Abstract
Background & Aims
MicroRNAs (miRNAs) have been implicated in the development and progression of human cancers. We investigated the roles and mechanisms of miR-26a in human cholangiocarcinoma.
Methods
We used in situ hybridization and quantitative reverse transcriptase polymerase chain reaction to measure expression of miR-26a in human cholangiocarcinoma tissues and cell lines (eg, CCLP1, SG231, HuCCT1, TFK1). Human cholangiocarcinoma cell lines were transduced with lentiviruses that expressed miR-26a1 or a scrambled sequence (control); proliferation and colony formation were analyzed. We analyzed growth of human cholangiocarcinoma cells that overexpress miR-26a or its inhibitor in severe combined immune-deficient mice. Immunoblot, immunoprecipitation, DNA pull-down, immunofluorescence, and luciferase reporter assays were used to measure expression and activity of glycogen synthase kinase (GSK)-3β, β-catenin, and related signaling molecules.
Results
Human cholangiocarcinoma tissues and cell lines had increased levels of miR-26a compared with the noncancerous biliary epithelial cells. Overexpression of miR-26a increased proliferation of cholangiocarcinoma cells and colony formation in vitro, whereas miR-26 depletion reduced these parameters. In severe combined immune-deficient mice, overexpression of miR-26a by cholangiocarcinoma cells increased tumor growth and overexpression of the miR-26a inhibitor reduced it. GSK-3β messenger RNA was identified as a direct target of miR-26a by computational analysis and experimental assays. miR-26a–mediated reduction of GSK-3β resulted in activation of β-catenin and induction of several downstream genes including c-Myc, cyclinD1, and peroxisome proliferator-activated receptor δ. Depletion of β-catenin partially prevented miR-26a-induced tumor cell proliferation and colony formation.
Conclusions
miR-26a promotes cholangiocarcinoma growth by inhibition of GSK-3β and subsequent activation of β-catenin. These signaling molecules might be targets for prevention or treatment of cholangiocarcinoma.
Keywords: Biliary Tract, Prostaglandin, COX-2, Post-Transcription Gene Regulation
Cholangiocarcinoma is a highly malignant cancer of the biliary tract with a poor prognosis.1–9 The incidence and mortality of cholangiocarcinoma is rising worldwide, and currently there is no effective chemoprevention or treatment. The tumor often arises from background conditions that cause long-standing inflammation, injury, and reparative biliary epithelial cell proliferation, such as primary sclerosing cholangitis, clonorchiasis, hepatolithiasis, or complicated fibropolycystic diseases. The pathogenesis of cholangiocarcinoma is complex and involves alterations of a number of signaling cascades and molecules, including Wnt/β-catenin10–14 and cyclooxygenase-2 (COX-2)-derived prostaglandin E2 (PGE2) pathways.5
β-catenin is a key mediator in Wnt regulation of multiple cellular functions in embryogenesis and tumorigenesis.15–18 In the absence of a Wnt signal, β-catenin exists within a cytoplasmic complex (β-catenin destruction complex) along with glycogen synthase kinase 3β (GSK-3β), adenomatous polyposis coli, and axin, where it is phosphorylated and targeted for degradation by the proteasome. Activation of Wnt signaling disrupts this destruction complex, leading to cytoplasmic accumulation of β-catenin and allowing its translocation into the cell nucleus. In the nucleus, β-catenin associates with T-cell factor (TCF)/lymphoid enhancer factor (LEF) that stimulates transcription of target genes important for proliferation, differentiation, and apoptosis.15–18 Recent studies have shown that accumulation of nuclear β-catenin is induced by COX-2/PGE2, in addition to the canonical Wnt/Frizzled signaling, in human colon cancer cells19,20 and cholangiocarcinoma cells.10
MicroRNAs (miRNAs) are noncoding RNAs of 20–22 nucleotides involved in the regulation of gene expression at a post-transcriptional level by binding to the target sites of messenger RNAs (mRNAs). Recent studies suggest an important role of miRNAs in a number of human and animal cancers21,22; however, the potential implication of miRNAs in cholangiocarcinogenesis remains largely unknown. Given the recently documented importance of miRNAs in protein regulation and tumorigenesis, we postulated that GSK-3β/β-catenin pathway might be regulated by miRNAs during cholangiocarcinoma growth. To explore this possibility, we performed computational analysis using TargetScan 5.1 and this approach led us to identify miR-26a as a noncoding RNA that directly binds to the 3′-untranslated region (UTR) of the GSK-3β mRNA. The objective of the current study was to validate the effect of miR-26a on GSK-3β in cholangiocarcinoma cells and to examine the role of this mechanism in cholangiocarcinogenesis and tumor progression. Our findings demonstrate a novel role of miR-26a–mediated β-catenin activation in human cholangiocarcinoma.
Materials and Methods
In situ hybridization for miR-26a was performed in the formalin-fixed and paraffin-embedded tissue specimens surgically resected from patients diagnosed with cholangiocarcinoma by using the MiRCURY LNA microRNA ISH Optimization Kit (Exiqon, Vedbaek Denmark), with the approval of the Institutional Review Board.
Four human cholangiocarcinoma cell lines, including CCLP1,23 SG231,24 HuCCT1,25 andTFK1,26 and 1 noncancerous cholangiocyte cell line (H69) were used in this study. The CCLP1, SG231, and HuCCT1 cells were cultured according to our methods as described previously.10,27–29 The TFK1 cells were cultured in RPMI-1640 containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin as described previously.26 The H69 cells were cultured in Bronchial Epithelial Cell Basal Medium (Lonza, Basel, Switzerland) supplemented with growth factors in BEGM SingleQuot Kit and 10% heat-inactivated fetal bovine serum.
Human cholangiocarcinoma cell lines were transduced with miR-26a1 lentivirus or miRNA-scramble control lentiviral vector and the cells were analyzed for proliferation and colongenic formation.
A severe combined immune-deficient (SCID) mice tumor xenograft model was used to determine the effect of miR-26a on cholangiocarcinoma growth in vivo. The tumor xenografts were established by inoculating 1.5 × 106 miR-26a-overexpressed or control CCLP1 cells into the flanks of mice (6 mice per group), and the animals were observed for 4 weeks for tumor formation.
Western blotting, immunoprecipitation, DNA pull-down, immunofluorescence, and luciferase reporter activity assays were performed to determine the expression and activity of glycogen synthase kinase-3β (GSK-3β)/β-catenin and related signaling molecules.
The methods are described in more detail in the Supplementary Material.
Results
Expression ofmiR-26a Is Increased in Human Cholangiocarcinoma Tissues and Cell Lines
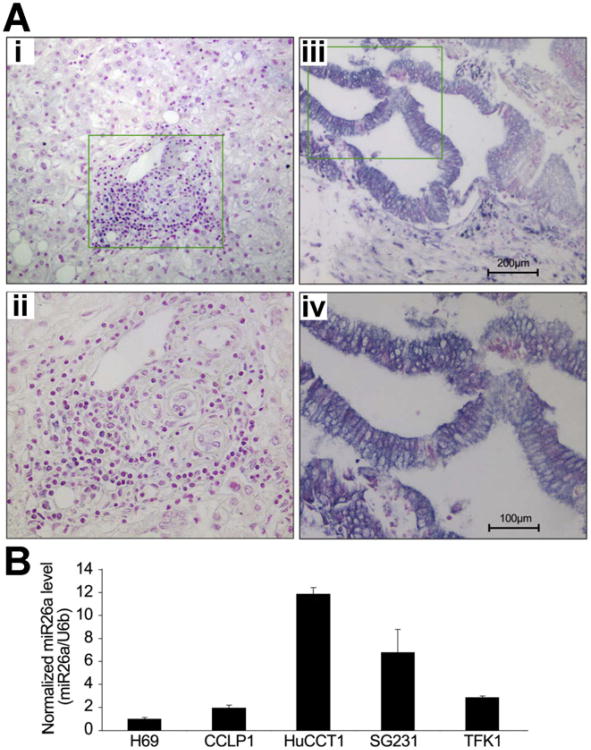
Human cholangiocarcinoma tissue samples and non-neoplastic bile duct epithelia were subjected to in situ hybridization using locked nucleic acid-modified probe against miR-26a. Formalin-fixed, paraffin-embedded tumor and liver tissues from 21 patients who underwent surgical resections for cholangiocarcinoma were analyzed. In cholangiocarcinoma tissues, miR-26a is expressed in 19 of 21 cases (90.5%); in normal bile ducts and non-neoplastic peribiliary glands, miR-26a is expressed in 7 of 21 cases (33.3%). As shown in Figure 1A and Supplementary Table 1, the staining frequency and intensity of miR-26a is significantly higher in cholangiocarcinoma cells (19.0% + + +, 28.6% ++, 42.9 +, 9.5% −) compared to the non-neoplastic bile duct epithelial cells (0% + + +, 4.8% + +, 28.6% +, 66.7% −) (P < .001). Consistent with these observations, quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) analysis showed higher levels of miR-26a expression in 4 human cholangiocarcinoma cell lines (ie, CCLP1, HuCCT1, SG231, TFK1) compared to the noncancerous human biliary epithelial cell line H69 (Figure 1B). These findings provide novel evidence for overexpression of miR-26a in human cholangiocarcinoma tissues and cell lines.
