Miltenyi Biotec), and plated on fibronectin-coated plates in EGM-2 media (Lonza) supplemented with 20% fetal bovine serum (Invitrogen), penicillin, and streptomycin (Invitrogen), which will be referred to as EGM-2/FBS-20%. CD133 negative cells were plated in the same manner. These cells were later sorted for endothelial cells using CD31 antibody-coated magnetic Dynal beads (Invitrogen). RNA was later isolated from both cell populations (CD133+, or HemSC, and CD31+, or HemEC) as described below.
Mesenchymal stem cells (MSCs) were purchased at passage 2 from Lonza. Human umbilical venous endothelial cells (HUVECs) and human dermal microvascular endothelial cells (HdMECs) were generously provided from the Kitajewski laboratory.
Cell culture and treatment with propranolol
HemECs and HemSCs were maintained on fibronectin-coated 10cm plates in EGM-2/FBS-20%. Media was changed every other day, and cells were passaged when 90% confluent. Cells at passage number 10-20 were used for all experiments. For experimentation, fresh propranolol (Sigma) was added to culture media during media changes at the indicated concentrations.
RNA isolation and RT-PCR
Total RNA isolation was performed with the RNeasy Mini Prep Kit (Qiagen). Quantity and quality of RNA was assessed by spectrophotometry (Fisher). cDNA was synthesized by reverse transcription of 2μg of total RNA, performed using the Superscript II Reverse Transcriptase kit (Invitrogen).
Primers
Primers (Eurofins) used were for β1 adrenergic receptor (ADRB1), β2 adrenergic receptor (ADRB2), peroxisome proliferator-activated receptor delta (PPARδ), peroxisome proliferator-activated receptor gamma (PPARγ), CCAAT/enhancer binding protein α (C/EBPα), CCAAT/enhancer binding protein β (C/EBPβ), CCAAT/enhancer binding protein δ (C/EBPδ), retinoid X receptor α (RXRα), and retinoid × receptor γ (RXRγ). ADRB1, forward: 5′ TCT TTT GTG TGT GCG TGT GA 3′, reverse: 5′ ATG CTT CTC CCT TCC CCT AA 3′. ADRB2, forward: 5′ GAT TTC AGG ATT GCC TTC CA 3′, reverse 5′ CTT GAT GGC CCA CAA AGT CT 3′. PPARδ, forward: 5′ CTT GCC CTC CCT TTC TCT CT 3′, reverse 5′ AGG TGT GCA AAA GCA GAG GT 3′. PPARγ, forward: 5′ GCT GGC CTC CTT GAT GAA TA 3′, reverse: 5′ TTG GGC TCC ATA AAG TCA CC 3′. CEBPα, forward: 5′ TGG ACA AGA ACA GCA ACG AG 3′, REVERSE 5′ TTG TCA CTG GTC AGC TCC AG 3′. CEBPβ, forward: 5′ TTT CGA AGT TGA TGC AAT CG 3′, reverse: 5′ CAA CAA GCC CGT AGG AAC AT 3′. CEBPδ, forward: 5′ ACT CAG CAA CGA CCC ATA CC 3′, reverse: 5′ CGC TCC TAT GTC CCA AGA AA 3′. RXRα, forward: 5′ TGG ACG CCT TCT CCA TAG TC 3′, reverse: 5′ GAC ACT TTC TTC CCC ACC AA 3′. RXRγ, forward: 5′ TGT GGT CAA CAG TGT CAG CA 3′, reverse: 5′ GTC TCC ACA GAT GGC ACA GA 3′.
Pcr
PCR of cDNA was performed using the Eppendorf Mastercycler gradient (Eppendorf) and the Platinum Taq DNA Polymerase kit (Invitrogen). PCR reaction was undertaken at a volume of 20μl with an initial heating step of 94°C for 2 minutes, followed by heating at 94°C for 45 sec, annealing at 60°C for 1 min, and extension at 72°C for 1 min for a total of 35 cycles. PCR product was run on a 1% agarose gel (BioRad).