Figure 1.
Expression of miR-26a in human cholangiocarcinoma tissues and cell lines. (A) In situ hybridization for miR-26a in human cholangiocarcinoma tissues was performed as described in the Materials and Methods section. Positive signals were shown as dark blue; nuclei were counterstained as red. (i,ii) Negative staining in the normal bile duct epithelial cells in the portal tracts; (iii,iv) positive miR-26a staining in human cholangiocarcinoma cells. Panels ii and iv (200×) represent high magnifications of panels i and iii (100×), respectively, from the highlighted areas. (B) qRT-PCR for mature miR-26a in a human cholangiocyte cell line (H69) and 4 human cholangiocarcinoma cell lines (ie, CCLP1, HuCCT1, SG231, TFK1). Results represent the mean ratio between miR-26a and the control miRNA U6b from 3 experiments.
miR-26a Promotes Cholangiocarcinoma Cell Growth, In Vitro
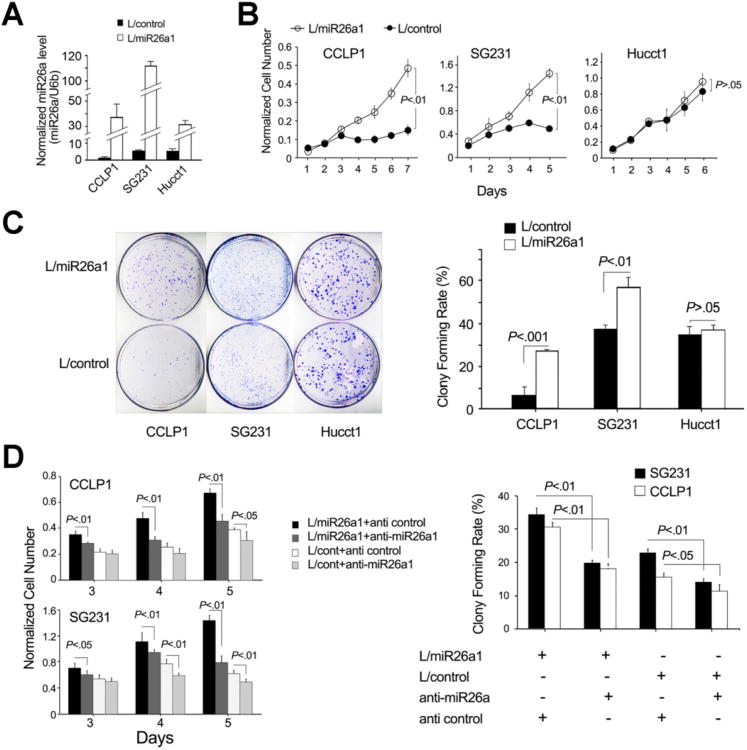
To investigate the role of miR-26a in cholangiocarcinoma cell growth, we constructed human cholangiocarcinoma cell lines with stable overexpression of miR-26a by infecting the parental cell lines with lentivirus particles carrying the miR-26a1 gene (this vector also carries the enhanced green fluorescent protein gene under the control of the same promoter). High infection efficiency was confirmed by the expression of enhanced green fluorescent protein in nearly all transduced cells (Supplementary Figure 1). Successful increase of miR-26a expression in the established cell lines was verified by qRT-PCR. As shown in Figure 2A, the cellular level of miR-26a was significantly higher in miR-26a–overexpressed cells than in miRNA-scramble control cells. These cells (with and without miR-26a overexpression) were then used to determine their growth curve and colony-formation capacity. Overexpression of miR-26a significantly increased the growth of CCLP1 and SG231 cholangiocarcinoma cell lines when compared to their corresponding controls (Figure 2B). miR-26a overexpression was also found to increase colony-formation efficiency in the CCLP1 and SG231 cells (Figure 2C). Accordingly, CCLP1 and SG231 cells with miR-26a depletion showed decreased cell growth and colony-formation capacity (Figure 2D). The control lentivirus vector was found to have no effect on cell growth or colony formation (Supplementary Figure 2). These data indicate that miR-26a is able to enhance the growth and colonogenic potential in CCLP1 and SG231 cells.
Figure 2.
miR-26a promotes cholangiocarcinoma cell proliferation and colony formation in vitro. Human cholangiocarcinoma cell lines (ie, CCLP1, SG231, and HuCCT1) were infected with miR-26a1 lentivirus (indicated as L/miR-26a1) and control lentivirus (indicated as L/control), respectively, and the stably transduced cells were analyzed for proliferation and colonogenic potential as described in the Materials and Methods section. (A) miR-26a levels in cells infected with miR-26a1 and miRNA-scramble control lentivirus were measured by qRT-PCR. Results represent mean ± standard error of mean (SEM) of the miR-26a/U6b ratio normalized to the baseline level in the CCLP-1 scramble control cells (n = 4). (B) Cell proliferation assay (WST-1). Data are presented as mean ± SEM from 4 independent experiments. (C) Colonogenic assays. Data were obtained from 3 independent experiments. (D) Inhibition of miR-26a prevents cholangiocarcinoma cell proliferation and colonogenic potential. miR-26a1–overex-pressed or miRNA-scramble control cells were transfected with anti–miR-26a1 or scramble control, respectively; 24–48 h after transfection, cells were analyzed for proliferation and colonogenic formation. Data are expressed as mean ± SEM from 3 experiments.
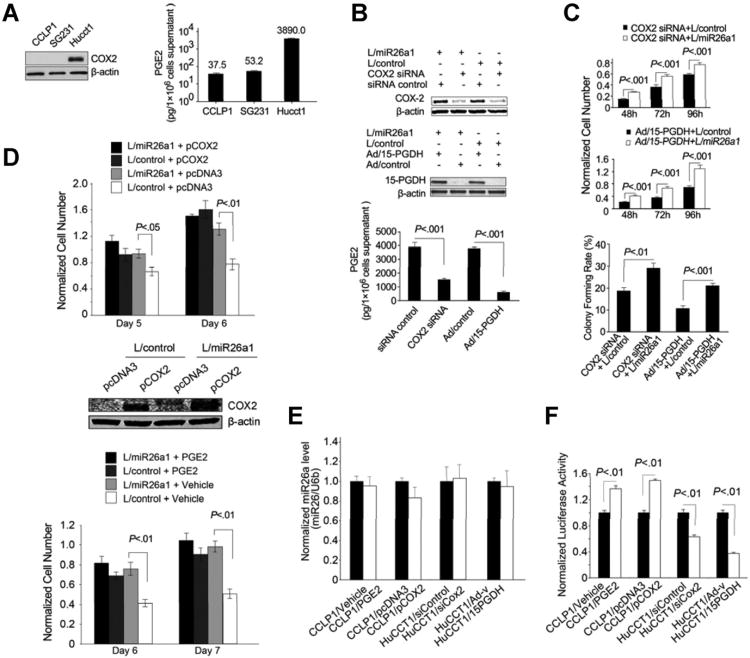
We observed that miR-26a overexpression did not significantly alter the growth rate or colony-formation efficiency in HuCCT1 cells; this phenomenon is likely due to the high COX-2 expression and PGE2 production in these cells. As shown in Figure 3A, the HuCCT1 cells express a much higher level of COX-2 and produce a higher amount of PGE2 compared to the CCLP1 and SG231 cells. Given the documented role of COX-2–derived PGE2 in cholangiocarcinoma growth,5 we reasoned that enhanced COX-2 and PGE2 signaling in HuCCT1 cells may render the cells resistant to miR-26a–mediated growth stimulation. To further evaluate this possibility, we examined the effect of miR-26a in HuCCT1 cells with COX-2 depletion or inhibition. Specifically, HuCCT1 cells with or without miR-26a overexpression were transfected with the COX-2 small interfering RNA (siRNA) or transduced with the adenoviral vector encoding 15-hydroxyprostaglandin dehydrogenase (15-PGDH; an enzyme that converts PGE2 to its inactive 15-keto metabolite and serves as a functional inhibitor of COX-2); these cells were then analyzed for their growth response and colony-formation capacity. As shown in Figure 3B and C, miR-26a—overexpressed HuCCT1 cells exhibit significantly increased proliferation and colony-forming capacity after COX-2 knockdown or PGE2 degradation by 15-PGDH. These findings indicate that high levels of COX-2 and PGE2 in HuCCT1 cells masquerade the miR-26—mediated growth stimulatory effect. To further evaluate the impact of COX-2 and PGE2 signaling, we transfected miR-26a–overexpressed CCLP1 cells with COX-2 expression plasmid or treated CCLP1 cells with PGE2 to determine cell growth parameters. As shown in Figure 3D, miR-26 was unable to induce CCLP1 cell growth when COX-2 was overexpressed or when the cells were treated with exogenous PGE2. These findings suggest that the status of COX-2 and PGE2 signaling in cholangiocarcinoma cells is an important factor that determines the cell response to miR-26. It is of note that the cellular level of miR-26a was not influenced by PGE2 treatment or altered expression of COX-2/15-PGDH (Figure 3E). Consistent with the documented role of COX-2–derived PGE2 for activation of β-catenin,10,19,20 our data showed that forced overexpression of COX-2 or treatment with exogenous PGE2 increased the activity of β-catenin in CCLP1 cells (with low endogenous COX-2/PGE2), whereas COX-2 knockdown or 15-PGDH overexpression decreased β-catenin activity in HuCCT1 cells (with high endogenous COX-2/PGE2) (Figure 3F). These observations suggest that COX-2–derived PGE2 signaling might influence miR-26 actions in cholangiocarcinoma cells via activation of the β-catenin signaling pathway.
Figure 3.
The effect of miR-26a in cholangiocarcinoma cells is influenced by COX-2 and PGE2. (A) High levels of COX-2 expression and PGE2 production in HuCCT1 cells compared to CCLP1 and SG231 cells. Representative Western blots for COX-2 and β-actin are shown at the left panel. Production of PGE2 in culture supernatant of 1 × 106 cells is shown at the right panel (data represent mean results from 3 experiments). (B) miR-26a1-overexpressed or miRNA-scramble control HuCCT1 cells were transfected with COX-2 siRNA or transduced with an adenoviral vector expression 15-PGDH (Ad/15-PGDH) or the control adenoviral vector (Ad/control). Successful alteration of COX-2 and 15-PGDH was confirmed by Western blotting analysis (upper and mid panels). Culture media were collected to measure the production of PGE by enzyme immunoassay and data are presented as mean ± standard error of mean (SEM) from 3 independent experiments (lowerpanel). (C) Cell proliferation and colonogenic assays. miR-26a1-overexpressed HuCCT1 cells were transfected with COX-2 siRNA or transduced with 15-PGDH adenoviral vector and their respective controls. Cells were then analyzed for proliferation (n = 3) and colonogenic capacity (n = 3). (D) WST-1 assay of miR-26a–overexpressed or miRNA-scramble control CCLP1 cells transfected with COX-2 expression plasmid or treated with PGE2 (10 μM). The values represent mean ± SEM from 4 independent experiments. The efficiency of COX-2 overexpression was confirmed by Western blotting analysis. (E) qRT-PCR analysis for miR-26a. The level of miR-26a was not influenced by PGE2 treatment or altered expression of COX-2 or 15-PGDH. Data are presented as mean ± SEM from 3 independent experiments. (F) The effect of COX-2/PGE2 on β-catenin transcription activity. CCLP1 and HuCCT1 cells were transfected with the TCF/LEF luciferase reporter plasmid. The CCLP1 cells were co-transfected with COX-2 expression vector or treated cells with PGE2, whereas the HuCCT1 cells were transfected with COX-2 siRNA or transduced with 15-PGDH adenovirus. Cells were cultured in serum-free Opti-MEM medium for 24 h, and the cell lysates were obtained to measure the luciferase activity by a luminometer. Data are presented as mean ± SEM from 3 independent experiments.
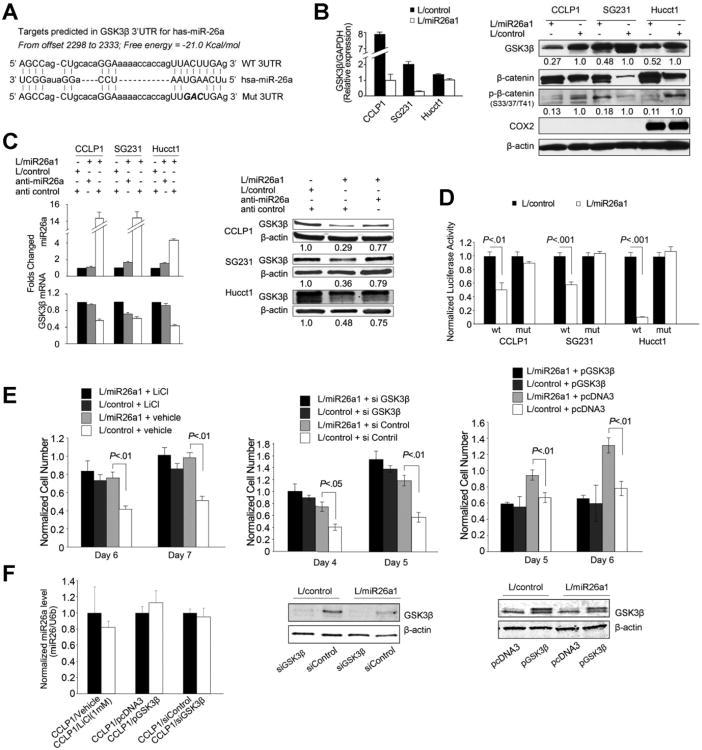
GSK-3β Is a Direct Target of miR-26a in Cholangiocarcinoma Cells
As illustrated in Figure 4A, complementary sequence of miR-26a was found in the 3′UTR of GSK-3β mRNA. qRT-PCR and Western blotting analyses showed that miR-26a overexpression decreased the levels of GSK-3β mRNA and protein (Figure 4B). Treatment with specific miR-26a inhibitor prevented miR-26a-induced reduction of GSK-3β mRNA and protein (Figure 4C). miR-26a overexpression decreased the GSK-3β 3′UTR luciferase reporter activity; this effect was abolished when the 3 nucleotides in the miR-26a seed binding site of the GSK-3β 3′UTR were mutated (Figure 4D). Consistent with these observations, alteration of GSK-3β level or activity impaired the pro-proliferation function of miR-26a. As shown in Figure 4E and F, inhibition of GSK-3β by lithium chloride or GSK-3β siRNA prevented miR-26a-induced cell growth; transfection with GSK-3β open reading frame plasmid without 3′-UTR (cannot be targeted by miR-26a) also abolished miR-26a–induced cell growth (alteration of GSK-3β level or activity did not affect the cellular level of miR-26a). These results demonstrate that GSK-3β is a direct target of miR-26a.