Quantitative real-time PCR
Real time qPCR was performed in triplicate utilizing the CFX Real-Time PCR Detection System (Biorad) and ABsolute Blue QPCR SYBR Green Mix (Thermo Fisher Scientific). PCR reaction was undertaken with initial heat activation at 50°C for 2 minutes followed by 95°C for 2 minutes. Amplification was conducted by heating at 95°C at 15 seconds, followed by an annealing step at 60°C (40 seconds) and extension at 72°C (30 seconds) for 50 cycles. The results were normalized to β-actin expression levels and the triplicate results averaged for each sample, as described previously(14).
Propranolol
Propranolol hydrochloride was purchased from Sigma-Aldrich (Cat# P0084) and reconstituted at 50mg/ml in pH 3.0 H2O (hereafter referred to as “vehicle”).
Proliferation assay
1.0 × 104 HemECs and HemSCs were seeded on fibronectin-coated (Sigma-Aldrich F1141) 24-well plates (BD Falcon) in EGM-2/FBS-20%. After 6 hours, media was changed and cells were treated with 50μM propranolol, 100μM propranolol or the corresponding volume of vehicle. Media was changed every other day; fresh propranolol or vehicle was added during media changes. HemSCs were counted after 3 days of culture, and HemECs were counted after 4 days. Cell numbers were assessed using the colorimetric Cell Counting Kit-8 (Dojindo) and the Versamax tunable microplate reader (Molecular Devices).
Proliferation assays for MSCs, HUVECs, and HdMECs were performed as described above.
Annexin V measurement by fluorescence-activated cell sorting
HemECs and HemSCs were grown to 75% confluence in EGM-2/FBS-20% media. Cells were then exposed to vehicle or 100μM propranolol for 48 hours. Levels of Annexin V expression by HemECs and HemSCs were measured using the Annexin V-FITC Apoptosis Kit (BioVision) according to the instructions of the manufacturer. The FACSCalibur flow cytometer (BD Biosciences) was used for antibody detection.
Adipogenic differentiation
3.0 × 104 HemSCs or MSCs were seeded on fibronectin-coated 8-well Nunc Lab-Tek II chamber slides (Thermo Scientific) in EGM-2/FBS-20% media overnight to ensure confluence. Media was removed, cells washed once with phosphate buffered saline, and cells cultured in adipogenic media (DMEM low glucose [Invitrogen], 10% FBS, 1% penicillin/streptomycin [Invitrogen], 5μg/ml insulin [Sigma], 1μM dexamethasone [Sigma], 0.5mM isobutylmethylxanthine [Sigma], 60μM indomethacin [Sigma]) for 4 days in the presence of either 50μM or 100μM propranolol, or vehicle. Adipogenic differentiation was assessed by oil red O staining for lipid droplets after 4 days of treatment in adipogenic media. Photos were taken using the Eclipse E800 microscope (Nikon).
In addition, 106 HemSC cells were seeded in fibronectin-coated 10cm plates in EGM-2/FBS-20%, and either 50μM or 100μM of propranolol, or vehicle, was added. RNA was collected after 24 hours of treatment, and gene transcripts were assessed by qPCR.
Results
HemECs and HemSCs express β-adrenergic receptors
As propranolol exerts at least some of its effects via the β-adrenergic receptors, we determined their expression in HemECs and HemSCs isolated from IHs. Both IH cell types expressed β1 and β2 adrenergic receptor transcripts, with β2 adrenergic receptor expression easily detected (Figure 2). These results suggest that propranolol exerts its effects via inhibition of β adrenergic receptors.
Figure 2.