Figure 4.
GSK-3β is a direct target of miR-26a in cholangiocarcinoma cells. (A) Putative miR-26a binding sequence in the 3′-UTR of GSK-3β mRNA. 3′-UTR fragments of GSK-3β containing wild-type or mutated (3 mutated nucleotides were indicated by italic type) miR-26a binding site were cloned into pMIR-REP-dCMV vector to obtain GSK-3β 3′-UTR luciferase reporter plasmids as described in the Materials and Methods section. (B) miR-26a reduces GSK-3β mRNA and protein levels in cholangiocarcinomia cells. (Left) miR-26a and GSK-3β mRNA levels as determined by qRT-PCR in miR-26a1–overexpressed and miRNA-scramble control cells. Data are shown as mean ± standard error of mean (SEM) from 3 independent experiments. (Right) Representative Western blots showing the protein levels of GSK-3β, β-catenin, phospho-β-catenin, COX-2, and β-actin in miR-26a–overexpressed and control cells. The numbers under the bands indicate the relative expression levels of individual proteins (the levels in control cells were set as 1.0). (C) Anti–miR-26a inhibits miR-26a–induced reduction of GSK-3β mRNA and protein. (Left) qRT-PCR assays of miR-26a and GSK-3β mRNA in miR-26a1–overexpressed cells treated with anti–miR-26a or the scramble control. Data are shown as mean ± SEM from 3 independent experiments. (Right) Representative Western blots for GSK-3β and β-actin in miR-26a1–overexpressed cells treated with or without anti–miR-26a. The numbers under individual bands show the relative expression level of GSK-3β protein (the level of GSK-3β in control cells were set as 1.0). (D) GSK-3β 3′-UTR luciferase reporter activity assay. miR-26a–overexpressed or control cells were transfected with either wild-type or mutant GSK-3β 3′-UTR reporter plasmids (indicated as wt or mut on the X-axis). Twenty-four hours post transfection, cell lysates were obtained to determine luciferase activity by using a luminometer. Renilla luciferase plasmid was used as the internal control. (E) Alteration of GSK-3β level and activity prevents miR-26a–induced cell pro-proliferation. miR-26a–overexpressed or control CCLP1 cells were treated with lithium chloride (as indicated in the left panel), transfected with the GSK-3β siRNA (as indicated in the mid panel), or transfected with the GSK-3β open reading frame (ORF) expression plasmid (as indicated in the right panel). Cells were then analyzed for proliferation by WST-1 assay. Data represent mean ± SEM from 3 individual experiments. Representative Western blots for GSK-3β in cells transfected with GSK-3β siRNA or expression plasmid are also shown. (F) qRT-PCR for miR-26a in CCLP1 cells treated with lithium chloride or transfected with the GSK-3β siRNA or expression plasmid. Data are presented as mean ± SEM from 3 independent experiments. The level of miR-26a was not altered by lithium chloride or GSK-3β level.
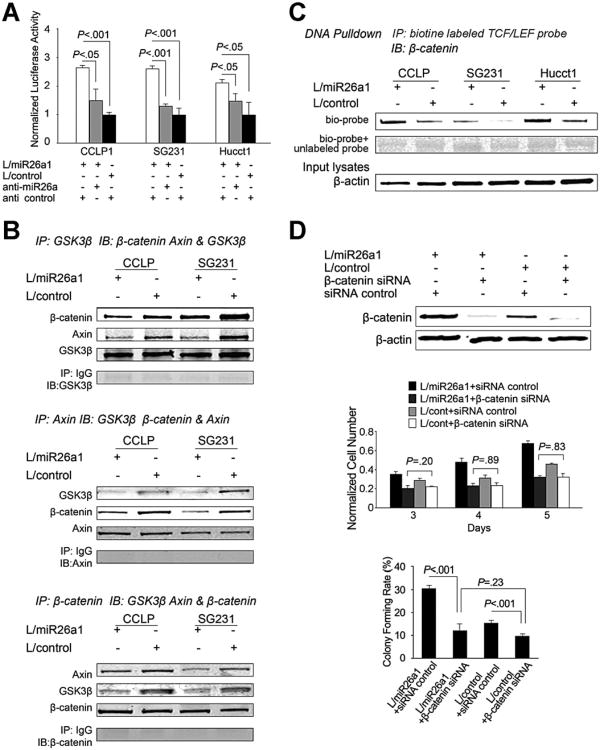
miR-26a Increases β-Catenin Activity in Cholangiocarcinoma Cells
Given that β-catenin is targeted for degradation via phosphorylation of its serine (Ser33/37/45) and threonine (Thr41) residues by GSK-3β,18,30 we measured the levels of β-catenin and phospho-β-catenin in cholangiocarcinoma cells with or without miR-26a overexpression. As shown in Figure 4B, miR-26a-mediated reduction of GSK-3β is associated with decreased phospho-β-catenin (Ser33/37/Thr41) and accumulation of total β-catenin. Luciferase reporter activity assays showed that miR-26a overexpression significantly increased β-catenin reporter activity and the effect was blocked by treatment with the miR-26a anti-miR (Figure 5A). As β-catenin degradation occurs in a multiprotein complex containing Axin and GSK-3β,31 we performed co-immunoprecipitation assays to determine the association of β-catenin with GSK-3β and Axin in CCLP1 and SG231 cells. As shown in Figure 5B, miR-26a overexpression reduced the formation of the GSK-3β/β-catenin/Axin binding complex. DNA pull-down using biotinylated TCF/LEF oligonucleotide showed that miR-26a overexpression increased β-catenin binding to its DNA response element (Figure 5C). Consistent with these observations, immunofluorescence staining showed more β-catenin nuclear accumulation in miR-26a–overexpressed CCLP1 cells compared to the vector control cells (Supplementary Figure 3). The importance of β-catenin in miR-26a–induced tumor cell growth is further supported by the observation that siRNA depletion of β-catenin prevented miR-26a–induced cell proliferation and colony-formation capacity (Figure 5D).
Figure 5.
miR-26a inhibits β-catenin degradation and enhances its transcription activity. (A) The effect of miR-26a on β-catenin transcription activity. miR-26a1–overexpressed and control cells were transfected with the TCF/LEF luciferase reporter plasmid. The cell lysates were obtained 24 h after trans-fection to measure luciferase activity by using a luminometer. Renilla luciferase expression plasmid pRL-TK was used as internal control. Data are presented as mean ± standard error of mean (SEM) from 3 independent experiments. (B) The effect of miR-26a on the formation of β-catenin degradation complex. Equal amounts of cell lysate from CCLP1 and SG231 cells infected with miR-26a1 lentivirus or control lentivirus was subjected to immunoprecipitation and immunoblotting by using specific antibodies as indicated for each panel. (C) DNA pull-down assay. Equal amounts of cell lysates were pulled down with biotinylated TCF/LEF DNA probe or cold probe followed by immunoblotting with anti-β-catenin antibody. (D) β-catenin knockdown partly prevents miR-26a-induced cell proliferation and colony formation in CCLP1 cell line. miR-26a1–overexpressed or control cells were transfected with β-catenin siRNA or control RNA, and the cells were analyzed for proliferation and colony formation. Data represent mean ± SEM from 3 independent experiments in triplicate. The efficiency of β-catenin depletion was confirmed by Western blotting analysis.
miR-26a Enhances Cholangiocarcinoma Growth in SCID Mice
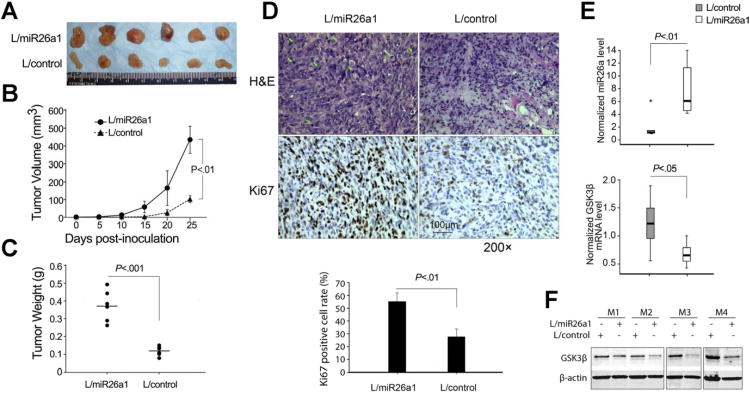
To further examine the effect of miR-26a on cholangiocarcinoma growth in vivo, miR-26a-overexpressed and scramble control CCLP1 cells were injected subcutaneously into the flank of SCID mice and the animals were closely monitored for tumor growth. As shown in Figure 6A and B, miR-26a–overexpressed tumors were larger in size and had higher tumor volume compared to the vector control tumors. An approximately 3-fold increase in tumor weight was observed in miR-26a–overexpressed tumors compared to the controls (0.37 ± 0.09 g vs 0.12 ± 0.02 g; P < .001) (Figure 6C). More prominent mitosis was observed in miR-26a–overexpressed tumors compared to the controls. Immunohistochemical staining for the cell proliferation marker Ki67 showed higher percentages of Ki67-positive tumor cells in miR-26a–overexpressed tumors (55.48% ± 7.04%) compared to the control tumors (27.65% ± 6.25%) (P < .01) (Figure 6D). qRT-PCR and Western blot analyses of the tumor tissues confirmed elevated miR-26a with reduced GSK-3β mRNA/protein in miR-26–overexpressed tumors (Figure 6E and F). Increased expression of miR-26a in cholangiocarcinoma tissues from the SCID mice was also confirmed by in situ hybridization using the locked nucleic acid–modified probe (same procedure as for analysis of human cholangiocarcinoma tissue samples) (Supplementary Figure 4).
Figure 6.
miR-26a promotes cholangiocarcinoma growth in vivo. miR-26a1–overexpressed or scramble control CCLP1 cells (1.5 × 106) were inoculated subcutaneously into SCID mice (n = 6), and the mice were closed monitored for tumor growth. Twenty-five days post inoculation, the mice were sacrificed and the tumors were recovered. (A) Photography of xenograft tumor masses from SCID mice. (B) The volume of xenograft tumors. Data represent mean ± standard deviation (SD) from 6 SCID mice. (C) The weight of xenograft tumors. Data represent mean ± SD from 6 SCID mice. (D) Histopathological analysis of the xenograft tumor tissues recovered from the SCID mice. (Upper panel) Representative H&E stain and Ki67 immunostain. (Lowerpanel) Percentages of Ki67-positive cells (data are expressed as mean ± SD, n = 6). (E) Levels of miR-26a and GSK-3β mRNA in xenograft tumor tissues as determined by qRT-PCR. (F) Western blot analysis of GSK-3β protein in miR-26a1–overexpressed and control xenograft tumor tissues. M1–4 denote individual tumors grown in different mice.
miR-26a Knockdown Inhibits Cholangiocarcinoma Growth in SCID Mice
As a parallel approach, we established SG231 and CCLP1 cell lines with stable knockdown of miR-26a; these cells were then injected subcutaneously into the flank of SCID mice and the animals were monitored for tumor growth. As shown in Supplementary Figure 5, the miR-26a knockdown tumors were smaller in size and weight compared to the scramble control tumors. An approximately 2- to 3-fold decrease of tumor weight was observed in miR-26a knockdown tumors compared to the controls (0.32 ± 0.15 g vs 0.62 ± 0.18 g; P < .05 for SG231 and 0.18 ± 0.06 g vs 0.57 ± 0.12 g; P < .001 for CCLP1). The tumor volume and tumor weight were not different between the scramble control lentivirus group and the non-viral group.
miR-26a Regulates β-Catenin Downstream Signaling Molecules
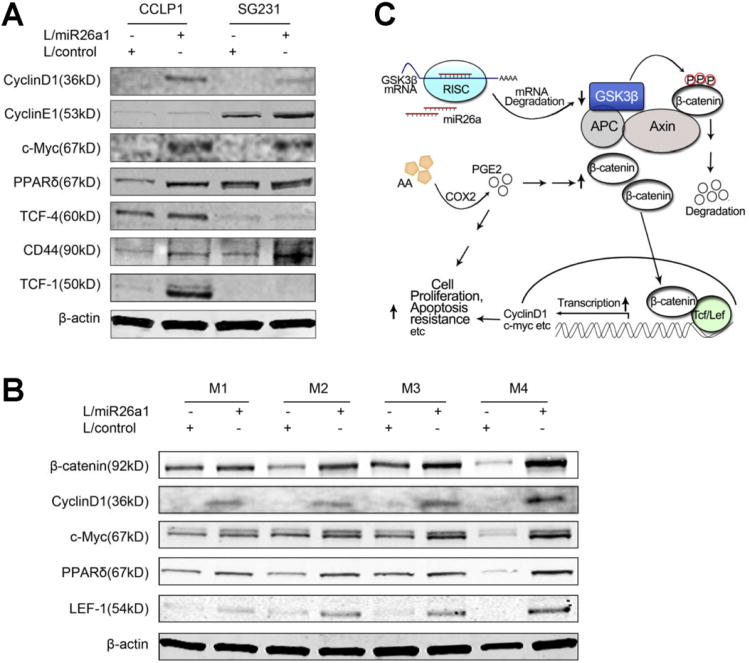
Given that β-catenin activates the transcription of a number of downstream genes implicated in tumor growth, such as c-Myc, CyclinD1, CyclinE1, peroxisome proliferator-activated receptor δ, CD44, TCF-1, TCF-4, and LEF-1,18,32–36 we further examined the potential effect of miR-26a on these molecules in our system. As shown in Figure 7A, the levels of these molecules were all increased in CCLP1 and SG231 cells stably overexpressing miR-26a, in vitro. Accordingly, the levels of β-catenin, CyclinD1, c-Myc, peroxisome proliferator-activated receptor δ, and LEF-1 were also increased in the xenograft tumor tissues with miR-26a overexpression (Figure 7B). These findings demonstrate that miR-26a induces the expression of (β-catenin downstream genes in human cholangiocarcinoma cells. Given that β-catenin and its downstream molecules are also implicated in cell migration,37,38 we further performed wound-healing assays to determine whether miR-26a might influence cholangiocarcinoma cell migration. As shown in Supplementary Figure 6, miR-26a overexpression enhanced CCLP1 cell migration at 8 h (45.40% ± 8.50% vs 21.52% ± 4.00% in mock control; P < .01) and 24 h (83.3% ± 7.78% vs 61.13% ± 2.27% in mock control; P < .05). Taken together, these findings suggest a key role of miR-26a in cholangiocarcinoma cell proliferation and migration.
Figure 7.
The effect of miR-26a on Wnt/β-catenin downstream molecules. (A) Western blotting with indicated antibodies in cultured CCLP1 and SG231 cells stably transduced with miR-26a or control lentivirus. β-actin was used as the loading control. (B) Western blotting with indicated antibodies in xenograft tumor tissues derived from miR-26a– overexpressed and vector control CCLP1 cells. β-actin was used as the loading control. (C) Schematic illustration of the key mechanism for miR-26a–induced cholangiocarcinoma growth.