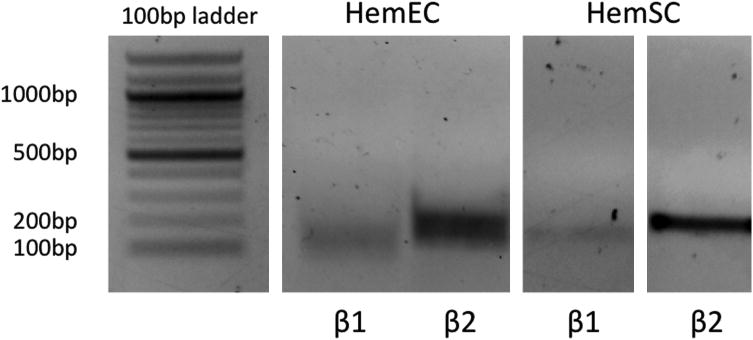
ADRB1 (β1) and ADRB2 (β2) genes are expressed in HemECs and HemSCs by PCR. In both cell types, ADRB2 expression is greater than ADRB1 expression. PCR was done for 35 cycles and products run on a 1% agarose gel.
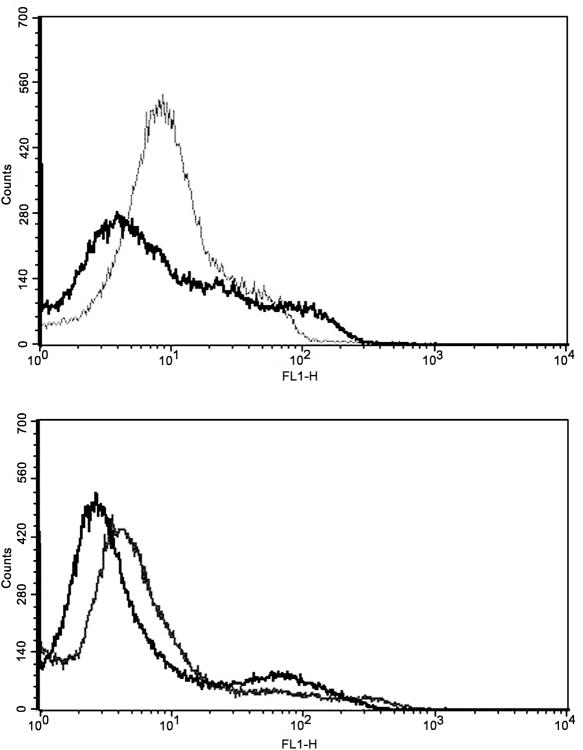
Propranolol induced apoptosis in HemECs, but not HemSCs
To determine whether apoptosis in HemECs and HemSCs is induced by propranolol, HemECs and HemSCs were treated with 100μM propranolol or vehicle for 48 hours and analyzed by FACS for levels of Annexin V, a cell surface marker of early apoptosis. Levels of Annexin V were increased in HemECs treated with propranolol, indicating HemECs were undergoing apoptosis (Figure 3, top panel). Annexin V levels were unchanged between groups in HemSCs (Figure 3, bottom panel). Thus, in vitro propranolol treatment of HemECs, but not HemSCs, promoted apoptosis.
Figure 3.
FACS analysis of HemECs and HemSCs treated with 100μM propranolol for 48 hours. (Top panel) Propranolol treatment of HemECs increased Annexin V levels on the cell surface. (Bottom panel) Annexin V levels were not altered in HemSCs, suggesting a lack of apoptotic response in HemSCs. Black line indicates control; gray line indicates propranolol treatment.
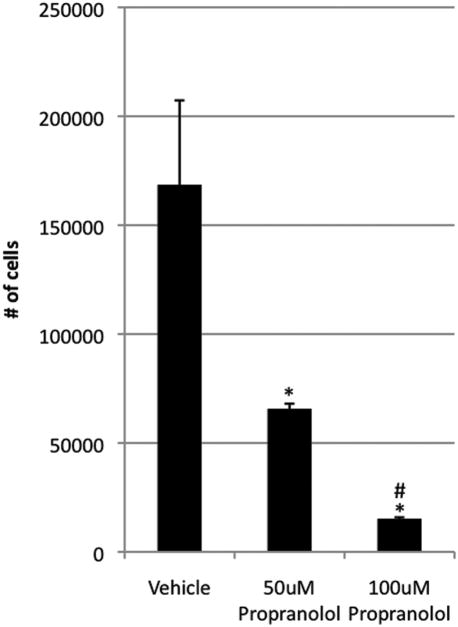
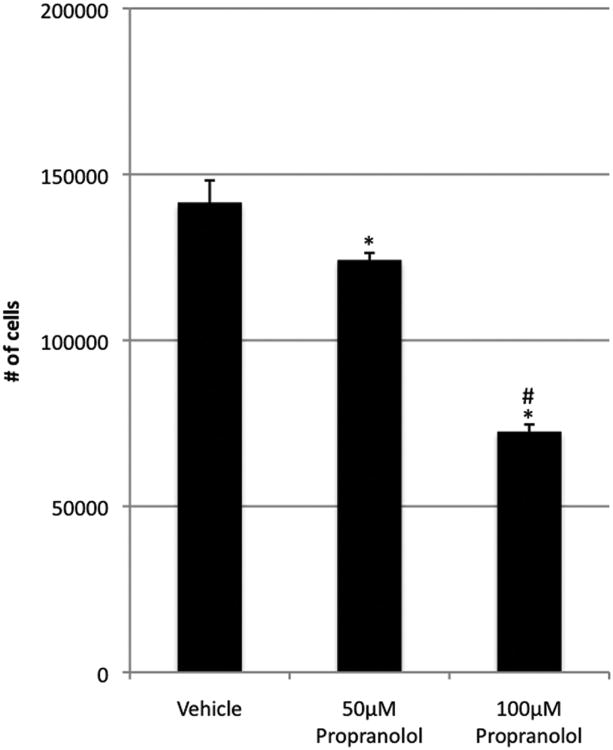
Propranolol inhibited proliferation of HemECs and HemSCs
HemECs and HemSCs were treated with 50μM and 100μM propranolol and proliferation determined at 72 and 96 hours. At 96 hours, HemEC proliferation significantly decreased in a dose-dependent manner when compared with controls (p<0.05) (Figure 4). Of note, there were 1.5 × 104 cells in the 100μM group, showing minimal increase in cell number from the initial 104 that were seeded. At 72 hours, HemSCs also underwent a significant dose-dependent decrease in proliferation when compared with controls (p<0.03 at 50μM and p<0.002 at 100μM) (Figure 5). Unlike HemECs, HemSCs proliferated—albeit poorly—even at 100μM of propranolol, as all groups showed at least 5-fold increase in cell count compared to the initial number of cells seeded. Taken together, these data demonstrate that propranolol exerts an anti-proliferative effect on both HemECs and HemSCs, in addition to its pro-apoptotic effect HemECs.
Figure 4.
Effect of varying concentrations of propranolol on 96 hours of HemEC proliferation in EGM-2/20%-FBS. Propranolol significantly inhibits proliferation of HemECs in a dose-dependent manner. Data are representative of three independent experiments. *p < 0.05 relative to control. **p < 0.05 relative to 50μM propranolol.
Figure 5.
Effect of varying concentrations of propranolol on 72 hours of HemSC proliferation in EGM-2/20%-FBS. Propranolol significantly inhibits proliferation of HemSCs in a dose-dependent manner. Data are representative of three independent experiments. *p < 0.05 relative to control. **p < 0.05 relative to 50μM propranolol.
Control endothelial cells (HUVECs and HdMECs) showed significantly decreased cellular proliferation at 100μM of propranolol, which is consistent with previously reported data (data not shown) (12, 13). MSCs showed significantly decreased proliferation at 100μM of propranolol (p=0.002). At 50μM of propranolol, there was a trend towards decreased proliferation, although this was not statistically significant (p =0.054; supplementary Figure 1).