Discussion
The current study provides the first evidence that miR-26a enhances cholangiocarcinoma progression by targeting GSK-3β. Elevated expression of miR-26a in human cholangiocarcinoma tissues and cell lines is documented by in situ hybridization and qRT-PCR. The role of miR-26a in cholangiocarcinoma growth is highlighted by the observations that miR-26a overexpression promoted cholangiocarcinoma cell proliferation, clonogenic formation and migration; anti-miR-26 inhibited cholangiocarcinoma cell proliferation and clonogenic formation; and overexpression of miR-26a enhanced cholangiocarcinoma growth in SCID mice. We have found that GSK-3β is a direct target of miR-26a in cholangiocarcinoma cells, and this conclusion is supported by the following observations: complementary sequence of miR-26a is identified in the 3′UTR of GSK-3β mRNA; miR-26a overexpression reduced GSK-3β mRNA and protein and this effect was attenuated by miR-26a inhibitor; miR-26a1 overexpression decreased GSK-3β 3′UTR luciferase report activity and this effect was abolished by mutation of the miR-26a seed binding site. The role of GSK-3β in miR-26a-mediated cholangiocarcinoma growth is further supported by the observations that alteration of GSK-3β level or activity by overexpression, siRNA, or lithium chloride impairs miR-26a–induced cholangiocarcinoma cell proliferation. Our findings are consistent with a recent study by Mohamed et al showing that miR-26a targets GSK-3β in human airway smooth muscle cells.39
Our data indicate that miR-26a–mediated inhibition of GSK-3β leads to β-catenin activation in cholangiocarcinoma cells, as highlighted by the following experimental evidences: miR-26a overexpression decreased the phosphorylation of β-catenin; miR-26a overexpression caused β-catenin accumulation and nuclear translocation; miR-26a overexpression increased β-catenin reporter activity, and this effect was blocked by anti-miR inhibition of miR-26a; miR-26a overexpression reduced the formation of β-catenin degradation complex; miR-26a overexpression enhanced β-catenin binding to its DNA response element. The importance of β-catenin in miR-26a–in-duced tumor cell growth is also supported by the observation that siRNA depletion of β-catenin prevented miR-26a–induced cholangiocarcinoma proliferation and colony-formation capacity. Consistent with these findings, miR-26 overexpression was found to enhance the expression of several β-catenin downstream genes in cholangiocarcinoma cells.
Immunohistochemical studies have documented β-catenin activation in human cholangiocarcinoma tissues10–14 (characterized by increased nuclear and cytoplasmic staining and reduced plasma membrane staining). However, the exact mechanism for β-catenin activation in cholangiocarcinoma has not been completely defined. As β-catenin mutation in human cholangiocarcinoma has not been reported, it is likely that activation of other growth-promoting signaling pathways may be responsible for β-catenin activation during cholangiocarcinogenesis. Our findings in this study demonstrate a key role of miR-26a for β-catenin activation in human cholangiocarcinoma cells. This finding is noteworthy, given that miR-26a is commonly up-regulated in human cholangiocarcinoma, as demonstrated in the present study, and that β-catenin is a key molecule implicated in cholangiocarcinogenesis. Thus, miR-26a overexpression is a new mechanism that activates β-catenin in human cholangiocarcinoma, and this pathway may play an important role in cholangiocarcinogenesis. Furthermore, we observed that miR-26a–mediated cholangiocarcinoma growth is influenced by COX-2/PGE2 which is also commonly up-regulated in cholangiocarcinoma tissues.40–42 Elevated expression of miR-26 and COX-2 may coordinately regulate GSK-3β and β-catenin pathway and promote cholangiocarcinogenesis.
miRNAs are increasingly recognized as a key player in carcinogenesis.21,22 Many human or mouse miRNAs are located near cancer susceptibility loci43,44 and miRNA alterations are involved in all stages of tumor development, including initiation, progression, and metasta-sis.45–47 To our knowledge, the current study is the first to describe the expression, function, and mechanism of miR-26a in human cholangiocarcinoma. There are 2 miR-26a genes in human genome, hsa-miR-26a-1 and hsa-miR-26a-2, that both produce the same mature form of miR-26a. miR-26a-1 is located on the intron of carboxy-terminal domain (CTD; RNA polymerase II, polypeptide A) small phosphatase-like gene, whereas miR-26a-2 is located on the intron of CTD small phosphatase 2 gene. Both CTD small phosphatase-like and CTD small phosphatase 2 belong to the small CTD phosphatase family. Previous studies have shown that CTD phosphatase counteract GSK-3β function by dephosphorylating Snail protein (a substrate of GSK-3β), thus assisting epithelial-mesenchy-mal transition.48 Therefore, both miR-26a and its host gene productions show function against GSK-3β, which represents an example of functional cooperation between miRNAs and their host genes. It remains to be further investigated whether miR-26a host genes might play a role in the regulation of cholangiocarcinoma growth.
In summary, this study provides novel evidence that miR-26a enhances cholangiocarcinoma progression by targeting the GSK-3β and β-catenin pathway. Our findings suggest that these signaling molecules might represent potential targets for future prevention and treatment of human cholangiocarcinoma.
Supplementary Material
Supplementary Figure 1. Expression of enhanced green fluorescent protein (eGFP) in stably transduced cell lines. Immunofluorescent microscopy for eGFP was performed in 3 human cholangiocarcinoma cell lines (ie, CCLP1, SG231, and HuCCT) stably transduced with the miR-26a1 lentivirus (L/miR-26a1, in which the eGFP and has-miR-26a1 genes were fused and controlled by CMV promoter). (A, B, C) L/miR-26a1–transduced cells; (D, E, F) noninfected control cells.
Supplementary Figure 2. Cholangiocarcinoma cells with or without stable transduction of control lentivirus. (A) qRT-PCR analysis for miR-26a and GSK3β mRNAs in cholangiocarcinoma cells with or without scramble control lentivirus transduction. Data are shown as mean ± standard error of mean (SEM) from 3 experiments. (B) Representative Western blots showing the protein levels of GSK-3β, β-catenin, and phosphorylated β-catenin in cholangiocarcinoma cells with or without scramble control lentivirus transduction. (C) TCF/LEF luciferase activity assay. Cholangiocarcinoma cells with or without scramble control lentivirus infection were transfected with the TCF/LEF luciferase reporter plasmid. The cell lysates were obtained 24 h after transfection to measure the luciferase activity by using a luminometer. Renilla luciferase expression plasmid pRL-TK was used as internal control. Data are presented as mean ± SEM from 3 independent experiments. (D) Cell proliferation assay. Cholangiocarcinoma cells with or without scramble control lentivirus infection were analyzed by the WST-1 assay. All cells were cultured in OPTI-MEM medium (Invitrogen) with 2% fetal bovine serum unless otherwise indicated. Three hours after WST-1 reagent incubation, the absorption was measured by an enzyme-linked immunosorbent assay reader under wavelength of 450 nm. Data are presented as mean ± SEM from 3 independent experiments. (E) Colonogenic assay in cholangiocarcinoma cells with or without scramble control lentivirus infection. One thousands cells were plated in 10-cm dishes and cultured for 10 to 14 days. The colonies were stained with 0.1% crystal violet.
Supplementary Figure 3. Representative immunofluorescence staining for β-catenin in CCLP1 cells transduced with miR-26a1 or control lentivirus. miR-26a–overexpressed cells show more β-catenin accumulation in the nuclei compared to the miRNA-scramble control cells.
Supplementary Figure 4. miR-26a expression in xenograft tumor tissues. In situ hybridizations for miR-26a and U6 small nuclear RNA were performed by using locked nucleic acid probe as described in the Materials and Methods section. Locked nucleic acid miRNA-scramble probe was used as the negative control.