Propranolol enhanced adipogenesis in HemSCs
To determine the effect of propranolol on adipogenesis, HemSCs were cultured in adipogenic differentiation media in the presence of vehicle, 50μM or 100μM propranolol. HemSCs died in adipogenic medium by day 3 after 100μM propranolol treatment, and day 7 after 50μM propranolol treatment, whereas HemSCs treated with vehicle survived and continued to differentiate for 2 weeks (data not shown).
On day 4, HemSCs treated with 50μM propranolol were stained with Oil Red O to visualize lipid droplets within the cytoplasm. Propranolol-treated HemSCs had a more robust response to adipogenic induction when compared to vehicle-treated HemSCs, displaying an increase in number and density of red vacuoles within the cell cytoplasm (Figure 6). These results suggest that while propranolol treatment accelerated adipogenic differentiation, the differentiation process was dysregulated and led to increased cell death. This effect of accelerated adipogenesis at low dosage (50μM; supplementary Figure 2) and cell death at high dosage (100μM) was also seen with MSCs (data not shown).
Figure 6.
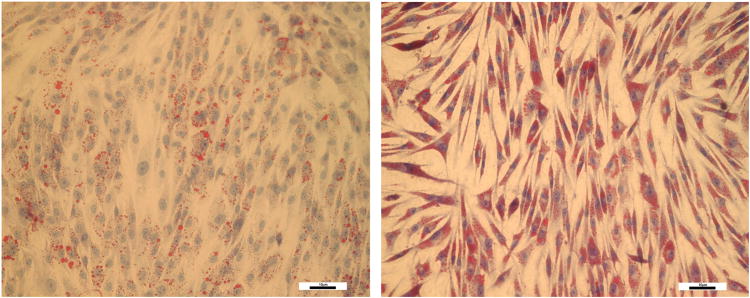
HemSCs cultured in adipogenic media in the presence of vehicle (left) and 50μM propranolol (right) for four days and stained for presence of lipid vacuoles by Oil Red O staining. Note minimal presence of intracytoplasmic lipid vacuoles in control cells. By contrast, there was increased presence of lipid vacuoles in HemSCs exposed to propranolol. (Magnification 20×). Scale bar represents 10μM.
Propranolol increased expression of adipogenesis-associated genes in HemSCs
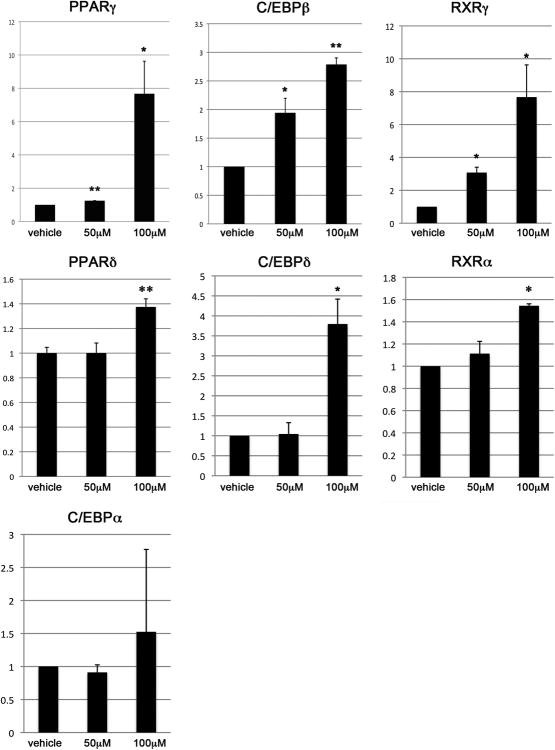
Transcripts of genes associated with adipogenesis were analyzed after HemSC grown in EGM-2/20%-FBS were treated with 50μM propranolol, 100μM propranolol or vehicle for 24 hours. We examined genes involved in adipogenesis and lipid uptake, including PPARδ and PPARγ, and transcription factors known to be upregulated during adipogenesis, including C/EBPα, C/EBPβ and C/EBPδ. Finally, the retinoid × receptors RXRα and RXRγ, were analyzed as they are nuclear receptors known to heterodimerize with PPAR proteins.
HemSCs treated with 100μM propranolol showed significantly higher transcript levels of the nuclear receptor PPARδ (1.4-fold, p<0.01) and PPARγ (7.7-fold, p<0.05). Expression of C/EBPβ and C/EBPδ transcripts were increased 2.8-fold (p<0.01), and 3.8-fold (p<0.05) respectively. Finally, the retinoid X receptors RXRα and RXRγ were upregulated 1.6-fold and 7.7-fold (p<0.05) respectively. HemSC treated with 50μM propranolol demonstrated increases in transcript levels of PPARγ (1.2-fold, p<0.01), C/EBPβ (1.9 fold, p<0.05), and RXRγ (3.1-fold, p<0.05) only. These increases were less than those seen in the 100μM propranolol group. In contrast to the 100μM propranolol group, transcript levels of PPARδ, C/EBPδ and RXRα did not differ from controls. Transcript levels of C/EBPα, a gene associated with terminal adipogenesis, was unchanged in both treatment groups. These results show that, even in the absence of adipogenic stimuli, propranolol treatment selectively induces the expression of adipogenic genes in HemSCs (Figure 7).
Figure 7.
Treatment with either 50μM or 100μM of propranolol resulted in significantly increased transcript levels of PPARγ, C/EBPβ, and RXRγ, (top panel). Treatment with 100μM, but not 50μM, of propranolol resulted in increased transcript levels of PPARδ, C/EBPδ, and RXRα (middle panel). There were no changes in transcript levels of C/EBPα with either 50μM or 100μM of propranolol (bottom panel). *p<0.05, **p<0.01.
Discussion
Propranolol has been extensively used to treat problematic IH since 2008. Like corticosteroids, its efficacy was discovered serendipitously. How propranolol exerts its effects is unknown. Several studies have demonstrated anti-angiogenic (12, 13) and apoptotic (15, 16) effects of propranolol on endothelial cells, but none of the studies used cells derived from IHs. While these studies have probed the effects of propranolol in endothelial cells, none have used IH-derived HemEC and HemSC cells. Additionally, while it has been speculated that β-adrenergic signaling may play a role in the differentiation of HemSCs, and that propranolol may induce preferential differentiation of HemSCs into adipocytes, there has been no scientific evidence in support of this thus far (17, 18). To our knowledge, this is the first study that attempts to delineate the effects of propranolol in both endothelial and stem cells derived from IHs.
We demonstrated that propranolol caused apoptosis in HemECs but did not have this effect on HemSCs. It is possible that HemSCs continue to proliferate—albeit at a slow rate—in IHs and can explain the phenomenon of rebound growth after propranolol therapy has been tapered.
In basal media, before induction of adipogenesis, treatment with propranolol caused significant increase in fat gene transcripts. When HemSCs were differentiated in induction media, treatment with 50μM propranolol initially accelerated adipogenesis. However, by day 7, no cells survived. In 100μM of propranolol, cell death occurred by day 3. These data suggest that propranolol primed HemSCs towards an adipogenic fate in basal medium. However, when induced, HemSCs treated with propranolol had accelerated adipogenesis followed by cell death. One possibility is that propranolol may also target a yet unknown specific adipocyte survival factor, in addition to its pro-adipogenic effects. Another possibility is that propranolol targets differentiated IH cells to cause apoptosis, as shown in pro-apoptotic effects of propranolol in HemECs and HemSCs in adipogenic media.