Supplementary Figure 5. The effect of miR-26a inhibition. SG231 and CCLP1 cells were stably transduced with the lentivirus expressing miR-26a–specific inhibitor (L/26a in) or the scramble control lentivirus vector (L/control). Cells without viral vector transduction were used as an additional control. (A) The level of miR-26a in cultured cells as determined by qRT-PCR analysis. The level of miR-26a in noninfected cells was set as 1.0. Data are presented as mean ± standard error of mean (SEM) from 3 experiments. (B) Representative Western blots for GSK-3β in cultured cells (with β-actin as the loading control). (C–E) The effect of miR-26a inhibitor on cholangiocarcinoma growth in SCID mice. miR-26a inhibitor expressed, vector control, or noninfected CCLP1 and SG231 cells (5 × 106 for CCLP1, 2 × 106 for SG231) were inoculated subcutaneously into SCID mice (n = 6), and the mice were closely monitored for tumor growth. The mice were sacrificed 35 (for CCLP1) or 45 days (for SG231) post inoculation to recover the tumors. (C) The growth curves of xenograft tumors (data represent mean ± standard deviation [SD] from 6 SCID mice). (D) Photography of xenograft tumors recovered from SCID mice. (E) The weight of xenograft tumors (data represent mean ± SD from 6 SCID mice).
Supplementary Figure 6. Wound-healing assay. (A) Representative photographs from CCLP1 cells stably transduced with the miR-26a1 lentivirus (L/miR-26a1) and the control lentivirus (L/control) taken at 0, 8, and 24 h after scratch. (B) The mean wound healing rate for miR-26a–overexpressed and control cells at 0, 8, and 24-h time points.
Supplementary Table 1. miR-26a Level in Human Cholangiocarcinoma Tissues.
NOTE. P < .001, Wilcoxon signed-ranks test(2-tailed).
Acknowledgments
Funding: Supported by National Institutes of Health grants CA102325, CA106280, CA134568, and DK077776 (to T.W.).
Abbreviations used in this paper
- COX-2
cyclooxygenase-2
- CTD
C-terminal domain
- GSK-3β
glycogen synthase kinase-3β
- LEF
lymphoid enhancer factor
- miRNA
microRNA
- mRNA
messenger RNA
- 15-PGDH
15-hydroxyprostaglandin dehydrogenase
- PGE2
prostaglandin E2
- qRT-PCR
quantitative reverse transcriptase polymerase chain reaction
- SCID
severe combined immune deficienct
- siRNA
small interfering RNAs
- TCF
T cell factor
- UTR
untranslated region
Footnotes
Supplementary Material: Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2012.03.045.
Conflicts of interest: The authors disclose no conflicts.
References
- 1.Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48:308–321. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gores GJ. Cholangiocarcinoma: current concepts and insights. Hepatology. 2003;37:961–969. doi: 10.1053/jhep.2003.50200. [DOI] [PubMed] [Google Scholar]
- 3.Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5–15. doi: 10.1002/hep.20537. [DOI] [PubMed] [Google Scholar]
- 4.Berthiaume EP, Wands J. The molecular pathogenesis of cholangiocarcinoma. Semin Liver Dis. 2004;24:127–137. doi: 10.1055/s-2004-828890. [DOI] [PubMed] [Google Scholar]
- 5.Wu T. Cyclooxygenase-2 and prostaglandin signaling in cholangiocarcinoma. Biochim Biophys Acta. 2005;1755:135–150. doi: 10.1016/j.bbcan.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology. 2005;128:1655–1667. doi: 10.1053/j.gastro.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 7.Malhi H, Gores GJ. Cholangiocarcinoma: modern advances in understanding a deadly old disease. J Hepatol. 2006;45:856–867. doi: 10.1016/j.jhep.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 9.Patel T. Cholangiocarcinoma—controversies and challenges. Nat Rev Gastroenterol Hepatol. 2011;8:189–200. doi: 10.1038/nrgastro.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim K, Han C, Xu L, et al. Cyclooxygenase-2-derived prostaglandin E2 activates beta-catenin in human cholangiocarcinoma cells: evidence for inhibition of these signaling pathways by omega 3 polyunsaturated fatty acids. Cancer Res. 2008;68:553–560. doi: 10.1158/0008-5472.CAN-07-2295. [DOI] [PubMed] [Google Scholar]
- 11.Ashida K, Terada T, Kitamura Y, et al. Expression of E-cadherin, alpha-catenin, beta-catenin, and CD44 (standard and variant isoforms) in human cholangiocarcinoma: an immunohistochemical study. Hepatology. 1998;27:974–982. doi: 10.1002/hep.510270412. [DOI] [PubMed] [Google Scholar]
- 12.Sugimachi K, Taguchi K, Aishima S, et al. Altered expression of beta-catenin without genetic mutation in intrahepatic cholangiocarcinoma. Mod Pathol. 2001;14:900–905. doi: 10.1038/modpathol.3880409. [DOI] [PubMed] [Google Scholar]
- 13.Tokumoto N, Ikeda S, Ishizaki Y, et al. Immunohistochemical and mutational analyses of Wnt signaling components and target genes in intrahepatic cholangiocarcinomas. Int J Oncol. 2005;27:973–980. [PubMed] [Google Scholar]
- 14.Settakorn J, Kaewpila N, Burns GF, et al. FAT, E-cadherin, beta catenin, HER 2/neu, Ki67 immuno-expression, and histological grade in intrahepatic cholangiocarcinoma. J Clin Pathol. 2005;58:1249–1254. doi: 10.1136/jcp.2005.026575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoppler S, Kavanagh CL. Wnt signalling: variety at the core. J Cell Sci. 2007;120:385–393. doi: 10.1242/jcs.03363. [DOI] [PubMed] [Google Scholar]
- 16.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 18.Moon RT, Kohn AD, De Ferrari GV, et al. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 19.Castellone MD, Teramoto H, Williams BO, et al. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 20.Shao J, Jung C, Liu C, Sheng H. Prostaglandin E2 stimulates the beta-catenin/T cell factor-dependent transcription in colon cancer. J Biol Chem. 2005;280:26565–26572. doi: 10.1074/jbc.M413056200. [DOI] [PubMed] [Google Scholar]
- 21.Ryan BM, Robles AI, Harris CC. Genetic variation in microRNA networks: the implications for cancer research. Nat Rev Cancer. 2010;10:389–402. doi: 10.1038/nrc2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimizu Y, Demetris AJ, Gollin SM, et al. Two new human cholangiocarcinoma cell lines and their cytogenetics and responses to growth factors, hormones, cytokines or immunologic effector cells. Int J Cancer. 1992;52:252–260. doi: 10.1002/ijc.2910520217. [DOI] [PubMed] [Google Scholar]
- 24.Storto PD, Saidman SL, Demetris AJ, et al. Chromosomal breakpoints in cholangiocarcinoma cell lines. Genes Chromosomes Cancer. 1990;2:300–310. doi: 10.1002/gcc.2870020408. [DOI] [PubMed] [Google Scholar]
- 25.Miyagiwa M, Ichida T, Tokiwa T, et al. A new human cholangiocellular carcinoma cell line (HuCC–T1) producing carbohydrate antigen 19/9 in serum-free medium. Vitro Cell Dev Biol. 1989;25:503–510. doi: 10.1007/BF02623562. [DOI] [PubMed] [Google Scholar]
- 26.Saijyo S, Kudo T, Suzuki M, et al. Establishment of a new extra-hepatic bile duct carcinoma cell line, TFK-1. Tohoku J Exp Med. 1995;177:61–71. doi: 10.1620/tjem.177.61. [DOI] [PubMed] [Google Scholar]
- 27.Wu T, Han C, Lunz JG, 3rd, et al. Involvement of 85-kd cytosolic phospholipase A(2) and cyclooxygenase-2 in the proliferation of human cholangiocarcinoma cells. Hepatology. 2002;36:363–373. doi: 10.1053/jhep.2002.34743. [DOI] [PubMed] [Google Scholar]
- 28.Han C, Leng J, Demetris AJ, et al. Cyclooxygenase-2 promotes human cholangiocarcinoma growth: evidence for cyclooxygenase-2-independent mechanism in celecoxib-mediated induction of p21waf1/cip1 and p27kip1 and cell cycle arrest. Cancer Res. 2004;64:1369–1376. doi: 10.1158/0008-5472.can-03-1086. [DOI] [PubMed] [Google Scholar]
- 29.Han C, Wu T. Cyclooxygenase-2-derived prostaglandin E2 promotes human cholangiocarcinoma cell growth and invasion through EP1 receptor-mediated activation of the epidermal growth factor receptor and Akt. J Biol Chem. 2005;280:24053–24063. doi: 10.1074/jbc.M500562200. [DOI] [PubMed] [Google Scholar]