Given that propranolol is a widely-used, well-tolerated medication with no reported side effect of endothelial cell death in humans, it seems counterintuitive to think that propranolol could be directly causing apoptosis of endothelial cells in blood vessels of IHs, as our study and others have suggested. One explanation is that propranolol targets actively proliferating endothelial cells but not quiescent endothelium. Another explanation is that HemECs are distinct from other microvascular endothelial cells and may have a unique response to propranolol. Aside from brain and placental endothelium, only IH endothelium expresses Glut-1 (19). Another possibility is that the perivascular microenvironment of HemECs is different from that found in normal vasculature; perhaps the microenvironment of normal vasculature protects endothelial cells from the deleterious effects of propranolol through juxtacrine or paracrine signaling that is absent in the IH microenvironment.
While both HemSCs and HemECs expressed β-adrenergic receptor transcripts, it is unclear if the observed effects of propranolol in this study were via inhibition of β-adrenergic receptor signaling. Another unanswered question is whether this effect is specific to propranolol, or whether other beta antagonists are equally efficacious. Finally, studies of proliferation and adipogenesis were performed on mesenchymal stem cells (MSCs), which demonstrated behavior similar to HemSCs, suggesting that propranolol's effect may not be specific to HemSCs. Further studies with other stem cells and beta-blockers will help answer some of these questions. Nonetheless, it is important to understand the biological mechanisms of actions of medications that are used to treat IHs in order that the most effective medical therapy is given with the lowest potential adverse effect. Further study of the mechanism of propranolol's effects on IHs could yield more effective, targeted therapy in the future.
Supplementary Material
Supplemental Figure 1. Propranolol significantly inhibits proliferation of MSCs at 100μM. There was a trend towards decreased proliferation at 50μM, but this was not statistically significant. Data are representative of two independent experiments. *p < 0.050 relative to control. **p < 0.0005 relative to 50μM propranolol.
Supplemental Figure 2. MSCs cultured in adipogenic media in the presence of vehicle (left) and 50μM propranolol (right) for four days and stained for presence of lipid vacuoles by Oil Red O staining. Similar to results seen with HemSCs, there was increased presence of lipid vacuoles in the propranolol-treated MSCs. (Magnification 20×). Scale bar represents 10μM.
Acknowledgments
This study was supported by the National Institutes of Health Grants 5K08HL102068-02 (JKW), 1R01CA136673 (CJS), 1R01HL062454 (JKK).
Footnotes
This study has been presented at the Northeastern Society of Plastic Surgeons meeting, October 2011, Amelia Island, Florida
Financial disclosures: The authors have no financial disclosures.
Propranolol used in this study was ordered from Sigma Aldrich.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frieden IJ, Haggstrom AN, Drolet BA, et al. Infantile hemangiomas: current knowledge, future directions. Proceedings of a research workshop on infantile hemangiomas, April 7-9, 2005, Bethesda, Maryland, USA. Pediatr Dermatol. 2005;22:383–406. doi: 10.1111/j.1525-1470.2005.00102.x. [DOI] [PubMed] [Google Scholar]
- 2.Haggstrom AN, Drolet BA, Baselga E, et al. Prospective study of infantile hemangiomas: clinical characteristics predicting complications and treatment. Pediatrics. 2006;118:882–887. doi: 10.1542/peds.2006-0413. [DOI] [PubMed] [Google Scholar]