- 30.Willert K, Jones KA. Wnt signaling: is the party in the nucleus? Genes Dev. 2006;20:1394–1404. doi: 10.1101/gad.1424006. [DOI] [PubMed] [Google Scholar]
- 31.Liu CM, Li YM, Semenov M, et al. Control of beta-catenin phos-phorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 32.Sansom OJ, Meniel VS, Muncan V, et al. Myc deletion rescues Apc deficiency in the small intestine. Nature. 2007;446:676–679. doi: 10.1038/nature05674. [DOI] [PubMed] [Google Scholar]
- 33.Botrugno OA, Fayard E, Annicotte JS, et al. Synergy between LRH-1 and beta-catenin induces G1 cyclin-mediated cell proliferation. Mol Cell. 2004;15:499–509. doi: 10.1016/j.molcel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 34.He TC, Chan TA, Vogelstein B, et al. PPARdelta is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99:335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanwar SS, Yu Y, Nautiyal J, et al. The Wnt/beta-catenin pathway regulates growth and maintenance of colonospheres. Mol Cancer. 2010;9:212. doi: 10.1186/1476-4598-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He TC, Sparks AB, Rago C, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 37.Ma L, Young J, Prabhala H, et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12:247–256. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 39.Mohamed JS, Lopez MA, Boriek AM. Mechanical stretch up-regulates microRNA-26a and induces human airway smooth muscle hypertrophy by suppressing glycogen synthase kinase-3beta. J Biol Chem. 2010;285:29336–29347. doi: 10.1074/jbc.M110.101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Endo K, Yoon BI, Pairojkul C, et al. ERBB-2 overexpression and cyclooxygenase-2 up-regulation in human cholangiocarcinoma and risk conditions. Hepatology. 2002;36:439–450. doi: 10.1053/jhep.2002.34435. [DOI] [PubMed] [Google Scholar]
- 41.Hayashi N, Yamamoto H, Hiraoka N, et al. Differential expression of cyclooxygenase-2 (COX-2) in human bile duct epithelial cells and bile duct neoplasm. Hepatology. 2001;34:638–650. doi: 10.1053/jhep.2001.28198. [DOI] [PubMed] [Google Scholar]
- 42.Chariyalertsak S, Sirikulchayanonta V, Mayer D, et al. Aberrant cyclooxygenase isozyme expression in human intrahepatic cholangiocarcinoma. Gut. 2001;48:80–86. doi: 10.1136/gut.48.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sevignani C, Calin GA, Nnadi SC, et al. MicroRNA genes are frequently located near mouse cancer susceptibility loci. Proc Natl Acad Sci U S A. 2007;104:8017–8022. doi: 10.1073/pnas.0702177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calin GA, Sevignani C, Dumitru CD, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 46.Nicoloso MS, Spizzo R, Shimizu M, et al. MicroRNAs—the micro steering wheel of tumour metastases. Nat Rev Cancer. 2009;9:293–302. doi: 10.1038/nrc2619. [DOI] [PubMed] [Google Scholar]
- 47.Esquela-Kerscher A, Slack FJ. Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 48.Wu Y, Evers BM, Zhou BP. Small C-terminal domain phosphatase enhances snail activity through dephosphorylation. J Biol Chem. 2009;284:640–648. doi: 10.1074/jbc.M806916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Expression of enhanced green fluorescent protein (eGFP) in stably transduced cell lines. Immunofluorescent microscopy for eGFP was performed in 3 human cholangiocarcinoma cell lines (ie, CCLP1, SG231, and HuCCT) stably transduced with the miR-26a1 lentivirus (L/miR-26a1, in which the eGFP and has-miR-26a1 genes were fused and controlled by CMV promoter). (A, B, C) L/miR-26a1–transduced cells; (D, E, F) noninfected control cells.
Supplementary Figure 2. Cholangiocarcinoma cells with or without stable transduction of control lentivirus. (A) qRT-PCR analysis for miR-26a and GSK3β mRNAs in cholangiocarcinoma cells with or without scramble control lentivirus transduction. Data are shown as mean ± standard error of mean (SEM) from 3 experiments. (B) Representative Western blots showing the protein levels of GSK-3β, β-catenin, and phosphorylated β-catenin in cholangiocarcinoma cells with or without scramble control lentivirus transduction. (C) TCF/LEF luciferase activity assay. Cholangiocarcinoma cells with or without scramble control lentivirus infection were transfected with the TCF/LEF luciferase reporter plasmid. The cell lysates were obtained 24 h after transfection to measure the luciferase activity by using a luminometer. Renilla luciferase expression plasmid pRL-TK was used as internal control. Data are presented as mean ± SEM from 3 independent experiments. (D) Cell proliferation assay. Cholangiocarcinoma cells with or without scramble control lentivirus infection were analyzed by the WST-1 assay. All cells were cultured in OPTI-MEM medium (Invitrogen) with 2% fetal bovine serum unless otherwise indicated. Three hours after WST-1 reagent incubation, the absorption was measured by an enzyme-linked immunosorbent assay reader under wavelength of 450 nm. Data are presented as mean ± SEM from 3 independent experiments. (E) Colonogenic assay in cholangiocarcinoma cells with or without scramble control lentivirus infection. One thousands cells were plated in 10-cm dishes and cultured for 10 to 14 days. The colonies were stained with 0.1% crystal violet.
Supplementary Figure 3. Representative immunofluorescence staining for β-catenin in CCLP1 cells transduced with miR-26a1 or control lentivirus. miR-26a–overexpressed cells show more β-catenin accumulation in the nuclei compared to the miRNA-scramble control cells.
Supplementary Figure 4. miR-26a expression in xenograft tumor tissues. In situ hybridizations for miR-26a and U6 small nuclear RNA were performed by using locked nucleic acid probe as described in the Materials and Methods section. Locked nucleic acid miRNA-scramble probe was used as the negative control.
Supplementary Figure 5. The effect of miR-26a inhibition. SG231 and CCLP1 cells were stably transduced with the lentivirus expressing miR-26a–specific inhibitor (L/26a in) or the scramble control lentivirus vector (L/control). Cells without viral vector transduction were used as an additional control. (A) The level of miR-26a in cultured cells as determined by qRT-PCR analysis. The level of miR-26a in noninfected cells was set as 1.0. Data are presented as mean ± standard error of mean (SEM) from 3 experiments. (B) Representative Western blots for GSK-3β in cultured cells (with β-actin as the loading control). (C–E) The effect of miR-26a inhibitor on cholangiocarcinoma growth in SCID mice. miR-26a inhibitor expressed, vector control, or noninfected CCLP1 and SG231 cells (5 × 106 for CCLP1, 2 × 106 for SG231) were inoculated subcutaneously into SCID mice (n = 6), and the mice were closely monitored for tumor growth. The mice were sacrificed 35 (for CCLP1) or 45 days (for SG231) post inoculation to recover the tumors. (C) The growth curves of xenograft tumors (data represent mean ± standard deviation [SD] from 6 SCID mice). (D) Photography of xenograft tumors recovered from SCID mice. (E) The weight of xenograft tumors (data represent mean ± SD from 6 SCID mice).
Supplementary Figure 6. Wound-healing assay. (A) Representative photographs from CCLP1 cells stably transduced with the miR-26a1 lentivirus (L/miR-26a1) and the control lentivirus (L/control) taken at 0, 8, and 24 h after scratch. (B) The mean wound healing rate for miR-26a–overexpressed and control cells at 0, 8, and 24-h time points.
Supplementary Table 1. miR-26a Level in Human Cholangiocarcinoma Tissues.
NOTE. P < .001, Wilcoxon signed-ranks test(2-tailed).