- 3.Chamlin SL, Haggstrom AN, Drolet BA, et al. Multicenter prospective study of ulcerated hemangiomas. J Pediatr. 2007;151:684–689. 689 e681. doi: 10.1016/j.jpeds.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 4.Boon LM, Burrows PE, Paltiel HJ, et al. Hepatic vascular anomalies in infancy: a twenty-seven-year experience. J Pediatr. 1996;129:346–354. doi: 10.1016/s0022-3476(96)70065-3. [DOI] [PubMed] [Google Scholar]
- 5.Arneja JS, Mulliken JB. Resection of amblyogenic periocular hemangiomas: indications and outcomes. Plast Reconstr Surg. 2010;125:274–281. doi: 10.1097/PRS.0b013e3181c49708. [DOI] [PubMed] [Google Scholar]
- 6.Ceisler E, Blei F. Ophthalmic issues in hemangiomas of infancy. Lymphat Res Biol. 2003;1:321–330. doi: 10.1089/153968503322758148. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz SR, Blei F, Ceisler E, et al. Risk factors for amblyopia in children with capillary hemangiomas of the eyelids and orbit. J AAPOS. 2006;10:262–268. doi: 10.1016/j.jaapos.2006.01.210. [DOI] [PubMed] [Google Scholar]
- 8.Bitar MA, Moukarbel RV, Zalzal GH. Management of congenital subglottic hemangioma: trends and success over the past 17 years. Otolaryngol Head Neck Surg. 2005;132:226–231. doi: 10.1016/j.otohns.2004.09.136. [DOI] [PubMed] [Google Scholar]
- 9.Grimmer JF, Mulliken JB, Burrows PE, et al. Radiofrequency ablation of microcystic lymphatic malformation in the oral cavity. Arch Otolaryngol Head Neck Surg. 2006;132:1251–1256. doi: 10.1001/archotol.132.11.1251. [DOI] [PubMed] [Google Scholar]
- 10.Khan ZA, Boscolo E, Picard A, et al. Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J Clin Invest. 2008;118:2592–2599. doi: 10.1172/JCI33493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leaute-Labreze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–2651. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- 12.Lamy S, L M, Lord-Dufour S, Béliveau R. Propranolol suppresses angiogenesis in vitro: inhibition of proliferation, migration and differentiation of endothelial cells. Vascul Pharmacol. 2010;53:200–208. doi: 10.1016/j.vph.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Annabi B, Lachambre MP, Plouffe K, et al. Propranolol adrenergic blockade inhibits human brain endothelial cells tubulogenesis and matrix metalloproteinase-9 secretion. Pharmacol Res. 2009;60:438–445. doi: 10.1016/j.phrs.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sommers Smith SK, Smith DM. Beta blockade induces apoptosis in cultured capillary endothelial cells. Vitro Cell Dev Biol Anim. 2002;38:298–304. doi: 10.1290/1071-2690(2002)038<0298:BBIAIC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: insights into the molecular mechanisms of action. Br J Dermatol. 2010;163:269–274. doi: 10.1111/j.1365-2133.2010.09848.x. [DOI] [PubMed] [Google Scholar]
- 17.Frieden IJ, Drolet BA. Propranolol for infantile hemangiomas: promise, peril, pathogenesis. Pediatr Dermatol. 2009;26:642–644. doi: 10.1111/j.1525-1470.2009.00977.x. [DOI] [PubMed] [Google Scholar]
- 18.Greenberger S, Boscolo E, Adini I, et al. Corticosteroid suppression of VEGF-A in infantile hemangioma-derived stem cells. N Engl J Med. 2010;362:1005–1013. doi: 10.1056/NEJMoa0903036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.North PE, Waner M, Mizeracki A, et al. GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol. 2000;31:11–22. doi: 10.1016/s0046-8177(00)80192-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Propranolol significantly inhibits proliferation of MSCs at 100μM. There was a trend towards decreased proliferation at 50μM, but this was not statistically significant. Data are representative of two independent experiments. *p < 0.050 relative to control. **p < 0.0005 relative to 50μM propranolol.
Supplemental Figure 2. MSCs cultured in adipogenic media in the presence of vehicle (left) and 50μM propranolol (right) for four days and stained for presence of lipid vacuoles by Oil Red O staining. Similar to results seen with HemSCs, there was increased presence of lipid vacuoles in the propranolol-treated MSCs. (Magnification 20×). Scale bar represents 10μM.